Abstract
Rationale
Oleoyl glycine (OlGly), a recently discovered fatty acid amide that is structurally similar to N- acylethanolamines, which include the endocannabinoid, anandamide (AEA), as well as endogenous peroxisome proliferator-activated receptor alpha (PPARα) agonists oleoylethanolamide (OEA) and palmitoylethanolamide (PEA), has been shown to interfere with nicotine reward and dependence in mice.
Objectives and methods
Behavioral and molecular techniques were used to investigate the ability of OlGly to interfere with the affective properties of morphine and morphine withdrawal (MWD) in male Sprague–Dawley rats.
Results
Synthetic OlGly (1–30 mg/kg, intraperitoneal [ip]) produced neither a place preference nor aversion on its own; however, at doses of 1 and 5 mg/kg, ip, it blocked the aversive effects of MWD in a place aversion paradigm. This effect was reversed by the cannabinoid 1 (CB1) receptor antagonist, AM251 (1 mg/kg, ip), but not the PPARα antagonist, MK886 (1 mg/kg, ip). OlGly (5 or 30 mg/kg, ip) did not interfere with a morphine-induced place preference or reinstatement of a previously extinguished morphine-induced place preference. Ex vivo analysis of tissue (nucleus accumbens, amygdala, prefrontal cortex, and interoceptive insular cortex) collected from rats experiencing naloxone-precipitated MWD revealed that OlGly was selectively elevated in the nucleus accumbens. MWD did not modify levels of the endocannabinoids 2-AG and AEA, nor those of the PPARα ligands, OEA and PEA, in any region evaluated.
Conclusion
Here, we show that OlGly interferes with the aversive properties of acute naloxone-precipitated morphine withdrawal in rats. These results suggest that OlGly may reduce the impact of MWD and may possess efficacy in treating opiate withdrawal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A recently discovered endogenous signaling lipid, N-oleoyl glycine (OlGly), that may play a role in drug withdrawal and reward processes has been identified (Donvito et al. 2019). OlGly is structurally similar to the N-acylethanolamines, which include the endocannabinoid, anandamide (AEA), as well as endogenous peroxisome proliferator-activated receptor alpha (PPARα) agonists, oleoylethanolamide (OEA), and palmitoylethanolamide (PEA) (Bradshaw et al. 2009). We (Donvito et al. 2019) have recently reported that OlGly does not bind CB1 or CB2 receptors in vitro and does not produce the tetrad (Martin et al. 1991) of behaviors (antinociception, hypothermia, catalepsy, and hypomobility) characteristic of CB1 agonists, but it weakly inhibits FAAH (IC50 8.65 μM) and also binds PPARα.
Recently, exogenous OlGly administration has been demonstrated to alter nicotine reward and withdrawal responses (Donvito et al. 2019) in mice. It has been shown that cigarette smokers following damage to the insular cortex display cessation of nicotine craving (Naqvi et al. 2007). Using an established mouse brain injury model, Donvito et al. (2019) found profound increases in OlGly in the insular cortex of brain-damaged mice. Then, they evaluated the potential of synthetic OlGly to alter nicotine reward effects and mecamylamine-precipitated withdrawal associated behaviors in nicotine-dependent mice. OlGly itself produced neither a conditioned place preference (CPP) nor a conditioned place aversion (CPA), but it interfered with both a nicotine-induced CPP and reduced precipitated withdrawal somatic responses and a withdrawal induced CPA in nicotine-dependent mice. The effects of OlGly in blocking nicotine-induced reward were mediated by PPARα receptors, not CB1 receptors; however, the mechanism by which OlGly interfered with nicotine withdrawal was not investigated. OlGly did not modify the strength of a morphine-induced CPP in mice. Therefore, Donvito et al. (2019) speculated that the release of OlGly in the insular cortex may interfere with nicotine dependence, which is consistent with a growing body of evidence that PPARα are critical for nicotine dependence (e.g., Mascia et al. 2011; Justinova et al. 2015).
Here, we extend the investigation of the effects of OlGly on drug reward and aversion by evaluating its potential to interfere with acute naloxone-precipitated morphine withdrawal (MWD)-induced CPA, morphine-induced CPP, and reinstatement of a previously extinguished morphine-induced CPP. Experimental morphine withdrawal can be produced by terminating chronic exposure to morphine or by administering an opiate antagonist to morphine-pretreated animals. Even after a single exposure to a high dose of morphine, administration of naloxone several hours later produces withdrawal symptoms in humans (Heishman et al. 1990; June et al. 1995) and other animals (Eisenberg 1982; Martin and Eades 1964). The withdrawal is apparently not only due to behavioral symptoms of abstinence but also to its ability to serve as an aversive motivational stimulus. The aversive properties of naloxone are dramatically enhanced when it is preceded 24–48 h by a high dose of morphine (20 mg/kg, sc; Parker and Joshi 1998; Parker et al. 2002; Shoblock and Maidment 2006; Wills et al. 2016). Rats injected with naloxone (1 mg/kg, sc) 24 h after saline did not display a CPA; however, rats injected with morphine 24 h prior to naloxone displayed a dramatic naloxone-induced CPA, presumably because morphine treatment promotes constitutive activity of the mu opiate receptors uncovering the aversive inverse agonist property of naloxone (Shoblock and Maidment 2006). Indeed, Wills et al. (2016) used this paradigm to investigate the role of the endocannabinod system in the regulation of the affective properties of MWD. Here, we extend the use of this paradigm to determine if OlGly would also interfere with the aversive affective properties of naloxone-precipitated MWD.
OlGly is structurally similar to endocannabinoids, and considerable evidence suggests that activation of the endocannabinoid system (eCB) ameliorates signs of opiate dependence (see Wills and Parker 2016). The eCB system consists of two receptors (CB1 and CB2), the eCBs (AEA; Devane et al. 1992 and 2-arachidonyl glycerol [2-AG]; Mechoulam et al. 1995) and the enzymes that regulate their synthesis and degradation (Ahn et al. 2008). Systemically administered AEA and 2-AG are rapidly degraded by fatty acid amide hydrolase (FAAH; Cravatt et al. 1996) and monoacylglycerol lipase (MAGL; Dinh et al. 2002), respectively. Blocking these catabolic enzymes produces a prolonged elevation of the levels of the respective eCB. Indeed, elevation of 2-AG in the insular cortex (the site of elevated OlGly by TBI in mice; Donvito et al. 2019) and in the basolateral amygdala (a region implicated in the aversive effects of MWD; Koob 2009a, b) by intracranial administration of the MAGL inhibitor, MJN110, attenuated acute naloxone-precipitated MWD-induced CPA in rats (Wills et al. 2016).
Here, we evaluated the potential of OlGly to interfere with the aversive properties associated with MWD and the rewarding effects of morphine using respective CPA and CPP paradigms. First, we evaluated the potential of OlGly administered alone to produce a CPA or CPP in rats. Given that it did not affect place conditioning, we then evaluated its potential to interfere with naloxone-precipitated MWD CPA. Having determined that OlGly prevented the MWD CPA, we next determined whether the PPARα antagonist, MK886, or the CB1 antagonist, AM251, would reverse this effect. We next evaluated the potential of OlGly to block morphine reward in a rat CPP paradigm. We also determined if OlGly would interfere with the potential of a morphine prime to reinstate a previously established morphine CPP. Finally, we evaluated the potential of naloxone-precipitated MWD to elevate OlGly and other N-acylethanolamines in the nucleus accumbens, amygdala, prefrontal cortex, and interoceptive insular cortex (regions implicated in the neurobiology of MWD, see Wills and Parker 2016).
Materials and methods
Animals
The subjects were 221 male Sprague–Dawley rats (200 to 250 g on arrival in the laboratory) purchased from Charles River Labs, St Constant, Quebec. Animals were pair-housed in an opaque Plexiglas cage while receiving food and water ad libitum. They were exposed to a 12/12-h reverse light/dark cycle where the lights turn on at 7 p.m. All experiments were conducted during the rats’ dark cycle. The colony room housing all of the rats was kept at 21 °C. All animal procedures were approved by the Animal Care Committee of the University of Guelph and adhere to the guidelines of the Canadian Council of Animal Care.
Drugs
Morphine and naloxone (OVC laboratory) were prepared with saline at a concentration of 20 and 1 mg/ml, respectively, before injecting subcutaneously (sc) at a volume of 1 ml/kg. OlGly (prepared by R. Mechoulam) and AM251 (Cayman Labs) were dissolved in a vehicle mixture of ethanol, Tween 80, and physiological saline in a 1:1:18 ratio. Oleoyl glycine and AM251 were both first dissolved in ethanol, Tween 80 was then added to the solution, and the ethanol was evaporated off with a nitrogen stream; after which, the saline was added. The final vehicle (VEH) consisted of 1:9 (Tween/saline). Oleoyl glycine was prepared at a concentration of 1 mg/mL, 5 mg/mL, or 30 mg/mL. AM251 (1 mg/kg; a dose that produces neither a place preference nor aversion, Wills et al. 2016; Sigma labs) was prepared at a concentration of 1 mg/mL. MK886 (1 mg/kg; a dose shown to prevent the effects of PPARα agonists, Rock et al. 2017; Sigma labs) was mixed in a vehicle of 1:9 Tween 80/saline and was administered intraperitoneal (ip).
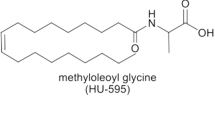
Synthesis of oleoyl glycine
To a solution of oleic acid (1 g, 3.54 mmol) and N,N-dimethylformamide (266 μL, 3.64 mmol) in dry methylene chloride (10 mL), oxalyl chloride (2.0 M solution in methylene chloride, 3.5 mL, 7 mmol) was added dropwise under nitrogen atmosphere. The reaction mixture was stirred for 1 h, and then, the solvent was evaporated under a nitrogen flow. The crude material in methylene chloride (10 mL) was added to a solution of glycine (800 mg, 10.62 mmol) and 2 N potassium hydroxide in an ice bath. Then, the reaction mixture was stirred for 1 h, water (10 mL) was added, and the mixture was acidified to pH 3 with 1 N HCl. The product was extracted with ether (3 × 50 mL) and dried (MgSO4), and solvent was evaporated under reduced pressure. The crude material was chromatographed on silica gel (eluting with chloroform: methanol) to yield a crystalline solid. Melting point 84 °C (degradation); LC-MS: (M-H)+ = 338 m/z; NMR (CDCl3, ppm): 5.9 (s, 1H), 5.35–5.32 (t, 2H), 4.06–4.04 (d, 2H), 2.28–2.23 (t, 2H), 2.01–1.98 (m, 4H), 1.66–1.62 (m, 2H), 1.30–1.26 (m, 22H), 0.9–0.85 (t, 3H). The compound is stable at room temperature and in freezer (4 °C) up to 2 years.
Extraction and quantification of OlGly, AraGly, 2-AG, AEA, OEA, and PEA
Brain tissues were frozen in liquid nitrogen immediately after dissection, which took place within 5 min from sacrifice. Tissues were dounce-homogenized and extracted with chloroform/methanol/Tris-HCl 50 mM pH 7.5 (2:1:1, v/v) containing internal deuterated standards for AEA, 2-AG, PEA, OEA, and AraGly quantification by isotope dilution (5 pmol for [2H]8AEA; 50 pmol for [2H]52-AG, [2H]4 PEA and [2H]4 OEA; 10 pmol for [2H]8AraGly). Then, the lipid extract was purified by open bed chromatography on silica. Fractions were eluted within increasing amounts of CH3OH in CHCl3 and the fraction 9:1 (v/v) was analyzed by LC-APCI-MS for AEA, 2-AG, PEA, and OEA levels, which were calculated on the basis of their area ratio with the internal deuterated standard signal areas (Bisogno et al. 1997). The 7:3 fraction was used for N-acylglycine identification and quantification by LC-MS-IT-TOF (Shimadzu Corporation, Kyoto, Japan) equipped with an ESI interface, using multiple reaction monitoring (MRM). The chromatograms of the high-resolution [M + H]+ values were extracted and used for calibration and quantification. LC analysis was performed in the isocratic mode using a Phenomenex Kintex C18 column (10 cm × 2.1 mm, 5 μm) and CH3OH/water/acetic acid (85:15:0.1 by vol.) as the mobile phase with a flow rate of 0.15 mL/min. Identification of N-acylglycines was carried out using ESI ionization in the positive mode with nebulizing gas flow of 1.5 mL/min and curved desolvation line temperature of 250 °C. OlGly was quantified using the peak of deuterated AraGly as internal standard.
Behavioral procedures
Apparatus
A place conditioning apparatus with removable floors was used as described by Wills et al. (2016). The conditioning apparatus was a rectangular box (60 × 25 × 25 cm) made of black Plexiglas and a wire mesh lid. During conditioning, removable metal floors characterized by either a hole surface (1 cm in diameter spaced 1 cm apart from each other) or a grid surface (1/2 cm horizontal bars spaced 1 cm apart) were placed upon a black rubber mat on top of the black Plexiglas surface. The different floors serve as contextual cues that differentiate the treatment floor and the VEH floor. During the test and pretest trials, black metal floors split into two equal halves (half hole and half grid surface) were placed into the conditioning boxes. The tactile stimulus properties of the two floor halves were identical to their matching floor counterparts used in conditioning. Ethovision software (Noldus, Inc., Netherlands) was used to automatically capture the movement of the rat among the floors which was collected by a video camera attached to the ceiling.
Experiment 1: potential of OlGly to produce a CPP or CPA
In each experiment, rats received a 10-min drug-free pretest trial to measure baseline floor preferences. Ethovision software tracked the movement of rats throughout the trial to determine how much time was spent on each floor. Rats with a bias of floor preference of 200 s or more were excluded from further testing, with n’s indicating the number of rats included in the conditioning trials. There were no significant differences in time spent on the hole or the grid floor in any experiment. Floors and conditioning boxes were washed between each trial.
One day following the pretest, rats received two conditioning trial cycles (as in experiments 2 and 3) with OlGly or VEH. On each trial cycle, they received ip injections of 1 mg/kg (n = 12), 5 mg/kg (n = 12), 30 mg/kg (n = 12) OlGly or VEH (24 h apart; with VEH and OlGly trial in counterbalanced order) 20 min (Donvito et al. 2019) prior to placement in the conditioning box lined with the grid or hole floor (counterbalanced) for 20 min while their locomotion was tracked by Ethovision. Three days after the final conditioning day, the rats received a 10-min drug-free test trial with the split grid/hole floor, and the time spent on each floor was automatically collected.
Experiment 2: effect of systemic OlGly on the establishment of a naloxone precipitated MWD-CPA
Following the pretest, rats received two 3-day conditioning trial cycles in order to attain a naloxone precipitated MWD-induced CPA (as described by Wills et al. 2016). On day 1 of each cycle, the floor opposite to the assigned drug floor was paired with a sc saline injection. The rats were injected (ip) with the VEH and 10 min later were injected ip with saline. Ten minutes after a saline injection, the rats were placed into the conditioning box with the assigned saline-paired floor for 20 min. On day 2 of each cycle, the rats received a high dose of morphine (20 mg/kg; sc), 24 h after the saline conditioning trial the previous day. After the injection, subjects were placed in an empty Plexiglas cage and monitored for signs of respiratory distress and stimulated when necessary until they recovered and were returned to the home cage. On day 3 of the cycle, 24 h post-morphine injections, the rats were injected with VEH (n = 12), 1 mg/kg OlGly (n = 10), 5 mg/kg OlGly (n = 10), or 30 mg/kg OlGly (n = 12) 10 min prior to receiving an sc injection of naloxone. Ten minutes later, they were placed into the conditioning box with the assigned naloxone-paired floor for 20 min. Four days later, all rats underwent a second 3-day conditioning cycle. Five days following the last naloxone trial, a 10-min drug-free test trial was performed. The test trial consisted of the same procedures as the pretest trial, but rats were given a sc saline injection 10 min prior to the test. During the test trial, Ethovision tracked the amount of time the rats spent on each floor surface.
Experiment 3: mechanism of action of OlGly interference with MWD CPA
The rats were treated exactly as in Experiment 2 except that on the naloxone trials they received an injection of VEH, MK886, or AM251 40 min (Donvito et al. 2019) prior to an injection of OlGly or VEH. Ten minutes later, they were injected with naloxone and placed in the MWD chamber. The groups were VEH-VEH (n = 12), VEH-OlGly (n = 12), MK886-VEH (n = 12), MK886-OlGly (n = 12), AM251-VEH (n = 11), and AM251-OlGly (n = 12).
Experiment 4: effect of systemic OlGly on the establishment of a morphine induced CPP
Rats received four 2-day conditioning trial cycles in order to produce a morphine-induced CPP. During each conditioning trial cycle, all rats received an sc injection of morphine (10 mg/kg) on 1 day and saline on the other day (in a counterbalanced order), 10 min prior to being placed into the conditioning chamber with a morphine- or saline-paired floor, respectively, for a duration of 30 min. On the morphine conditioning trial, the rats were administered an ip injection of VEH (n = 11), 5 mg/kg OlGly (n = 11), or 30 mg/kg OlGly (n = 10) 10 min prior to the morphine injection. On the saline conditioning trial, all rats were injected with VEH 10 min prior to the saline injection. Three days after the final conditioning day, the rats received a 10-min drug-free test trial with the split grid/hole floor. All rats received a sc administration of saline 10 min prior to each test trial.
Experiment 5: effect of systemic OlGly on the reinstatement of a previously established morphine-induced CPP
Rats received conditioning trials as in Experiment 4, except they were not given the pretreatment injections prior to each conditioning trial. Following conditioning, the rats received a total of four 10-min test/extinction trials, 24 h apart, until the place preference extinguished. Twenty-four hours following the final test/extinction trial, the rats were administered VEH (n = 12), 1 mg/kg (n = 12), or 5 mg/kg (n = 12) OlGly 10 min prior to receiving an sc injection of 5 mg/kg morphine and 10 min later were given the reinstatement test trial.
Experiment 6: ex vivo study—effect of naloxone-precipitated MWD on OlGly, AraGly, 2-AG, AEA, OEA, and PEA in the nucleus accumbens, amygdala, prefrontal cortex, and interoceptive insular cortex
Prior to tissue collection, rats were subjected to naloxone-precipitated MWD. Following 1-week habituation in the colony room on day 1, rats were given an sc injection of saline or morphine (20 mg/kg) and 24 h later (day 2) received an sc injection of saline or naloxone (1 mg/kg) creating two groups: saline (n = 6) and naloxone-precipitated MWD (n = 6). Tissue was collected 20 min after the saline or naloxone injection. The time of tissue collection was based upon the time in which rats were halfway through conditioning in the MWD-CPA experiments 2 and 3. Briefly, rats were decapitated, the brains extracted, and the bilateral nucleus accumbens, amygdala (BLA and CEA combined), prefrontal cortex, and interoceptive insular cortex were removed and flash frozen in isopentane. The tissue was stored at − 80 °C until being shipped to the DiMarzo laboratory in Naples, Italy, where it was analyzed for levels of OlGly, AraGly, 2-AG, AEA, OEA, and PEA.
Data analysis
In experiment 1, the mean sec spent on the OlGly-paired and the saline-paired floor during the preference test was entered into a 3 × 2 mixed factor analysis of variance (ANOVA) with the between-group factor of OlGly dose and the within-group factor of floor. Activity during the conditioning trials was analyzed as a 3 × 2 × 2 mixed factor ANOVA with the between-group factor of OlGly dose and the within-groups factors or drug-paired floor and trial. In experiments 2–4, the mean sec spent on the drug (MWD or morphine)-paired and the saline-paired floor by group was entered into a mixed factor ANOVA. In experiment 5, the mean sec spent on the morphine-paired floor and saline-paired floor during the test/extinction trial, the fourth test/extinction trial, and the reinstatement test trial were entered into a mixed factor ANOVA. In experiment 6, the level (pmol/g) of endogenous OlGly, AraGly, 2-AG, AEA, OEA, and PEA levels in the nucleus accumbens, amygdala, prefrontal cortex, and interoceptive insular cortex of the rats treated with MWD and saline were analyzed by independent t tests. Significance was defined as p < 0.05.
Results
Experiment 1: potential of OlGly to produce a CPP or CPA
OlGly did not produce a significant preference or aversion for the drug-paired floor. However, 30 mg/kg, but not 1 or 5 mg/kg, OlGly decreased activity during conditioning trials. Table 1 provides the mean distance (cm) traveled during conditioning on each trial and the mean seconds spent on each floor during the CPP test of experiment 1. The 3 × 2 × 2 mixed factor ANOVA of the activity data revealed significant effects of group, F(2, 33) = 6.6, p < 0.01; conditioning cycle, F(1, 33) = 6.5, p < 0.01; drug trial, F(1, 33) = 17.5, p < 0.001; group × drug trial, F(2, 33) = 18.2, p < 0.001. Subsequent paired t tests revealed that group 30 mg/kg OlGly (t(11) = 5.2; p < 0.001), but not 1 or 5 mg/kg OlGly, displayed less activity on the pooled OlGly trials than on the pooled VEH trials. For the CPP test, the 3 × 2 mixed factor ANOVA revealed no significant effects (group × floor, F(2,33) = 2.0).
Experiment 2: effect of systemic OlGly on the establishment of a MWD-CPA
At 1 and 5 mg/kg, ip, but not 30 mg/kg, ip, OlGly significantly interfered with the establishment of the naloxone-precipitated MWD-induced CPA. Figure 1 presents the mean (± SEM) number of seconds spent on the saline floor and the MWD floor among the OlGly pretreatment groups. . The mean seconds spent on the saline paired floor and the MWD-paired floor were entered into a 4 × 2 mixed factor ANOVA with the between-group factor of OlGly dose and the within-group factor of floor. The analysis revealed a significant main effect of floor, F(1, 40) = 17.1, p < 0.001 and a dose × floor interaction, F(3, 40) = 3.0; p = 0.043. The interaction was assessed by paired t tests for the floor among each dose group; group VEH (p < 0.001) and 30 mg/kg OlGly (p < 0.05) spent significantly less time on the MWD-paired floor than the saline-paired floor, but not group 1 or 5 mg/kg OlGly.
Mean (± SEM) time spent in seconds on the saline-paired floor and the MWD-paired floor during the drug-free test trial of experiment 2 by rats treated with VEH (n = 12), 1 mg/kg OlGly (n = 10), 5 mg/kg OlGly (n = 10), or 30 mg/kg OlGly (n = 12) during each MWD trial in experiment 2. Asterisks indicate a significant difference between the saline and morphine withdrawal paired floors. *p < 0.05, ***p < 0.001
Experiment 3: mechanism of action of OlGly interference with MWD CPA
The CB1 antagonist, AM251, but not the PPARα antagonist, MK886, prevented OlGly interference of naloxone-precipitated MWD-induced place aversion. Figure 2 presents the mean number of seconds that the rats in each group spent on the MWD-paired floor and the saline-paired floor. The 6 × 2 ANOVA revealed a significant effect of floor, F(1, 65) = 65.6; p < 0.001, and a group by floor interaction, F(3, 65) = 3.5; p < 0.01, subsequent paired t tests for each group revealed that, except for group VEH-OlGly and MK886-OlGly, all groups displayed a significant (ps < 0.001) aversion to the MWD-paired floor. Therefore, the CB1 antagonist interfered with the suppression of MWD by OlGly, whereas the PPARα antagonist did not.
Mean (± SEM) time spent in seconds on the saline-paired floor and the MWD-paired floor during the drug-free test trial by rats treated with VEH, AM251(1 mg/kg), or MK886 (1 mg/kg) 40 min prior to VEH or OlGly (5 mg/kg) during each MWD trial in experiment 2 (n’s = 11–12/group). Asterisks indicate a significant difference between the saline and morphine withdrawal paired floors. ***p < 0.001
Experiment 4: effect of OlGly on the establishment of a morphine- induced CPP
At 5 or 30 mg/kg, OlGly did not modify the establishment of a morphine-induced CPP. Figure 3 presents the mean (± SEM) number of sec spent on the saline-paired and the MWD-paired floor during the drug-free test trial by rats that received VEH, 5 mg/kg, or 30 mg/kg OlGly during each MWD conditioning trial in experiment 4. A 3 × 2 mixed factor ANOVA with between-group factor of pretreatment drug (VEH, 5 mg/kg OlGly, 30 mg/kg OlGly) and the within-group factor of floor (morphine, saline) revealed a significant effect of floor, F(1, 31) = 5.62, p = 0.025, but no significant pretreatment drug by floor interaction. Overall, all rats displayed a morphine-induced CPP, but systemic OlGly administrations did not alter that preference.
Mean (± SEM) time spent in seconds on the saline-paired floor and the morphine-paired floor during the drug-free test trial by rats that received VEH (n = 11), 5 mg/kg OlGly (n = 11), or 30 mg OlGly (n = 10) during the morphine conditioning trial in experiment 4. Asterisks indicate a significant overall preference for the morphine paired floor across groups. *p = 0.025
Experiment 5: effect of OlGly on the reinstatement of a previously extinguished morphine-induced CPP
OlGly did not interfere with a morphine-prime reinstatement of an extinguished CPP. Figure 4 presents the mean sec spent on the morphine-paired floor and saline-paired floor on the first test/extinction trial (top section), the final (fourth) test/extinction trial (middle section), and on the reinstatement trial (bottom section) of experiment 5. A 3 × 2 mixed factor ANOVA for the first test trial revealed only a significant effect of floor, F(1, 33) = 43.4; p < 0.001, with rats showing a preference for the morphine-paired floor. On the final, 4th, test/extinction trial, the 3 × 2 mixed factor ANOVA revealed no significant effects (floor, F[1, 33] = 0.5). However, on the following day, a morphine-prime reinstated the morphine CPP in all groups; the 3 × 2 mixed factor ANOVA revealed only a significant effect of floor, F(1,33) = 49.2; p < 0.001.
Experiment 6: ex vivo study—effects of naloxone-precipitated MWD on endogenous OlGly, AraGly, 2-AG, AEA, OEA, and PEA levels in the nucleus accumbens, amygdala, prefrontal cortex, and interoceptive insular cortex
Naloxone-precipitated MWD dramatically elevated OlGly in the nucleus accumbens and slightly elevated AraGly in the amygdala. There were no significant effects of MWD on endocannabinoid (AEA and 2-AG) or N-acylethanolamine (OEA and PEA) levels in any brain region. Figure 5 presents the mean OlGly (5A) and AraGly (5B) pmol/g of tissue measured from the nucleus accumbens, amygdala, prefrontal cortex, and the interoceptive insular cortex of rats pretreated with saline- or naloxone-precipitated MWD. Independent t tests revealed that rats treated with naloxone-precipitated MWD displayed significantly greater levels of OlGly in the nucleus accumbens (mean ± SEM = 533 ± 77 pmol/g) than rats treated with saline (mean ± SEM = 216 ± 30 pmol/g), t(10) = 4.1; p = 0.002. Additionally, rats treated with naloxone-precipitated MWD (mean = 283 ± 27 pmol/g) displayed slightly higher levels of AraGly (mean = 193.3 ± 25 pmol/g) in the amygdala (but no other brain region) than those treated with saline, t(10) = 2.4; p = 0.034. Table 2 presents the mean pmol/g of tissue levels of 2-AG (5A), AEA (5B), OEA (5C), and PEA (5D) in each brain region measures of rats treated with saline or naloxone-precipitated MWD. There were no significant differences of these lipids in any brain region (Fig. 5).
Discussion
Although ip administration of OlGly alone was neither aversive nor rewarding, 1 and 5 mg/kg, but not 30 mg/kg, OlGly interfered with the aversive effects of naloxone-precipitated MWD-induced CPA learning in Sprague–Dawley rats. The observation that 30 mg/kg OlGly produced locomotor inhibitory effects, but did not interfere with naloxone-precipitated MWD, suggests a biphasic effect on the aversive affective properties of MWD. This apparent inverted U-shaped curve is not uncommon for endocannabinoids, such as anandamide (Sulcova et al. 1998). The finding that AM251, but not MK866, blocked OlGly attenuation of the aversive effects of MWD indicates a CB1 receptor mechanism of action. It is important to note that Donvito et al. (2019) did not evaluate the effect of either a CB1 or PPARα antagonist on the actions of OlGly on nicotine withdrawal. However, they did demonstrate that OlGly attenuation of CPP was mediated by PPARα (Donvito et al. 2019). The failure of MK866 to reverse the MWD-induced CPA should be interpreted cautiously, as only a single dose was examined. Nonetheless, this dose of MK866 significantly reversed the anti-nausea effects of FAAH inhibitors (Rock et al. 2015, 2017). Consistent with the report of Donvito et al. (2019) in mice, OlGly (5 and 30 mg/kg, ip) did not modify morphine CPP learning in rats. Here, we extended this work by demonstrating that the OlGly (1 and 5 mg/kg, ip) did not modify the potential of a morphine prime to reinstate a previously extinguished morphine CPP. Its effects appear to be selective to the aversive properties of MWD.
Donvito et al. (2019) reported that OlGly did not produce CB1 receptor mediated effects either in vivo (e.g., mouse “tetrad” test of cannabimimetic activity) or in vitro (CB1 and CB2 binding assays). Additionally, OlGly did not exert positive allosteric modulatory activity in vivo, although it weakly inhibited FAAH. Thus, it is possible that indirect activation of CB1 receptors subsequent to inhibition of AEA inactivation contributed to the effects of OlGly observed in the present study. Two arguments are consistent with this hypothesis: (1) even potent FAAH inhibitors do not exert cannabimimetic activity in the mouse “tetrad”; (2) FAAH inhibitors reduce the symptoms of morphine withdrawal (Ramesh et al. 2011; Gamage et al. 2015; Manwell et al. 2009) without affecting morphine reward (Manwell et al. 2009; Luchicchi et al. 2010), or the priming effect of morphine to reinstate a previously extinguished CPP (McCallum et al. 2010).
Considerable evidence implicates the action of the endocannabinoid system in regulating the aversive effects of MWD in animal models (Wills et al. 2016). THC (Bhargava 1976a, b), AEA (Vela et al. 1995), and 2-AG (Yamaguchi et al. 2001) reduce the intensity of MWD somatic symptoms in mice. Ramesh et al. (2011) reported that both FAAH (PF-3845) and MAGL (JZL184) inhibitors significantly attenuated naloxone-precipitated MWD somatic symptoms in mice, although the MAGL inhibitor was more effective than was the FAAH inhibitor. Additionally, MAGL (but not FAAH inhibition [Wills et al. 2014]) blocked the naloxone-precipitated MWD-induced place aversion in rats (Wills et al. 2016). The effect of MAGL inhibition on MWD-induced place aversion was mediated by elevated 2-AG in the BLA and in the interoceptive insular cortex (IC) (Wills et al. 2016), which have both been implicated in the negative reinforcement associated with MWD (Koob 2009a, b; Contreras et al. 2007; Naqvi et al. 2007). Indeed, Li et al. (2013) reported that inactivation of the interoceptive IC prevents the acquisition of naloxone precipitated MWD CPA. Therefore, it is conceivable that the release of eCBs (AEA and 2-AG) and related lipids (such as OlGly) in the interoceptive IC may homeostatically regulate the impact of opiate withdrawal.
Our finding that OlGly is significantly increased in the nucleus accumbens (an area implicated in MWD; Koob 2009a, b) in rats undergoing acute naloxone-precipitated MWD suggests that it may play a neuroprotective role in overcoming the aversive properties of withdrawal in these animals. Future research with intracranial delivery of OlGly directly to the nucleus accumbens shell and core is warranted. On the other hand, AraGly was moderately elevated in the amygdala, a region implicated in the emotional regulation of drug withdrawal (Koob 2009a, b). This compound has been implicated in anti-inflammatory effects (Burstein et al. 2011) and neuroprotection (Cohen-Yeshurun et al. 2011). More importantly, AraGly inhibits FAAH more potently than OlGly (Arreaza et al. 1997; Huang et al. 2001; Bradshaw et al. 2009), and this effect, if exerted in the amygdala, may lead to facilitated extinction (Marsicano et al. 2002; Varvel et al. 2007; Manwell et al. 2009) and enhanced acquisition (Wise et al. 2009) of aversive memories and anxiolytic responses (Naidu et al. 2007; Scherma et al. 2008; Hill et al. 2010). Therefore, we cannot rule out a protective role also for this mediator in MWD, exerted at the level of the amygdala. However, given the lack of groups of rats treated with saline-naloxone and morphine-saline, these data should be considered preliminary. We are planning a complete evaluation (with all controls) of OlGly and other N-acylethanolamines released in the nucleus accumbens, amygdala, prefrontal cortex, and interoceptive insular cortex by naloxone-precipitated MWD, as well as the effect of exogenous OlGly treatment on these effects.
Several issues remain unresolved regarding the potential of OlGly to modify MWD and nicotine reward and dependence in rodent models. Indeed, although OlGly interfered with nicotine-induced place preference through a PPARα mechanism in mice, the mechanism by which OlGly interferes with nicotine withdrawal has not been evaluated (Donvito et al. 2019). It will be of interest to determine if OlGly also interferes with the aversive affective properties of naloxone-precipitated MWD in mice (as it does in rats) and if so, whether it is CB1 mediated. Extending the findings from MWD produced by acute morphine to chronic morphine may facilitate cross-species comparisons. It is also unknown if OlGly interferes with nicotine reward in rat models by a PPARα mechanism. It will be of interest to determine if OlGly interferes with the display of somatic MWD effects, as it does affective MWD effects, which are mediated by different neural circuitries (Koob 2009a, b). The affective signs of MWD produced by chronic exposure to morphine via osmotic minipumps are elicited by lower doses of antagonists than the classic physical manifestations of withdrawal (Gellert and Sparber 1977; Higgins and Sellers 1994; Schulteis et al. 1994). Finally, it will be of interest to examine whether OlGly affects the development of tolerance to effects of morphine.
Conclusion
The observations that OlGly interferes with nicotine withdrawal and nicotine reward in mice (Donvito et al. 2019) as well as acute naloxone-precipitated MWD in rats shown here suggest that it may play a role in protecting against the dysregulating effects of drugs of abuse. That is, the mammalian body may react to drug-induced dysregulation by synthesizing an endogenous compound (OlGly) to combat it. Thus, OlGly may represent an endogenous regulatory agent to reduce the impact of nicotine rewarding effects and opiate and nicotine withdrawal aversive effects on the mammalian brain in a similar manner that OEA has been reported to protect against the impact of alcoholism (Bilbao et al. 2016).
References
Ahn K, McKinney MK, Cravatt BF (2008) Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev 108(5):1687–1707
Arreaza G, Devane WA, Omeir RL, Sajnani G, Kunz J, Cravatt BF, Deutsch DG (1997) The cloned rat hydrolytic enzyme responsible for the breakdown of anandamide also catalyzes its formation via the condensation of arachidonic acid and ethanolamine. Neurosci Lett 234:59–62
Bhargava HN (1976a) Inhibition of naloxone-induced withdrawal in morphine dependent mice by l-trans-Δ9-tetrahydrocannabinol. Eur J Pharmacol 36:259–262
Bhargava HN (1976b) Inhibition of naloxone-induced withdrawal in morphine dependent mice by 1-thetrahydrocannabinol. Eur J Pharmacol 36:259–262
Bilbao A, Serrano A, Cippitelli A, Pavón FJ, Giuffrida A, Suárez J, Garcia-Marchena N, Baixeras E, Gomez de Heras R, Ciccocioppo R, Cravatt BF, Parsons LH, Piomelli D, Rodriguez de Fonseca F (2016) Role for the satiety factor Oleoylethanolamide in alcoholism. Addict Biol 21:859–872
Bisogno T, Sepe N, De Petrocellis L, Di Marzo V (1997) Biosynthesis of 2-arachidonyl-glycerol, a novel cannabimimetic eicosanoid, in mouse neuroblastoma cells. Adv Exp Med Biol 433:201–204
Bradshaw HB, Rimmerman N, Hu SSJ, Burstein S, Walker JM (2009) Novel endogenous N-acyl glycines. Identification and characterization. Vitam Horm 81:191–205
Burstein SH, McQuain CA, Ross AH, Salmonsen RA, Zurier RE (2011) Resolution of inflammation by N-arachidonoylglycine. J Cell Biochem 112:3227–3233
Cohen-Yeshurun A, Trembovler V, Alexandrovich A, Ryberg E, Greasley PJ, Mechoulam R, Shohami E, Leker RR (2011) N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis. J Cereb Blood Flow Metab 31:1768–1777
Contreras M, Ceric F, Torrealba F (2007) Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 318:655–658
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87
Devane WA, Hanuš L, Breuer A, Pertwee RG, Lesley A, Griffin G, Gibson D, Mandelbaum A, Etinger A, Stevenson LA (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D (2002) Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A 99:10819–10824
Donvito G, Piscitelli F, Muldoon P, Jackson A, Vitale RM, D’Aniello E, Giordano C, Ignatowska-Jankowska BM, Mustafa MA, Guida F, Petrie GN, Parker L, Smoum R, Sim-Selley L, Maione S, Lichtman AH, Damaj MI, Di Marzo V, Mechoulam R (2019) N-Oleoyl-glycine reduces nicotine reward and withdrawal in mice. Neuropharmacology 148:320–321
Eisenberg RM (1982) Further studies on the acute dependence produced by morphine in opiate naive rats. Life Sci 31:1531–1540
Gamage TF, Ignatowska-Jankowska BM, Muldoon PP, Cravatt BF, Damaj MI, Lichtman AH (2015) Differential effects of endocannabinoid catabolic inhibitors on morphine withdrawal in mice. Drug Alcohol Depend 146:7–16
Gellert VF, Sparber SB (1977) A comparison of the effects of naloxone upon body weight loss and suppression of fixed-ratio operant behavior in morphine-dependent rats. J Pharmacol Exp Ther 201:44–54
Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA (1990) Acute opioid physical dependence in humans: effect of naloxone at 6 and 24 hours postmorphine. Pharmacol Biochem Behav 36:393–399
Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TTY, Gray JM, Hillard CJ, Gorzalka BB, Viau V (2010) Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A 107:9406–9411
Higgins GA, Sellers EM (1994) Antagonist-precipitated opioid withdrawal in rats: evidence for dissociations between physical and motivational signs. Pharmacol Biochem Behav 48:1–8
Huang SM, Bisogno T, Petros TJ, Chang SY, Zavitsanos PA, Zipkin RE, Sivakumar R, Coop A, Maeda DY, De Petrocellis L et al (2001) Identification of a new class of molecules, the arachidonyl amino acids,and characterization of one member that inhibits pain. J Biol Chem 276:42639–42644
June HL, Stitzer ML, Cone E (1995) Acute physical dependence: time course and relation to human plasma morphine concentrations. Clin Pharmacol Ther 57:270–280
Justinova Z, Panlilio LV, Moreno-Sanz G, Redhi GH, Auber A, Secci ME, Mascia P, Bandiera T, Armirotti A, Bertorelli R, Chefer SI, Barnes C, Yasar S, Piomelli D, Goldberg SR (2015) Effects of fatty acid amide hydrolase (FAAH) inhibitors in non-human primate models of nicotine reward and relapse. Neuropsychopharmacology 40:2185–2197
Koob GF (2009a) Brain stress systems in the amygdala and addiction. Brain Res 1293:61–75
Koob GF (2009b) Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry 42(Suppl 1):S32–S41
Li CL, Zhu N, Meng XL, Li YH, Sui N (2013) Effects of inactivating the agranular or granular insular cortex on the acquisition of the morphine-induced conditioned place preference and naloxone-precipitated conditioned place aversion in rats. J Psychopharmacol 27:837–844
Luchicchi A, Lecca S, Carta S, Pillolla G, Muntoni AL, Yasar S, Goldberg SR, Pistis M (2010) Effects of fatty acid amide hydrolase inhibition on neuronal responses to nicotine, cocaine and morphine in the nucleus accumbens shell and ventral tegmental area: involvement of PPAR-α nuclear receptors. Addict Biol 15:277–288
Manwell LA, Satvat E, Lang ST, Allen CP, Leri F, Parker LA (2009) FAAH inhibitor, URB-597, promotes extinction and CB1 antagonist, SR141716, inhibits extinction of conditioned aversion produced by naloxone-precipitated morphine withdrawal, but not extinction of conditioned preference produced by morphine in rats. Pharmacol Biochem Behav 94:154–162
Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascioll MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:530–534
Martin WR, Eades CG (1964) A comparison between acute and chronic physical dependence in the chronic spinal dog. J Pharmacol Exp Ther 146:385–394
Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ (1991) Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav 40:471–478
Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le Foll B, Tanda G, Piomelli D, Goldberg SR (2011) Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry 69:633–641
McCallum AL, Limebeer CL, Parker LA (2010) Reducing endocannabinoid metabolism with the fatty acid amide hydrolaseinhibitor, URB597, fails to modify reinstatement of morphine-induced conditioned floor preference and naloxone-precipitated morphine withdrawal-induced conditioned floor avoidance. Pharmacol Biochem Behav 96:496–500
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90
Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH (2007) Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology 192:61–70
Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007) Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534
Parker LA, Cyr JA, Santi AN, Burton PD (2002) The aversive properties of acute morphine dependence persist 48 h after a single exposure to morphine: evaluation by taste and place conditioning. Pharmacol Biochem Behav 72:87–92
Parker LA, Joshi A (1998) Naloxone-precipitated morphine withdrawal induced place aversions: effect of naloxone at 24 hours postmorphine. Pharmacol Biochem Behav 61:331–333
Ramesh D, Ross GR, Schlosburg JE, Owens R, Abdullah R, Kinsey SG, Long JZ, Nomura DK, Sim-Selley LJ, Cravatt BF, Akbarali HI, Lichtman AH (2011) Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther 339:173–185
Rock EM, Limebeer CL, Ward JM, Cohen A, Grove K, Niphakis MJ, Cravatt BF, Parker LA (2015) Fatty acid amide hydrolase (FAAH) inhibition interferes with acute nausea by a PPARα mechanism and anticipatory nausea by a CB1 receptor mechanism in a double dissociation. Psychopharmacology 232:3841–3848
Rock EM, Guillermo MS, Limebeer CL, Petrie G, Angelini R, Piomelli D, Parker LA (2017) Suppression of acute and anticipatory nausea by peripherally restricted FAAH inhibitor in animal models: role of PPARa and CB1 receptors. Br J Pharmacol 174:3837–3847
Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, Mikics E, Haller J, Yasar S, Tanda G, Goldberg SR (2008) The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology 54:129–140
Schulteis G, Markou A, Gold LH, Stinus L, Koob GF (1994) Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantiative dose-response analysis. J Pharmacol Exp Ther 271:1391–1398
Shoblock JR, Maidment NT (2006) Constitutively active mu opioid receptors mediate the enhanced conditioned aversvie effect of naloxone in morphine-dependent mice. Neuropsychopharmacology 31:171–177
Sulcova E, Mechoulam R, Fride E (1998) Biphasic effects of anandamide. Pharmacol Biochem Behav 59:347–352
Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH (2007) Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology 32:1032–1041
Vela G, Ruiz-Gayo M, Fuentes JA (1995) Anandamide decreases naloxone-precipitated withdrawal signs in mice chronically treated with morphine. Neuropharmacology 34:665–668
Wills KL, Parker LA (2016) Effect of pharmacological modulation of the endocannabinoid system on opiate withdrawal: a review of the preclinical animal literature. Front Pharmacol 7:1–9
Wills KL, Petrie GN, Millett G, Limebeer CL, Rock EM, Niphakis MJ, Cravatt BF, Parker LA (2016) Double dissociation of monoacylglycerol lipase inhibition and CB1 antagonism in the central amygdala, basolateral amygdala, and the interoceptive insular cortex on the affective properties of acute naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology 41:1865–1873
Wills KL, Vemuri K, Kalmar A, Lee A, Limebeer CL, Makriyannis A, Parker LA (2014) CB1 antagonism: interference with affective properties of acute naloxone-precipitated morphine withdrawal in rats. Psychopharmacology 231:4291–4300
Wise LE, Harloe JP, Lichtman AH (2009) Fatty acid amide hydrolase (FAAH) knockout mice exhibit enhanced acquisition of an aversive, but not of an appetitive, Barnes maze task. Neurobiol Learn Mem 92:597–601
Yamaguchi T, Hagiwara Y, Tanaka H, Sugiura T, Waku K, Shoyama Y, Watanabe S, Yamamoto T (2001) Endogenous cannabinoid, 2-arachidonoylglycerol, attenuates naloxone-precipitated withdrawal signs in morphine-dependent mice. Brain Res 909:121–126
Funding
The research reported here was funded by research grants from the Natural Sciences and Engineering Research Council (NSERC 920157) and the Canadian Institutes for Health Research (CIHR 388239) to LAP, NIH grants R01DA039942, P30DA033934, and VCU School of Pharmacy start-up funds to AHL.
Author information
Authors and Affiliations
Contributions
GP, KW, ER, MS, and AH performed behavioral experiments. FP and VD performed the molecular analyses. RS and RM synthesized OlGly. CL prepared all drugs and collected all tissues. GP, KW, AL, VD, RM, and LP designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
All animal procedures were approved by the Animal Care Committee of the University of Guelph and adhere to the guidelines of the Canadian Council of Animal Care.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Petrie, G.N., Wills, K.L., Piscitelli, F. et al. Oleoyl glycine: interference with the aversive effects of acute naloxone-precipitated MWD, but not morphine reward, in male Sprague–Dawley rats. Psychopharmacology 236, 2623–2633 (2019). https://doi.org/10.1007/s00213-019-05237-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05237-9