Abstract
Rationale and objective
Paeoniflorin has been reported to exhibit antidepressant-like effects in several animal model depression; and it also exerts a neuroprotective effect. In the present study, we investigated the effects of paeoniflorin administration on depression-like behaviors and cognitive abilities in mice subjected to chronic unpredictable mild stress (CUMS), an animal model associated with depressive disorders and cognitive deficits.
Methods
We administered paeoniflorin (20 mg/kg), which is the main active constituent extracted from Paeonia lactiflora Pall. and exerts multiple pharmacological actions, to CUMS mice. Subsequently, animals were subjected to tests of depression-like behavior including the sucrose preference test, the forced swimming test and the tail suspension test. The Morris water maze (MWM) task was applied to evaluate learning and memory capacity. Hippocampal CA1 long-term potentiation (LTP) was recorded. Dendritic spine density and the expression levels of brain-derived neurotrophic factor (BDNF) and postsynaptic density protein 95 (PSD95) in the hippocampus were also investigated.
Results
The administration of paeoniflorin protected against CUMS-induced depression-like behavior. Paeoniflorin also improved the performance of CUMS mice in the MWM. The impairment of hippocampal CA1 LTP caused by CUMS was also reversed. Furthermore, paeoniflorin administration prevented decreases in dendritic spine density and in the expression of BDNF and PSD95 in the hippocampus of CUMS mice.
Conclusion
Our observations suggest that paeoniflorin is a potential antidepressant that protects against cognitive impairment in depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression, one of the most common psychiatric disorders, is characterized by disturbances of emotional learning and memory (Duman et al. 2016). Clinical observation has shown that depressed patients exhibit memory bias, tending to store and retrieve negative information disproportionately, which indicates that alterations of learning and memory in depression are not mere consequences of depression but core elements of this psychiatric disorder (Kizilbash et al. 2002). As the most significant susceptibility factor for depression, chronic stress induces sustained activation of HPA axis and high level of glucocorticoid (Krishnan and Nestler 2008). Rodent experiments showed that both HPA activation and glucocorticoid are closely associated with depression and decreases of synaptic number and function (Duman et al. 2016).
Chronic unpredictable mild stress (CUMS) is a well-accepted animal model mimicking the development and progress of stress-associated clinical depression and related cognitive deficits (Willner 1990). CUMS also induces impairment of hippocampus-dependent cognitive abilities. CUMS mice exhibited longer latency times during the learning process, dramatically fewer platform crossings, and less time swimming in the target quadrant than normal mice in the Morris water maze (MWM) task (Xi et al. 2011). Hippocampal long-term potentiation (LTP), one of the most important forms of synaptic plasticity, is also impaired in CUMS mice (Qiao et al. 2014).
Currently available antidepressants have limited activity against depression-related cognitive impairment. Several antidepressants are available in the pharmaceutical market, including serotonin/norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), and monoamine oxidase inhibitors (MAOIs) (Lane 2015; Sharma et al. 2015). However, the protective effect of regular antidepressant drugs against cognitive deficits is not obvious in either clinical observations or laboratory animal studies (Gorenstein et al. 2006; Naudon et al. 2007). Furthermore, patients taking these antidepressants may suffer from adverse effects, including suicidal tendencies (Clayton et al. 2016), sexual problems, and sleep disorders (Wilson and Argyropoulos 2005). Therefore, there is a strong need for antidepressants with fewer adverse effects and greater protective effects on cognition. Herbal medicines have been used as treatments against depression for many years, and researchers have also implied that some compounds from herbal extracts are functional antidepressants (Liu et al. 2015). Combined with modern medical technology, compounds extracted from these traditional herbs provide a prospective alternative in the treatment of depression.
Paeonia lactiflora Pall., commonly known as the peony, has been used as an herbal medicine in East Asia for over 1000 years (Wang et al. 2014) for antispasmodic, analgesic, antifebrile, hepatoprotective, vasodilating, antiseptic, and anti-aging purposes (He and Dai 2011; Qiu et al. 2016). Paeonia lactiflora Pall. is also a component herb of several traditional formulae used to treat depression-like disorders in China (Mao et al. 2012). One of the main active components of Paeonia lactiflora Pall. is paeoniflorin (Tanaka et al. 2013), which exhibits anti-oxidation, anti-inflammation, anticonvulsant, and antithrombotic properties (Abdel-Hafez et al. 1998; Abdel-Hafez et al. 1999; Ye et al. 2001).
Studies have shown that paeoniflorin exhibits neuroprotective activity in several brain injury or stress models. Paeoniflorin protects against Aβ-induced neurotoxicity by preventing mitochondrial dysfunction (Zheng et al. 2016). Administration of paeoniflorin to a rat model of vascular dementia (VD) for 28 days significantly suppressed brain damage, as indicated by decreased expression levels of NSE and S100-β (Zhang et al. 2016b; Zheng et al. 2016). Paeoniflorin also exhibits antistroke activity in a rat model of cerebral ischemia (Liu et al. 2005). Furthermore, there is increasing evidence that paeoniflorin can ameliorate declines in learning and memory capacity in several animal models. Paeoniflorin improved cognitive function in AD mice and ameliorated abnormalities in their escape distance and escape latency in the MWM test (Gu et al. 2016). Paeoniflorin also ameliorates memory disruption mediated by the adenosine A1 receptor and modulates adenosine-mediated inhibition of LTP in the hippocampus (Tabata et al. 2001). Administration of paeoniflorin could significantly attenuate the learning and memory impairments induced by cerebral hypoperfusion (Luo et al. 2018).
Thus, there is a strong demand for the development of drug candidates that exhibit both antidepressant and cognition-enhancing activity. The fact that paeoniflorin exhibits antidepressant effects in several animal models of depression and protects memory in many brain disease models makes it interesting to explore the protective activity of paeoniflorin on depression-impaired learning and memory. Therefore, we hypothesize that paeoniflorin has antidepressant activity and beneficial effects on cognitive deficits in depression.
To test this hypothesis, we applied the CUMS model to evaluate whether the administration of paeoniflorin could protect against synaptic plasticity defects induced by chronic stress while exerting an antidepressant effect.
Materials and methods
Animals
In all the experimental procedures, we used 20–25 g C57BL/6 wild-type male mice purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., and all experimental mice were 2 to 3 months old. All animals were fed with standard chow fodder and water ad libitum and were maintained under standard housing conditions (22 ± 2 °C ambient temperature, 50 ± 5% relative humidity) and a 12 h light/12 h dark cycle, with the lights turning on at 07:00 AM. Animals for experiments were group-housed (4–5 mice per cage, the dimensions of the cages were 295 × 190 × 125 mm), and a 7-day acclimation period was allowed. All procedures involving animals were conducted in accordance with the ARRIVE guidelines, and the Animal Care and Use Committee of Kunming Medical University formulated and approved the protocol.
Chronic unpredictable mild stress
CUMS is a classical animal model of major depressive disorder (Willner et al. 1987). The CUMS procedure used in our study was slightly modified but otherwise carried out following the previous description in our report (He et al. 2016a). Originally, 11 animals in each group were group housed (4–5 mice per cage) to become familiarized with the experimental environment for 1 week. Then, the animals were randomly grouped, and mice in the CUMS group were separately housed. The CUMS procedure included 10 mild stressors: 5 min of warm water swimming (37 ± 2 °C), 10 min of cage shaking (180 rpm), 5 min of frigid water swimming (13 ± 1 °C), 12 h of water deprivation, 1 min of tail pinching, 8 h of cage tilt (45°), 24 h of reversed light/dark, 12 h of food deprivation, 8 h of moist bedding, and no stress (24 h). CUMS mice were given a random stressor per day, while the control animals were left undisturbed. The stress period lasted for 35 days to develop depression-like behaviors.
Behavioral measurements
Sucrose preference test
The declining response to incentive stimulation, evaluated by the SPT, is a major symptom of depression (Willner et al. 1987). Animals were given 2 bottles containing 1% sucrose for 24 h to habituate to the sucrose solution before testing. In the next 24 h, the mice were deprived of water and food. In the SPT test, 2 bottles were offered to the animals: 1 bottle with normal water and another with 1% sucrose in the water. Mice were allowed to drink freely for 10 h. Consumed amounts of 2 liquid were weighed and recorded, and the formula (sucrose intake / (sucrose intake + water intake) × 100) was used to calculate the percentage of sucrose intake. Each group contained 11 mice (n = 11), and the observers were blinded to the group allocation of the tested mice.
Forced swimming test
The forced swimming test is a reliable test to assess depression-like behaviors, we performed it as described previously (Porsolt et al. 1977) with slight modification. The mice were individually placed into a transparent cylinder (25 cm high; 16 cm diameter) filled with 23–25 °C water. The immobility time during the testing duration (total 6 min) was recorded on video and manually scored by observers after the experiment. Mice’s slight movements to keep their head above the water without struggling are classified as immobility. Each group contained 11 mice (n = 11), and the observers were blinded to the group allocation of the tested mice.
Tail suspension test
The tail suspension test is another reliable test for recognizing the signs of depression. The method was adopted from previous studies (Steru et al. 1985). Adhesive tape was used to attach the tail of a mouse to the center of a shelf and hung the mice 35 cm above the ground. The testing duration was 6 min, and the immobility duration was recorded. The situation in which mice hung passively and remained completely motionless was judged as immobile. Each group contained 11 mice (n = 11), and the observers were blinded to the group allocations of the tested mice.
Water maze test
Spatial learning and memory function was evaluated by the Morris water maze (MWM) (Morris et al. 1982). The Morris water maze was composed of a circular tank (200 cm diameter) filled with water (23 ± 1 °C) made opaque with washable white paint. Markers in different shapes and colors were posted on the curtain around the pool to aid navigation. Two imaginary perpendicular lines crossing the center of the tank divided the tank into 4 quadrants. The top of the escape platform (10 cm diameter) was 1 cm below the water surface in a designated quadrant. Twenty-four hours before spatial training, animals were allowed for free swim to familiarize with the testing environment for 60 s. During the hidden platform training trial, each mouse was placed into the water along the wall, and we ensured that it was facing the wall. Mice were pseudorandomly dropped at 1 of the 4 quadrants, and we ensured that each of the four quadrants had served as a starting point in daily trials. The training lasted 4 days, and the animals were given 4 trials per day. In each trial, mice needed to swim in search of the platform until they climbed onto it and stayed for 20 s, at which time the trial would stop. If mice were unable to locate the platform during the trial (within 90 s), the tester would guide the animals to the platform and ensure that the mice stayed on the platform for 20 s. During the probe test on day 5, animals were allowed up to 60 s to swim to the former location of the hidden platform. Ten mice from each group were used for the MWM test. A video-based tracking system measured animal activity (WaterMaze; ANY-maze Instruments).
Long-term potentiation
Preparation of hippocampal slices
Hippocampal slices were prepared as previously reported in our laboratory (He et al. 2016a). Briefly, mice were anesthetized with ether and decapitated, and then the brain was dissected rapidly and sliced into 400-μm sections in oxygenated (95% O2 and 5% CO2) ice-cold artificial cerebrospinal fluid (aCSF) (mM: 1 NaH2PO4, 1.5 MgSO4, 126 NaCl, 2.5 CaCl2, 2.5 KCl, 26 NaHCO3, and 10 glucose; pH 7.4). Oxygenated aCSF was supplied to the slices at room temperature for 1 h before use.
Electrophysiological recording
Hippocampus slices were perfused with oxygenated aCSF at a speed of 2 ml/min in a recording chamber (PSMI; Harvard Apparatus). A bipolar electrode (Frederick Haer Co., Bowdoinham, ME, USA) was used to stimulate the Schaffer collaterals. The field excitatory postsynaptic potentials (fEPSPs) were recorded with a glass microelectrode with 1–4 MΩ resistance containing aCSF and acquired with a multiclamp 700A amplifier (Axon Instruments, Molecular Devices), filtered at 5 kHz, and digitized at 10 kHz. Stimulus intensity was adjusted to evoke approximately 40% of the maximal response for baseline recordings. LTP was elicited by applying theta burst stimulation (TBS; 4 trains of 10 bursts of 4 stimuli with 20 s, 200 ms, and 10 ms intervals between trains, bursts, and stimuli, respectively) at the same stimulus intensity for the baseline values. Slices from 5 randomly selected animals were analyzed (n = 5 animals/group). The best slice from each mouse was applied for electrophysiological experiment; the second slice will be applied to experiment when the hippocampus slice condition is good. Data from different slices of the same animal will be averaged to produce one value per animal.
All electrophysiological data were analyzed with Clampfit version 10.0 (Axon Instruments) and further processed with Origin 5.1 (Microcal Software Northampton). Experimenters were blinded to the mice’s group allocations during the LTP measurements.
Golgi staining
The mice were anesthetized and decapitated, and the cerebral hemispheres were rinsed with double-distilled water. Subsequently, the hemispheres were processed for Golgi staining with a Golgi staining kit (FD NeuroTechnologies) following the manufacturer’s instructions. Briefly, hemispheres were immersed in solution C for 24 h at 4 °C after soaking in solutions A and B for 14 days at room temperature, and then hippocampal coronal sections (100 μm) were cut and mounted on slides. The dendritic spines were viewed using confocal microscopy (Zeiss, Germany) and the LSM Image Browser software (Version 4.2, Zeiss). Neurons fulfill the following criteria will be analyzed: (1) the neurons were isolated from the surrounding neurons; (2) all the dendrites were visible within the plane of focus. Dendritic spine density was measured 100–200 μm apart from soma and 20 μm dendritic length in 2 segments from pyramidal cells in the hippocampal CA1 area. Brain slices from five other mice except for LTP and five neurons per slice were analyzed (3 slices/animals, n = 5 animals/group). Observers were blinded to the mice’s group allocations during Golgi staining.
Western blot assay
Western blotting was conducted as described in our previous study (He et al. 2016b). Hippocampi were isolated and homogenized in RIPA buffer (Thermo Fisher) containing phosphatase and protease inhibitor cocktail (Thermo Scientific). The lysates were dissolved in 2× Laemmli sample buffer (Bio-Rad) and boiled at 95 °C for 5 min. Then, each sample of lysates (20 μL) was fractionated by SDS-PAGE and electroblotted onto PVDF membranes. After blocking with 5% skim milk in TBS-T, the membranes were incubated with primary antibodies, including anti-BDNF (Cell Signaling Technology), anti-PSD95 (Cell Signaling Technology), and anti-β-actin (Sigma). Secondary antibodies were purchased from Sigma. Hippocampus were isolated from the brains of 5 mice (n = 5 animals/group). Observers were blinded to the mice’s group allocations during the Western blot assay.
Compound
In this study, paeoniflorin (Sigma) was dissolved in 0.9% normal saline and diluted to the desired concentration (20 mg/kg) for intraperitoneal injection. According to a previous report, paeoniflorin at this concentration significantly reversed depressive behaviors in mice (Qiu et al. 2013a; Tao et al. 2016).
Statistical analysis
Two-way ANOVA was used to analyze the effects of CUMS stimulation and paeoniflorin treatment, and post hoc Tukey’s tests were applied to analyze the interaction between groups. Three-way ANOVA was applied for electrophysiological data, repeated measures three-way ANOVA and multivariate ANOVA were applied for MWM escape latency over time. All values are expressed as the mean ± SEM. p < 0.05 was considered significant.
The study has been designed to minimize the number of mice per experiment while still retaining biological statistical significance. The estimates of the necessary sample size(s) required were based on previous publications: n = 11 for CUMS (Li et al. 2018; Wu et al. 2007), n = 10 for Morris water maze (Hui et al. 2016; Luo et al. 2014), n = 5 for hippocampus LTP (Costa-Mattioli et al. 2007; Holderbach et al. 2007), n = 5 for spine density (Higuchi et al. 2016; Yu et al. 2019), n = 5 for Western blot (Wang et al. 2018; Zhang et al. 2016a). To further evaluate the samples size for each experiment, statistical power were calculated using “G*power 3.1” with an α level of 5%.
Results
Paeoniflorin protects against depression-like behavior caused by chronic unpredictable mild stress
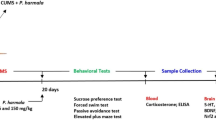
We first determined whether application of paeoniflorin could protect against depression-like behaviors in CUMS model mice. The experimental design is shown in Fig. 1 a. Briefly, animals were randomly divided into 4 groups and then treated with saline, paeoniflorin, CUMS + saline, or CUMS + paeoniflorin. CUMS stimulations were applied for 5 weeks, and paeoniflorin was applied from 14 days until the end of the experiment. A significant interaction between paeoniflorin treatment and stress was detected on the SPT (Treatment × Model: F(1, 40) = 6.02, p < 0.05, statistical power = 0.87), FST (Treatment × Model: F(1, 40) = 44.03, p < 0.01, statistical power = 0.99), and TST (Treatment × Model: F(1, 40) = 28.03, p < 0.01, statistical power = 0.99). These data showed that depression-like behavior was dramatically increased after 5 weeks of CUMS stimulation. The CUMS-exposed mice showed a significant reduction in sucrose consumption percentage (Model: F(1, 40) = 28.51, p < 0.01; CUMS + saline vs. control + saline: p < 0.01, Tukey’s tests; Fig. 1 b). As illustrated in Fig. 1 c and d, the immobility duration of CUMS mice was significant elevated in comparison with that of the control group during the FST (Model: F(1, 40) = 59.08, p < 0.01; CUMS + saline vs. control + saline: p < 0.01, Tukey’s tests; Fig. 1 c), as well as TST (Model: F(1, 40) = 3.18, p > 0.05; CUMS + saline vs. control + saline: p < 0.01, Tukey’s tests; Fig. 1 d). Interestingly, after the paeoniflorin treatment, the sucrose consumption percentage in the CUMS group was significantly reversed (Treatment: F(1, 40) = 4.18, p < 0.05; CUMS + paeoniflorin vs. CUMS + saline: p < 0.05, Tukey’s tests; Fig. 1 b). Paeoniflorin treatment effectively reversed the increased immobility time induced by CUMS (Treatment: F(1, 40) = 23.59, p < 0.01; CUMS + paeoniflorin vs. CUMS + saline: p < 0.01, Tukey’s tests; Fig. 1 c) in FST. Paeoniflorin could also prevent the CUMS-evoked increase in immobility time in the TST (Treatment: F(1, 40) = 13.35, p < 0.01; CUMS + paeoniflorin vs. CUMS + saline: p < 0.01, Tukey’s tests; Fig. 1 d). Paeoniflorin treatment had no effect on depression-like behaviors in control mice (control + paeoniflorin vs. control + saline: all p > 0.05, Tukey’s tests; Fig. 1). Therefore, paeoniflorin treatment effectively protected against CUMS-increased depression-like behaviors, and paeoniflorin had no effect on depression-like behaviors in control mice.
Effects of paeoniflorin on depression-like behaviors in CUMS mice. a Illustration of the protocols for CUMS, paeoniflorin administration, behavioral tests, and other sets of experiments. Forty-four mice were randomly divided into the control group, the paeoniflorin-treated control group, the CUMS group and the paeoniflorin-treated CUMS group (n = 11 in each group). The CUMS and paeoniflorin-treated CUMS groups experienced the same CUMS stimulations for 35 days, and paeoniflorin (20 mg/kg) or saline was injected daily i.p. from the 14th day of CUMS stimuli to the end of MWM. FST and TST were carried out at the end of the CUMS procedure, and then all the mice were trained and tested in the MWM. b CUMS mice consumed less sucrose than control mice in SPT. Interestingly, 3 weeks of administration of paeoniflorin (66.60 ± 14.51) prevented the decrease in sucrose consumption caused by CUMS (52.91 ± 6.77). n = 11 for all groups. Data are presented as the mean ± SEM. **, p < 0.01, CUMS group vs. control group; #, p < 0.05, CUMS + paeoniflorin group vs. CUMS + saline group. c In FST, paeoniflorin treatment (162.20 ± 18.29) protected against the increase in immobility time in CUMS animals (258.00 ± 32.32). n = 11 for all groups. Data are presented as the mean ± SEM. **, p < 0.01, CUMS group vs. control group; ##, p < 0.01, CUMS + paeoniflorin group vs. CUMS + saline group. d In TST, paeoniflorin treatment (124.50 ± 26.78) protected against the increase in immobility time of CUMS animals (216.10 ± 38.55). n = 11 for all groups. Data are presented as the mean ± SEM. **, p < 0.01, CUMS group vs. control group; ##, p < 0.01, CUMS + paeoniflorin group vs. CUMS + saline group
Paeoniflorin ameliorates the impairment of spatial learning and memory by chronic stress
To investigate the influence of paeoniflorin on CUMS-impaired spatial cognition performance, we administered the MWM task. As shown in Fig. 2 a, the escape latencies in all 4 groups improved with increasing trial training in the MWM, three-way repeated measures ANOVA analysis revealed the change in escape latency time during training (Time × Treatment × Model: F(3, 108) = 6.51, p < 0.01; Time × Treatment: F(3, 108) = 7.77, p < 0.01; Time × Model: F(3, 108) = 4.54, p < 0.01; Treatment × Model: F(1, 36) = 1.56, p > 0.05; Time: F(3, 108) = 161.81, p < 0.01; Treatment: F(1, 36) = 1.27, p > 0.05; Model: F(1, 36) = 3.44, p > 0.05). Furthermore, multivariate analysis of variance (ANOVA) showed that there was a significant difference between the 4 groups during the training (4 days) on the 3rd day (group: F(3, 36) = 5.52, p < 0.01, multivariate ANOVA) and the 4th day (group: F(3, 36) = 7.40, p < 0.01, multivariate ANOVA). The latency of CUMS group was significantly different from that of the control group on the 3rd day (CUMS + saline vs. control + saline: p < 0.05, Tukey’s tests; Fig. 2 a) and the 4th day (CUMS + saline vs. control + saline: p < 0.01, Tukey’s tests; Fig. 2 a). Interestingly, the use of paeoniflorin significantly reversed the decrease in latency time in CUMS group on the 3rd day (CUMS + paeoniflorin vs. CUMS + saline: p < 0.05, Tukey’s tests; Fig. 2 a) and the 4th day (CUMS + paeoniflorin vs. CUMS + saline: p < 0.01, Tukey’s tests; Fig. 2 a). Two-way ANOVA showed a significant interaction during the probe test between the paeoniflorin treatment and stress in the time that mice spent in the target quadrant (Treatment × Model: F(1, 36) = 4.57, p < 0.05, statistical power = 0.59; Fig. 2 b), as well as the number of times that mice crossed the former location of the removed hidden platform (Treatment × Model: F(1, 36) = 8.27, p < 0.01, statistical power = 0.84; Fig. 2 c). From the data shown in Fig. 2 b, CUMS-exposed mice spent less time in the target quadrant than control mice did (Model: F(1, 36) = 21.47, p < 0.01; CUMS + saline vs. control + saline: p < 0.01, Tukey’s tests; Fig. 2 b), and the CUMS group also showed a significant reduction in the number of transits across the former location of the platform area (Model: F(1, 36) = 11.01, p < 0.01; CUMS + saline vs. control + saline: p < 0.01, Tukey’s tests; Fig. 2c). In addition, it is noteworthy that after paeoniflorin treatment, CUMS mice spent more time in the target quadrant (Treatment: F(1, 36) = 12.80, p < 0.01; CUMS + paeoniflorin vs. CUMS + saline: p < 0.01, Tukey’s tests; Fig. 2 b). Similarly, paeoniflorin treatment increased the number of crossings (Treatment: F(1, 36) = 2.40, p > 0.05; CUMS + paeoniflorin vs. CUMS + saline: p < 0.05, Tukey’s tests; Fig. 2 c). Paeoniflorin treatment had no effect on spatial learning in control mice (control + paeoniflorin vs. control + saline: all p > 0.05, Tukey’s tests; Fig. 2). The above data suggested that paeoniflorin significantly improved CUMS-impaired spatial learning and memory in MWM.
The impairment of spatial learning by chronic stress was rescued by paeoniflorin treatment. a During the hidden platform training, all mice showed improvement in escape latency. During the last 2 days of hidden platform training, the CUMS group showed significantly less improvement than the control group in escape latency. Paeoniflorin treatment reversed the impaired improvement in escape latency of CUMS mice but had no obvious effects on mice in the control group. n = 10 for all groups. Data are presented as the mean ± SEM. *, p < 0.05; **, p < 0.01, CUMS group vs. control group; #, p < 0.05; ##, p < 0.01, CUMS + paeoniflorin group vs. CUMS + saline group. b The probe test revealed that CUMS-exposed mice (11.97 ± 1.45) spent less time in the target quadrant compared with mice in the control group (22.44 ± 2.08), but the application of paeoniflorin reversed the decreased time (20.81 ± 1.43). n = 10 for all groups. Data are presented as the mean ± SEM. **, p < 0.01, CUMS group vs. control group; ##, p < 0.01, CUMS + paeoniflorin group vs. CUMS + saline group. c The CUMS group had a lower platform cross number (2.80 ± 0.36) during the probe test than the mice in the control group (5.60 ± 0.60). Paeoniflorin treatment increased the platform crossing number (4.80 ± 0.29) of mice in the CUMS group. There was no significant difference between saline-treated and paeoniflorin-treated mice. n = 10 for all groups. Data are presented as the mean ± SEM. **, p < 0.01, CUMS group vs. control group; #, p < 0.05, CUMS + paeoniflorin group vs. CUMS + saline group
Paeoniflorin treatment restores hippocampal long-term potentiation in CUMS mice
To investigate whether the suppression of hippocampal LTP in CUMS model mice can be rescued by paeoniflorin treatment, we recorded hippocampal CA1 area LTP in control mice, paeoniflorin-treated control mice, CUMS mice, and paeoniflorin-treated CUMS model mice by 4 trains of TBS stimuli. LTP was successfully induced in control mice (151.80 ± 15.88%) and control mice treated with paeoniflorin (156.00 ± 4.62%) but reduced in CUMS mice (107.70 ± 4.60%), and the suppression was rescued in paeoniflorin-treated CUMS model mice (156.20 ± 12.36%), Fig. 3a and b. Three-way ANOVA revealed that LTP was significantly suppressed in CUMS mouse hippocampal slices (Time × Treatment × Model: F(5, 168) = 0.01; Time × Treatment: F(5, 168) = 0.03; Time × Model: F(5, 168) = 0.01; Treatment × Model: F(1, 168) = 0.17; Time: F(5, 168) = 0.02; Treatment: F(1, 168) = 2.38; Model: F(1, 168) = 128.40, all factors and interactions: p < 0.01; CUMS vs. control, CUMS vs. paeoniflorin, both p < 0.01, statistical power = 0.87). No clear difference was found among control, paeoniflorin-treated control mice, and paeoniflorin-treated CUMS mice. These results indicated that CUMS led to a suppression of LTP and that paeoniflorin treatment could protect against the suppression of LTP by CUMS.
LTP impairment caused by CUMS stimulation was reversed by paeoniflorin treatment. a Time course of the effects of high-frequency stimulation (HFS) on the fEPSP initial slope in the control group, paeoniflorin-treated control group, CUMS group, paeoniflorin-treated CUMS group. n = 5 for all groups. b Comparison of average normalized fEPSP slopes from 40 to 60 min after HFS among control + saline (151.80 ± 15.88%), control + paeoniflorin (156.00 ± 4.62%), CUMS + saline (107.70 ± 4.60%), CUMS + paeoniflorin (156.20 ± 12.36%) groups. Data are presented as the mean ± SEM. **, p < 0.01, CUMS group vs. control group; ##, p < 0.01, CUMS + paeoniflorin group vs. CUMS + saline group
Paeoniflorin treatment rescues CUMS-impaired dendritic spine density
Changes in dendritic spine morphology are strongly related to cognitive abilities. To investigate the effect of CUMS on dendritic spine density, we first used Golgi staining to determine the number of dendritic spines in hippocampal CA1 pyramidal cells of all groups. Two-way ANOVA measures revealed a change in dendritic spine density in hippocampal pyramidal neurons (Treatment × Model: F(1, 16) = 8.40, p < 0.05, statistical power = 0.86). Dendritic spine density (number/20 μm) was decreased in CUMS mice (Model: F(1, 16) = 7.85, p < 0.05; CUMS + saline vs. control + saline: p < 0.01, Tukey’s tests; Fig. 4 a and b) compared to control mice. Interestingly, paeoniflorin treatment rescued the decrease in spine density (Treatment: F(1, 16) = 6.31, p < 0.05; CUMS + paeoniflorin vs. CUMS + saline: p < 0.01, Tukey’s tests; Fig. 4 a and b).
The decrease in dendritic spine number of hippocampal CA1 pyramidal cells in CUMS mice was protected by paeoniflorin treatment. a Golgi staining showed dendrite spines of adult pyramidal neurons in the CA1 hippocampus in control + saline, control + paeoniflorin, CUMS + saline, CUMS + paeoniflorin-treated mice. Scale bar = 5 μm. b Quantification of spine density of the Golgi-stained neurons in control + saline (32.00 ± 2.24), control + paeoniflorin (31.20 ± 2.44), CUMS + saline (20.20 ± 1.46), CUMS + paeoniflorin-treated mice (31.40 ± 2.02), and 5 neurons per mouse were analyzed. n = 5 for all groups. Data are presented as the mean ± SEM. **, p < 0.01, CUMS group vs. control group; ##, p < 0.01, CUMS + paeoniflorin group vs. CUMS + saline group
BDNF and PSD95 expression changes are involved in the protective effects of paeoniflorin against CUMS
Numerous evidence show that BDNF plays an essential role in the structural plasticity induced by depression (Brunoni et al. 2008; Castren and Rantamaki 2010). BDNF regulates several important signaling pathways, including the expression level of PSD95. Both BDNF and PSD95 expression levels were suppressed in the CUMS model; and a supply of BDNF exhibited antidepressant activities, inducing an increase in PSD95 (Qiao et al. 2017). We detected the expression levels of BDNF and PSD95 to determine their roles in the protective function of paeoniflorin in CUMS. Hippocampus BDNF protein levels were analyzed with two-way ANOVA (Treatment × Model: F(1, 16) = 1.82, p > 0.05, statistical power = 0.86). Tukey’s test showed that there was a significant decrease in BDNF protein levels in the hippocampus of CUMS mice (Model: F(1, 16) = 31.41, p < 0.01; CUMS + saline vs. control + saline: p < 0.01, Tukey’s tests; Fig. 5 a and b). Paeoniflorin treatment significantly increased the hippocampal BDNF levels in CUMS-exposed mice (Treatment: F(1, 16) = 7.94, p < 0.05; CUMS + paeoniflorin vs. CUMS + saline: p < 0.05, Tukey’s tests; Fig. 5 a and b). PSD95 protein levels were analyzed by two-way ANOVA (Treatment × Model: F(1, 16) = 2.16, p > 0.05, statistical power = 0.83). Tukey’s test also indicated a significant decrease in PSD95 protein levels in CUMS mice in comparison to the control mice (Model: F(1, 16) = 9.10, p < 0.01; CUMS + saline vs. control + saline: p < 0.05, Tukey’s tests; Fig. 5 a and b). Paeoniflorin treatment significantly increased the hippocampal PSD95 levels in CUMS-exposed mice (Treatment: F(1, 16) = 7.25, p < 0.05; CUMS + paeoniflorin vs. CUMS + saline: p < 0.05, Tukey’s tests; Fig. 5 a and b). No significant difference between the paeoniflorin-treated control group and the saline-treated control group (control + paeoniflorin vs. control + saline; p > 0.05, Tukey’s tests) was observed. These data indicated that paeoniflorin could ameliorate BDNF and PSD95 expression, effects that are involved in its antidepressant-like functions.
Paeoniflorin treatment reversed CUMS stimulation and decreased BDNF and PSD95 expression. a The expression levels of BDNF and PSD95 in hippocampal tissues by Western blotting. The protein loading control for the samples was β-actin. b CUMS stimulation decreased BDNF expression (0.48 ± 0.05) compared to that of the control group (1.00 ± 0.10). Paeoniflorin treatment reversed BDNF expression (0.79 ± 0.05). CUMS also decreased PSD95 expression (0.65 ± 0.03) compared with that of the control group (1.00 ± 0.09), and paeoniflorin treatment reversed this process (0.98 ± 0.08). BDNF and PSD95 expression was normalized to β-action. n = 5 samples for each group. Data are presented as the mean ± SEM. *, p < 0.05; **, p < 0.01, CUMS group vs. control group; #, p < 0.05, CUMS + paeoniflorin group vs. CUMS + saline group
Discussion
In our study, we demonstrated that paeoniflorin, one of the major active components of Paeonia lactiflora Pall., remarkably protected against chronic-stress-induced depression-like behavior in a mouse CUMS model. Furthermore, paeoniflorin treatment significantly attenuated the impairment of hippocampus-related learning and memory performance in MWM and LTP in CUMS mice. We then found significant changes in synapse density and the expression of BDNF and PSD95 in CUMS mice before and after paeoniflorin administration; these changes may be involved in the protective function of paeoniflorin against the impairment of cognitive ability by CUMS.
Disturbances of emotional learning and memory are among the main features of depressive disorders. Hippocampal formation is highly sensitive to stress-induced morphological and functional changes. In the CUMS model, animals are exposed to several moderate stressors for a relatively long time. These stimulations lead to changes, such as inhibition of neurogenesis in the dentate gyrus (DG), decreases in LTP and impairment of hippocampus-dependent learning and memory (Bangasser and Shors 2007; Hayashi et al. 2008; Shors and Thompson 1992). The main interest and strength of the present work is that paeoniflorin displays both antidepressant and pro-cognitive action in the CUMS model. Except for a few of the currently available antidepressants, such as agomelatine (Martin et al. 2017) and vortioxetine (Wallace et al. 2014), reversed learning impairment in depression animals. Most current antidepressant drugs lack efficacy against cognitive deficits in depressive patients and laboratory animals (Gorenstein et al. 2006; Naudon et al. 2007). Here, in the current study, we found that paeoniflorin administration in CUMS mice also protected against stress-impaired learning and memory deficiency in MWM and impairment of hippocampal LTP. Previous studies have demonstrated that paeoniflorin is a cognitive enhancer that is capable of attenuating learning and memory dysfunction caused by chronic cerebral hypoperfusion in aged mice and preventing age-evoked learning behavior lesions in operant brightness discrimination tasks (Liu et al. 2006). In rats, unilateral lesion of the nucleus basalis magnocellularis, which induced spatial learning deficits, could also be ameliorated by paeoniflorin (Ohta et al. 1994). The present research furthered our understanding of the antidepressant activity of paeoniflorin.
Our finding is consistent with previous reports that paeoniflorin exhibits antidepressant-like effects in several depression models, including TST, FST, CUS (chronic unpredictable stress), and menopause depression model (Huang et al. 2015; Mao et al. 2008; Qiu et al. 2013a). Here, we further confirmed that long-term treatment with paeoniflorin protected against depression-like behaviors in a 35-day CUMS-induced mouse depression model. Traditional antidepressants, such as SSRIs, which show great potency in inhibiting 5-HT uptake, also blocked histaminic, cholinergic, and alpha-1 adrenergic receptor sites, and this action brought about strong unwanted side effects (Artigas et al. 2002; Mandrioli et al. 2012). Consistent with this observation, short-term or long-term expose of SSRIs, like fluoxetine (Jin et al. 2017), fluvoxamine (Ushijima et al. 2005), and sertraline (Mikail et al. 2012), reduces immobility time in FST or TST. Our observation that paeoniflorin did not show any effect on control mice as revealed by the FST and TST indicates relatively low potential side effects of paeoniflorin. However, one previous study reported that 7-day administration of total glycosides of peony decreased the immobility time of mice in the TST and FST (Mao et al. 2008). One explanation for this observation is that the total glycosides of peony they used contain 30% of paeoniflorin and 10% of albiflorin as determined by high-performance liquid chromatography. We noticed that a previously published paper (Wang et al. 2016) confirmed powerful antidepressant-like effects of albiflorin. The 7-day administration of albiflorin to normal animals decreased immobility in both FST and TST. Therefore, it is worth to compare the antidepressant activity of paeoniflorin and albiflorin in the decline in FST and TST immobility time in normal mice in future studies. In addition, the animals used in Mao’s study were male Institute of Cancer Research (ICR) mice; however, C57BL/6 wild-type male mice were used in our experiment.
The expression of BDNF, a critical neurotrophic factor, is closely regulated by neuronal activity, and depression is associated with reduced brain BDNF levels (Lee and Kim 2010). Decreases in BDNF are related to several neuronal dysfunctions in depression, including a decrease in PSD-95, dysregulation of synaptic plasticity, and impaired neurogenesis (Lee and Kim 2010; Qiao et al. 2017; Yu and Chen 2011). Increasing the expression of BDNF could develop remarkable antidepressant responses (Bjorkholm and Monteggia 2016). We observed that administration of paeoniflorin in CUMS mice also reversed expression changes in BDNF and PSD95 and spine density defects, which is a potential mechanism by which paeoniflorin protects against CUMS-induced synaptic plasticity deficiency and learning and memory impairment. Although it is still unclear how paeoniflorin affects the expression of BDNF and PSD95 during the depression process, reports have shown that paeoniflorin exhibits neuroprotective function through the Ca2+/CaMKII/CREB signaling pathway against (NMDA)-elicited excitotoxicity (Ip et al. 2016) or cerebral ischemia reperfusion injury (Zhang et al. 2017). When activated through phosphorylation at Ser133, CREB could further induce the expression of BDNF and PSD-95. Studies have also shown that paeoniflorin administration regulates neurotransmitters, such as increasing the expression of 5HT1AR and noradrenaline (NA), decreasing the expression levels of the adrenocorticotropic hormone (ACTH), corticotrophin releasing hormone (CRH), and cortisol (CORI) in rodent brain (Huang et al. 2015; Qiu et al. 2013b). Additionally, in vitro studies showed that paeoniflorin exerts neuroprotective effects against glutamate-induced neurotoxicity by inhibiting oxidative stress, Ca2+ overload (Mao et al. 2010), and apoptosis pathways (Chen et al. 2017). Although our research and others revealed the potential mechanism by which paeoniflorin protects against CUMS-induced depression-like behaviors and cognitive impairments, understanding the comprehensive mechanism still requires further work. Research aims to reveal the correlation between paeoniflorin and 5-HT, glutamate signaling, BDNF signaling, apoptosis pathways, and neurogenesis pathways will further our understanding.
In summary, paeoniflorin prevents CUMS-induced elevation of depression-like behaviors, impairment of cognitive abilities, deficiency of hippocampal LTP, and morphological changes in dendritic spines, as well as changes in the protein concentrations of BDNF and PSD-95. The current study will benefit further antidepressant application of paeoniflorin.
Abbreviations
- Pae:
-
Paeoniflorin
- CUMS:
-
Chronic unpredictable mild stress (CUMS)
- MWM:
-
Morris water maze
- SPT:
-
Sucrose preference test
- FST:
-
Forced swimming test
- TST:
-
Tail suspension test
- LTP:
-
Long-term potentiation
- BDNF:
-
Brain-derived neurotrophic factor
- PSD95:
-
Postsynaptic density protein 95
- aCSF:
-
Artificial cerebrospinal fluid
- Sal:
-
Saline
References
Abdel-Hafez AA, Meselhy MR, Nakamura N, Hattori M, Watanabe H, Mohamed TA, Mahfouz NM, el-Gendy MA (1998) Potent anticonvulsant paeonimetabolin-I derivatives obtained by incubation of paeoniflorin and thiol compounds with Lactobacillus brevis. Chem Pharm Bull (Tokyo) 46:1486–1487
Abdel-Hafez AA, Meselhy MR, Nakamura N, Hattori M, Watanabe H, Murakami Y, El-Gendy MA, Mahfouz NM, Mohamed TA (1999) Anticonvulsant activity of paeonimetabolin-I adducts obtained by incubation of paeoniflorin and thiol compounds with Lactobacillus brevis. Biol Pharm Bull 22:491–497
Artigas F, Nutt DJ, Shelton R (2002) Mechanism of action of antidepressants. Psychopharmacol Bull 36 Suppl(2):123–132
Bangasser DA, Shors TJ (2007) The hippocampus is necessary for enhancements and impairments of learning following stress. Nat Neurosci 10:1401–1403
Bjorkholm C, Monteggia LM (2016) BDNF - a key transducer of antidepressant effects. Neuropharmacology 102:72–79
Brunoni AR, Lopes M, Fregni F (2008) A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol 11:1169–1180
Castren E, Rantamaki T (2010) The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Developmental neurobiology 70:289–297
Chen A, Wang H, Zhang Y, Wang X, Yu L, Xu W, Xu W, Lin Y (2017) Paeoniflorin exerts neuroprotective effects against glutamateinduced PC12 cellular cytotoxicity by inhibiting apoptosis. Int J Mol Med 40:825–833
Clayton AH, Alkis AR, Parikh NB, Votta JG (2016) Sexual dysfunction due to psychotropic medications. Psychiatr Clin North Am 39:427–463
Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjevic K, Lacaille JC, Nader K, Sonenberg N (2007) eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 129:195–206
Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249
Gorenstein C, de Carvalho SC, Artes R, Moreno RA, Marcourakis T (2006) Cognitive performance in depressed patients after chronic use of antidepressants. Psychopharmacology 185:84–92
Gu X, Cai Z, Cai M, Liu K, Liu D, Zhang Q, Tan J, Ma Q (2016) Protective effect of paeoniflorin on inflammation and apoptosis in the cerebral cortex of a transgenic mouse model of Alzheimer’s disease. Mol Med Rep 13:2247–2252
Hayashi F, Takashima N, Murayama A, Inokuchi K (2008) Decreased postnatal neurogenesis in the hippocampus combined with stress experience during adolescence is accompanied by an enhanced incidence of behavioral pathologies in adult mice. Mol Brain 1:22
He DY, Dai SM (2011) Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora Pall., a traditional Chinese herbal medicine. Front Pharmacol 2:10
He ZY, Hu WY, Zhang M, Yang ZZ, Zhu HM, Xing D, Ma QH, Xiao ZC (2016a) Wip1 phosphatase modulates both long-term potentiation and long-term depression through the dephosphorylation of CaMKII. Cell Adhes Migr 10:237–247
He ZY, Wang WY, Hu WY, Yang L, Li Y, Zhang WY, Yang YS, Liu SC, Zhang FL, Mei R, Xing D, Xiao ZC, Zhang M (2016b) Gamma-H2AX upregulation caused by Wip1 deficiency increases depression-related cellular senescence in hippocampus. Sci Rep 6:34558
Higuchi F, Uchida S, Yamagata H, Abe-Higuchi N, Hobara T, Hara K, Kobayashi A, Shintaku T, Itoh Y, Suzuki T, Watanabe Y (2016) Hippocampal microRNA-124 enhances chronic stress resilience in mice. J Neurosci 36:7253–7267
Holderbach R, Clark K, Moreau JL, Bischofberger J, Normann C (2007) Enhanced long-term synaptic depression in an animal model of depression. Biol Psychiatry 62:92–100
Huang H, Zhao J, Jiang L, Xie Y, Xia Y, Lv R, Dong L (2015) Paeoniflorin improves menopause depression in ovariectomized rats under chronic unpredictable mild stress. Int J Clin Exp Med 8:5103–5111
Hui JJ, Xi GJ, Liu SS, Li XL, Geng LY, Teng GJ, Nie BB, Shan BC, Yan J, Dong L, Reynolds GP, Zhang ZJ (2016) Blood oxygen level-dependent signals via fMRI in the mood-regulating circuit using two animal models of depression are reversed by chronic escitalopram treatment. Behav Brain Res 311:210–218
Ip FC, Zhao YM, Chan KW, Cheng EY, Tong EP, Chandrashekar O, Fu GM, Zhao ZZ, Ip NY (2016) Neuroprotective effect of a novel Chinese herbal decoction on cultured neurons and cerebral ischemic rats. BMC Complement Altern Med 16:437
Jin ZL, Chen XF, Ran YH, Li XR, Xiong J, Zheng YY, Gao NN, Li YF (2017) Mouse strain differences in SSRI sensitivity correlate with serotonin transporter binding and function. Sci Rep 7:8631
Kizilbash AH, Vanderploeg RD, Curtiss G (2002) The effects of depression and anxiety on memory performance. Arch Clin Neuropsychol 17:57–67
Krishnan V, Nestler EJ (2008) The molecular neurobiology of depression. Nature 455:894–902
Lane RM (2015) Antidepressant drug development: focus on triple monoamine reuptake inhibition. J Psychopharmacol 29:526–544
Lee BH, Kim YK (2010) The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig 7:231–235
Li MX, Zheng HL, Luo Y, He JG, Wang W, Han J, Zhang L, Wang X, Ni L, Zhou HY, Hu ZL, Wu PF, Jin Y, Long LH, Zhang H, Hu G, Chen JG, Wang F (2018) Gene deficiency and pharmacological inhibition of caspase-1 confers resilience to chronic social defeat stress via regulating the stability of surface AMPARs. Mol Psychiatry 23:556–568
Liu DZ, Xie KQ, Ji XQ, Ye Y, Jiang CL, Zhu XZ (2005) Neuroprotective effect of paeoniflorin on cerebral ischemic rat by activating adenosine A1 receptor in a manner different from its classical agonists. Br J Pharmacol 146:604–611
Liu J, Jin DZ, Xiao L, Zhu XZ (2006) Paeoniflorin attenuates chronic cerebral hypoperfusion-induced learning dysfunction and brain damage in rats. Brain Res 1089:162–170
Liu L, Liu C, Wang Y, Wang P, Li Y, Li B (2015) Herbal medicine for anxiety, depression and insomnia. Curr Neuropharmacol 13:481–493
Luo J, Min S, Wei K, Cao J, Wang B, Li P, Dong J, Liu Y (2014) Propofol prevents electroconvulsive-shock-induced memory impairment through regulation of hippocampal synaptic plasticity in a rat model of depression. Neuropsychiatr Dis Treat 10:1847–1859
Luo XQ, Li A, Yang X, Xiao X, Hu R, Wang TW, Dou XY, Yang DJ, Dong Z (2018) Paeoniflorin exerts neuroprotective effects by modulating the M1/M2 subset polarization of microglia/macrophages in the hippocampal CA1 region of vascular dementia rats via cannabinoid receptor 2. Chin Med 13:14
Mandrioli R, Mercolini L, Saracino MA, Raggi MA (2012) Selective serotonin reuptake inhibitors (SSRIs): therapeutic drug monitoring and pharmacological interactions. Curr Med Chem 19:1846–1863
Mao QQ, Ip SP, Tsai SH, Che CT (2008) Antidepressant-like effect of peony glycosides in mice. J Ethnopharmacol 119:272–275
Mao QQ, Zhong XM, Feng CR, Pan AJ, Li ZY, Huang Z (2010) Protective effects of paeoniflorin against glutamate-induced neurotoxicity in PC12 cells via antioxidant mechanisms and Ca(2+) antagonism. Cell Mol Neurobiol 30:1059–1066
Mao QQ, Ip SP, Xian YF, Hu Z, Che CT (2012) Anti-depressant-like effect of peony: a mini-review. Pharm Biol 50:72–77
Martin V, Allaili N, Euvrard M, Marday T, Riffaud A, Franc B, Mocaer E, Gabriel C, Fossati P, Lehericy S, Lanfumey L (2017) Effect of agomelatine on memory deficits and hippocampal gene expression induced by chronic social defeat stress in mice. Sci Rep 8:45907
Mikail HG, Dalla C, Kokras N, Kafetzopoulos V, Papadopoulou-Daifoti Z (2012) Sertraline behavioral response associates closer and dose-dependently with cortical rather than hippocampal serotonergic activity in the rat forced swim stress. Physiol Behav 107:201–206
Morris RG, Garrud P, Rawlins JN, O'Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
Naudon L, Hotte M, Jay TM (2007) Effects of acute and chronic antidepressant treatments on memory performance: a comparison between paroxetine and imipramine. Psychopharmacology 191:353–364
Ohta H, Nishi K, Matsumoto K, Shimizu M, Watanabe H (1994) Paeoniflorin improves learning deficit in 4-arm baited radial maze performance in rats with unilateral nucleus basalis magnocellularis lesion. Phytomedicine 1:117–121
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336
Qiao H, An SC, Ren W, Ma XM (2014) Progressive alterations of hippocampal CA3-CA1 synapses in an animal model of depression. Behav Brain Res 275:191–200
Qiao H, An SC, Xu C, Ma XM (2017) Role of proBDNF and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Brain Res 1663:29–37
Qiu F, Zhong X, Mao Q, Huang Z (2013a) The antidepressant-like effects of paeoniflorin in mouse models. Exp Ther Med 5:1113–1116
Qiu FM, Zhong XM, Mao QQ, Huang Z (2013b) Antidepressant-like effects of paeoniflorin on the behavioural, biochemical, and neurochemical patterns of rats exposed to chronic unpredictable stress. Neurosci Lett 541:209–213
Qiu J, Chen M, Liu J, Huang X, Chen J, Zhou L, Ma J, Sextius P, Pena AM, Cai Z, Jeulin S (2016) The skin-depigmenting potential of Paeonia lactiflora root extract and paeoniflorin: in vitro evaluation using reconstructed pigmented human epidermis. Int J Cosmet Sci 38:444–451
Sharma H, Santra S, Dutta A (2015) Triple reuptake inhibitors as potential next-generation antidepressants: a new hope? Future Med Chem 7:2385–2406
Shors TJ, Thompson RF (1992) Acute stress impairs (or induces) synaptic long-term potentiation (LTP) but does not affect paired-pulse facilitation in the stratum radiatum of rat hippocampus. Synapse 11:262–265
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370
Tabata K, Matsumoto K, Murakami Y, Watanabe H (2001) Ameliorative effects of paeoniflorin, a major constituent of peony root, on adenosine A1 receptor-mediated impairment of passive avoidance performance and long-term potentiation in the hippocampus. Biol Pharm Bull 24:496–500
Tanaka R, Yamazaki M, Hasada K, Nagatsu A (2013) Application of quantitative 1H-NMR method to determination of paeoniflorin in Paeoniae radix. J Nat Med 67:657–661
Tao W, Wang H, Su Q, Chen Y, Xue W, Xia B, Duan J, Chen G (2016) Paeonol attenuates lipopolysaccharide-induced depressive-like behavior in mice. Psychiatry Res 238:116–121
Ushijima K, Sakaguchi H, Sato Y, To H, Koyanagi S, Higuchi S, Ohdo S (2005) Chronopharmacological study of antidepressants in forced swimming test of mice. J Pharmacol Exp Ther 315:764–770
Wallace A, Pehrson AL, Sanchez C, Morilak DA (2014) Vortioxetine restores reversal learning impaired by 5-HT depletion or chronic intermittent cold stress in rats. Int J Neuropsychopharmacol 17:1695–1706
Wang QS, Gao T, Cui YL, Gao LN, Jiang HL (2014) Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm Biol 52:1189–1195
Wang YL, Wang JX, Hu XX, Chen L, Qiu ZK, Zhao N, Yu ZD, Sun SZ, Xu YY, Guo Y, Liu C, Zhang YZ, Li YF, Yu CX (2016) Antidepressant-like effects of albiflorin extracted from Radix paeoniae Alba. J Ethnopharmacol 179:9–15
Wang Y, Xu J, Liu Y, Li Z, Li X (2018) TLR4-NF-kappaB signal involved in depressive-like behaviors and cytokine expression of frontal cortex and hippocampus in stressed C57BL/6 and ob/ob mice. Neural Plast 2018:7254016
Willner P (1990) Animal models of depression: an overview. Pharmacol Ther 45:425–455
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93:358–364
Wilson S, Argyropoulos S (2005) Antidepressants and sleep: a qualitative review of the literature. Drugs 65:927–947
Wu LM, Han H, Wang QN, Hou HL, Tong H, Yan XB, Zhou JN (2007) Mifepristone repairs region-dependent alteration of synapsin I in hippocampus in rat model of depression. Neuropsychopharmacology 32:2500–2510
Xi G, Hui J, Zhang Z, Liu S, Zhang X, Teng G, Chan KC, Wu EX, Nie B, Shan B, Li L, Reynolds GP (2011) Learning and memory alterations are associated with hippocampal N-acetylaspartate in a rat model of depression as measured by 1H-MRS. PLoS One 6:28686
Ye J, Duan H, Yang X, Yan W, Zheng X (2001) Anti-thrombosis effect of paeoniflorin: evaluated in a photochemical reaction thrombosis model in vivo. Planta Med 67:766–767
Yu H, Chen ZY (2011) The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin 32:3–11
Yu H, Zhong J, Niu B, Zhong Q, Xiao J, Xie J, Lin M, Zhou Z, Xu J, Wang H (2019) Inhibition of phosphodiesterase 4 by FCPR03 alleviates chronic unpredictable mild stress-induced depressive-like behaviors and prevents dendritic spine loss in mice hippocampi. Int J Neuropsychopharmacol 22:143–156
Zhang X, Song Y, Bao T, Yu M, Xu M, Guo Y, Wang Y, Zhang C, Zhao B (2016a) Antidepressant-like effects of acupuncture involved the ERK signaling pathway in rats. BMC Complement Altern Med 16:380
Zhang Y, Wang LL, Wu Y, Wang N, Wang SM, Zhang B, Shi CG, Zhang SC (2016b) Paeoniflorin attenuates hippocampal damage in a rat model of vascular dementia. Exp Ther Med 12:3729–3734
Zhang Y, Qiao L, Xu W, Wang X, Li H, Xu W, Chu K, Lin Y (2017) Paeoniflorin attenuates cerebral ischemia-induced injury by regulating Ca2+/CaMKII/CREB signaling pathway. Molecules 22:359
Zheng M, Liu C, Fan Y, Shi D, Zhang Y (2016) Protective effects of Paeoniflorin against MPP(+)-induced neurotoxicity in PC12 cells. Neurochem Res 41:1323–1334
Funding
This work was supported by the National Natural Science Program of China (81860254, Z.Y.H.; 81360175, M. Z), the Joint Program of Yunnan Province and Kunming Medical University (2017FE468-249, Z.Y.H. 2015FB006, W. Y. H), and the support provided by Yunnan Key Laboratory of Stem Cell and Regenerative Medicine (Z. Y. H., M. Z. and W.Y.H.) is also appreciated.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, SC., Hu, WY., Zhang, WY. et al. Paeoniflorin attenuates impairment of spatial learning and hippocampal long-term potentiation in mice subjected to chronic unpredictable mild stress. Psychopharmacology 236, 2823–2834 (2019). https://doi.org/10.1007/s00213-019-05257-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05257-5