Abstract
Rationale
Obsessive–compulsive disorder (OCD) is characterized by executive function impairment and by clinical responsivity to selective serotonin reuptake inhibitors (SSRIs). Executive planning deficits constitute a candidate endophenotype for OCD. It is not known whether this endophenotype is responsive to acute serotonin manipulation.
Objective
The study aimed to investigate the effects of acute SSRI administration on executive function in patients with OCD, first-degree relatives of patients with OCD, and healthy controls.
Methods
A randomized double-blind cross-over study assessed the effects of single-dose escitalopram (20 mg) and placebo on executive planning in 24 patients with OCD, 13 clinically unaffected first-degree relatives of patients with OCD, and 28 healthy controls. Performance on a Tower of London task measuring executive planning was assessed 4 h after oral administration of the pharmacological challenge/placebo and compared across and within groups using a mixed model analysis of variance.
Results
On the outcome measure of interest, i.e., the mean number of choices to obtain the correct solution, there was a marginally significant effect of group (F(2, 59) = 3.1; p = 0.052), with patients (least square (LS) mean 1.43; standard error [SE] 0.06; 95% confidence interval (CI), 1.31–1.55) and their relatives (LS mean 1.46; SE 0.08; 95% CI, 1.30–1.62) performing worse than matched healthy controls (LS mean 1.26; SE 0.05; 95% CI, 1.15–1.37) on placebo. There was a trend towards a significant group × treatment interaction (F(2, 58) = 2.8, p = 0.069), with post hoc tests showing (i) patients (p = 0.009; LS mean difference 0.23; SE 0.08) and relatives (p = 0.03; LS mean difference 0.22; SE 0.10) were more impaired compared to controls and (ii) escitalopram was associated with improved executive planning in patients with OCD (p = 0.013; LS mean difference 0.1; SE 0.04), but not other groups (both p > 0.1; controls: LS mean difference − 0.03; SE 0.04; relatives: LS mean difference 0.02; SE 0.05).
Conclusion
Our findings are consistent with a view that there is impaired executive planning in OCD and that this constitutes a behavioural endophenotype. In patients with OCD, but not in relatives, acute SSRI administration ameliorated this deficit. Further investigation is needed to understand common and differential involvement of neurochemical systems in patients with OCD and their relatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Obsessive–compulsive disorder (OCD) is a psychiatric condition characterized by intrusive thoughts, images, or impulses/urges and/or time-consuming repetitive behaviours, including repetitive mental acts, leading to significant functional impairment and distress (American Psychiatric Association 2013). Deficits in executive planning are a common finding in the OCD literature. Planning is key in cognitive functioning and requires individuals to identify and organize the necessary steps to achieve a goal. This type of cognitive ability is classically probed by using problem-solving tasks such as the Tower of London and its variants (Owen et al. 1990; Shallice 1982). For example, in a study using a computerized version of the Tower of London task, patients with OCD had significant planning deficits on difficult problems, versus controls (medium-large effect size) (Chamberlain et al. 2007). That study ensured that groups did not differ on confounding issues that are often overlooked in the neuropsychological literature, such as education levels, IQ, and trait impulsivity; furthermore, the patients were free from comorbidities. Patients with OCD exhibited decreased fronto-striatal activation (of dorsolateral prefrontal cortex and caudate nucleus) during performance of the Tower of London in an fMRI task (van den Heuvel et al. 2005). More recently, a similar fronto-striatal hypoactivation was demonstrated not only in patients with OCD but also in their clinically asymptomatic relatives, with reduced coupling between the right dorsolateral prefrontal cortex and the putamen being associated with longer times taken to solve the problems in the patients (Vaghi et al. 2017). Taken together, the literature thus indicates that executive planning is often impaired in OCD and that it is associated with aberrant functional connectivity of the fronto-striatal circuitry that is heavily implicated in the pathophysiology of OCD.
In recent years there has been an effort to investigate endophenotypes for OCD; these represent quantitative variables (e.g., cognitive or neurophysiological) associated with the disease while being distinct from the clinical phenotype itself (Gottesman and Gould 2003). Executive planning deficits are found not only in OCD but also in first-degree relatives of individuals with OCD, suggesting that such impairment represents an endophenotypic vulnerability to OCD (e.g., Zartaloudi et al. 2019). Moreover, OCD has a strong genetic component, with first-degree relatives having an approximately five-fold increased risk on average to also be affected with the disease (e.g., Grabe et al. 2006; Nestadt et al. 2000; Pauls et al. 1995). It has therefore been argued that deficits in certain executive functions may be promising endophenotypes for OCD. There has been consistent support for the use of selective serotonin reuptake inhibitors (SSRIs) as first-line pharmacologic interventions for OCD (e.g., Fineberg et al. 2020; Hirschtritt et al. 2017; Soomro et al. 2008). There is, however, mixed evidence as to whether first-line SSRI treatment leads to amelioration of cognitive deficits in OCD. For example, one study comparing neuropsychological performance in medicated vs. un-medicated adults with OCD found that there were no significant differences between these two groups on any of the cognitive domains that were studied, suggesting that deficits (including planning) persisted following chronic SRI treatment (Mataix-Cols et al. 2002). Another study of adult OCD patients found that SSRI administration was associated with cognitive decline in the acute phase (first 8 weeks) of treatment (Sayyah et al. 2016). In children and adolescents with OCD, however, there is tentative evidence that some cognitive deficits like memory, speed of information processing, and executive functions may normalize with treatment (Andres et al. 2008). Thus, the question remains whether and how acute serotonin manipulation can modulate cognition in OCD, and furthermore whether such effects extend to asymptomatic first-degree relatives—which would indicate the response to pharmacological challenge is itself an endophenotype.
This study therefore investigated response of executive function to acute SSRI administration in adult OCD patients, first-degree relatives of OCD patients, and healthy controls. We hypothesised that patients with OCD and their relatives would be impaired on the task compared to healthy controls. We further hypothesised that this deficit would be ameliorated by an acute SSRI-challenge in both groups. We selected escitalopram as the most selective of all the SSRIs, with little or no affinity for numerous other binding sites (Sanchez et al. 2003). Escitalopram binds to both the primary, high-affinity site on the serotonin transporter protein, which inhibits serotonin reuptake. It is likely that binding to the serotonin transporter initiates a series of effects in the brain beyond serotonin reuptake inhibition (Hedges and Woon 2007). The use of escitalopram as a pharmacological tool for exploring the role of serotonin in modulating normal emotional processing and in the pathophysiology of anxiety and depressive disorders has been suggested previously (Alves-Neto et al. 2010). In addition, clinical studies have shown that escitalopram causes few and mild side-effects and has a relatively rapid onset of action (Waugh and Goa 2003).
Methods
Ethics
The study protocol and patient information and consent forms were approved by the Health Research Ethics Committee of the Faculty of Medicine and Health Sciences, Stellenbosch University; by the Human Research Ethics Committee of the University of Cape Town; and acknowledged by the Medicines Control Council of South Africa. Written informed consent was obtained from all participants prior to study procedures being conducted.
Study design
A randomized double-blind counterbalanced cross-over study design was utilized to assess the effects of a single dose of the SSRI escitalopram (20 mg) and placebo on specific executive functions (i.e., executive planning) in adults with OCD, first-degree relatives of individuals with OCD, and matched healthy controls.
Clinical procedures
Information of the recruitment and inclusion/exclusion criteria of participants can be found in a previous publication (Lochner et al. 2016). Screening and diagnostic assessments are also described in this document.
In summary, patients with a primary diagnosis of OCD were included if they were at least moderately symptomatic on the Yale-Brown Obsessive–Compulsive Severity Scale (Y-BOCS), i.e., if they had a Y-BOCS total score > 16. The Y-BOCS is a clinician-rated 10-item measure of the severity of symptoms of OCD; each item is rated from 0 (no symptoms) to 4 (extreme symptoms), with a total range of 0 to 40 and separate subtotals for the severity of obsessions and compulsions. The scale has good inter-rater reliability, validity, and a high degree of internal consistency among all items. First-degree relatives of OCD patients and healthy controls were included if they had no significant current DSM-IV Axis I or II mental disorder or history of any substance or alcohol abuse/dependency.
Notably, patients were included only if they were either psychotropic medication free or on a psychotropic medication that was (1) limited to a single SSRI, (2) administered at a steady dose that was not higher than the optimal dose for OCD for the particular agent (e.g., 60 mg of fluoxetine), (3) taken for at least 2 months (8 weeks), and (4) stabilized according to the treating clinician. Patients on chronic SSRI were instructed to take their medication on the testing days, in addition to the challenge drug.
Pharmacological challenge
Participants were administered a single dose of escitalopram (20 mg) or placebo orally, in randomized order, on two separate testing days, approximately 1 week apart.
Neurocognitive assessments
Executive planning was measured using a computerized version of the Tower of London (TOL) task from the Cambridge Neuropsychological Test Automated Battery (CANTAB) (CANTABeclipse, Cambridge Cognition Ltd., Cambridge, UK). The same task was administered twice. The task version is also known as the One-Touch Stockings of Cambridge (OTS) task, and the version used had difficulty levels ranging from 1 to 5 move solutions. Three hours after ingestion of escitalopram/placebo, participants underwent magnetic resonance imaging (MRI) for an hour (these data will be reported separately). The TOL task was undertaken subsequently, approximately 4 h following ingestion of escitalopram or placebo. The rationale for using this timing schedule was based on the pharmacokinetic profile of escitalopram, with peak plasma concentration levels (PPCLs) being expected 3–4 h after oral dosing (Rao 2007).
During the administration of this computerized task, participants were presented with two sets of coloured balls, each arranged within three stacks, and instructed to calculate the minimum possible number of moves required to rearrange one set of coloured balls to match the other. They then indicated their calculation of the minimum number of moves required, by pressing the corresponding box for each choice (1–5) on the touch screen of the computer. When incorrect, the participants were instructed to try again. The key outcome measure on the task is the number of choices made by the participant to obtain the correct solution. A higher number of mean choices to obtain the correct solution suggests poorer performance on the task.
Data analyses
Demographic characteristics of the groups were compared using mixed model of analysis of variance (ANOVA) with post hoc t tests where any trend (p < 0.1) or significant effects of group were found. Post hoc tests were conducted for trend-significant main effects because (i) ANOVA is overly conservative given that two groups were expected to show similar performance rather than different performance (i.e., patients with OCD and relatives) and (ii) the sample size in the relatives group was relatively lower. Performance on the TOL task was analysed by means of a repeated measures analysis of variance using a mixed model approach (including a restricted maximum likelihood test, or REML). All data were analysed using Statistica 9.0 for Windows (Statsoft, Tulsa, Oklahoma). The following factors were included as variables in the analyses: group (i.e., OCD, relative or control), the type of treatment (escitalopram or placebo), and the task variables. A subset of the OCD sample was taking chronic SSRI medications at the time of their participation. In a subsequent analysis, we compared the performance on the study tasks between patients with OCD on chronic treatment with those not on any chronic psychotropic medication.
The outcome measure of interest was the mean number of choices to obtain the correct solution. Results from the analyses of the latency to the correct choice, expressed in milliseconds, or ms, are included in Supplement 1.
We employed a 5% threshold as the guideline for determining significant differences. Figures include letters (a, b, c) to indicate post hoc differences at a 5% significance level; i.e., means without overlapping letters are significantly different. Also, the length of the vertical (error) bars is the 95% confidence interval for the mean.
Results
Demographics and sample characteristics
Twenty-four (N = 24) patients with OCD, 13 clinically unaffected first-degree relatives of patients with OCD and 28 healthy controls, ages ranging between 19 and 64 years (mean age: 33.4, SD: 12), took part. The OCD cohort included patients who were not on any psychotropic medication at the time of participation (n = 13) and a group stabilized on SSRI treatment (n = 11). None of the medicated participants received more than one drug concurrently at the time of assessment. None of the relatives and healthy controls were taking any psychotropic medication. Y-BOCS scores of patients at screening ranged from 17 (moderate) to 32 (severe), with a mean (SD) of 23.3 (4.4) (Table 1).
Performance on the Tower of London task
On the outcome measure of interest for the executive planning task (Fig. 1), there was a marginally significant effect of group (F(2, 59) = 3.1; p = 0.052), with patients with OCD (least square (LS) mean 1.43; SE 0.06; 95% confidence interval (CI), 1.31–1.55) and their relatives (LS mean 1.46; SE 0.08; 95% CI, 1.30–1.62) performing worse than matched healthy controls (LS mean 1.26; SE 0.05; 95% CI, 1.15–1.37) on placebo and escitalopram combined.
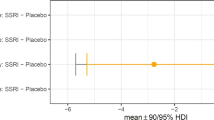
There was a trend towards a significant group × treatment interaction (F(2, 58) = 2.8, p = 0.069), with post hoc tests showing (i) patients (p = 0.009; LS mean difference 0.23; SE 0.08) and relatives (p = 0.03; LS mean difference 0.22; SE 0.10) were more impaired on the task overall (i.e., following either placebo or escitalopram challenge) compared to controls and (ii) escitalopram challenge was associated with significantly improved executive planning in OCD patients (p = 0.013; LS mean difference 0.1; SE 0.04), but not other groups (both p > 0.1; controls: LS mean difference −0.03; SE 0.04; relatives: LS mean difference 0.02; SE 0.05) (Fig. 2).
No significant effect of group or group by treatment interaction was found for the analyses of latency to the correct choice (p > 0.10, see Supplement 1).
The OCD group was subsequently subdivided depending on their treatment status. Comparison of the group not on chronic psychotropic medication and those stabilized on SSRI treatment in terms of their executive planning abilities showed no main effect of group (on chronic medication vs. off chronic medication) and no group × treatment condition interaction (both p > 0.1). Therefore, acute SSRI treatment had equivalent effects in medicated and un-medicated OCD patients.
Discussion
This study tested whether acute SSRI administration altered executive planning in patients with OCD, their first-degree relatives, and healthy controls. Consistent with our initial hypothesis, executive planning was significantly impaired in patients with OCD and symptom-free relatives of patients with OCD, compared to healthy controls. Partly consistent with our second hypothesis, we found mixed evidence for effects of acute SSRI administration on executive planning dysfunction: the group × treatment interaction was at a trend level but did not reach statistical significance; however, in post hoc tests, executive planning significantly improved following acute escitalopram in the OCD group, but not in the other groups.
Our finding of executive planning impairment in OCD and relatives is consistent with the literature. As noted earlier, a prior study identified reduced functional connectivity between prefrontal cortex and basal ganglia in OCD patients and their relatives, in comparison to controls (Vaghi et al. 2017). Our data confirmed that impaired executive planning on the TOL task may constitute a vulnerability marker (or candidate endophenotype) for OCD, since we found impairment in both OCD patients and their asymptomatic relatives. Based on prior neuroimaging findings (Vaghi et al. 2017), this decrement may reflect disruption of fronto-striatal connectivity. Our results are in keeping with a recent meta-analysis comparing executive functioning in unaffected relatives of patients with OCD and healthy controls; this identified significant impairments in relatives of OCD patients compared to healthy controls across a range of cognitive domains, particularly for executive planning (Hedge’s g = 0.37). (Zartaloudi et al. 2019).
The finding that a single dose of escitalopram ameliorated executive planning dysfunction in OCD is consistent with a role for the serotonin system in mediating such dysfunction. However, caution is needed in terms of linking this cognitive finding to the therapeutic role of SSRI in OCD. Acute SSRI treatment has been hypothesized to lead to inhibition of serotonin neuronal activity via actions at autoreceptors (e.g., Blier et al. 1990; Hajos et al. 1995), sometimes leading to an exacerbation of affective symptoms, including cognitive problems (Sayyah et al. 2016). In rodents acute low doses of SSRIs can have opposite effects to those of higher doses (Bari et al. 2010). In addition, acute pharmacological manipulations using SSRIs in healthy volunteers may improve some cognitive domains, while impairing others (Skandali et al. 2018). Thus, further work is needed to explore the effects of both acute and chronic SSRI treatment on cognition in OCD, across a range of domains. Notably, SSRIs not only alter serotonin levels but also exert an effect on other neurotransmitters such as dopamine (DA). Striatal DA levels may also affect performance on the TOL task (Reeves et al. 2007), with increasing DA leading to improved planning performance in healthy adults (Elliott et al. 1997). Further studies are therefore needed to fully delineate the neurochemical mechanisms involved in planning dysfunction in OCD and its amelioration under escitalopram.
Escitalopram did not significantly improve the executive planning deficit in relatives of patients with OCD, perhaps suggesting that in family members non-serotonergic neurobiological mechanisms underpin cognitive deficits. However, results for relatives of OCD patients should be considered preliminary given the small sample size, which may have limited the statistical power to detect effects of medication. OCD relatives were older than the other two cohorts; age was therefore included in an additional analysis as a covariate, with the findings appearing robust to this potentially confounding factor. Another potential limitation is the fact that our OCD sample was heterogeneous in terms of their OC symptoms, given that patients with particular OC symptom subtypes may be more prone to respond to SSRIs (Stein et al. 2008). Approximately half of the cohort of patients with OCD were taking chronic SSRI at the time of participation; the finding that such patients did not differ from those not on SSRIs in terms of executive planning may suggest that this dysfunction is an underlying deficit that persists despite chronic treatment (Nielen and Den Boer 2003). We also found that acute escitalopram administration improved executive planning in OCD patients irrespective of chronic treatment. There are a number of potential explanations. First, executive planning dysfunction in OCD may comprise both state and trait abnormalities, with the findings here suggesting that acute escitalopram administration improves state-related but not trait-related executive planning dysfunction. Thus, SSRI may only partially remediate the executive planning deficit. Second, the potentially cognitive enhancing effects of SSRIs may “wear off” after repeated administration of the drug, due to adaptive changes within the 5-HT system. When subsequently challenged with an additional dose of SSRI, in addition to the dose stabilized on, the patient may again manifest acute cognitive improvement. Third, the impact of acute SSRI administration on executive planning may have been brought about by primarily improving obsessive–compulsive (or anxiety) symptoms, which were present only in the patient group, and secondarily improving cognition.
In conclusion, our findings are in keeping with the hypothesis that impaired executive planning constitutes a behavioural endophenotype for OCD. In OCD, but not relatives, acute SSRI administration ameliorated this deficit, perhaps suggesting that in family members non-serotonergic neurobiological mechanisms underpin cognitive deficits. Further investigation is needed to consolidate these hypotheses; in particular longitudinal studies may be useful in determining whether chronic treatment of OCD can ameliorate cognitive impairments, perhaps by modulating fronto-striatal connectivity in OCD, and if so, for which domains and in which circuits. If the effect of SSRI on improving executive function in OCD is further substantiated, there may be implications for the clinical management of patients with this highly disabling disorder.
References
Alves-Neto WC, Guapo VG, Graeff FG, Deakin JFW, Del-Ben CM (2010) Effect of escitalopram on the processing of emotional faces. Braz J Med Biol Res 43(3):285–289. https://doi.org/10.1590/s0100-879x2010005000007
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (5th ed.). In American Journal of Psychiatry. https://doi.org/10.1176/appi.books.9780890425596.744053
Andres S, Lazaro L, Salamero M, Boget T, Penades R, Castro-Fornieles J (2008) Changes in cognitive dysfunction in children and adolescents with obsessive-compulsive disorder after treatment. J Psychiatr Res 42(6):507–514. https://doi.org/10.1016/j.jpsychires.2007.04.004
Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW (2010) Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology 35(6):1290–1301. https://doi.org/10.1038/npp.2009.233
Blier P, Serrano A, Scatton B (1990) Differential responsiveness of the rat dorsal and median raphe 5-HT systems to 5-HT1 receptor agonists and p-chloroamphetamine. Synapse 5(2):120–133. https://doi.org/10.1002/syn.890050206
Chamberlain SR, Fineberg NA, Blackwell AD, Clark L, Robbins TW, Sahakian BJ (2007) A neuropsychological comparison of obsessive-compulsive disorder and trichotillomania. Neuropsychologia 45(4):654–662. https://doi.org/10.1016/j.neuropsychologia.2006.07.016
Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW (1997) Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology 131(2):196–206. https://doi.org/10.1007/s002130050284
Fineberg NA, Hollander E, Pallanti S, Walitza S, Grünblatt E, Dell’Osso BM, Albert U, Geller DA, Brakoulias V, Janardhan Reddy YC, Arumugham SS, Shavitt RG, Drummond L, Grancini B, de Carlo V, Cinosi E, Chamberlain SR, Ioannidis K, Rodriguez CI, Garg K, Castle D, van Ameringen M, Stein DJ, Carmi L, Zohar J, Menchon JM (2020) Clinical advances in obsessive-compulsive disorder: a position statement by the International College of Obsessive-Compulsive Spectrum Disorders. Int Clin Psychopharmacol 35(4):173–193. https://doi.org/10.1097/YIC.0000000000000314
Gottesman II, Gould TD (2003) The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160(4):636–645. https://doi.org/10.1176/appi.ajp.160.4.636
Grabe HJ, Ruhrmann S, Ettelt S, Buhtz F, Hochrein A, Schulze-Rauschenbach S et al (2006) Familiality of obsessive-compulsive disorder in nonclinical and clinical subjects. Am J Psychiatry 163(11):1986–1992. https://doi.org/10.1176/ajp.2006.163.11.1986
Hajos M, Gartside SE, Sharp T (1995) Inhibition of median and dorsal raphe neurones following administration of the selective serotonin reuptake inhibitor paroxetine. Naunyn Schmiedeberg's Arch Pharmacol 351(6):624–629. https://doi.org/10.1007/bf00170162
Hedges DW, Woon FLM (2007) An emerging role for escitalopram in the treatment of obsessive-compulsive disorder. Neuropsychiatr Dis Treat 3(4):455–461
Hirschtritt ME, Bloch MH, Mathews CA (2017) Obsessive-compulsive disorder: advances in diagnosis and treatment. JAMA 317(13):1358–1367. https://doi.org/10.1001/jama.2017.2200
Lochner C, Chamberlain SR, Kidd M, Fineberg NA, Stein DJ (2016) Altered cognitive response to serotonin challenge as a candidate endophenotype for obsessive-compulsive disorder. Psychopharmacology 233(5):883–891. https://doi.org/10.1007/s00213-015-4172-y
Mataix-Cols D, Alonso P, Pifarre J, Menchon JM, Vallejo J (2002) Neuropsychological performance in medicated vs. unmedicated patients with obsessive-compulsive disorder. Psychiatry Res 109(3):255–264. https://doi.org/10.1016/s0165-1781(02)00024-0
Nestadt G, Samuels J, Riddle M, Bienvenu OJ 3rd, Liang KY, LaBuda M et al (2000) A family study of obsessive-compulsive disorder. Arch Gen Psychiatry 57(4):358–363. https://doi.org/10.1001/archpsyc.57.4.358
Nielen MMA, Den Boer JA (2003) Neuropsychological performance of OCD patients before and after treatment with fluoxetine: evidence for persistent cognitive deficits. Psychol Med 33(5):917–925. https://doi.org/10.1017/s0033291703007682
Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW (1990) Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 28(10):1021–1034. https://doi.org/10.1016/0028-3932(90)90137-d
Pauls DL, Alsobrook JP 2nd, Goodman W, Rasmussen S, Leckman JF (1995) A family study of obsessive-compulsive disorder. Am J Psychiatry 152(1):76–84. https://doi.org/10.1176/ajp.152.1.76
Rao N (2007) The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet 46(4):281–290. https://doi.org/10.2165/00003088-200746040-00002
Reeves SJ, Mehta MA, Montgomery AJ, Amiras D, Egerton A, Howard RJ, Grasby PM (2007) Striatal dopamine (D2) receptor availability predicts socially desirable responding. NeuroImage 34(4):1782–1789. https://doi.org/10.1016/j.neuroimage.2006.10.042
Sanchez C, Bergqvist PBF, Brennum LT, Gupta S, Hogg S, Larsen A, Wiborg O (2003) Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. Psychopharmacology 167(4):353–362. https://doi.org/10.1007/s00213-002-1364-z
Sayyah M, Eslami K, AlaiShehni S, Kouti L (2016) Cognitive function before and during treatment with selective serotonin reuptake inhibitors in patients with depression or obsessive-compulsive disorder. Psychiatry J 2016:5480391–5480394. https://doi.org/10.1155/2016/5480391
Shallice T (1982) Specific impairments of planning. Philos Trans R Soc Lond Ser B Biol Sci 298(1089):199–209. https://doi.org/10.1098/rstb.1982.0082
Skandali N, Rowe JB, Voon V, Deakin JB, Cardinal RN, Cormack F, Passamonti L, Bevan-Jones WR, Regenthal R, Chamberlain SR, Robbins TW, Sahakian BJ (2018) Dissociable effects of acute SSRI (escitalopram) on executive, learning and emotional functions in healthy humans. Neuropsychopharmacology 43(13):2645–2651. https://doi.org/10.1038/s41386-018-0229-z
Soomro GM, Altman D, Rajagopal S, Oakley-Browne M (2008) Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev 1:CD001765. https://doi.org/10.1002/14651858.CD001765.pub3
Stein DJ, Carey PD, Lochner C, Seedat S, Fineberg N, Andersen EW (2008) Escitalopram in obsessive-compulsive disorder: response of symptom dimensions to pharmacotherapy. CNS Spectr 13(6):492–498. https://doi.org/10.1017/S1092852900016722
Vaghi MM, Hampshire A, Fineberg NA, Kaser M, Bruhl AB, Sahakian BJ et al (2017) Hypoactivation and dysconnectivity of a frontostriatal circuit during goal-directed planning as an endophenotype for obsessive-compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 2(8):655–663. https://doi.org/10.1016/j.bpsc.2017.05.005
van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJLM, van Hartskamp J, Barkhof F, van Dyck R (2005) Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry 62(3):301–309. https://doi.org/10.1001/archpsyc.62.3.301
Waugh J, Goa KL (2003) Escitalopram: a review of its use in the management of major depressive and anxiety disorders. CNS Drugs 17(5):343–362. https://doi.org/10.2165/00023210-200317050-00004
Zartaloudi E, Laws KR, Bramon E (2019) Endophenotypes of executive functions in obsessive compulsive disorder? A meta-analysis in unaffected relatives. Psychiatr Genet 29(6):211–219. https://doi.org/10.1097/YPG.0000000000000241
Acknowledgements
We are grateful to the European College of Neuropsychopharmacology (ECNP) Obsessive-Compulsive and Related Disorders Research Network (OCRN), the International College of Obsessive-Compulsive Spectrum Disorders (ICOCS), and the European Cooperation in Science and Technology COST Action (CA16207) European Network for Problematic Usage of the Internet for providing networking support. Dr. Chamberlain’s consults for Promentis and Ieso Digital Health. Dr. Fineberg leads an NHS treatment service for OCD. She holds Board membership for various registered charities linked to OCD. She gives expert advice on psychopharmacology to the UK MHRA. Dr. Robbins consults for Cambridge Cognition, Unilever, Shionogi, Tacheda, Greenfield Bioventures, and Cassava.
Funding
The authors received support from the Medical Research Council of South Africa (Lochner and Stein), the Obsessive-Compulsive Foundation (Stein), and the National Research Foundation of South Africa (Lochner) and an unrestricted grant from Lundbeck H/S. Dr. Chamberlain’s role in this project was funded by a Wellcome Trust Clinical Fellowship (110049/Z/15/Z). Dr. Fineberg declares that in the past 3 years, she had held research or networking grants from the ECNP, UK NIHR, EU H2020, MRC, and University of Hertfordshire. In the past 3 years, she had accepted a paid speaking engagement in a webinar sponsored by Abbott. Previously, she had accepted paid speaking engagements in various industry supported symposia and have recruited patients for various industry-sponsored studies in the field of OCD treatment. Dr. Robbins is supported by Wellcome Trust Grant 146301/Z/14/Z/. He holds research grants from Shionogi and GlaxoSmithKline. Dr. Stein has received research grants and/or consultancy honoraria from Lundbeck and Sun.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 48 kb)
Rights and permissions
About this article
Cite this article
Lochner, C., Chamberlain, S.R., Kidd, M. et al. The effects of acute serotonin challenge on executive planning in patients with obsessive–compulsive disorder (OCD), their first-degree relatives, and healthy controls. Psychopharmacology 237, 3117–3123 (2020). https://doi.org/10.1007/s00213-020-05597-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05597-7