Abstract
Rationale
Sleep difficulties are one of the problems associated with adolescent binge drinking. However, the mechanisms underlying adolescent alcohol-associated sleep disturbances and potential targets for therapy remain under investigated. Orexin receptor antagonists may have therapeutic value in the treatment of insomnia, yet the use of this class of drugs in the treatment of sleep disturbances following adolescent alcohol exposure has not been studied.
Objectives
This study employed a model whereby ethanol vapor exposure occurred for 5 weeks during adolescence (AIE), and waking event-related oscillations (EROs) and EEG sleep were subsequently evaluated in young adult rats. The ability of two doses (10, 30 mg/kg PO) of a dual orexin receptor antagonist (DORA-12) to modify sleep, EEG, and EROs was investigated in AIE rats and controls.
Results
Adolescent vapor exposure was found to produce a fragmentation of sleep, in young adults, that was partially ameliorated by DORA-12. DORA-12 also produced increases in delta and theta power in waking EROs recorded before sleep, and deeper sleep as indexed by increases in delta and theta power in the sleep EEG in both ethanol and control rats. Rats given DORA-12 also fell asleep faster than vehicle-treated rats as measured by a dose-dependent reduction in the latency to both the first slow wave and REM sleep episodes.
Conclusions
This study showed that DORA-12 can affect the sleep disturbance that is associated with a history of adolescent ethanol exposure and also has several other sleep-promoting effects that are equivalent in both ethanol and control rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disturbance in sleep regulation is one of the health risks associated with adolescent alcohol and drug use. Adolescence is a time when both drug and alcohol-seeking behaviors and disturbances in sleep emerge; and adolescents may be particularly vulnerable to sleep disturbance associated with substance use (Bartel et al. 2015; Hasler and Clark 2013; Hasler et al. 2014a; Hasler et al. 2013; Hasler et al. 2014b, 2015).
Recently, animal models of alcohol-induced insomnia have been developed that allow for study of the effects of ethanol on sleep independent of factors that may confound human studies, such as premorbid conditions and other substance use. Investigations conducted in our laboratory as well as others, in rodents, have shown that chronic ethanol exposure can produce persistent and long-term changes in sleep, similar to what has been reported in humans with alcohol use disorders (AUD) (Criado and Ehlers 2010; Ehlers et al. 2013a; Ehlers and Slawecki 2000; Sanchez-Alavez et al. 2018; Thakkar et al. 2015). More recently, an animal model of sleep disturbance, resulting from alcohol administration during adolescence, has been developed (see (Criado et al. 2008b; Ehlers et al. 2011; Ehlers et al. 2018)). We have shown that alcohol exposure during adolescence, in this rat model, can result in persistent electrophysiological, behavioral, and neuroanatomical deficits in young adulthood (see (Ehlers et al. 2011; Ehlers et al. 2014; Ehlers et al. 2013b, c)) that parallel findings seen in human adolescent binge drinkers (Ehlers et al. 2019; Hanson et al. 2011; McQueeny et al. 2009; Schweinsburg et al. 2011; Squeglia et al. 2009). Although these animal models have been developed, they have been little used to study therapeutic targets.
A recent body of literature supports a prominent role of the hypothalamic peptide hypocretin/orexin (Hct/OX) in homeostatic control of a number of regulatory processes (see (de Lecea 2012; Li et al. 2014)). The Hct/OX system has not only been suggested to be an important regulator of the sleep wake cycle (Hoyer and Jacobson 2013; Inutsuka and Yamanaka 2013; Krystal et al. 2013; Mieda et al. 2013), but has also been shown to influence a range of other physiological processes including feeding and energy metabolism, reward/motivated behavior, the modulation of stress responses (Baldo et al. 2003; Cason et al. 2010; DiLeone et al. 2003; Martin-Fardon et al. 2010; Xu et al. 2013), as well as ethanol-seeking and drinking behaviors (Anderson et al. 2014; Brown et al. 2013; Brown et al. 2016; Jupp et al. 2011a, b; Kastman et al. 2016; Kim et al. 2012; Lawrence 2010; Lawrence et al. 2006; Martin-Fardon and Weiss 2012; Moorman 2018; Moorman and Aston-Jones 2009; Moorman et al. 2017; Srinivasan et al. 2012; Ubaldi et al. 2016; Walker and Lawrence 2017). However, few studies have investigated the ability of the Hct/OX system to modify alcohol-related sleep pathology (Sanchez-Alavez et al. 2019).
In the present study, we used an animal model of adolescent alcohol exposure to study the effects of two doses of a newly developed dual orexin receptor antagonist (DORA-12) on waking and sleep physiology (Gotter et al. 2014; Ramirez et al. 2013). For the evaluation of DORA-12 on waking electrophysiology, we used measures of event-related oscillations (EROs). EROs are oscillatory changes within the dynamics of ongoing EEG rhythms that are enhanced or synchronized by a time-locked sensory and/or cognitive stimulus (see (Anokhin 2014; Basar et al. 2000; Klimesch et al. 2007; Roach and Mathalon 2008)). EROs have been demonstrated to be sensitive measures of normal (Basar et al. 1999; Schack and Klimesch 2002) and abnormal cognitive functioning, as well as more importantly, endophenotypes for alcohol use disorders (Ehlers et al. 2015; Pandey et al. 2012; Rangaswamy and Porjesz 2014). We also studied the effects of this drug on sleep measures and the spectral content of the sleep EEG during slow wave (SW) and rapid eye movement (REM) sleep in young adult rats who experienced alcohol vapor or control conditions during their adolescence.
Materials and methods
Animal subjects
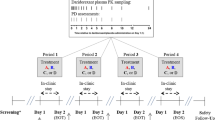
Forty-four adolescent male Wistar rats (Charles River, USA) arrived with their dams on postnatal day (PD) 21. They were triple-housed under a 12 h light/dark cycle (lights on 08:00), with water and food available ad libitum. The study was approved by The Scripps Research Institute’s Animal Care and Use Committee and adheres to the guidelines outlined in the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised 1996).
Ethanol vapor exposure
The methodology for ethanol vapor inhalation during adolescence has been previously described (see (Ehlers et al. 2011; Ehlers et al. 2013a; Ehlers et al. 2013c)). The chambers used to expose rats to ethanol were titrated to produce constant levels of high to moderate blood ethanol concentrations (BECs) between 175 and 225 mg/dL, which are levels that are similar to binge drinking seen in adolescence in some studies (Patrick et al. 2013). Rats in the ethanol group were exposed to vaporized 95% ethanol from 8 p.m. to 10 a.m. daily for a 5-week period (P22-57). Tail blood samples were collected during this time every 3–4 days to assess BECs (5-week average = 215.53 ± 6.45 mg/dL). Rats in the control group were handled identically including tail blood collection. An Analox micro-statAM1 (Analox Instr. Ltd., Lunenberg, MA) was used to estimate BECs. After the 5-week ethanol or control exposure, rats were housed in standard cages for the rest of the experiment.
Surgical procedure
The surgical procedures performed in this study have been previously described elsewhere (Ehlers et al. 2013b; Ehlers et al. 2018; Ehlers and Slawecki 2000). Briefly, rats were implanted (PD 55-71) with two screw electrodes in the calvarium, one overlying the frontal cortex (FCTX, AP: 1.5 mm, ML: ± 3.0 mm, FR1), and another over the parietal cortex (PCTX, AP: − 4.5 mm, ML: ± 4.5 mm) with a reference implanted over lambda, guided by the (Paxinos and Watson 1986) atlas. A multi-pin Plastics One® connector was used to make electrical connections.
Electrophysiological recordings
Two weeks after recovery from surgery, rats were habituated to the electrophysiological recording conditions. All ERO/EEG sessions began at 08:00. The EEG was recorded from the two leads (frontal cortex and parietal cortex) that were referenced to the lambda ground using a Sensorium preamplifier/amplifier unit (Shelburne, VT). Signals were digitized at a rate of 256 Hz using software described previously.
ERO collection and a 5 h EEG sleep recording were obtained for each session. EROs were elicited by auditory stimuli that were presented through a small speaker centered approximately 70 cm above the rat’s head. EROs were elicited by an acoustic “oddball” plus noise paradigm. The acoustic parameters were three square wave tones (rise/fall times, 1 ms): a frequent tone (50 ms, 2 kHz, 70 dB SPL) presented on 83% of the trials (n = 259), an infrequent tone (50 ms, 2 kHz, 80 dB SPL) presented on 10.3% of the trials (n = 32), and a noise burst (50 ms, noise, 100 dB SPL) presented on 6.7% of the trials (n = 21). Signals were digitized at a rate of 256 Hz and transferred to a PC for offline analyses. Immediately following the ERO session, the 5-h sleep EEG was obtained.
Acute DORA-12 administration
At PD 92, the pharmacology experiments were begun. Rats were randomized for the order of the dose: vehicle or DORA-12 (Merck): low dose (10 mg/kg) or high dose (30 mg/kg) using a Latin square design. The vehicle for this compound was a Vitamin E TPGS (D-a-Tocopherol polyethylene glycol 1000 succinate) 20% solution, in sterile culture grade water that was sonicated for 30 min prior to administration. To maximize bioavailability of the compound, vehicle or DORA-12 was administered by oral gavage. Intragastric administration was performed by gently inserting a ball-tipped stainless steel curved needle into the esophagus. Approximately 60 min post-gavage, the 5-h sleep recordings were begun. To avoid carry over effects, at least a week elapsed between drug doses. The vehicle was given in an equivalent volume to the DORA-12 doses.
Event-related oscillation analyses
Event-related oscillation (ERO) energy (peak magnitude of the S transform output, squared, in a time frequency region of interest) was obtained from the auditory paradigm for each stimulus. Methods for these analyses have been described in detail elsewhere (Ehlers and Criado 2009; Ehlers et al. 2012). The ERO trials were digitized at a rate of 256 Hz. Trials containing excessive artifact were eliminated prior to averaging (< 5% of the trials). An artifact rejection program was utilized to eliminate individual trials in which the EEG exceeded ± 400 μV. Data from single trials generated by the stimuli were entered into the time frequency analyses algorithm. The S-transform (ST), a generalization of the Gabor transform (Gabor 1946), was used.
To quantify S-transform magnitudes, a region of interest (ROI) was identified by specifying the band of frequencies and the time interval contained in the rectangular ROI. The time-frequency points saved from each S transformation are from 100 ms before to 900 ms after the onset of the stimulus, and from 1 Hz through 50 Hz at intervals of 0.5 Hz. Energy is the square of the magnitude of the S-transform output in a time frequency region of interest. The S-transform magnitude squared for a time/frequency interval is proportional to volts squared.
Rectangular regions of interest (ROIs) were defined by specifying, for each ROI, a time interval relative to the stimulus onset time over a specific frequency band. The ROI frequencies were delta (1–4 Hz), theta (4–7 Hz), and beta (13–30 Hz). The ROI time intervals were delta (200–500 ms), theta (10–400 ms), and beta (0–300 ms). ROI time intervals were selected based on ERO energy in specific event-related potential (ERP) component locations (N1, P3) in previous ERP studies (Ehlers et al. 1998). Using mean values over trials, the maximum values were calculated for each ROI, for the two electrode locations.
Sleep EEG analyses
Slow-wave sleep (SWS) (1–4 Hz) was scored for the 5-h EEG recording sessions for the vehicle (control), DORA-12 10 mg/kg, and DORA-12 30 mg/kg P.O. SWS episodes were defined as an increase in EEG power that exceeded twice the amplitude of waking baseline EEG power lasting longer than 8 s. Rapid eye movement (REM) sleep was visually identified as synchronized theta activity (4–8 Hz) that was preceded by an episode of SWS in the absence of muscle activity. Sleep patterns were identified and analyzed for SWS and REM states. Measures included (1) latency to the onset of the first episode of SW and REM sleep, (2) the mean duration of all SW and REM sleep episodes, and (3) the total number of SW and REM sleep episodes.
Spectral characteristics of the EEG were also quantitated for the 5-h recording period as described previously (Ehlers and Havstad 1982; Ehlers et al. 2018). EEG signals were band-pass filtered (0.53–70 Hz), digitized, artifact removed, and Fourier transformed to generate the power spectrum. EEG analyses focused on two frequency bands: delta (1–4 Hz) and theta (4–8 Hz) activity. Spectral power was calculated separately for the first slow wave sleep epoch and for the average of all the SWS epochs over the entire 5-h recording session.
Statistical analyses
Data analyses were based on the two aims of the study which were to test the effects of vehicle and two doses of DORA-12 on (1) waking electrophysiology as indexed by event-related oscillations (EROs), and (2) sleep physiology as indexed by REM and SW sleep parameters, and EEG sleep spectral characteristics, in the ethanol and control treatment groups. For the ERO analyses, energy in the three time-frequency regions of interest (delta, theta, beta) were compared in response to the infrequent tone, in leads frontal cortex (FCTX) and parietal cortex (PCTX) in the alcohol vapor and control animals for the three drug conditions using a group (ethanol, control) × three condition (vehicle, DORA-12 10 mg/kg, DORA-12 30 mg/kg) ANOVA. Due to non-normal distributions of the data, alcohol vapor and control animals were compared on the REM and SW sleep measures (latency to onset, mean duration of episodes, number of episodes) for the treatment condition (vehicle, DORA-12 10 mg/kg, DORA-12 30 mg/kg) using Friedman’s test and between the two alcohol exposure groups using Mann-Whitney U (MWU). Spectral power in the sleep EEG in two frequency bands (delta, theta) over all the SW and REM sleep episodes in leads FCTX and PCTX were also evaluated for the three drug conditions using a two group (ethanol, control) × three condition (vehicle, DORA-12 10 mg/kg, DORA-12 30 mg/kg) ANOVA. Five animals were not used in the analysis due to decreasing function of electrodes leads over the duration of testing. Post hoc analyses were used when significant main effects were found. Significance was set at p < 0.05.
Results
Effects of DORA-12 on waking EROs
Our first aim evaluated the response to vehicle and two doses of the DORA-12 as indexed by waking ERO energy, in the control and EtOH-exposed groups in frontal (FCTX) and parietal (PCTX) cortex. Repeated measure ANOVA revealed an effect of condition (vehicle (VEH), DORA-12 10 mg/kg, 30 mg/kg) on ERO energy in delta frequency band, to the infrequent tone, in frontal cortex (condition: F(2,38) = 34.0, p < 0.001) and parietal cortex (condition: F(2,38) = 47.6, p < 0.001). Post hocs revealed that these results were significant when vehicle was compared to the 10 mg/kg dose (F = 39.5, p < 0.0001) or the 30 mg/kg dose (F = 45.2, p < 0.0001) and when the low dose was compared to the high dose (F = 8.5, p < 0.006) in frontal cortex as well as in parietal cortex (VEH vs. 10: F = 69.2, p < 0.0001; VEH vs. 30: F = 73.1, p < 0.0001; 10 vs. 30: 12.0, p < 0.001) (see Fig. 1). In addition, repeated measures ANOVA revealed condition effects on ERO energy in the theta frequency band, in both frontal cortex (condition: F(2,38) = 34.6, p = 0.001) and parietal cortex (condition: F(2,38) = 25.5, p < 0.001). Post hocs revealed that these results were significant when vehicle was compared to the 10 mg/kg dose (F = 47.8, p < 0.0001) or the 30 mg/kg dose (F = 52.0, p < 0.0001) and when the low dose was compared to the high dose (F = 4.9, p < 0.03) in frontal cortex as well as in parietal cortex (VEH vs. 10: F = 20.7, p < 0.0001; VEH vs. 30: F = 56.4, p < 0.0001; 10 vs. 30: 5.2, p < 0.03) (see Fig. 1).
Event related oscillation (ERO) energy in the delta and theta time-frequency regions of interest (ROI) during waking in frontal and parietal cortex. Delta and theta power at three time points in rats exposed to ethanol vapor during adolescence or air controls: Vehicle (VEH), 10 mg/kg DORA-12(LD), and 30 mg/kg DORA-12(HD). Significant post hoc results shown, Asterisk indicates p ≤ 0.05 dose effect. Error bars = S.E.M
Effects of DORA-12 on REM and SW sleep parameters
We evaluated whether DORA-12 had an effect on sleep parameters using non-parametric tests. An overall effect of group was found for DORA-12 for both the mean duration of slow wave sleep episodes (MWU = 1263, p < 0.017) and for the total number of slow wave sleep episodes (MWU = 1279, p < 0.02). As seen in Fig. 2, the adolescent ethanol exposure group had both shorter duration of their SWS episodes and a larger number of episodes overall as compared to the air control group resulting in a fragmentation of sleep. Post-hoc analyses revealed that this effect was only significant for the duration of SWS episodes in the VEH condition (MWU = 120, p < 0.05). Significant effects of condition were found, using the Friedman test, for two of the SWS variables as seen in Fig. 2 (latency to first SWS: Chi-square: 41.2, p < 0.001, total number of SWS episodes: Chi-square: 18.5, p < 0.001). Post hoc analyses using the Wilcoxon test showed that both doses of DORA decreased the latency to the onset of the first SWS episode (VEH vs. 10: Z = − 4.6, p < 0.0001; VEH vs. 30: Z = − 5.1, p < 0.0001) in a dose-dependent fashion (10 vs. 30: Z = − 2.0, p < 0.04), as seen in Fig. 2. Both doses of DORA-12 were also found to increase the number of slow wave sleep episodes (VEH vs. 10: Z = − 4.0, p < 0.0001; VEH vs. 30: Z = − 2.4, p < 0.016, 10 vs. 30: Z = − 2.26, p < 0.024).
Slow wave sleep (SWS) measures following vehicle and two doses of DORA-12 in control and adolescent alcohol-exposed rats. Latency to the first slow wave sleep episode, mean duration, and total number of all sleep episodes shown following vehicle (VEH), 10 mg/kg DORA-12, and 30 mg/kg, DORA-12. Significant post hoc results shown, plus sign indicates p ≤ 0.05 group effect. Asterisk indicates p ≤ 0.05 dose effect. Error bars = S.E.M
REM parameters were also found to significantly differ both as a function of group and condition. AIE animals were found to have significantly more REM episodes overall (MWU: 1275, p < 0.02), although the post hocs were not significant between the groups within condition levels. Significant effects of condition were found, using the Friedman test, for all three REM variables as seen in Fig. 3 (latency to first REM: Chi-square: 40.2, p < 0.001, mean length of REM episodes: Chi-square = 6.8, p < 0.03, total number of REM episodes: Chi-square: 6.7, p < 0.04). Post hoc analyses using the Wilcoxon test showed that both doses of DORA-12 decreased the latency to the onset of the first REM episode (VEH vs. 10: Z = − 4.9, p < 0.0001; VEH vs. 30: Z = − 5.1, p < 0.0001) in a dose-dependent fashion (10 vs. 30: Z = − 2.0, p < 0.04), as seen in Fig. 3. The high dose of DORA-12 was also found to reduce the mean duration of REM episodes when compared to VEH (Z = − 2.6, p < 0.01).
Rapid eye movement (REM) sleep latency and duration measurements following vehicle and two doses of DORA-12 in adolescent alcohol exposed and control rats. Latency to first REM episode, mean duration, and total number of all REM episodes shown following vehicle (VEH), 10 mg/kg DORA-12, and 30 mg/kg, DORA-12. Significant post hoc results shown, asterisk indicates p ≤ 0.05 dose effect. Error bars = S.E.M
Effects of DORA-12 on sleep EEG spectra
An evaluation of the EEG sleep spectra during the first SWS episode demonstrated that DORA-12 produced increases in EEG delta power in frontal (F = 25.6, p < 0.0001) and parietal (F = 24.5, p < 0.0001) cortex, as seen in Fig. 4. Post hoc analyses showed that VEH was significantly different from the low and high dose (FCTX: VEH vs. 10: F = 35.1, p < 0.001; VEH vs. 30: F = 42.6, p < 0.0001; PCTX: VEH vs. 10: F = 33.5, p < 0.001; VEH vs. 30: F = 36.6, p < 0.0001), in delta power during the first slow wave sleep episode, but low and high dose were not different from each other in either cortical region. However, when the amount of power in the delta frequencies was assessed over all SW sleep episodes, DORA-12 was found to produce a slight decrease in frontal cortex only (F = 4.4, p < 0.018), and post hocs revealed that it was only significant when comparing VEH to the lowest dose (F = 10.8, p < 0.002) (data not shown).
Delta and theta power in the EEG during the first slow wave sleep (SWS) episode in frontal and parietal cortex. Delta and theta power during the first slow wave sleep (SWS) at three time points in rats exposed to ethanol vapor during adolescence or air controls following: vehicle (VEH), 10 mg/kg DORA-12(LD), and 30 mg/kg DORA-12(HD). Significant post hoc results shown, asterisk indicates p ≤ 0.05 dose effect. Error bars = S.E.M
An evaluation of the EEG sleep spectra during the first SWS episode demonstrated that DORA-12 also produced increases in EEG theta power in frontal (F = 8.9, p < 0.001) and parietal cortex (F = 9.0, p < 0.001), as seen in Fig. 4. Post hoc analyses showed that VEH was significantly different from the low dose (FCTX: F = 13.9, p < 0.001; PCTX: 14.6, p < 0.001), and high dose (FCTX: F = 7.62, p < 0.009; PCTX: 4.8, p < 0.04). The parietal cortex was also significantly different between the low dose and high dose (6.9, p < 0.01). DORA-12 did not produce changes in EEG theta power when the mean of all SWS episodes were evaluated, in either lead (data not shown).
Spectral changes in REM sleep as a function of dose of DORA-12 were also determined and the only findings were a decrease in mean delta power for all REM episodes, over the entire recording period, in both leads (FCTX: F = 6.6, p < 0.002; PCTX: F = 4.1, p < 0.023). Post hoc analyses found that it was significant when VEH was compared to low dose (FCTX: F = 11.2, p < 002; PCTX: F = 7.6, p < 0.009) and high dose (FCTX: F = 7.8, p < 008; PCTX: F = 4.4, p < 0.04), but not when the two doses were compared (data not shown).
Discussion
Alcohol use during early adolescence has been associated with long-term health consequences, as well as elevated risk for alcohol use disorders in young adulthood (Dawson et al. 2008; Ehlers et al. 2006). Preclinical models allow for study of ethanol exposure during adolescence on sleep independent of factors that may confound human studies such as differences in genetic risk, psychiatric comorbidity, as well as environmental and cultural factors. Studies from our laboratory have shown that adolescent alcohol exposure can lead to a disruption in slow-wave sleep (SWS) (Criado et al. 2008a; Ehlers et al. 2013a; Ehlers et al. 2018). The present study is a replication and confirmation of those studies, in that we found that young adult rats with a history of adolescent alcohol vapor exposure demonstrated a fragmentation of sleep that consisted of a decrease in the duration and an increase in the number of slow wave sleep episodes. These findings are also similar to what we have reported previously in adult animals exposed to chronic alcohol exposure (Sanchez-Alavez et al. 2018), although the findings appear to be somewhat less pronounced in adolescents as compared to adults.
Although preclinical models of adolescent alcohol exposure allow for the identification of alcohol-specific sleep deficits, as opposed to premorbid conditions, they have seldom been utilized to identify therapeutic targets. The treatment of insomnia in adolescents and young adults is particularly problematic since this is a time during development when mental alertness is particularly important and risk for developing addiction is high (see (de Zambotti et al. 2018)). We have demonstrated that Gabapentin, which binds the α2δ auxiliary subunit of the voltage-gated calcium channels, can produce dose-dependent increases in slow wave sleep, and ameliorate the effects of chronic alcohol exposure on sleep fragmentation, in both an adult rat model of alcohol-induced sleep disturbance (Sanchez-Alavez et al. 2018), and also ameliorate the deficits seen in slow wave power in adolescent ethanol-exposed animals (Ehlers et al. 2018). Gabapentin has been shown to improve sleep and measures of recovery in individuals with AUD in several clinical trials (Brower 2015; Brower et al. 2008; Mason et al. 2014), and may have less potential for addiction liability than benzodiazepine-type hypnotics; however, it may be too sedating for use in teens.
More recently, therapeutic drugs that target orexin/hypocretin receptors have been developed and shown to have some limited use in insomnia (Keks et al. 2017; Kripke 2015; Neubauer et al. 2018; Patel et al. 2015; Winrow and Renger 2014). These drugs may prove to have efficacy in the treatment of adolescent insomnia because they may have less potential for addiction and unacceptable side effects. In one study, in Japan, the tolerability, efficacy, and safety of suvorexant, a dual orexin receptor antagonist, was evaluated in adolescents with insomnia (Kawabe et al. 2017). In that study, suvorexant was found to improve the subjective sleep quality, although 40% of those prescribed the drug discontinued its use during the study.
A recent review has proposed that suvorexant may be particularly suited for the treatment of alcohol use disorders (Campbell et al. 2018). However, the effects of suvorexant in patients with AUD are currently unknown and preclinical studies of the treatment of alcohol-induced sleep pathology with suvorexant are limited. In one study, adult rats were exposed to chronic ethanol vapor exposure and the effects of suvorexant were evaluated on EEG and sleep parameters. In that study, suvorexant was found to hasten the onset of SW and REM sleep but exacerbated the sleep fragmentation observed as a function of alcohol exposure (Sanchez-Alavez et al. 2019). Enhanced fragmentation of sleep has also been shown following almorexant administration in a murine model of narcolepsy as well as cataplexy (Black et al. 2013). In the present study, a newly developed dual orexin receptor antagonist (DORA-12) (Gotter et al. 2014; Ramirez et al. 2013) was administered to adult rats that had been exposed to chronic alcohol exposure during adolescence and their controls. Chronic ethanol vapor exposure during adolescence, in the rat, was found to produce a fragmentation of sleep in young adults that was partially ameliorated by DORA-12. DORA-12 was also found to produce several significant effects on sleep and waking EEG that were equivalent in the alcohol vapor and control rats. Significant increases in delta and theta power in waking EROs, recorded just before sleep time, as well as deeper sleep as indexed by increases in delta and theta power in the sleep EEG were seen in both alcohol and control rats. Rats given DORA-12 also fell asleep faster than rats given vehicle as measured by a dose-dependent reduction in the latency to both the first slow wave and REM episodes. These data suggest that DORA-12 may have some efficacy in the treatment of alcohol-associated insomnia and may also demonstrate some overall superiority in its ability to produce deeper sleep, as compared to suvorexant, in preclinical studies in rats.
Adolescent alcohol exposure most likely influences multiple neurotransmitter systems and brain circuits that could ultimately lead to disrupted sleep in young adults, in addition to the hypocretin/orexin system (Veatch 2006). We have shown that persistent reductions in cell counts of ChAT-immunoreactive (ChAT-IR) neurons in the basal forebrain, an area important in sleep regulation, are found in adolescent ethanol vapor-exposed rats (Ehlers et al. 2011). However, whether ChAT-IR reductions are responsible for the fragmentation of sleep found in adolescent ethanol exposed rats is currently unknown. A reasonable hypothesis is that adolescent alcohol exposure influences multiple sleep systems, as has been reported in adult animals exposed to ethanol (see (Ehlers et al. 2013a; Ehlers et al. 2013b; Sanchez-Alavez et al. 2018; Sharma et al. 2010; Sharma et al. 2017; Thakkar et al. 2010)).
Taken together, these studies suggest that adolescent alcohol exposure, in the rat, can result in a fragmentation of sleep. This study also showed that DORA-12 can affect the sleep disturbance that is associated with a history of adolescent ethanol exposure but also has several other sleep-promoting effects that are equivalent in both ethanol and control rats. DORA-12 was found to decrease the latency to onset of sleep and produce deeper sleep. While these studies describe potential treatment targets for alcohol-induced sleep pathology, however, they do not mimic the complex environmental and genetic risks for insomnia seen in adolescent and young adult humans (de Zambotti et al. 2018), nor do they model the range of drinking levels observed in adolescent humans (Ehlers et al. 2019).
Change history
03 October 2020
The title of this article is ���Effect of a dual orexin receptor antagonist (DORA-12) on sleep and event-related oscillations in rats exposed to ethanol vapor during adolescence���.
References
Anderson RI, Becker HC, Adams BL, Jesudason CD, Rorick-Kehn LM (2014) Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Front Neurosci 8:33. https://doi.org/10.3389/fnins.2014.00033
Anokhin AP (2014) Genetic psychophysiology: advances, problems, and future directions. Int J Psychophysiol 93:173–197. https://doi.org/10.1016/j.ijpsycho.2014.04.003
Baldo BA, Daniel RA, Berridge CW, Kelley AE (2003) Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 464:220–237. https://doi.org/10.1002/cne.10783
Bartel KA, Gradisar M, Williamson P (2015) Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med Rev 21:72–85. https://doi.org/10.1016/j.smrv.2014.08.002
Basar E, Basar-Eroglu C, Karakas S, Schurmann M (1999) Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett 259:165–168. https://doi.org/10.1016/s0304-3940(98)00934-3
Basar E, Basar-Eroglu C, Karakas S, Schurmann M (2000) Brain oscillations in perception and memory. Int J Psychophysiol 35:95–124
Black SW, Morairty SR, Fisher SP, Chen TM, Warrier DR, Kilduff TS (2013) Almorexant promotes sleep and exacerbates cataplexy in a murine model of narcolepsy. Sleep 36:325–336. https://doi.org/10.5665/sleep.2442
Brower KJ (2015) Assessment and treatment of insomnia in adult patients with alcohol use disorders. Alcohol 49:417–427. https://doi.org/10.1016/j.alcohol.2014.12.003
Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA (2008) A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 32:1429–1438. https://doi.org/10.1111/j.1530-0277.2008.00706.x
Brown RM, Khoo SY, Lawrence AJ (2013) Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol 16:2067–2079. https://doi.org/10.1017/S1461145713000333
Brown RM, Kim AK, Khoo SY, Kim JH, Jupp B, Lawrence AJ (2016) Orexin-1 receptor signalling in the prelimbic cortex and ventral tegmental area regulates cue-induced reinstatement of ethanol-seeking in iP rats. Addict Biol 21:603–612. https://doi.org/10.1111/adb.12251
Campbell EJ, Marchant NJ, Lawrence AJ (2018) A sleeping giant: Suvorexant for the treatment of alcohol use disorder? Brain Res. https://doi.org/10.1016/j.brainres.2018.08.005
Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G (2010) Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav 100:419–428. https://doi.org/10.1016/j.physbeh.2010.03.009
Criado JR, Ehlers CL (2010) Effects of adolescent ethanol exposure on event-related oscillations (EROs) in the hippocampus of adult rats. Behav Brain Res 210:164–170. https://doi.org/10.1016/j.bbr.2010.02.021
Criado JR, Wills DN, Walker BM, Ehlers CL (2008a) Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol 42:631–639. https://doi.org/10.1016/j.alcohol.2008.08.001
Criado JR, Wills DN, Walker BM, Ehlers CL (2008b) Electrophysiological effects of dizocilpine (MK-801) in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res 32:1752–1762. https://doi.org/10.1111/j.1530-0277.2008.00760.x
Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF (2008) Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res 32:2149–2160. https://doi.org/10.1111/j.1530-0277.2008.00806.x
de Lecea L (2012) Hypocretins and the neurobiology of sleep-wake mechanisms. Prog Brain Res 198:15–24. https://doi.org/10.1016/B978-0-444-59489-1.00003-3
de Zambotti M, Goldstone A, Colrain IM, Baker FC (2018) Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev 39:12–24. https://doi.org/10.1016/j.smrv.2017.06.009
DiLeone RJ, Georgescu D, Nestler EJ (2003) Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 73:759–768. https://doi.org/10.1016/s0024-3205(03)00408-9
Ehlers CL, Criado JR (2009) Event-related oscillations in mice: effects of stimulus characteristics. J Neurosci Methods 181:52–57. https://doi.org/10.1016/j.jneumeth.2009.04.015
Ehlers CL, Havstad JW (1982) Characterization of drug effects on the EEG by power spectral time series analysis. Psychopharmacol Bull 18:43–47
Ehlers CL, Slawecki CJ (2000) Effects of chronic ethanol exposure on sleep in rats. Alcohol 20:173–179. https://doi.org/10.1016/s0741-8329(99)00077-4
Ehlers CL, Garcia-Andrade C, Wall TL, Sobel DF, Phillips E (1998) Determinants of P3 amplitude and response to alcohol in Native American Mission Indians. Neuropsychopharmacology 18:282–292. https://doi.org/10.1016/S0893-133X(97)00160-7
Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC (2006) Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol Clin Exp Res 30:1856–1865. https://doi.org/10.1111/j.1530-0277.2006.00222.x
Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT (2011) Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience 199:333–345. https://doi.org/10.1016/j.neuroscience.2011.10.011
Ehlers CL, Wills DN, Havstad J (2012) Ethanol reduces the phase locking of neural activity in human and rodent brain. Brain Res 1450:67–79. https://doi.org/10.1016/j.brainres.2012.02.039
Ehlers CL, Desikan A, Wills DN (2013a) Developmental differences in EEG and sleep responses to acute ethanol administration and its withdrawal (hangover) in adolescent and adult Wistar rats. Alcohol 47:601–610. https://doi.org/10.1016/j.alcohol.2013.09.040
Ehlers CL, Liu W, Wills DN, Crews FT (2013b) Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience 244:1–15. https://doi.org/10.1016/j.neuroscience.2013.03.058
Ehlers CL, Oguz I, Budin F, Wills DN, Crews FT (2013c) Peri-adolescent ethanol vapor exposure produces reductions in hippocampal volume that are correlated with deficits in prepulse inhibition of the startle. Alcohol Clin Exp Res 37:1466–1475. https://doi.org/10.1111/acer.12125
Ehlers CL, Desikan A, Wills DN (2014) Event-related potential responses to the acute and chronic effects of alcohol in adolescent and adult Wistar rats. Alcohol Clin Exp Res 38:749–759. https://doi.org/10.1111/acer.12299
Ehlers CL, Wills DN, Phillips E, Havstad J (2015) Low voltage alpha EEG phenotype is associated with reduced amplitudes of alpha event-related oscillations, increased cortical phase synchrony, and a low level of response to alcohol. Int J Psychophysiol 98:65–75. https://doi.org/10.1016/j.ijpsycho.2015.07.002
Ehlers CL, Sanchez-Alavez M, Wills D (2018) Effect of gabapentin on sleep and delta and theta EEG power in adult rats exposed to chronic intermittent ethanol vapor and protracted withdrawal during adolescence. Psychopharmacology 235:1783–1791. https://doi.org/10.1007/s00213-018-4888-6
Ehlers CL, Phillips E, Wills D, Benedict J, Sanchez-Alavez M (2019) Phase locking of event-related oscillations is decreased in both young adult humans and rats with a history of adolescent alcohol exposure. Addict Biol. https://doi.org/10.1111/adb.12732
Gabor D (1946) Theory of Communication. Part 1: The analysis of information. J Inst Electr Eng III: Radio Commun Eng 93:429–441. https://doi.org/10.1049/ji-3-2.1946.0074
Gotter AL, Garson SL, Stevens J, Munden RL, Fox SV, Tannenbaum PL, Yao L, Kuduk SD, McDonald T, Uslaner JM, Tye SJ, Coleman PJ, Winrow CJ, Renger JJ (2014) Differential sleep-promoting effects of dual orexin receptor antagonists and GABAA receptor modulators. BMC Neurosci 15:109. https://doi.org/10.1186/1471-2202-15-109
Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA (2011) Impact of adolescent Alcohol and drug use on neuropsychological functioning in young adulthood: 10-year outcomes. J Child Adolesc Subst Abuse 20:135–154. https://doi.org/10.1080/1067828X.2011.555272
Hasler BP, Clark DB (2013) Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcohol Clin Exp Res 37:558–565. https://doi.org/10.1111/acer.12003
Hasler BP, Sitnick SL, Shaw DS, Forbes EE (2013) An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Res 214:357–364. https://doi.org/10.1016/j.pscychresns.2013.08.005
Hasler BP, Martin CS, Wood DS, Rosario B, Clark DB (2014a) A longitudinal study of insomnia and other sleep complaints in adolescents with and without alcohol use disorders. Alcohol Clin Exp Res 38:2225–2233. https://doi.org/10.1111/acer.12474
Hasler BP, Soehner AM, Clark DB (2014b) Circadian rhythms and risk for substance use disorders in adolescence. Curr Opin Psychiatry 27:460–466. https://doi.org/10.1097/YCO.0000000000000107
Hasler BP, Soehner AM, Clark DB (2015) Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol 49:377–387. https://doi.org/10.1016/j.alcohol.2014.06.010
Hoyer D, Jacobson LH (2013) Orexin in sleep, addiction and more: is the perfect insomnia drug at hand? Neuropeptides 47:477–488. https://doi.org/10.1016/j.npep.2013.10.009
Inutsuka A, Yamanaka A (2013) The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front Endocrinol (Lausanne) 4:18. https://doi.org/10.3389/fendo.2013.00018
Jupp B, Krivdic B, Krstew E, Lawrence AJ (2011a) The orexin(1) receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res 1391:54–59. https://doi.org/10.1016/j.brainres.2011.03.045
Jupp B, Krstew E, Dezsi G, Lawrence AJ (2011b) Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin(1) receptors. Br J Pharmacol 162:880–889. https://doi.org/10.1111/j.1476-5381.2010.01088.x
Kastman HE, Blasiak A, Walker L, Siwiec M, Krstew EV, Gundlach AL, Lawrence AJ (2016) Nucleus incertus Orexin2 receptors mediate alcohol seeking in rats. Neuropharmacology 110:82–91. https://doi.org/10.1016/j.neuropharm.2016.07.006
Kawabe K, Horiuchi F, Ochi M, Nishimoto K, Ueno SI, Oka Y (2017) Suvorexant for the treatment of insomnia in adolescents. J Child Adolesc Psychopharmacol 27:792–795. https://doi.org/10.1089/cap.2016.0206
Keks NA, Hope J, Keogh S (2017) Suvorexant: scientifically interesting, utility uncertain. Australas Psychiatry 25:622–624. https://doi.org/10.1177/1039856217734677
Kim AK, Brown RM, Lawrence AJ (2012) The role of orexins/hypocretins in alcohol use and abuse: an appetitive-reward relationship. Front Behav Neurosci 6:78. https://doi.org/10.3389/fnbeh.2012.00078
Klimesch W, Sauseng P, Hanslmayr S, Gruber W, Freunberger R (2007) Event-related phase reorganization may explain evoked neural dynamics. Neurosci Biobehav Rev 31:1003–1016. https://doi.org/10.1016/j.neubiorev.2007.03.005
Kripke DF (2015) Is suvorexant a better choice than alternative hypnotics? F1000Res 4: 456. https://doi.org/10.12688/f1000research.6845.1
Krystal AD, Benca RM, Kilduff TS (2013) Understanding the sleep-wake cycle: sleep, insomnia, and the orexin system. J Clin Psychiatry 74:3–20. https://doi.org/10.4088/JCP.13011su1c
Lawrence AJ (2010) Regulation of alcohol-seeking by orexin (hypocretin) neurons. Brain Res 1314:124–129. https://doi.org/10.1016/j.brainres.2009.07.072
Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B (2006) The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148:752–759. https://doi.org/10.1038/sj.bjp.0706789
Li J, Hu Z, de Lecea L (2014) The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol 171:332–350. https://doi.org/10.1111/bph.12415
Martin-Fardon R, Weiss F (2012) N-(2-methyl-6-benzoxazolyl)-N'-1,5-naphthyridin-4-yl urea (SB334867), a hypocretin receptor-1 antagonist, preferentially prevents ethanol seeking: comparison with natural reward seeking. Addict Biol 19:233-236. https://doi.org/10.1111/j.1369-1600.2012.00480.x.
Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F (2010) Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res 1314:145–161. https://doi.org/10.1016/j.brainres.2009.12.027
Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A (2014) Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 174:70–77. https://doi.org/10.1001/jamainternmed.2013.11950
McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF (2009) Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res 33:1278–1285. https://doi.org/10.1111/j.1530-0277.2009.00953.x
Mieda M, Tsujino N, Sakurai T (2013) Differential roles of orexin receptors in the regulation of sleep/wakefulness. Front Endocrinol (Lausanne) 4:57. https://doi.org/10.3389/fendo.2013.00057
Moorman DE (2018) The hypocretin/orexin system as a target for excessive motivation in alcohol use disorders. Psychopharmacology 235:1663–1680. https://doi.org/10.1007/s00213-018-4871-2
Moorman DE, Aston-Jones G (2009) Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol—preferring Sprague-Dawley rats. Alcohol 43:379–386. https://doi.org/10.1016/j.alcohol.2009.07.002
Moorman DE, James MH, Kilroy EA, Aston-Jones G (2017) Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res 1654:34–42. https://doi.org/10.1016/j.brainres.2016.10.018
Neubauer DN, Pandi-Perumal SR, Spence DW, Buttoo K, Monti JM (2018) Pharmacotherapy of insomnia. J Cent Nerv Syst Dis 10:1-7. https://doi.org/10.1177/1179573518770672
Pandey AK, Kamarajan C, Rangaswamy M, Porjesz B (2012) Event-related oscillations in alcoholism research: a review. J Addict Res Ther S7:3844. https://doi.org/10.4172/2155-6105.S7-001.
Patel KV, Aspesi AV, Evoy KE (2015) Suvorexant: a dual orexin receptor antagonist for the treatment of sleep onset and sleep maintenance insomnia. Ann Pharmacother 49:477–483. https://doi.org/10.1177/1060028015570467
Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O'Malley PM, Johnston LD (2013) Extreme binge drinking among 12th-grade students in the United States: prevalence and predictors. JAMA Pediatr 167:1019–1025. https://doi.org/10.1001/jamapediatrics.2013.2392
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd. edn. Academic Press, Sydney
Ramirez AD, Gotter AL, Fox SV, Tannenbaum PL, Yao L, Tye SJ, McDonald T, Brunner J, Garson SL, Reiss DR, Kuduk SD, Coleman PJ, Uslaner JM, Hodgson R, Browne SE, Renger JJ, Winrow CJ (2013) Dual orexin receptor antagonists show distinct effects on locomotor performance, ethanol interaction and sleep architecture relative to gamma-aminobutyric acid-A receptor modulators. Front Neurosci 7:254. https://doi.org/10.3389/fnins.2013.00254
Rangaswamy M, Porjesz B (2014) Understanding alcohol use disorders with neuroelectrophysiology. Handb Clin Neurol 125:383–414. https://doi.org/10.1016/B978-0-444-62619-6.00023-9
Roach BJ, Mathalon DH (2008) Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull 34:907–926. https://doi.org/10.1093/schbul/sbn093
Sanchez-Alavez M, Wills DN, Amodeo L, Ehlers CL (2018) Effect of Gabapentin on Sleep and Event-Related Oscillations (EROs) in Rats Exposed to Chronic Intermittent Ethanol Vapor and Protracted Withdrawal. Alcohol Clin Exp Res 42:624–633. https://doi.org/10.1111/acer.13588
Sanchez-Alavez M, Benedict J, Wills DN, Ehlers CL (2019) Effect of suvorexant on event-related oscillations and EEG sleep in rats exposed to chronic intermittent ethanol vapor and protracted withdrawal Sleep 42. https://doi.org/10.1093/sleep/zsz020.
Schack B, Klimesch W (2002) Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci Lett 331:107–110. https://doi.org/10.1016/s0304-3940(02)00846-7
Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF (2011) Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction 106:564–573. https://doi.org/10.1111/j.1360-0443.2010.03197.x
Sharma R, Engemann S, Sahota P, Thakkar MM (2010) Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. J Neurochem 115:782–794. https://doi.org/10.1111/j.1471-4159.2010.06980.x
Sharma R, Sahota P, Thakkar MM (2017) Lesion of the basal forebrain cholinergic neurons attenuates sleepiness and adenosine after alcohol consumption. J Neurochem 142:710–720. https://doi.org/10.1111/jnc.14054
Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF (2009) Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav 23:715–722. https://doi.org/10.1037/a0016516
Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, Hopf FW, Bonci A, Bartlett SE (2012) The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration. PLoS One 7:e44726. https://doi.org/10.1371/journal.pone.0044726
Thakkar MM, Engemann SC, Sharma R, Mohan RR, Sahota P (2010) Sleep-wakefulness in alcohol preferring and non-preferring rats following binge alcohol administration. Neuroscience 170:22–27. https://doi.org/10.1016/j.neuroscience.2010.07.005
Thakkar MM, Sharma R, Sahota P (2015) Alcohol disrupts sleep homeostasis. Alcohol 49:299–310. https://doi.org/10.1016/j.alcohol.2014.07.019
Ubaldi M, Giordano A, Severi I, Li H, Kallupi M, de Guglielmo G, Ruggeri B, Stopponi S, Ciccocioppo R, Cannella N (2016) Activation of hypocretin-1/orexin—a neurons projecting to the bed nucleus of the stria terminalis and paraventricular nucleus is critical for reinstatement of alcohol seeking by neuropeptide S. Biol Psychiatry 79:452–462. https://doi.org/10.1016/j.biopsych.2015.04.021
Veatch LM (2006) Disruptions in sleep time and sleep architecture in a mouse model of repeated ethanol withdrawal. Alcohol Clin Exp Res 30:1214–1222. https://doi.org/10.1111/j.1530-0277.2006.00134.x
Walker LC, Lawrence AJ (2017) CRF and the nucleus incertus: a node for integration of stress signals. Nat Rev Neurosci 18:158. https://doi.org/10.1038/nrn.2016.158
Winrow CJ, Renger JJ (2014) Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol 171:283–293. https://doi.org/10.1111/bph.12261
Xu TR, Yang Y, Ward R, Gao L, Liu Y (2013) Orexin receptors: multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cell Signal 25:2413–2423. https://doi.org/10.1016/j.cellsig.2013.07.025
Acknowledgments
The authors wish to acknowledge the technical support of Mellany Santos and Philip Lau. National Institutes of Health (NIH) funding for this study was provided by the National Institute on Alcoholism and Alcohol Abuse (NIAAA) U01 AA19969, AA006059 to CLE. DORA-12 was provided by Merck pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest. The experiments comply with the current laws of the country in which they were performed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ehlers, C.L., Benedict, J., Wills, D. et al. PSPH-D-18-00526: Effect of a dual orexin receptor antagonist (DORA-12) on sleep and event-related oscillations in rats exposed to ethanol vapor during adolescence. Psychopharmacology 237, 2917–2927 (2020). https://doi.org/10.1007/s00213-019-05371-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05371-4