Abstract
Aim
To investigate whether circulating T cells including regulatory T cells (Treg) and derived cytokines contribute to the immune imbalance observed in schizophrenia.
Methods
Forty patients with schizophrenia and 40 age, sex, body mass index, education, and smoking status–matched healthy controls (HC) are included in the study. We stained cells with anti-CD14, anti-CD3, anti-CD4, anti-CD8, anti-CD19, anti-CD20, and anti-CD16/56. Peripheral blood mononuclear cells (PBMCs) were isolated and stained with the human FoxP3 kit containing anti-CD4/anti-CD25 and intracellular anti-Foxp3. PBMCs were cultured for 72 h and stimulated with anti-CD3/anti-CD28. Cytokines (IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF-α, and IL-17A) were measured from the culture supernatant and plasma using the Th1/Th2/Th17 cytokine bead array kit.
Results
In comparison with HC, Treg percentages in schizophrenia were higher (1.17 ± 0.63 vs 0.81 ± 0.53, P = 0.005) in unstimulated but lower in the stimulated condition (0.73 ± 0.69 vs 0.97 ± 0.55, P = 0.011). Activated T cell percentages were higher in schizophrenia than HC in unstimulated (2.22 ± 0.78 vs 1.64 ± 0.89, P = 0.001) and stimulated (2.25 ± 1.01 vs 1.72 ± 1.00, P = 0.010) conditions. The culture supernatant levels of IL-6 (7505.17 ± 5170.07 vs 1787.81 ± 1363.32, P < 0.001), IL-17A (191.73 ± 212.49 vs 46.43 ± 23.99, P < 0.001), TNF-α (1557 ± 1059.69 vs 426.57 ± 174.62, P = 0.023), and IFN-γ (3204.13 ± 1397.06 vs 447.79 ± 270.13, P < 0.001); and plasma levels of IL-6 (3.83 ± 3.41vs 1.89 ± 1.14, P = 0.003) and IL-17A (1.20 ± 0.84 vs 0.83 ± 0.53, P = 0.033) were higher in patients with schizophrenia than HC.
Conclusion
Our explorative study shows reduced level of Foxp3 expressing Treg in a stimulated condition with induced levels of proinflammatory cytokines in patients with schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
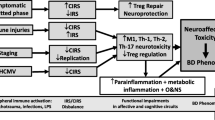
Accumulating evidence suggest that the immune system may play an important role in the pathogenesis of schizophrenia. Immune imbalance observed in patients with schizophrenia includes changes in circulating CD4+ T lymphocytes (Miller et al. 2013; Miller and Goldsmith 2017). Recent studies have indicated that the central nervous system can be influenced by the neuroinflammation mediated by CD4+ T cells (Louveau et al. 2015; Najjar and Pearlman 2015). Neurotransmitters such as dopamine are particularly capable of modulating the functions of T cells (Contreras et al. 2016; Prado et al. 2012). In support of this view, dopaminergic receptor (DR) gene variants were mainly associated with the count of CD4+ T cells but not with the count of CD8+ T cells (Cosentino et al. 2015). CD4+ CD25+FoxP3 regulatory T cells (Treg) have a function in maintaining peripheral tolerance, suppressing the activation of the immune system, and thereby limiting chronic inflammation (Debnath and Berk 2014). Treg cells are especially sensitive to dopamine and cAMP levels in lymphocytes; and the functions of Treg cells are mediated by DR signaling (Levite et al. 2017; Osorio-Barrios et al. 2018). Furthermore, Treg cells show a neuroprotective response, possibly by upregulating the expression of neurotrophic factors and downregulating the synthesis of proinflammatory cytokines and reactive oxygen species (Gendelman and Appel 2011), which may be associated with an impairment of the higher-order brain functions and may also lead to the progressive brain changes (Anderson et al. 2013).
Several studies reported increased relative T cell counts and functions (Henneberg et al. 1990; Sperner-Unterweger et al. 1999), diminished T cell proliferation and functions (Craddock et al. 2007; Steiner et al. 2010), or no differences between patients with schizophrenia and healthy controls (Rudolf et al. 2004). Few studies investigating the numbers of Treg cells showed that the T cell system was associated with the activation of both proinflammatory (high percentages of activated CD3+CD25+ T cells and Th17 cells) and anti-inflammatory (high percentages of Treg cells and IL-4-containing lymphocytes) states in the patients with recent-onset schizophrenia (Drexhage et al. 2011). One study showed a proportional shift toward naive T cells from antigen-experienced CD4+ T cell and decreased Treg populations in treatment-resistant patients with schizophrenia (Fernandez-Egea et al. 2016). In contrast, a recent study found that Treg cells were increased in medicated patients with schizophrenia (Kelly et al. 2018).
However, the altered numbers of T cell and responses in schizophrenia still remain unclear. It appears that this discrepancy may partially be explained by differences in the methodology and sampling, such as differences in clinical stages of schizophrenia (Meyer 2011). In addition, several confounding factors such as age, sex, body mass index (BMI), smoking, antipsychotic use, and socioeconomic status may influence the analysis of T cell functions in schizophrenia (Miller et al. 2013; Miller and Goldsmith 2017). The analysis of cytokines in cellular supernatants is advantageous to serum/plasma sampling with a more precise measurement of the cellular source of cytokines (do Prado et al. 2013). A recent meta-analysis proposed that the validation of the cell sources (e.g., TH1 vs TH2 vs TH17 cells) of specific cytokines might clarify the inconsistency of immune findings in schizophrenia (Miller and Goldsmith 2017). Also, peripheral blood mononuclear cell (PBMC) cultures may offer a better platform with the stimulated condition than the measurement of cytokines in serum/plasma (Koola 2016). The investigation of the T cell stimulation, which closely mimics T cell activation from antigen-presenting cells (T cell in vivo proliferation) and T cell ex vivo expansion, may provide new insights to the immune responses in patients with schizophrenia (Frauwirth and Thompson 2002).
Given that, we aimed to investigate a specific subset of T cells that may influence the inflammatory state observed in patients with schizophrenia. Specifically, we measured the following in patients with stable-chronic schizophrenia and compared with healthy controls: (a) the distribution of various lymphocyte subsets and regulatory T cells in PBMC culture, (b) the proliferation of regulatory T cells during T cell stimulation with anti CD3/CD28 co-culture, (c) Th1/Th2/Th17 cytokines in culture supernatants and plasma.
Material and methods
Participants
This cross-sectional study was conducted in the Department of Psychiatry, Istanbul, Bezmialem Vakıf University, at April 2017 to June 2017. The age range of all participants was between 18 and 65 years. Weight and height were measured to calculate body mass index (BMI). Exclusion criteria for all participants were as follows: alcohol or drug abuse or dependence (except for nicotine use), history of head trauma, being a shift worker, having a severe medical illness (including infections, allergies, autoimmune disorders, heart disease, epilepsy, hepatic or renal diseases, diabetes, aplastic anemia), and medication use that may influence immune markers (e.g., non-steroid anti-inflammatories, antibiotics) in the last 4 weeks.
Fifty-seven patients diagnosed with schizophrenia were eligible for the current study. Of these patients, 18 patients were referred from local community psychiatry service and 39 were followed up at our outpatient clinic. Seven patients refused study participation. Clinical stability, defined as no change in medication dosage in the past 6 months and no hospitalization in the past 1 year, was an inclusion criterion. Seven patients had an acute psychotic episode in the last 6 months. Three patients had major medication change in the last 2 months. After exclusion of these patients, 40 clinically stable patients diagnosed with schizophrenia were confirmed with the Structured Clinical Interview for DSM-IV-Patient Edition (First et al. 1997) by two senior psychiatrists and medical records. The Positive and Negative Syndrome Scale (PANSS) was used to assess psychopathology scores. The mean of duration of illness was 17.5 ± 10.2 years in patients with schizophrenia. All patients were on antipsychotics at the time of sample collection: clozapine (n = 7, 17.5%), other atypical antipsychotics (n = 11, 27.5%), typical antipsychotics (n = 8, 17%), atypical antipsychotic long-acting injection (n = 14, 35%), and typical/atypical antipsychotic combination (n = 21, 52.5%). The average cumulative chlorpromazine equivalent dose (CPZe) was 713.35 ± 430.45 mg.
Potential healthy controls (HC) were selected from a pool of administrative hospital staff who share the same residential area codes with patients. A senior psychiatrist and a psychiatrist in training interviewed 67 HC for mental disorders and evaluated their electronic health records. HCs were matched in terms of age, sex, ethnicity, education, BMI, and smoking habits. Participants with a life-time diagnosis of major mental disorder that might be associated with immune-related changes (i.e., psychotic disorders, major depressive disorder, bipolar disorders, and obsessive-compulsive disorder) were excluded. Nine participants were diagnosed with chronic systemic diseases. Eight participants did not match with patients’ BMI. Two participants took anti-inflammatory agents over the last 2 weeks. Six participants refused to give blood sample. After exclusion of these participants, 40 HCs were enrolled in the study.
Blood collection and cell isolation
Twenty milliliters (10 ml with each sample in heparinized and EDTA tubes) of peripheral blood were collected by venipuncture between 8:00 and 10:00 am. No fasting was required. Heparinized tubes were extracted freshly between 10:00 am and 1:00 pm on the same day for the peripheral blood mononuclear cell (PBMC) isolation. Blood samples were diluted 1/1 (v/v) with sterile PBS. PBMCs were isolated by density-gradient centrifugation for 20 min at 2000 rpm. The buffy coat was collected, washed twice with complete RPMI 1640 (cRPMI) medium (RPMI supplemented with 10% FBS and 1% penicillin/streptomycin) by centrifugation at 1500 rpm for 5 min. Supernatant discarded and remaining cell pellet was resuspended with cRPMI medium for culturing. Cells were counted by means of microscopy (× 100) and viability always exceeded 95%, as judged from their ability to exclude Trypan Blue (Sigma). The other 10 ml of blood were immediately centrifuged at 3000g for 10 min for the analyses of cytokines (IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF-α, and IL-17A), and the plasma was collected and stored at − 80 °C until assayed.
PBMC culture conditions
Investigating the function of cell activation during T cell stimulation may provide new insights into understanding normal immune responses (T cell in vivo proliferation), as well as T cell ex vivo expansion, which is a critical requirement for recent study protocols. Anti-CD3 and anti-CD28 is a cocktail of antibodies that bind to CD3 and CD28 on the surface of all T cells, which triggers intracellular signaling pathway and enhances proliferation of T cells (Trickett and Kwan 2003). Therefore, we decided to use anti-CD3/CD28 co-culture to investigate CD4+CD25+Foxp3 cells’ frequency and cytokine profiles of T cell in during stimulation (Huang et al. 2017). Briefly, 5 × 105 cells were placed in 48 well plates in 500-μL culture medium and stimulated with anti-CD3/anti-CD28 (CD mix). All cells were incubated for 72 h in 37 °C incubator with 5% CO2.
Immunophenotyping
A large panel of lymphocyte and monocyte subpopulations was identified by multi-color flow cytometry in the whole venous blood. To evaluate monocyte and specific lymphocyte subsets, cells were stained for 30 min with the combinations of the following monoclonal human antibodies: anti-CD14, anti-CD3 FITC, anti-CD4 PE, anti-CD8 APC, anti-CD19 PE, anti-CD20 APC, anti-CD1656 PE (all from BD Biosciences). After incubation, FACS Lysing Solution was added and incubated for 10 min in the dark at room temperature. Immediately after staining, cells were washed, resuspended, and analyzed by flow cytometry. The instrument was checked for sensitivity and overall performance with Cytometer Setup and Tracking beads (BD Bioscience) before data acquisition. A minimum of 10,000 lymphocytes was identified by size (forward scatter [FSC]) and granularity (side scatter (SSC)) and acquired with a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed using the Cell Quest software (BD Biosciences).
Intracellular detection of FoxP3
CD4+CD25+Foxp3+ cells were analyzed in PBMCs. The cells were assessed via flow cytometry using Human regulatory T cell Kit according to the manufacturer’s instructions (eBiosciences). Briefly, cells were washed for twice with cold 1× PBS. Pellet was stained with anti-CD4 and anti-CD25 for 20 min in dark room. After incubation, cells were washed and pellet was diluted with lysis buffer, and lysate was stained with Foxp3 antibody. After incubation period, a minimum of 100,000 events on lymphocyte population was acquired for analysis. The same protocol was applied for PBMCs in culture after 72 h of the culture period.
Supernatant and plasma cytokine determinations
Multiple soluble cytokines (IL-2, IL-10, IL-4, IL-6, IFN-γ, TNF-α, IL-17A) were simultaneously measured by flow cytometry using the Cytometric Bead Array (CBA) Human Th1/Th2/Th17 Kit (BD Biosciences) from culture supernatants and plasma according to the manufacturer’s protocol. The samples were thawed in the same day and processed together. Samples were acquired in a FACSCanto II flow cytometer (BD Biosciences). The instrument had been checked for sensitivity and overall performance with Cytometer Setup and Tracking beads (BD Biosciences) prior to the data acquisition, and the quantitative results were generated using the FCAP Array v1.0.1 software (Soft Flow Inc., Pecs, Hungary). Results were expressed as picograms per milliliter. The detection limits for these assays ranged from 2.4 to 4.9 pg/mL (IL-2, IL-10, IL-4, IL-6, IFN-γ, TNF-α) and 18.9 pg/mL for IL-17A. Undetectable plasma samples were measured for several times.
Statistics
Statistical analysis was performed using the SPSS 22.0 package for Macintosh (SPSS Inc., USA). Data were tested for normal distribution using the Kolmogorov-Smirnov test. The majority of the variables were non-normally distributed. Therefore, non-parametric, Mann-Whitney U tests were carried out to identify significant expression differences between patients and HC. Furthermore, two-tailed Wilcoxon rank-sum tests were performed to identify within-subject differences between unstimulated and stimulated percentages of CD4+CD25+ Foxp3 and CD4+CD25+ in patients and HC. The specific tests used were mentioned in the tables and figure legends. The statistical significance level was set at P = 0.05 (two-tailed).
Results
Demographic data
Demographic and clinical characteristics of the sample are summarized in Table 1.
Immune cell subsets
PBMCs from patients with schizophrenia and HC were stained for surface markers in ex vivo condition. Monocyte and lymphocyte subpopulations were evaluated by the expression of the membrane-bound molecules CD14+, CD3+, CD4+, CD8+, CD19+, CD20+, and CD16+CD56+ by the activation marker CD25. Gating strategy is shown in Supp. Figure 1.
In comparison with HC, patients with schizophrenia presented lower percentages of CD3+ T cells (Z = − 1.862, P = 0.001), and particularly lower percentages of CD3+CD4+ Th cells (Z = − 3.618, P < 0.001). Additionally, the percentage of CD19+ B cell (Z = − 1.650, P = 0.031) was higher in patients with schizophrenia than in HC. Table 2 reports all unstimulated and stimulated results of immune cell subsets.
The percentages of CD4+CD25+ Foxp3 (Z = − 2.820, P = 0.005) and CD4+CD25+ (Z = − 3.407, P = 0.001) were higher in patients with schizophrenia than in HC in unstimulated condition. In contrast, CD4+CD25+ Foxp3 cells were lower in patients with schizophrenia than in HC after in vitro stimulation (Z = − 2.555, P = 0.011). The percentage of activated CD4+CD25+ remained higher in patients with schizophrenia than in HC after in vitro stimulation (Z = 2.565, P = 0.010). Analysis of CD4+CD25+ (Fig. 1a, b) and CD4+CD25+FoxP3 cells (Fig. 1c, d) by flow cytometry is illustrated in Fig. 1.
Analysis of CD4+CD25+ and CD4+CD25+FoxP3 cells by flow cytometry. a Representative dot plots of CD4+CD25+-activated T cells of gated peripheral lymphocytes in baseline (day 0) and in stimulated (day 3) culture condition. b Box plots show the percentages of CD4+CD25+-activated T cells of gated peripheral lymphocytes in baseline (P = 0.001) and in stimulated (P = 0.010) culture condition. c The histogram graphic demonstrates FoxP3 expression in CD4+CD25+-activated T cell in baseline and in stimulated culture condition. d Box plots show the percentages of CD4+CD25+FoxP3 of gated peripheral lymphocytes in baseline (P = 0.005) and in stimulated culture condition (P = 0.011). HC, healthy controls; SZ, schizophrenia. Calculated by Mann-Whitney test, statistically significant differences are indicated P < 0.05
Within-subject comparison showed that the percentages of CD4+CD25+ Foxp3 cells decreased after stimulation in patients with schizophrenia (Z = − 2.877, P = 0.004), but not in HC (Z = − 1.788 P = 0.074). The stimulation did not influence the percentages of CD4+CD25+ cells in patients with schizophrenia (Z = − 0.56 P = 0.955) or HC (Z = − 0.27 P = 0.979).
Cytokine production
In culture supernatants, patients with schizophrenia had higher levels of IL-6 (Z = − 4.815, P < 0.001), IL-17A (Z = − 6.002, P < 0.001), TNF-α (Z = − 2.230, P = 0.023), and IFN-γ (F = 79.954, df = 77, P < 0.001) than HC. We found a trend-significant higher IL-2 in patients with schizophrenia than in HC (Z = − 1.919, P = 0.056) (Table 3).
In plasma samples, patients with schizophrenia had higher levels of IL-6 (Z = − 3.014, P = 0.003) and IL-17A (Z = − 2.137, P = 0.033) than HC (Table 4).
Correlation between lymphocyte subsets, cytokines, and symptom severity
In our sample, the percentages of Treg cell were not correlated with clinical data and other immunological parameters (Table 5). Both CD19+ and CD20+ percentages were positively correlated with PANSS total and global scores. The supernatant levels of IL-4 and IL-10 were negatively correlated with PANSS negative scores (Table 5). There were no significant differences in Treg and other immune cells and cytokine concentrations between patients on clozapine (n = 7) and the rest (data not shown).
Discussion
To the best of our knowledge, this is the first study investigating the percentages of the Treg cells and levels of Th1/Th2/Th17 cytokines both plasma (unstimulated) and stimulated (co-culture 72 h with CD3+CD28+) condition in stable patients with schizophrenia. The main finding of our study is that although patients had an increased level of Treg cell in unstimulated condition, Treg cell levels in stimulated condition decreased in parallel with higher cytokine production (IL-6, IL-17A, TNF-α and IFN-γ) compared with HC.
We showed that patients with schizophrenia had higher percentages of natural regulatory T cells (CD4+ CD25+ FoxP3+) than HC in unstimulated culture condition. In line with this finding, the higher percentages of Treg cells have been found in first episode psychosis (Drexhage et al. 2011) and remitted patients with schizophrenia (Kelly et al. 2018). However, one study showed decreased levels of Treg in a sample of 18 treatment-resistant clozapine-treated patients with schizophrenia compared with those in healthy controls (Fernandez-Egea et al. 2016). The discrepancy might be explained by the heterogeneous nature of schizophrenia spectrum disorder and the differences in the course of illness (e.g., FEP, treatment-resistant, and clinically stable schizophrenia). Although Treg cells are known to play a pivotal role in immune regulation, only a few studies investigated Treg cells in schizophrenia. For instance, Treg cells prevent immune responses against self-antigens, and they can inhibit both Th1- and Th2-type responses (Sakaguchi et al. 2008). Therefore, the increased level of Treg cells can be speculated as a response to an immune response to balance the high proinflammatory state in the disease and do not support the concept that the T cell system is in a pure proinflammatory state, but instead, there is a dysregulation or imbalance between pro- and anti-inflammatory states in patients with schizophrenia (Corsi-Zuelli et al. 2019).
However, we found that the level of Treg cells was decreased in stimulated condition. To the best of our knowledge, this is the first study that investigates Treg cells in patients with schizophrenia in a stimulated condition and shows reduced levels of Foxp3 expressing Treg cells. T cells in patients with schizophrenia show abnormalities not only in distribution but also in function, such as altered responses to mitogenic stimulation and diminished T cell proliferation and functions (Craddock et al. 2007; Herberth et al. 2010; Steiner et al. 2010). Additionally, it is known that the lymphocytes and the gene expression patterns of central nervous system (CNS) are correlated (Sullivan et al. 2006); and several researchers suggest that pathways of cell cycle, intracellular signaling, and oxidative stress metabolism might be responsible for abnormal cell function (Bowden et al. 2006; Craddock et al. 2007; Herberth et al. 2011). Furthermore, T cell hypo-responsiveness is associated with compensation for the immune system by increased efforts of proinflammatory forces (Craddock et al. 2007). Decreased levels of Treg cells have also been found following Toxoplasma gondii-driven Th1-inflammatory response (Oldenhove et al. 2009), suggesting that the decreased number of Treg cells and loss of Foxp3 expression can trigger several autoimmune conditions (Sawant and Vignali 2014). Accordingly, schizophrenia has been associated with a 29% increase in risk of prior autoimmune and infectious diseases (Benros et al. 2011). Epidemiological studies show an association between prenatal maternal infection and increased risk of schizophrenia in the offspring (Meyer 2013). Decreased expression of Treg in schizophrenia might be under consideration for the increased rate of autoimmune diseases, in-utero exposure to infection, decreased T cell immune response, lower type IV delayed skin hypersensitivity reactions (Riedel et al. 2007), and lower antibody responses to vaccination with hepatitis B in schizophrenia (Russo et al. 1994).
In our study, activated T cell (CD4+CD25+Foxp3−) percentages in both unstimulated and stimulated culture conditions were higher in patients with schizophrenia than in HC. Recent preclinical evidence suggests that proinflammatory cytokines of Th1, Th2, and Th17 lineages mediate the antagonistic downregulation of Foxp3, and in some instances, also the conversion of Treg cell to effector T cell (Wei et al. 2007; Yang et al. 2008). Foxp3 deletion in mature Treg leads to a loss of function and conversion into proinflammatory cytokine-producing effector T cell (Williams and Rudensky 2007), but these immune mechanisms have been not investigated thoroughly in schizophrenia. Furthermore, IL-6 in conjunction with IL-1 and IL-23 induces ROR γ t and downregulates Foxp3+ leading to the consequent secretion of IL-17A by the lack of Foxp3 (Lal et al. 2009; Yang et al. 2008). In stimulated condition, we found an increased effort of Th1, Th2, and Th17 cell axis with a greater tendency toward production of proinflammatory cytokines (IL-6, IL-17A), lower Treg (CD4+CD25+Foxp3+) function, and higher activated T cell (CD4+CD25+Foxp3−) percentages in patients with schizophrenia than in HC. Our results are in line with the preclinical literature showing that the higher levels of IL-6 might cause Foxp3 loss of function and also explain the higher secretion of IL-17A.
Patients with schizophrenia had higher IL-17A levels than HC in the stimulated culture supernatant and plasma. Few studies reported increased IL-17A concentrations in the first episode psychosis (FEP), but findings were not consistent likely due to small sample sizes and heterogeneity (Borovcanin et al. 2012; Ding et al. 2014; Noto et al. 2015). A recent meta-analysis conducted in a total of 313 patients with drug-naïve FEP and 238 HC from 5 studies did not find significant differences between groups for IL-17A concentrations (Fang et al. 2018). One study found significantly decreased levels of IL-17A in chronic patients with schizophrenia compared with HC (Dimitrov et al. 2013). In contrast, Himmerich et al. demonstrated that antipsychotics might increase IL-17A production (Himmerich et al. 2011). Taken together, antipsychotic treatment may have a direct impact on the levels of IL-17A. Interestingly, a very recent study has found an increased Th17 percentage in psychotic patients with 22q11.2 Deletion syndrome was compared with non-psychotic patients with 22q11.2 deletion syndrome (Vergaelen et al. 2018). Furthermore, Th17 percentage was associated with the presence of positive psychotic symptoms. In a mouse model, researchers showed that increased infection-induced levels of peripheral IL-17A in the mother caused irregularities in the neuron layers of the brain cortex in offspring that were linked to deficits in social interaction, behavior, and communication (Choi et al. 2016). Furthermore, they demonstrated that offspring lacking an essential regulator of the IL-17A pathway did not show these behavioral deficits, providing further support for the hypothesis of the role of T cells, more specifically, Th17 cells in association with psychotic symptoms.
We found increased levels of IFN-γ, which is profoundly secreted from Th1 cells. In accordance, increased levels of Th1 and Th17, and outnumbered Th2 have been reported previously (Drexhage et al. 2011). Further, we found increased TNF-α in patients. Our findings are in line with a previous meta-analysis that suggests that elevated levels of IFN-γ and TNF-α may be trait markers of schizophrenia (Miller et al. 2011). Additionally, we showed increased IL-6 in patients with schizophrenia. However, no significant differences in IL-6 levels between stable-chronic patients and control subjects have been found in the small number of studies in the stable patients (Miller et al. 2011). A more recent transdiagnostic meta-analysis of cytokines in patients with schizophrenia (acute and chronic), bipolar disorder (BD) and major depressive disorder (MDD) revealed that IL-6 were significantly higher in schizophrenia (chronic), euthymic (but not depressed) BD, and MDD than in controls (Goldsmith et al. 2016). Additionally, CSF levels of IL-6 were higher in patients with schizophrenia and MDD than in HC (Wang and Miller 2018). The increased pattern in these cytokines in patients with chronic schizophrenia, BD, and MDD raises the possibility of common underlying pathways for the chronic inflammation, such as the NF-κB signaling pathway (Altinoz et al. 2018).
Increased relative numbers of B cells have been reported in schizophrenia (Fernandez-Egea et al. 2016; Al-Diwani et al. 2017). In line with these findings, we found increased percentages of CD19+ B cell in patients with schizophrenia. Moreover, there was an association between B immune cells (CD19+ and CD20+) and PANNS scores (total and global), in accordance with previous findings showing an association between B cell (CD19+) counts and psychosis severity (Steiner et al. 2010). Also, recent genome-wide association findings have shown that the MHC and B cell markers CD19 and CD20 achieve genome-wide significance for schizophrenia, and further remain significant even after excluding the extended MHC region (Schizophrenia Working Group of the Psychiatric Genomics Consortium 2014). Lastly, we found that IL-4 and IL-10 were negatively associated with PANNS negative scores. A prior study found that serum levels of IL-4 and IL-10 were associated with the severity of negative symptoms in patients with early onset schizophrenia (Simsek et al. 2016). Also, previous findings consistently show a positive correlation between TNF-α and IL-6 and negative symptoms in patients with schizophrenia (Goldsmith et al. 2016). However, we did not find a large or statistically significant association of PANNS subscales with TNF-α and IL-6.
The potential confounders, including age, sex, BMI, smoking, socioeconomic status, and the time of the day of blood sampling, are particularly important in the present context of immunological research in schizophrenia, and could account for differences in immune cell parameters between different populations at different stages of illness (Miller et al. 2013). Therefore, we carefully conducted our study and matched our patient group with healthy controls. Furthermore, it has been suggested that the use of frozen peripheral blood cells may confound the interpretation of the data (Meyer 2011). Altered apoptotic mechanisms have been implicated in schizophrenia (Jarskog 2006). Given that, it is plausible to assume that the freeze-thaw cycles may significantly affect recovery and survival of immune cells differently between the patients with schizophrenia and HC. We, therefore, performed our PBMC cultures within 3 h after blood collection without freezing. However, this study is limited in some respects. First, schizophrenia is a heterogeneous disorder and presents with a variety of symptoms. As we conducted our study in chronic and clinically stable patients, our results cannot be generalized to the entire psychosis spectrum. Second, the sample size was modest (n = 40); the design was cross-sectional; and all patients were on antipsychotics. Third, the function of FoxP3 and the clonal proliferation of Treg cells in the body remain largely unclear in patients with schizophrenia; and further research is therefore needed to conclusively reveal the molecular mechanisms of Treg cell. Finally, given the explorative nature of the study, we did not correct statistical significance level for multiple testing. The multiplicity adjustment rarely solves the problem of exploratory data analysis, and in fact, may lead to a false sense of confidence in the “statistically significant” findings, which in turn are given particular importance (Bender and Lange 2001). Similarly, although dimensionality reduction techniques may help with reducing the number of tests, the interpretability and comparability of findings with previous and future research would not be easy. To overcome this issue, pre-registered confirmatory studies are needed in the field of psychoneuroimmunology.
Conclusion
Our findings show a high level of cytotoxic T cell activation with dysfunction of Treg cells in schizophrenia. In this regard, it is plausible to argue that cellular mechanisms involved in Treg cell responses to antigen might be less efficient in schizophrenia. Some evidence suggests that schizophrenia is associated with reduced potency to exhibit anti-inflammatory responses in the CNS. When proinflammatory stimulus is not sufficiently counteracted by appropriate anti-inflammatory response, it may lead to long-term detrimental consequences for cognitive and behavioral functions in patients with schizophrenia. Therefore, anti-inflammatory signaling in schizophrenia need to be studied within a context-dependent manner, which may provide opportunities for further exploration of the benefits associated with anti-inflammatory strategies for treatment.
References
Al-Diwani AAJ, Pollak TA, Irani SR, Lennox BR (2017) Psychosis: an autoimmune disease? Immunology 152:388–401. https://doi.org/10.1111/imm.12795
Altinoz MA, Ince B, Tek C, Srihari VH, Guloksuz S (2018) The NF-kappaB signaling pathway: an important therapeutic target in psychiatric disorders. Mol Psychiatry 23:490–491. https://doi.org/10.1038/mp.2016.207
Anderson G et al (2013) Immuno-inflammatory, oxidative and nitrosative stress, and neuroprogressive pathways in the etiology, course and treatment of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 42:1–4. https://doi.org/10.1016/j.pnpbp.2012.10.008
Bender R, Lange S (2001) Adjusting for multiple testing--when and how? J Clin Epidemiol 54(4):343–349
Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB (2011) Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry 168:1303–1310. https://doi.org/10.1176/appi.ajp.2011.11030516
Borovcanin M, Jovanovic I, Radosavljevic G, Djukic Dejanovic S, Bankovic D, Arsenijevic N, Lukic ML (2012) Elevated serum level of type-2 cytokine and low IL-17 in first episode psychosis and schizophrenia in relapse. J Psychiatr Res 46:1421–1426. https://doi.org/10.1016/j.jpsychires.2012.08.016
Bowden NA, Weidenhofer J, Scott RJ, Schall U, Todd J, Michie PT, Tooney PA (2006) Preliminary investigation of gene expression profiles in peripheral blood lymphocytes in schizophrenia. Schizophr Res 82:175–183. https://doi.org/10.1016/j.schres.2005.11.012
Choi GB et al (2016) The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science (New York, NY) 351:933–939. https://doi.org/10.1126/science.aad0314
Contreras F et al (2016) Dopamine receptor D3 signaling on CD4+ T cells favors Th1- and Th17-mediated immunity. Journal of immunology (Baltimore, Md : 1950) 196:4143–4149. https://doi.org/10.4049/jimmunol.1502420
Corsi-Zuelli F et al (2019) Cytokine profile in first-episode psychosis, unaffected siblings and community-based controls: the effects of familial liability and childhood maltreatment. Psychol Med:1–9. https://doi.org/10.1017/s0033291719001016
Cosentino M, Ferrari M, Kustrimovic N, Rasini E, Marino F (2015) Influence of dopamine receptor gene polymorphisms on circulating T lymphocytes: a pilot study in healthy subjects. Hum Immunol 76:747–752. https://doi.org/10.1016/j.humimm.2015.09.032
Craddock RM, Lockstone HE, Rider DA, Wayland MT, Harris LJ, McKenna PJ, Bahn S (2007) Altered T-cell function in schizophrenia: a cellular model to investigate molecular disease mechanisms. PLoS One 2:e692. https://doi.org/10.1371/journal.pone.0000692
Debnath M, Berk M (2014) Th17 pathway–mediated immunopathogenesis of schizophrenia: mechanisms and implications. Schizophr Bull 40:1412–1421. https://doi.org/10.1093/schbul/sbu049
Dimitrov DH et al (2013) Differential correlations between inflammatory cytokines and psychopathology in veterans with schizophrenia: potential role for IL-17 pathway. Schizophr Res 151:29–35. https://doi.org/10.1016/j.schres.2013.10.019
Ding M et al (2014) Activation of Th17 cells in drug naive, first episode schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 51:78–82. https://doi.org/10.1016/j.pnpbp.2014.01.001
do Prado CH, Rizzo LB, Wieck A, Lopes RP, Teixeira AL, Grassi-Oliveira R, Bauer ME (2013) Reduced regulatory T cells are associated with higher levels of Th1/TH17 cytokines and activated MAPK in type 1 bipolar disorder. Psychoneuroendocrinology 38:667–676. https://doi.org/10.1016/j.psyneuen.2012.08.005
Drexhage R, Hoogenboezem T, Cohen D, Versnel M, Nolen W, van Beveren M, Drexhage H (2011) An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychoph 14:746–755. https://doi.org/10.1017/S1461145710001653
Fang X, Zhang Y, Fan W, Tang W, Zhang C (2018) Interleukin-17 alteration in first-episode psychosis: a meta-analysis. Mol Neuropsychiatry 3:135–140. https://doi.org/10.1159/000481661
Fernandez-Egea E et al (2016) Peripheral immune cell populations associated with cognitive deficits and negative symptoms of treatment-resistant schizophrenia. PLoS One 11:e0155631. https://doi.org/10.1371/journal.pone.0155631
First MB, Spitzer RL, Goibbon M, Williams JBW (1997) Structured clinical interview for DSM-IV axis I disorders (SCID-I), clinical version, Washington, DC
Frauwirth KA, Thompson CB (2002) Activation and inhibition of lymphocytes by costimulation. J Clin Invest 109:295–299. https://doi.org/10.1172/jci14941
Gendelman HE, Appel SH (2011) Neuroprotective activities of regulatory T cells. Trends Mol Med 17:687–688. https://doi.org/10.1016/j.molmed.2011.08.005
Goldsmith DR, Rapaport MH, Miller BJ (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709. https://doi.org/10.1038/mp.2016.3
Henneberg A, Riedl B, Dumke HO, Kornhuber HH (1990) T-lymphocyte subpopulations in schizophrenic patients. Eur Arch Psychiatr Neurol Sci 239:283–284
Herberth M et al (2010) Differential effects on T-cell function following exposure to serum from schizophrenia smokers. Mol Psychiatry 15:364–371. https://doi.org/10.1038/mp.2008.120
Herberth M et al (2011) Impaired glycolytic response in peripheral blood mononuclear cells of first-onset antipsychotic-naive schizophrenia patients. Mol Psychiatry 16:848–859. https://doi.org/10.1038/mp.2010.71
Himmerich H, Schonherr J, Fulda S, Sheldrick AJ, Bauer K, Sack U (2011) Impact of antipsychotics on cytokine production in-vitro. J Psychiatr Res 45:1358–1365. https://doi.org/10.1016/j.jpsychires.2011.04.009
Huang EE et al (2017) The maintenance of telomere length in CD28+ T cells during T lymphocyte stimulation. Sci Rep 7:6785. https://doi.org/10.1038/s41598-017-05174-7
Jarskog LF (2006) Apoptosis in schizophrenia: pathophysiologic and therapeutic considerations. Curr Opin Psychiatr 19:307–312. https://doi.org/10.1097/01.yco.0000218603.25346.8f
Kelly DL et al (2018) Increased circulating regulatory T cells in medicated people with schizophrenia. Psychiatry Res 269:517–523. https://doi.org/10.1016/j.psychres.2018.09.006
Koola MM (2016) Methodological issues in cytokine measurement in schizophrenia. Indian J Psychol Med 38:6–9. https://doi.org/10.4103/0253-7176.175086
Lal G et al (2009) Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. Journal of immunology (Baltimore, Md : 1950) 182:259–273. https://doi.org/10.4049/jimmunol.182.1.259
Levite M, Marino F, Cosentino M (2017) Dopamine, T cells and multiple sclerosis (MS). J Neural Transm (Vienna, Austria 1996) 124:525–542. https://doi.org/10.1007/s00702-016-1640-4
Louveau A et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341. https://doi.org/10.1038/nature14432
Meyer U (2011) Anti-inflammatory signaling in schizophrenia. Brain Behav Immun 25:1507–1518. https://doi.org/10.1016/j.bbi.2011.05.014
Meyer U (2013) Developmental neuroinflammation and schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 42:20–34. https://doi.org/10.1016/j.pnpbp.2011.11.003
Miller BJ, Goldsmith DR (2017) Towards an immunophenotype of schizophrenia: progress, potential mechanisms, and future directions. Neuropsychopharmacology 42:299–317. https://doi.org/10.1038/npp.2016.211
Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B (2011) Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70:663–671. https://doi.org/10.1016/j.biopsych.2011.04.013
Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A (2013) Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 73:993–999. https://doi.org/10.1016/j.biopsych.2012.09.007
Najjar S, Pearlman DM (2015) Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 161:102–112. https://doi.org/10.1016/j.schres.2014.04.041
Noto C et al (2015) Effects of depression on the cytokine profile in drug naive first-episode psychosis. Schizophr Res 164:53–58. https://doi.org/10.1016/j.schres.2015.01.026
Oldenhove G et al (2009) Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31:772–786. https://doi.org/10.1016/j.immuni.2009.10.001
Osorio-Barrios F, Prado C, Contreras F, Pacheco R (2018) Dopamine receptor D5 signaling plays a dual role in experimental autoimmune encephalomyelitis potentiating Th17-mediated immunity and favoring suppressive activity of regulatory T-cells. Front Cell Neurosci 12:192. https://doi.org/10.3389/fncel.2018.00192
Prado C et al (2012) Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity. J Immunol 188:3062–3070. https://doi.org/10.4049/jimmunol.1103096
Riedel M, Spellmann I, Schwarz MJ, Strassnig M, Sikorski C, Moller HJ, Muller N (2007) Decreased T cellular immune response in schizophrenic patients. J Psychiatr Res 41:3–7. https://doi.org/10.1016/j.jpsychires.2005.11.007
Rudolf S, Schlenke P, Broocks A, Peters M, Rothermundt M, Arolt V, Kirchner H (2004) Search for atypical lymphocytes in schizophrenia. World J Biol Psychiatr 5:33–37
Russo R, Ciminale M, Ditommaso S, Siliquini R, Zotti C, Ruggenini AM (1994) Hepatitis B vaccination in psychiatric patients. Lancet 343:356
Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133:775–787. https://doi.org/10.1016/j.cell.2008.05.009
Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. https://doi.org/10.1038/nature13595
Simsek S, Yildirim V, Cim A, Kaya S (2016) Serum IL-4 and IL-10 levels correlate with the symptoms of the drug-naive adolescents with first episode, early onset schizophrenia. J Child Adolesc Psychopharmacol 26:721–726. https://doi.org/10.1089/cap.2015.0220
Sperner-Unterweger B, Whitworth A, Kemmler G, Hilbe W, Thaler J, Weiss G, Fleischhacker WW (1999) T-cell subsets in schizophrenia: a comparison between drug-naive first episode patients and chronic schizophrenic patients. Schizophr Res 38:61–70
Steiner J et al (2010) Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci 260:509–518. https://doi.org/10.1007/s00406-010-0098-x
Sullivan PF, Fan C, Perou CM (2006) Evaluating the comparability of gene expression in blood and brain. 141b:261–268. https://doi.org/10.1002/ajmg.b.30272
Trickett A, Kwan YL (2003) T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods 275:251–255. https://doi.org/10.1016/s0022-1759(03)00010-3
Vergaelen E et al (2018) A pilot study on immuno-psychiatry in the 22q11.2 deletion syndrome: a role for Th17 cells in psychosis? Brain Behav Immun 70:88–95. https://doi.org/10.1016/j.bbi.2018.03.022
Wang AK, Miller BJ (2018) Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia. Bipolar Disord Depress Schizophr Bull 44:75–83. https://doi.org/10.1093/schbul/sbx035
Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX (2007) Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 104:18169–18174. https://doi.org/10.1073/pnas.0703642104
Williams LM, Rudensky AY (2007) Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol 8:277–284. https://doi.org/10.1038/ni1437
Yang XO et al (2008) Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 29:44–56. https://doi.org/10.1016/j.immuni.2008.05.007
Author information
Authors and Affiliations
Contributions
Cigdem Sahbaz, MD, managed the conceptualization, literature searches, statistical analyses, and designing figures and wrote the first draft of the manuscript; Tunc Akkoc, PhD, planned and managed experimental analysis; Sinan Guloksuz, MD, PhD, supervised interpreting the data and writing. All authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics declarations
The Medical Ethical Review Committee of the Bezmialem Vakif University approved the study, which was conducted according to the latest version of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PPTX 49 kb)
Rights and permissions
About this article
Cite this article
Sahbaz, C., Zibandey, N., Kurtulmus, A. et al. Reduced regulatory T cells with increased proinflammatory response in patients with schizophrenia. Psychopharmacology 237, 1861–1871 (2020). https://doi.org/10.1007/s00213-020-05504-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05504-0