Abstract
Background
There is evidence that post-training exposure to nicotine, cocaine, and their conditioned stimuli (CS), enhance memory consolidation in rats. The present study assessed the effects of blocking noradrenergic and dopaminergic receptors on nicotine and cocaine unconditioned and conditioned memory modulation.
Methods
Males Sprague–Dawley rats tested on the spontaneous object recognition task received post-sample exposure to 0.4 mg/kg nicotine, 20 mg/kg cocaine, or their CSs, in combination with 5–10 mg/kg propranolol (PRO; beta-adrenergic antagonist) or 0.2–0.6 mg/kg pimozide (PIM; dopamine D2 receptor antagonist). The CSs were established by confining rats in a chamber (the CS +) after injections of 0.4 mg/kg nicotine, or 20 mg/kg cocaine, for 2 h and in another chamber (the CS −) after injections of vehicle, repeated over 10 days (5 drug/CS + and 5 vehicle/CS − pairings in total). Object memory was tested 72 h post sample in drug-free animals.
Results
Co-administration of PRO or PIM blocked the memory-enhancing effects of post-training injections of nicotine, cocaine, and, importantly, exposure to their CSs.
Conclusions
These data suggest that nicotine, cocaine as well as their conditioned stimuli share actions on overlapping noradrenergic and dopaminergic systems to modulate memory consolidation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is evidence that post-training administration of nicotine and cocaine enhance memory consolidation; a neural process of memory stabilization (McGaugh 2000; Melichercik et al. 2012; Rkieh et al. 2014; White 2002). Recently, we also reported that conditioned stimuli (CSs) paired with the effects of nicotine and cocaine have very similar effects on memory consolidation. Thus, using the spontaneous object recognition task (OR), rats that were exposed to contextual nicotine or cocaine CSs following the sample phase of OR displayed enhanced object memory when tested 72 h later (Wolter et al. 2019). It is well known that drug-paired CSs generate emotional, cognitive, and physiological responses which promote drug-seeking and -taking behaviors (Deroche-Gamonet et al. 2003; Tessari et al. 2007). For example, drug-free exposure to these CSs can enhance operant responding (Rescorla and Solomon 1975; Tunstall and Kearns 2017), attract animals to drug-associated contexts in place conditioning (for review, see Tzschentke 1998), and mimic other behavioral responses such as conditioned locomotion (Baidoo et al. 2020; Brown et al. 1992; Wolter et al. 2019, 2020). The current question of interest is whether drug CSs activate the same neurochemical systems of memory modulation that are directly stimulated by the drugs themselves.
One of these is the noradrenergic (NA) system. It is well known that emotional experiences are better remembered (Cahill et al. 1994; Kobayashi and Yasoshima 2001), and there is extensive experimental evidence in various species that fear, emotional arousal, and epinephrine enhance memory consolidation (Holahan and White 2002, 2004; Liang et al. 1990; McGaugh 2013) and that their effects can be reversed by propranolol (PRO), a beta-adrenergic antagonist (Cahill et al. 2000; McGaugh 2013; Roozendaal et al. 2008; Wolter et al. 2020). Moreover, nicotine and cocaine elevate levels of NA in several regions involved in memory functions such as the hippocampus, amygdala, striatum, and the nucleus accumbens (Arqueros et al. 1978; Brazell et al. 1991; Florin et al. 1994; Fu et al. 2003; Mitchell et al. 1989; Verheij et al. 2014). Although the neurochemical systems activated by nicotine or cocaine CSs during memory consolidation have not been systemically explored yet, there is evidence that the basolateral amygdala (BLA) is required for the establishment and expression of responses to drug CSs (Hsu et al. 2002) and that BLA NA mediates the facilitation of memory consolidation by fear CSs (Goode et al. 2016; Holahan and White 2002, 2004).
Dopamine (DA) is also likely to be involved in conditioned modulation of memory consolidation. A number of studies have found that both D1-like (D1 and D5) and D2-like (D2, D3, and D4) receptors (Mishra et al. 2018) modulate memory encoding and consolidation (Castellano et al. 1994; de Lima et al. 2011; Keshavarzian et al. 2018; Rossato et al. 2013; Yamasaki and Takeuchi 2017). Furthermore, stimuli known to enhance DA, such as exposure to novelty, optogenetic stimulation of ventral tegmental area (VTA) DA neurons, and infusions of DA agonists into the amygdala and medial pre-frontal cortex, all enhance memory consolidation (Duszkiewicz et al. 2019; Kim et al. 2012; Lisman and Grace 2005; Rossato et al. 2013; Tang et al. 2020). Finally, both nicotine and cocaine enhance DA levels in limbic structures involved in memory formation, although via different mechanisms (Bocklisch et al. 2013; Dani and Bertrand 2007; Hadjiconstantinou and Neff 2011; Rossi et al. 2005).
Therefore, the current study explored the roles of NA and DA receptors in the unconditioned and conditioned effects of cocaine and nicotine on consolidation of object recognition (OR) memory. OR is based on the natural tendency of rats to explore novel objects (Ennaceur and Delacour 1988; Winters et al. 2004), and it was selected because of our previous demonstration that object memory 72 h after sample exposure is significantly improved by post-training administration of cocaine (Rkieh et al. 2014) and other drugs (Baidoo et al. 2020; Wolter et al. 2019, 2020). PRO was selected because the beta-noradrenergic receptors have been implicated in memory consolidation by various laboratories (Cahill et al. 1994, 2000; Villain et al. 2016; Wolter et al. 2020). Also, our group has demonstrated that PRO blocked the enhancement of object memory consolidation induced by exposure to a heroin-paired CS (Wolter et al., 2020). Finally, we began our investigation of DA receptors involvement with the D2 receptor antagonist pimozide (PIM) because D2-like receptors have been implicated in the reinforcing effects of drugs on behavior, conditioned drug responses, and drug’s effects on learning and memory (Beninger and Phillips 1980; Castellano et al. 1994; Horvitz and Ettenberg 1991; Introini-Collison and Baratti 1986; White and Major 1978).
Materials and Methods
Subjects
A total of 113 male Sprague–Dawley rats (Charles River, Quebec, Canada) weighing between 225 and 250 g at the beginning of the experiments were individually housed in standard rat cages (polycarbonate; 50.5 cm × 48.5 cm × 20 cm) with standard environmental enrichment, and were maintained on a reverse light–dark schedule (lights off at 07:00; on at 19:00). All testing and injections were performed during their dark period. Rats had access to ~ 25 g per day of standard rat chow, and water was available ad libitum in home cages. All procedures adhered to the guidelines of the Canadian Council on Animal Care and were approved by the University of Guelph Animal Care Committee.
Apparatus
Conditioning chambers
The chambers (30 cm × 40 cm × 26 cm) used for contextual CS conditioning were made of semi-transparent Plexiglas (University of Guelph, ON, Canada), differed in visual (half of the chambers had vertical black and white stripes and the other half had a checkered pattern) and tactual (half of the chambers included a ceramic tile on the floor) cues, and were covered by black wire mesh to enable automatic video tracking (EthoVision v11.5; Noldus, The Netherlands).
Spontaneous object recognition (OR) task
This memory task is based on the natural tendency of rats to explore novel objects (Ennaceur and Delacour 1988; Winters et al. 2004) and was selected because of our previous demonstration that recognition of objects 72 h after sample exposure is improved by post-sample cocaine, nicotine, or exposure to cocaine- or nicotine-contextual CSs (Wolter et al. 2019). The Y-apparatus used for OR has been described previously by Winters et al. (2004). The objects used were of varying sizes, tactile qualities, visual qualities, shape, and height. On each object recognition trial, the rats experienced a new set of never-before-seen objects.
Procedures
Experiment 1
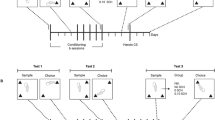
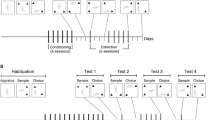
A group of 12 rats was used to assess the effect of immediate post-training 0.4 mg/kg nicotine and co-administration with 0, 5 or 10 mg/kg PRO. The rats were first habituated to the Y-apparatus for 5 min on two consecutive days 24 h prior to testing. Each OR trial consisted of two phases: a sample phase and a choice phase, separated by a 72-h retention interval. This retention interval was chosen as a “suboptimal” condition in which drug-naïve rats do not express a memory (Melichercik et al. 2012; Rkieh et al. 2014; Wolter et al. 2019, 2020).
During the sample phase, two identical novel objects were placed into the Y-apparatus at the end of each arm. The rats were placed in the start box, and the guillotine door was opened. Exploration during the sample phase was restricted to 25 s of total exploration (sum of exploration times of both objects) or if 180 s had elapsed, whichever came first. If animals failed to explore objects during the sample phase, they were removed from the experiment. Object exploration was defined as directing the nose to the object at < 2 cm and/or touching the object with the nose. The rats were immediately injected after the conclusion of the sample phase with vehicle, 0.4 mg/kg nicotine or nicotine combined with 5 or 10 mg/kg PRO. All animals were tested at each dose of nicotine and PRO co-administration, and the order of doses was counterbalanced using a Latin Square Design. Following the 72-h retention interval, the rats experienced the choice phase; the Y-apparatus contained a copy of the original sample object in one arm and a novel object in the other. The choice pairs, the novel side, as well as the designated sample and novel objects were counterbalanced. Here, it should be noted that a “delay” control group exposed to nicotine, cocaine, or their CSs 6 h after the sample phase was not included because these data have already been published in Wolter et al. (2019).
A separate group of 16 rats was used to assess the effect of 10 mg/kg PRO on post-training exposure to compartments previously paired with 0.4 mg/kg nicotine in the CS + . All rats were habituated to two conditioning chambers (vehicle in the CS − and 0.4 mg/kg nicotine in the CS +) for 30 min, 24 h prior to the beginning of conditioning. At the beginning of conditioning, rats received either vehicle or 0.4 mg/kg nicotine and were immediately placed in the CS − or CS + chamber for 2 h, respectively. The chambers of the apparatus used as CS − and CS + were counterbalanced across rats. All rats received a total of 5 conditioning sessions in the CS − and 5 conditioning sessions in the CS + , alternating over 10 successive days. The rats were also habituated to the Y-apparatus on days 9 and 10 of conditioning and were exposed to the sample phase prior to the first test of conditioned locomotion on day 11. Conditioned locomotion was assessed on four separate tests. The first test occurred the day after the last conditioning session and half of the animals were placed in the CS − and the other half in the CS + . The second test occurred 72 h later and the same animals were tested in the alternate chamber. The final two tests followed the same testing conditions, but the rats were injected with 10 mg/kg PRO prior to exposure to the CS − and the CS + .
Experiment 2
A group of eight rats was used to assess the effect of immediate post-training 0.4 mg/kg nicotine and co-administration with 0, 0.2 or 0.6 mg/kg PIM on object recognition memory. The OR experimental procedures used in this experiment were the same as in experiment 1. Another group of 12 rats was included in this experiment to assess the effect of immediate post-training 0.2 mg/kg PIM on OR memory using a 24-h retention interval. A 24-h retention interval has been established as a sufficiently short interval at which normal rats perform OR successfully when tested in a Y-apparatus (Winters et al. 2004, 2008; Wolter et al. 2020). Therefore, this group was included as a control to verify whether post-training PIM could block object memory. An assessment of PRO alone using a 24-h delay was not included in this study because it was tested by Wolter et al. (2020) and was not found to impact OR memory.
A separate group of 12 rats was used to assess the effect of immediate 0.2 mg/kg PIM on post-training exposure to the CS + paired with 0.4 mg/kg nicotine, as described in experiment 1.
Experiment 3
A group of 12 rats was used to assess the effect of immediate post-training 20 mg/kg cocaine and co-administration with 0, 5 or 10 mg/kg PRO. The OR experimental procedures used in this experiment were the same as in experiment 1. A separate group of 12 rats was used to assess the effect of immediate 10 mg/kg PRO on post-training exposure to the CS + paired with 20 mg/kg cocaine as in experiment 1.
Experiment 4
A group of 17 rats was used to assess the effect of immediate post-training 20 mg/kg cocaine and co-administration with 0, 0.2 and 0.6 mg/kg PIM using a 72-h retention interval. The OR experimental procedures used in this experiment were the same as in experiment 1.
A separate group of 12 rats was used to assess the effect of immediate 0.2 mg/kg PIM on post-training exposure to the CS + paired with 20 mg/kg cocaine as in experiment 1.
Drugs
All drugs were injected intraperitoneally (IP). Vehicle (sterile 0.9% saline or 6 mg/ml tartaric acid) was administered at 1 ml/kg. Nicotine hydrogen tartrate salt at 0.4 mg/kg (Sigma) and cocaine hydrochloride at 20 mg/kg (Dumex, Toronto, ON, Canada) were dissolved in sterile 0.9% physiological saline. The doses of these two drugs were selected because of their known stimulatory properties (Zavala et al. 2008) and their facilitatory effects on object recognition memory consolidation (Melichercik et al. 2012; Rkieh et al. 2014). Propranolol hydrochloride (PRO) at 5 and 10 mg/kg (Sigma Aldrich) was dissolved in 0.9% physiological saline. The range of doses of PRO were selected on the basis of previous memory consolidation studies (Cahill et al. 1994; Lee and Ma 1995; McGaugh 2004). Pimozide (PIM) at 0.2 and 0.6 mg/kg was dissolved in 6 mg/ml tartaric acid and injected at a volume of 1 ml/kg. This range of doses was selected on the basis of place conditioning and memory consolidation studies (Blackburn et al. 1987; Ichihara et al. 1989; White and Major 1978).
Data analysis
The discrimination ratio (DR) is a ratio of object preference, where a score of 0 means the rat shows no preference between the two objects, a positive score indicates preference of the novel object, and a negative score indicates preference for the familiar object (Eq. (1)):
A sample DR was also calculated for the sample phase (Eq. (2)):
to rule out exploration preferences in the Y-apparatus. Total object exploration was used as a control to rule out non-specific drug effects on object exploration. The choice DR and total object exploration in each phase were analyzed using a repeated measures one-way ANOVA and Student–Newman–Keuls post hoc analyses to probe for significant main effects within choice DRs for the acute cocaine and nicotine experiments. Paired sample t tests were performed to assess the choice DRs within the nicotine and cocaine contextual CS experiments. In addition, paired-sample t tests were used to compare sample and choice DRs in each condition of an experiment, a DR of 0 in the sample phase is expected when two identical objects are equally novel. Hence, a significant difference between the sample and choice phase DR indicates discrimination between the familiar and novel objects in the choice phase and is interpreted as an intact memory. All statistical analyses were performed using SigmaPlot (v.12.5; Systat Software), with an \({\alpha }\) = 0.05. A minimum exploration time was not employed in these calculations. The exact values of non-significant analyses are not reported.
Results
Experiment 1
Both 5 and 10 mg/kg PRO blocked the memory-enhancing effect of 0.4 mg/kg nicotine on object recognition memory. Figure 1A represents mean (SEM) DR calculated during the sample and choice phases of OR following immediate post-sample injections of 0.4 mg/kg nicotine co-administered with 0, 5, or 10 mg/kg PRO. The ANOVA was significant [F(2,35) = 5.09, P < 0.05] and post hoc comparisons indicated that when rats were injected with 0.4 mg/kg nicotine and 0 mg/kg PRO, their choice DRs were significantly higher than when nicotine was co-administered with 5 or 10 mg/kg PRO. This finding was confirmed by the planned comparisons between sample and choice DRs, which were significant only when rats received 0 mg/kg PRO [t(11) = 3.45, P < 0.01]. The analysis of total object exploration was non-significant (see Table 1).
A Mean (SEM) discrimination ratios from the sample and choice phases by the same rats (n = 12) following post-sample injections of 0.4 mg/kg nicotine co-administered with 0, 5, or 10 mg/kg PRO. The * denotes a significant difference compared to 0 mg/kg PRO choice phase discrimination ratio. The # denotes a significant difference of the choice phase DR compared to sample DR within dose. B Mean (SEM) discrimination ratios from the sample and choice phases of OR displayed by the same rats (n = 16) following injections of 0 mg/kg PRO or 10 mg/kg PRO prior to confinement to the CS + paired with 0.4 mg/kg nicotine. The * denotes a significant difference compared to 0 mg/kg PRO choice phase DR. The # denotes a significant difference compared to the sample phase DR
PRO at 10 mg/kg also blocked the effects of the CS + previously paired with 0.4 mg/kg nicotine on object recognition memory. Figure 1B represents mean (SEM) discrimination ratio calculated during the sample and choice phase of OR following post-sample confinement to the nicotine CS + . The t test on choice DRs was significant [t(15) = − 3.95, P < 0.01] indicating that the mean choice DR was higher when the rats were injected with 0 mg/kg PRO than when they were injected with 10 mg/kg PRO. Planned comparisons between the sample and choice phase DRs were significant [t(15) = − 3.00, P < 0.01] only when rats were injected with 0 mg/kg PRO. The analysis of total object exploration was non-significant (see Table 1).
Experiment 2
Both 0.2 and 0.6 mg/kg PIM blocked the effects of 0.4 mg/kg nicotine on object recognition memory. Figure 2A represents mean (SEM) discrimination ratio calculated during the sample and choice phases of OR following immediate post-sample injections of 0.4 mg/kg nicotine co-administered with 0, 0.2, or 0.6 mg/kg PIM. The ANOVA of the choice DRs was significant [F(2,23) = 3.90, P < 0.05] and post hoc comparisons indicated that rats had higher choice DRs when they received 0 in comparison to 0.2 or 0.6 mg/kg PIM. Furthermore, planned comparisons between the sample and choice phase DRs were significant [t(7) = − 3.16, P < 0.05] only when rats received 0 mg/kg PIM. The analysis of total object exploration was non-significant (data not shown).
A Mean (SEM) discrimination ratios from the sample and choice phases by the same rats (n = 8) following post-sample injections of 0.4 mg/kg nicotine co-administered with 0, 0.2, or 0.6 mg/kg PIM. The # denotes a significant difference compared to sample DR within dose. B Mean (SEM) discrimination ratios from the sample and choice phases of OR displayed by the same rats (n = 12) following injections of 0 or 0.2 mg/kg PIM prior to confinement into the CS + paired with 0.4 mg/kg nicotine. The * denotes a significant difference compared to 0 mg/kg PIM choice phase DR. The # denotes a significant difference of the choice phase DR compared to the sample phase DR
PIM at 0.2 mg/kg also blocked the effect of the CS + previously paired with 0.4 mg/kg nicotine on object recognition memory. Figure 2B represents mean (SEM) discrimination ratio calculated during the sample and choice phases of OR following post-sample confinement into the CS + . The analysis was significant [t(11) = 4.93, P < 0.01] indicating that choice DRs were higher when rats were injected with 0 mg/kg PIM compared to 0.2 mg/kg PIM prior to confinement into the CS + . Further, planned comparisons between sample and choice DRs were significant [t(11) = − 3.01, P < 0.05] only when rats were injected with 0 mg/kg PIM. The analysis of total object exploration was significant [t(11) = 5.43, P < 0.01] during the choice phase indicating that rats injected with 0.2 mg/kg PIM in the CS + explored objects less than when they were injected with 0 mg/kg PIM (see Table 1).
The acute post-sample administration of 0.2 mg/kg PIM did not alter 24-h DRs. The comparison between sample and choice DRs was significant [t(9) = − 3.61, P < 0.01] indicating that when rats were injected with 0.2 mg/kg pimozide post-training and assessed after a 24-h retention interval (n = 12), their choice DRs (M = 0.42, SEM = 0.08) were higher than their sample DRs (M = 0.05, SEM = 0.14).
Experiment 3
Both 5 and 10 mg/kg PRO blocked the effect of 20 mg/kg cocaine on object recognition memory. Figure 3A represents mean (SEM) discrimination ratio calculated during the sample and choice phases of OR following immediate post-sample injections of 20 mg/kg cocaine co-administered with 0, 5, or 10 mg/kg PRO. The ANOVA was significant [F(2,35) = 14.01, P < 0.01] and post hoc comparisons further indicated that rats co-administered with 0 mg/kg PRO had higher choice DRs than when they were injected with 5 or 10 mg/kg PRO. Further, planned comparisons between the sample and choice phase DRs were significant [t(11) = − 6.11, P < 0.01] only when rats were injected with 0 mg/kg PRO. The analysis of total object exploration was non-significant (see Table 2).
A Mean (SEM) discrimination ratios from the sample and choice phases by the same rats (n = 12) following post-sample injections of 20 mg/kg cocaine co-administered with 0, 5, or 10 mg/kg PRO. The * denotes a significant difference compared to 0 mg/kg PRO choice phase discrimination ratio. The # denotes a significant difference of the choice phase DR compared to sample DR within dose. B Mean (SEM) discrimination ratios from the sample and choice phases of OR displayed by the same rats (n = 12) following injections of 0 or 10 mg/kg PRO prior to confinement to the CS + paired with 20 mg/kg cocaine. The # denotes a significant difference of the choice phase DR compared to the sample phase DR
PRO at 10 mg/kg also blocked the effects of the CS + previously paired with 20 mg/kg cocaine on object recognition memory. Figure 3B represents mean (SEM) discrimination ratio calculated during the sample and choice phase of OR following post-sample confinement in the CS + . Although the paired-samples t test was non-significant, planned comparisons between the sample and choice phase DRs were significant [t(11) = − 3.16, P < 0.01] only when rats were injected with 0 mg/kg PRO. The analysis of total object exploration was non-significant (see Table 2).
Experiment 4
Both 0.2 and 0.6 mg/kg PIM blocked the memory-enhancing effect of 20 mg/kg cocaine on object recognition memory. Figure 4A represents mean (SEM) discrimination ratio calculated during the sample and choice phases of OR following immediate post-sample injections of 20 mg/kg cocaine co-administered with 0, 0.2, or 0.6 mg/kg PIM. The ANOVA was significant [F(2,32) = 14.89, P < 0.01] and post hoc comparisons indicated that rats injected with 0 mg/kg PIM had higher choice DRs compared to when the same rats were co-administered with 0.2 or 0.6 mg/kg PIM post-training. Furthermore, planned comparisons between sample and choice DRs indicated that rats had significantly higher [t(11) = − 3.16, P < 0.01] choice DRs compared to sample when they were injected with 0 mg/kg PIM. The analysis of total object exploration was non-significant (see Table 2).
A Mean (SEM) discrimination ratios from the sample and choice phases by the same rats (n = 17) following post-sample injections of 20 mg/kg cocaine co-administered with 0, 0.2, or 0.6 mg/kg PIM. The # denotes a significant difference compared to sample DR within dose. B Mean (SEM) discrimination ratios from the sample and choice phases of OR displayed by the same rats (n = 12) following injections of 0 or 0.2 mg/kg PIM prior to confinement to the CS + previously paired with 20 mg/kg cocaine. The * denotes a significant difference compared to 0 mg/kg PIM choice phase DR. The # denotes a significant difference of the choice phase DR compared to the sample phase DR
PIM at 0.2 mg/kg also blocked the effects of the cocaine CS + on object recognition memory. Figure 4B represents the mean (SEM) discrimination ratio calculated during the sample and choice phases of OR following immediate post-sample confinement into the CS + . The t test of choice DRs was significant [t(11) = 2.77, P < 0.05] indicating that choice DRs were higher when rats were injected with 0 mg/kg PIM than when the same rats were injected with 0.2 mg/kg PIM. Further, planned comparisons between the sample and choice phase DRs were significant [t(11) = − 3.71, P < 0.01] indicating that when rats were injected with 0 mg/kg PIM prior to confinement in the CS + their choice DR was higher than the sample DR. The analysis of total object exploration was significant [t(11) = 3.32, P < 0.01] during the choice phase indicating that rats explored objects significantly less when they had been injected with 0.2 mg/kg PIM prior to confinement into the CS + (see Table 2).
Discussion
The present study assessed the effects of blocking noradrenergic and dopaminergic receptors on nicotine and cocaine unconditioned and conditioned memory modulation. The nicotine and cocaine contextual conditioned stimuli (CS +) were established by confining rats for 2 h in a chamber after injections of 0.4 mg/kg nicotine or 20 mg/kg cocaine. The effects on memory consolidation were evaluated by injecting rats with either nicotine or cocaine, or by exposing them to the drug CSs, post-sample during the object recognition task. It was found that co-administration of propranolol (PRO) and pimozide (PIM) blocked the enhancement of discrimination ratios induced by post-sample administration of nicotine, cocaine, or exposure to their contextual CSs. These data suggest that the memory-enhancing effects of nicotine and cocaine and their conditioned stimuli share actions on adrenergic and dopaminergic systems of memory consolidation.
The first set of experiments replicated the findings reported by Wolter et al. (2019) in which nicotine, cocaine, and exposure to their contextual CSs enhanced choice phase discrimination ratios in rats. Importantly, these are within-subjects experiments which control for non-specific effects that the drugs, or the exposure to the drug CSs, may have on memory. Importantly, the memory-enhancing effects of nicotine, cocaine, and their CSs, were all blocked by post-sample injections of the beta-NA receptor antagonist PRO. This result is interpreted as a blockade of the enhancement of memory consolidation by cocaine, nicotine, and their contextual CSs, as we have previously reported that this dose of PRO has no effect on 24-h retention intervals (Wolter et al. 2020). However, it should also be acknowledged that other studies have found different results with PRO that may be dependent on the dose, injection method or infusion site, test conditions as well as testing apparatus (open field vs. Y-apparatus) (Roozendaal et al. 2008; Winters et al. 2004).
The second set of experiments explored the role of the dopamine D2 receptor using PIM. Similar to the results above, PIM blocked the enhancement of choice DRs induced by post-training nicotine, cocaine, and exposure to their contextual CSs. Interestingly, PIM also altered total object exploration of choice discrimination ratios in the CS + (see Tables 1 and 2); however, it is unlikely that this reduction affected memory because post-training injections of PIM did not alter choice DRs when evaluated with a 24-h retention interval, indicating that the post-training effect of PIM were selective to unconditioned and conditioned enhancement of memory consolidation.
Although our experiments did not explore the central site of action of PRO and PIM in modulating nicotine, cocaine, and their CSs on memory consolidation, there is substantial evidence pointing to an involvement of the BLA, the hippocampus (HPC), and the perirhinal cortex (PRh). In fact, both nicotine and cocaine self-administration enhance NA and DA in the BLA (Di Ciano and Everitt 2004; Fu et al. 2003), and exposure to nicotine and cocaine CSs have very similar effects (Fotros et al. 2013; Khaled et al. 2014; Sharp 2019). Furthermore, the BLA is involved in memory enhancement induced by nicotine and cocaine (Barros et al. 2005; Cestari et al. 1996), NA and DA agonists and antagonists infused into the BLA impact memory consolidation (Castellano et al. 1991; Ferry et al. 1999; Gibbs et al. 2010; Heath et al. 2015; McGaugh and Roozendaal 2002; Roozendaal et al. 1999, 2002; Stern and Alberini 2013), and the BLA is involved in memory enhancement by emotional CSs via NA mechanisms (Goode et al. 2016; Holahan and White 2004). The HPC is known to be involved in the consolidation of drug-related memories (Kutlu and Gould 2016; Melichercik et al. 2012) through afferents from the NA locus coeruleus and mesolimbic DA system (Hansen 2017; Koch et al. 2011; Lisman and Grace 2005; Lodge and Grace 2008), and injections of nicotine or cocaine enhance levels of NA and DA in the HPC (Fitzgerald 2013; Fotros et al. 2013; Kramar et al. 2014; Placzek et al. 2009; Rossi et al. 2005). Moreover, inactivation of the HPC impairs responses to drug CSs (Atkins et al. 2012; Fuchs et al. 2005; Kutlu and Gould 2016). Finally, the PRh is required for the consolidation of object memories (Winters et al. 2004) and although cholinergic and glutamatergic systems regulate PRh-dependent memories (Brophey and Raptis 2003; Melichercik et al. 2012; Winters and Bussey 2005), modulations of its efferents from the mesolimbic system, locus coeruleus, and the BLA have also been reported to alter memory (Albasser et al. 2015; Balderas et al. 2013; Holmes et al. 2013; Laing and Bashir 2014).
In conclusion, this study expands upon the hypothesis of White (1996) and the findings of Wolter et al. (2019) suggesting that psychomotor stimulants such as cocaine and nicotine share overlapping neurochemical systems with their contextual CSs to enhance memory consolidation. Although this study only employed two relatively non-selective compounds at a limited range of doses, and did not investigate central sites of action, it does provide evidence to justify exploration of how visual/tactual/olfactory conditioned environmental stimuli gain the ability to mimic the actions of pharmacological stimuli on cognitive processes. Furthermore, this data suggest the possibility that drug CSs may not only perpetuate addiction-like behaviors by causing drug-like or drug-opposite responses (Stewart et al. 1984), but they also can have cognitive effects on memory that could play a role in perpetuating the maintenance addictive behaviors by enhancing the consolidation of memories linked to drug-seeking and -taking.
References
Albasser MM, Olarte-Sánchez CM, Amin E et al (2015) Perirhinal cortex lesions in rats: novelty detection and sensitivity to interference. Behav Neurosci 129(3):227–243. https://doi.org/10.1037/bne0000049
Arqueros L, Naquira D, Zunino E (1978) Nicotine-induced release of catecholamines from rat hippocampus and striatum. Biochem Pharmacol 27(23):2667–2674. https://doi.org/10.1016/0006-2952(78)90040-0
Atkins A, Mashoon Y, Kantak KM (2012) Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Bone 23(1):1–7. https://doi.org/10.1038/jid.2014.371
Baidoo N, Wolter M, Holahan MR, et al. (2020) The effects of morphine withdrawal and conditioned withdrawal on memory consolidation and c-Fos expression in the central amygdala. Addiction Biology (January 2020): 1–11. DOI: https://doi.org/10.1111/adb.12909
Balderas I, Moreno-Castilla P, Bermudez-Rattoni F (2013) Dopamine D1 receptor activity modulates object recognition memory consolidation in the perirhinal cortex but not in the hippocampus. Hippocampus 23(10):873–878. https://doi.org/10.1002/hipo.22143
Barros DM, Ramirez MR, Izquierdo I (2005) Modulation of working, short- and long-term memory by nicotinic receptors in the basolateral amygdala in rats. Neurobiol Learn Mem 83(2):113–118. https://doi.org/10.1016/j.nlm.2004.10.001
Beninger RJ, Phillips AG (1980) The effect of pimozide on the establishment of conditioned reinforcement. Psychopharmacology 153:147–153
Blackburn JR, Phillips AG, Fibiger HC (1987) Dopamine and preparatory behavior: I. Effects of Pimozide 101(3):352–360
Bocklisch C, Pascoli V, Wong JCY et al (2013) Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area christina. Science 341:1521–1526. https://doi.org/10.1126/science.1142365
Brazell MP, Mitchell SN, Gray JA (1991) Effect of acute administration of nicotine on in vivo release of noradrenaline in the hippocampus of freely moving rats: a dose-response and antagonist study. Neuropharmacology 30(8):823–833. https://doi.org/10.1016/0028-3908(91)90116-S
Brophey A, Raptis H (2003) Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron 38:237–252
Brown EE, George S, Robertson S et al (1992) Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci 12(10):4112–4121
Cahill L, Prins B, Weber M et al (1994) β-Adrenergic activation and memory for emotional events. Nature. https://doi.org/10.1038/371702a0
Cahill L, Pham CA, Setlow B (2000) Impaired memory consolidation in rats produced with β-adrenergic blockade. Neurobiol Learn Mem 74(3):259–266. https://doi.org/10.1006/nlme.1999.3950
Castellano C, Cestari V, Cabib S et al (1991) Post-training dopamine receptor agonists and antagonists affect memory storage in mice irrespective of their selectivity for D1 or D2 receptors. Behav Neural Biol 56(3):283–291. https://doi.org/10.1016/0163-1047(91)90439-W
Castellano C, Cestari V, Cabib S et al (1994) The effects of morphine on memory consolidation in mice involve both D1 and D2 dopamine receptors. Behav Neural Biol 61(2):156–161. https://doi.org/10.1016/S0163-1047(05)80069-X
Cestari V, Mele A, Oliverio A et al (1996) Amygdala lesions block the effect of cocaine on memory in mice. Brain Res 713(1–2):286–289
Dani JA, Bertrand D (2007) Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47(1):699–729. https://doi.org/10.1146/annurev.pharmtox.47.120505.105214
de Lima MNM, Presti-Torres J, Dornelles A et al (2011) Modulatory influence of dopamine receptors on consolidation of object recognition memory. Neurobiol Learn Mem 95(3):305–310. https://doi.org/10.1016/j.nlm.2010.12.007
Deroche-Gamonet V, Martinez A, Le Moal M et al (2003) Relationships between individual sensitivity to CS- and cocaine-induced reinstatement in the rat. Psychopharmacology 168(1–2):201–207. https://doi.org/10.1007/s00213-002-1306-9
Di Ciano P, Everitt BJ (2004) Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci 24(32):7167–7173. https://doi.org/10.1523/JNEUROSCI.1581-04.2004
Duszkiewicz AJ, Mcnamara CG, Takeuchi T, et al. (2019) Novelty and dopaminergic modulation of memory persistence: a tale of two systems. Trends Neurosci 42(2). Elsevier Ltd: 102–114. https://doi.org/10.1016/j.tins.2018.10.002
Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31:47–59. https://doi.org/10.1016/S0166-4328(05)80315-8
Ferry B, Roozendaal B, McGaugh JL (1999) Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between β- and α1-adrenoceptors. J Neurosci. https://doi.org/10.1523/jneurosci.19-12-05119.1999
Fitzgerald PJ (2013) Elevated norepinephrine may be a unifying etiological factor in the abuse of a broad range of substances: alcohol, nicotine, marijuana, heroin, cocaine, and caffeine. Subst Abuse 7:171–183. https://doi.org/10.4137/SART.S13019
Florin SM, Kuczenski R, Segal DS (1994) Regional extracellular norepinephrine responses to amphetamine and cocaine and effects of clonidine pretreatment. Brain Res 654(1):53–62. https://doi.org/10.1016/0006-8993(94)91570-9
Fotros A, Casey KF, Larcher K, et al. (2013) Cocaine cue-induced dopamine release in amygdala and hippocampus: a high-resolution PET 18Fallypride study in cocaine dependent participants. Neuropsychopharmacology 38(9). Nature Publishing Group: 1780–1788. https://doi.org/10.1038/npp.2013.77
Fu Y, Matta SG, Kane VB et al (2003) Norepinephrine release in amygdala of rats during chronic nicotine self-administration: an in vivo microdialysis study. Neuropharmacology 45(4):514–523. https://doi.org/10.1016/S0028-3908(03)00201-6
Fuchs RA, Evans KA, Ledford CC et al (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30(2):296–309. https://doi.org/10.1038/sj.npp.1300579
Gibbs ME, Hutchinson DS and Summers RJ (2010) Noradrenaline release in the locus coeruleus modulates memory formation and consolidation; roles for α- and β-adrenergic receptors. Neuroscience 170(4). Elsevier Inc.: 1209–1222. https://doi.org/10.2147/CIA.S118152
Goode TD, Leong KC, Goodman J, et al. (2016) Enhancement of striatum-dependent memory by conditioned fear is mediated by beta-adrenergic receptors in the basolateral amygdala. Neurobiology of Stress 3. Elsevier Inc: 74–82. https://doi.org/10.1016/j.ynstr.2016.02.004
Hadjiconstantinou M and Neff NH (2011) Nicotine and endogenous opioids: neurochemical and pharmacological evidence. Neuropharmacology 60(7–8). Elsevier Ltd: 1209–1220. https://doi.org/10.1016/j.neuropharm.2010.11.010.
Hansen N (2017) The longevity of hippocampus-dependent memory is orchestrated by the locus coeruleus–noradrenergic system. Neural Plasticity 2017. https://doi.org/10.1155/2017/2727602
Heath FC, Jurkus R, Bast T et al (2015) Dopamine D1-like receptor signalling in the hippocampus and amygdala modulates the acquisition of contextual fear conditioning. Psychopharmacology 232(14):2619–2629. https://doi.org/10.1007/s00213-015-3897-y
Holahan MR and White NM (2002) Conditioned memory modulation, freezing, and avoidance as measures of amygdala-mediated conditioned fear. 275: 250–275. https://doi.org/10.1006/nlme.2001.4012
Holahan MR, White NM (2004) Amygdala inactivation blocks expression of conditioned memory modulation and the promotion of avoidance and freezing. Behav Neurosci 118(1):24–35. https://doi.org/10.1037/0735-7044.118.1.24
Holmes NM, Parkes SL, Killcross AS et al (2013) The basolateral amygdala is critical for learning about neutral stimuli in the presence of danger, and the perirhinal cortex is critical in the absence of danger. J Neurosci 33(32):13112–13125. https://doi.org/10.1523/JNEUROSCI.1998-13.2013
Horvitz JC, Ettenberg A (1991) Conditioned incentive properties of a food-paired conditioned stimulus remain intact during dopamine receptor blockade. Behav Neurosci 105(4):536–541. https://doi.org/10.1037/0735-7044.105.4.536
Hsu EH, Schroeder JP, Packard MG (2002) The amygdala mediates memory consolidation for an amphetamine conditioned place preference. Behav Brain Res 129(1–2):93–100. https://doi.org/10.1016/S0166-4328(01)00376-X
Ichihara K, Nabeshima T, Kameyama T (1989) Differential effects of pimozide and SCH 23390 on acquisition of learning in mice. Eur J Pharmacol 164:189–195
Introini-Collison IB, Baratti CM (1986) Opioid peptidergic systems modulate the activity of β-adrenergic mechanisms during memory consolidation processes. Behav Neural Biol 46(2):227–241. https://doi.org/10.1016/S0163-1047(86)90710-7
Keshavarzian E, Ghasemzadeh Z and Rezayof A (2018) The basolateral amygdala dopaminergic system contributes to the improving effect of nicotine on stress-induced memory impairment in rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry 86(May). Elsevier: 30–35. https://doi.org/10.1016/j.pnpbp.2018.05.008
Khaled MATM, Pushparaj A, Di Ciano P, et al. (2014) Dopamine D3 receptors in the basolateral amygdala and the lateral habenula modulate cue-induced reinstatement of nicotine seeking. Neuropsychopharmacology 39(13). Nature Publishing Group: 3049–3058. https://doi.org/10.1038/npp.2014.158
Kim KM, Baratta MV, Yang A, et al. (2012) Optogenetic mimicry of the transient activation of dopamine neurons by natural reward is sufficient for operant reinforcement. PLoS ONE 7(4). https://doi.org/10.1371/journal.pone.0033612
Kobayashi K, Yasoshima Y (2001) The central noradrenaline system and memory consolidation. Neuroscientist 7(5):371–376. https://doi.org/10.1177/107385840100700506
Koch G, Esposito Z, Codecà C, et al. (2011) Altered dopamine modulation of LTD-like plasticity in Alzheimer’s disease patients. Clinical Neurophysiology 122(4). International Federation of Clinical Neurophysiology: 703–707. https://doi.org/10.1016/j.clinph.2010.10.033.
Kramar CP, Chefer VI, Wise RA, et al. (2014) Dopamine in the dorsal hippocampus impairs the late consolidation of cocaine-associated memory. Neuropsychopharmacology 39(7). Nature Publishing Group: 1645–1653. https://doi.org/10.1038/npp.2014.11
Kutlu MG, Gould TJ (2016) Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learn Mem 23(10):515–533. https://doi.org/10.1101/lm.042192.116
Laing M and Bashir ZI (2014) β-Adrenoceptors and synaptic plasticity in the perirhinal cortex. Neuroscience 273. IBRO: 163–173. https://doi.org/10.1016/j.neuroscience.2014.04.070
Lee EH, Ma YL (1995) Amphetamine enhances memory retention and facilitates norepinephrine release from the hippocampus in rats. Brain Res Bull 37(4):411–416
Liang KC, Mcgaugh JL and Yao H-Y (1990) Involvement of amygdala pathways in the influence of post-training intra-amygdala norepinephrine and peripheral epinephrine on memory storage. Brain Research. Available at: https://ac-els-cdn-com.subzero.lib.uoguelph.ca/0006899390904006/1-s2.0-0006899390904006-main.pdf?_tid=8aebb597-37a1-4b4c-8a80-ae7334baa68e&acdnat=1547578826_9e20f835a592a55a4eeb9e7af566a132 (accessed 15 January 2019)
Lisman JE, Grace AA (2005) The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46(5):703–713. https://doi.org/10.1016/j.neuron.2005.05.002
Lodge DJ, Grace AA (2008) Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci 28(31):7876–7882. https://doi.org/10.1523/JNEUROSCI.1582-08.2008
McGaugh JL (2000) Memory—a century of consolidation. Science 287(5451):248–251. https://doi.org/10.1126/science.287.5451.248
McGaugh JL (2004) The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27:1–28. https://doi.org/10.1146/annurev.neuro.27.070203.144157
McGaugh JL (2013) Making lasting memories: remembering the significant. Proc Natl Acad Sci USA 110(SUPPL2):10402–10407. https://doi.org/10.1073/pnas.1301209110
McGaugh JL, Roozendaal B (2002) Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol 12(2):205–210
Melichercik AM, Elliott KS, Bianchi C, et al. (2012) Nicotinic receptor activation in perirhinal cortex and hippocampus enhances object memory in rats. Neuropharmacology 62(5–6). Elsevier Ltd: 2096–2105. https://doi.org/10.1016/j.neuropharm.2012.01.008.
Mishra A, Singh S and Shukla S (2018) Physiological and functional basis of dopamine receptors and their role in neurogenesis: possible implication for Parkinson’s disease. J Exp Neurosci 12. https://doi.org/10.1177/1179069518779829
Mitchell SN, Brazell MP, Joseph MH et al (1989) Regionally specific effects of acute and chronic nicotine on rates of catecholamine and 5-hydroxytryptamine synthesis in rat brain. Eur J Pharmacol 167(3):311–322
Placzek AN, Zhang TA, Dani JA (2009) Nicotinic mechanisms influencing synaptic plasticity in the hippocampus. Acta Pharmacol Sin 30(6):752–760. https://doi.org/10.1038/aps.2009.39
Rescorla RA, Solomon RL (1975) Two process learning theory: relationships between Pavlovian conditioning and instrumental learning. Psychol Rev 74(3):151–182
Rkieh N, Cloke JM, Gallagher N et al (2014) Drugs of abuse as memory modulators: a study of cocaine in rats. Psychopharmacology 231(11):2339–2348. https://doi.org/10.1007/s00213-013-3390-4
Roozendaal B, Nguyen BT, Power AE et al (1999) Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc Natl Acad Sci 96(20):11642–11647. https://doi.org/10.1073/pnas.96.20.11642
Roozendaal B, Carmi O and McGaugh JL (2002) Adrenocortical suppression blocks the memory-enhancing effects of amphetamine and epinephrine. Proceedings of the National Academy of Sciences 93(4). National Academy of Sciences: 1429–1433. https://doi.org/10.1073/pnas.93.4.1429.
Roozendaal B, Castello NA, Vedana G, et al. (2008) Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiology of Learning and Memory 90(3). NIH Public Access: 576–579. https://doi.org/10.1016/j.nlm.2008.06.010.
Rossato JI, Radiske A, Kohler CA, et al. (2013) Consolidation of object recognition memory requires simultaneous activation of dopamine D1/D5 receptors in the amygdala and medial prefrontal cortex but not in the hippocampus. Neurobiology of Learning and Memory 106. Elsevier Inc.: 66–70. https://doi.org/10.1016/j.nlm.2013.07.012
Rossi S, Singer S, Shearman E et al (2005) The effects of cholinergic and dopaminergic antagonists on nicotine-induced cerebral neurotransmitter changes. Neurochem Res 30(4):541–558. https://doi.org/10.1007/s11064-005-2689-x
Sharp BM (2019) Basolateral amygdala, nicotinic cholinergic receptors, and nicotine: pharmacological effects and addiction in animal models and humans. Eur J Neurosci 50(3):2247–2254. https://doi.org/10.1111/ejn.13970
Stern SA, Alberini CM (2013) Mechanisms of memory enhancement. Wiley Interdiscip Rev Syst Biol Med 5(1):37–53. https://doi.org/10.1002/wsbm.1196
Stewart J, de Wit H, Eikelboom R (1984) Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev 91(2):251–268. https://doi.org/10.1037/0033-295X.91.2.251
Tang W, Kochubey O, Kintscher M et al (2020) A VTA to basal amygdala dopamine projection contributes to signal salient somatosensory events during fear learning. J Neurosci 40(20):3969–3980. https://doi.org/10.1523/JNEUROSCI.1796-19.2020
Tessari M, Catalano A, Pellitteri M et al (2007) Correlation between serum ghrelin levels and cocaine-seeking behaviour triggered by cocaine-associated conditioned stimuli in rats. Addict Biol 12(1):22–29. https://doi.org/10.1111/j.1369-1600.2007.00052.x
Tunstall BJ, Kearns DN (2017) Cocaine can generate a stronger conditioned reinforcer than food despite being a weaker primary reinforcer. Addict Biol 21(2):282–293. https://doi.org/10.1111/adb.12195.Cocaine
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56(6):613–672. https://doi.org/10.1016/S0301-0082(98)00060-4
Verheij MMM, Karel P, Cools AR, et al. (2014) Reduced cocaine-induced serotonin, but not dopamine and noradrenaline, release in rats with a genetic deletion of serotonin transporters. European Neuropsychopharmacology 24(11). Elsevier: 1850–1854. https://doi.org/10.1016/j.euroneuro.2014.09.004.
Villain H, Benkahoul A, Drougard A et al (2016) Effects of propranolol, a β-noradrenergic antagonist, on memory consolidation and reconsolidation in mice. Front Behav Neurosci 10(March):1–14. https://doi.org/10.3389/fnbeh.2016.00049
White N (1996) Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction 91(7):921–949. https://doi.org/10.1111/j.1360-0443.1996.tb03586.x
White N (2002) The psychobiology of reinforcers. Annu Rev Psychol. https://doi.org/10.1146/annurev.psych.43.1.443
White N, Major R (1978) Effect of pimozide on the improvement in learning produced by self-stimulation and by water reinforcement I. Pharmacol Biochem Behav 8:565–571
Winters BD, Bussey TJ (2005) Glutamate receptors in perirhinal cortex mediate encoding, retrieval, and consolidation of object recognition memory. J Neurosci 25(17):4243–4251. https://doi.org/10.1523/JNEUROSCI.0480-05.2005
Winters BD, Forwood SE, Cowell RA et al (2004) Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci 24(26):5901–5908. https://doi.org/10.1523/JNEUROSCI.1346-04.2004
Winters BD, Saksida LM, Bussey TJ (2008) Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev 32(5):1055–1070. https://doi.org/10.1016/j.neubiorev.2008.04.004
Wolter M, Huff E, Speigel T et al (2019) Cocaine, nicotine, and their conditioned contexts enhance consolidation of object memory in rats. Learn Mem 26:46–55. https://doi.org/10.1101/lm.048579.118.Freely
Wolter M, Huff AE, Baidoo N, et al. (2020) Modulation of object memory consolidation by heroin and heroin-conditioned stimuli: role of opioid and noradrenergic systems. European Neuropsychopharmacology 33. Elsevier B.V.: 146–157. https://doi.org/10.1016/j.euroneuro.2020.01.010
Yamasaki M and Takeuchi T (2017) Locus coeruleus and dopamine-dependent memory consolidation. Neural Plasticity 2017. Hindawi. https://doi.org/10.1155/2017/8602690
Zavala AR, Browning JR, Dickey ED et al (2008) Region-specific involvement of AMPA/Kainate receptors in Fos protein expression induced by cocaine-conditioned cues. Eur Neuropsychopharmacol 18(8):600–611. https://doi.org/10.1016/j.euroneuro.2008.04.010
Acknowledgements
This research was funded by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wolter, M., Lapointe, T., Melanson, B. et al. Memory enhancing effects of nicotine, cocaine, and their conditioned stimuli; effects of beta-adrenergic and dopamine D2 receptor antagonists. Psychopharmacology 238, 2617–2628 (2021). https://doi.org/10.1007/s00213-021-05884-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05884-x