Abstract
Rationale
The influence of the main dopaminergic brain regions controlling copulation, the medial preoptic area (mPOA) and the nucleus accumbens (NAcc), on male rat sexual behavior expression has not been fully established.
Objective
This work analyzes the sexual effects of dopamine (DA) receptor activation in the mPOA or the NAcc of sexually active male rats, with an intact (sexually experienced) or a reduced (sexually exhausted) sexual motivation.
Methods
The non-specific DA receptor agonist apomorphine and the D2-like receptor agonist quinpirole were infused into the mPOA or the NAcc of sexually experienced or sexually exhausted male rats and their sexual behavior recorded.
Results
DA receptor activation neither in the mPOA nor in the NAcc modified the copulatory behavior of sexually experienced male rats. DA receptor stimulation in the NAcc, but not in the mPOA, reversed the characteristic sexual inhibition of sexually satiated rats, and D2-like receptors were found to participate in this effect.
Conclusion
The optimal sexual performance of sexually experienced male rats cannot be further improved by DA receptor activation at either brain region. In sexually satiated rats, which are sexually inhibited and have a diminished sexual motivation, NAcc DA receptor stimulation appears to play a key role in their capacity to respond to a motivational significant stimulus, the receptive female, with the participation of D2-like receptors. Activation of DA receptors with the same drug, at the same dose and in the same brain region, produces different effects on copulatory behavior that depend on the animal’s sexual motivational state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine (DA) has been found to be important for the assignment of motivational value to rewarding behaviors (Berridge and Kringelbach 2011), which are essential to species reproduction and survival (Kringelbach and Berridge 2009). Motivation plays a central role in the maintenance of rewarding behaviors, like copulation, which are triggered by salient environmental stimuli (Schultz et al. 1997). The role played by DA in the regulation of masculine sexual behavior has been extensively documented (for review, see Hull and Rodríguez-Manzo 2017). In the rat, two dopaminergic (DAergic) brain circuits are most directly involved in this regulation: the incertohypothalamic and the mesolimbic DAergic systems. In the first case, the medial preoptic area (mPOA) is a major target of these DAergic fibers (Bitran et al. 1988) and has been associated with the endocrine activation of copulation, genital reflexes’ regulation, copulatory performance, and sexual motivation; this brain region is essential for male sexual behavior expression of all known vertebrate species (Hull and Rodríguez-Manzo 2017). In the second case, the ventral tegmental area (VTA), origin of this DAergic pathway, and the nucleus accumbens (NAcc) (Melis and Argiolas 1995), a major output region of this circuit, are both involved in the processing of the rewarding effects of sexual behavior, and the regulation of its reinforcing properties (Kelley and Berridge 2002). Electrical stimulation of the mPOA (Malsbury 1971; Merari and Ginton 1975; Rodríguez-Manzo et al. 2000; Van Dis and Larsson 1971), the VTA (Markowski and Hull 1995; Rodríguez-Manzo and Pellicer 2007), and the NAcc (Rodríguez-Manzo and Pellicer 2010) all facilitate copulatory behavior expression in sexually experienced male rats. Besides, exposure to the scent of a sexually receptive female and copulation itself increase Fos protein expression (a marker of neuronal activation) in the mPOA (Kelliher et al. 1999; Robertson et al. 1991), the NAcc, and the VTA (Balfour et al. 2004), and raise DA levels in the NAcc (Mitchell and Gratton 1994) and in the mPOA (Blackburn et al. 1992; Hull et al. 1995).
Male rats allowed to freely copulate with a single sexually receptive female perform 5 to 12 successive ejaculations, before reaching a state of sexual inactivity known as sexual satiety (Beach and Jordan 1956; Rodríguez-Manzo and Fernández-Guasti 1994). This phenomenon is characterized by a long-lasting sexual behavior inhibition (around 72 h), which gradually fades away, requiring a 15-day period of sexual rest for full copulatory recovery (Rodríguez-Manzo et al. 2011). Twenty-four hours after copulation to satiety, the sexual inhibition is manifested in two different ways in the presence of a sexually receptive female: by the absence of sexual behavior or by the execution of one ejaculatory series, after which the male does no resume sexual activity (Rodríguez-Manzo and Fernández-Guasti 1994). Over time, studies have concluded that enhancement of DAergic transmission facilitates male sexual behavior expression (Pfaus 2009). Systemic administration of the non-specific DA receptor agonist apomorphine decreases the ejaculatory threshold of sexually experienced rats (Ahlenius and Larsson 1984; Paglietti et al. 1978) and increases the proportion of sexually sluggish males capable of ejaculating within a 30-min period (Tagliamonte et al. 1974).
Like DA, apomorphine interacts with all DA receptor subtypes, although with a higher affinity for the D2-like receptor family (0.5 nM < Ki < 50 nM) than for the D1-like family (50 nM < Ki < 500 nM). In particular, the highest affinity of this drug is for the D2 and D4 DA receptor subtypes (0.5 nM < Ki < 5 nM), followed by that for the D3 subtype (5 nM < Ki < 50 nM) (Missale et al. 1998).
D2-like receptor agonists, such as LY163502 (quinerolane), also decrease the ejaculatory threshold of sexually experienced rats and induce copulation in so-called non-copulating animals (Foreman and Hall 1987). The group of Elaine Hull was the first studying the effects of the intra-brain administration of DA receptor agonists on copulation, showing that intra-mPOA apomorphine facilitates copulation (Hull et al. 1986, 1989) and genital reflexes (Hull et al. 1992; Pehek et al. 1989) in rats, while a D2-like DA receptor agonist delays the onset of copulation, but decreases the ejaculatory threshold of sexually experienced rats (Moses et al. 1984). This group found no effect of intra-NAcc DA agonist infusion on male rat copulation (Hull et al. 1986; Moses et al. 1984). We recently reported that systemically administered DA receptor agonists had differential effects on copulation of sexually active (experienced) and temporarily sexually inactive (exhausted) male rats (Guadarrama-Bazante et al. 2014). Though, the influence of the two main DAergic brain regions controlling copulation, i.e., the mPOA and the NAcc, on sexual behavior expression of these two populations has not been established. Both brain regions have been related to the control of male sexual motivation, with some controversy surrounding this role in each case (Everitt 1990; Hull et al. 1986). To address this issue, here we analyze the effects of the direct infusion of same doses of apomorphine or quinpirole into the mPOA or the NAcc on copulation of sexually active rats, either with an intact (experienced) or a reduced (exhausted) sexual motivational state.
Experimental procedures
Animals
Two hundred twenty-three adult male Wistar rats (200–250 g b. wt.), raised at the Cinvestav animal facilities, were used for the study. They were housed in acrylic boxes (44 × 33 × 20 cm), eight per cage, in a room at 22 °C, under inverted light-dark cycle conditions (12 h light/12 h dark), lights turned off at 10:00 AM, and with free access to food and water. Males were subjected to independent sexual behavior tests, and those showing ejaculation latencies shorter than 15 min, in at least three tests, were considered sexually experienced. Sexual receptivity was induced in female rats by the sequential injection of estradiol benzoate (4 μg/rat) followed 44 h later by progesterone (2 mg/rat). The Local Committee of Ethics on Animal Experimentation approved all experimental procedures (Protocol 0230-16), which followed the principles of laboratory animal care (Kilkenny et al. 2013).
Sexual behavior observations
Sexual behavior tests were conducted during the dark phase of the cycle, as described elsewhere (Rodríguez-Manzo et al. 2011). To render rats sexually satiated, males were allowed to copulate ad libitum with a single receptive female until reaching the satiety criterion (i.e., 90 min after the last ejaculation without attaining another ejaculation). The satiated rats received the drug treatments 24 h after copulation to satiety and were tested for sexual behavior with a new receptive female.
Spontaneous locomotor activity test
To rule out possible non-specific effects of the treatments that could have interfered with copulation, spontaneous ambulation of male rats was recorded after the sexual behavior tests (Guadarrama-Bazante et al. 2014).
Surgery
Male rats were stereotaxically implanted with bilateral guide cannulae, as described earlier (Rodríguez-Manzo and Canseco-Alba 2017), directed to the mPOA or to the NAcc. Target coordinates for the mPOA were as follows: AP, − 0.3 mm from bregma; L, ± 0.6 mm; DV, − 8.2 mm, and for the NAcc: AP, + 1.2 mm from bregma; L, ± 1.0 mm; DV, − 6.6 mm (Paxinos and Watson 2009). Implanted animals were individually housed and handled daily to later allow drug microinjection without anesthesia.
Drugs
All drugs were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Estradiol benzoate and progesterone were dissolved in corn oil and s.c. injected in a volume of 1 ml/kg. Apomorphine and quinpirole were dissolved in sterile saline and infused in a volume of 0.5 μg/cannula.
Histology
Animals were deeply anesthetized with sodium pentobarbital (200 mg/kg, i.p), and intracardially perfused with 250 ml formaldehyde at 10%. Their brains were removed and 60 μm coronal sections were obtained using a freezing cryostat (Leica® CM 1100). Brain slices containing the mPOA or the NAcc were placed directly onto a glass slide and scanned, locating cannulae tips in the images to verify their correct location.
Statistics
Differences in the proportion of animals showing the different sexual behavior responses were established using the Fisher’s F test. The specific sexual behavior parameters and ambulatory behavior data were analyzed with a Kruskal-Wallis ANOVA followed by Dunn’s test. Paired comparisons were established with the Mann-Whitney U test. The Sigma Stat Program® (version 12.0) was used for all analyses. P < 0.05 values were considered statistically significant.
Experimental design
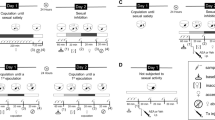
Sexually experienced, implanted males were randomly assigned to 1 of 14 independent groups, infused into the mPOA (n = 5–9 each) or into the NAcc (n = 7–12 each) with different doses of apomorphine (0.6, 2.0, or 6.0 μg/rat), quinpirole (0.3, 1.0, or 3.0 μg/rat), or vehicle (0.5 μl/cannula), and their sexual behavior recorded during 30 min. Fifteen additional groups of implanted, sexually experienced rats copulated to satiety received 24 h later apomorphine (0.6, 2.0, or 6.0 μg/rat), quinpirole (0.1, 0.3, 1.0, or 3.0 μg/rat), or vehicle (0.5 μl/cannula) into the mPOA (n = 7–9 each) or into the NAcc (n = 5–10 each), and their sexual behavior was recorded during 60 min.
Results
As expected, almost all sexually experienced rats, infused into the mPOA with vehicle or with the different doses of apomorphine, copulated to ejaculation and resumed copulation thereafter. The specific sexual behavior parameters of these apomorphine-treated rats were not significantly different from those of vehicle-infused sexually experienced rats (see Table 1). In sexually exhausted animals, the control group infused, 24 h after copulation to satiety, with vehicle into the mPOA showed the typical sexual inhibition of this condition, with a very small proportion of rats ejaculating (10%) and none of them resuming copulation after ejaculation. Intra-mPOA infusion of the different apomorphine doses to satiated rats slightly increased those proportions (around 30–40% copulating to ejaculation and 15–40% resuming copulation thereafter). These increases were not statistically significant.
All sexually experienced rats exhibited all the copulatory behaviors after the intra-mPOA infusion of the two lower quinpirole doses (0.3 and 1.0 μg/rat), while with the 3.0 μg/rat dose, two out of nine animals did not attain ejaculation, an effect that was not statistically significant. The sexual parameters of the quinpirole-infused animals were not different from those of the control males, infused with vehicle into the mPOA (see Table 1).
Figure 1 depicts the proportion of sexually satiated animals that copulated after intra-mPOA infusion of different doses of quinpirole, as well as the copulatory parameters of those animals that attained ejaculation in response to quinpirole treatment. It can be observed that quinpirole, at the dose of 0.3 μg/rat, statistically significantly increased the proportion of exhausted males showing all sexual behavior responses, as compared to vehicle-infused satiated rats [Fisher F test, P < 0.05 or P < 0.01). Thus, six out seven (85.7%) satiated rats ejaculated [Fisher F test, P < 0.01] and four out of seven (57%) resumed copulation after ejaculation [Fisher F test, P < 0.01]. The satiated rats infused with lower (0.1 μg/rat) and higher (1.0 and 3.0 μg/rat) quinpirole doses did not copulate.
Effects of intra-mPOA infusion of different doses of quinpirole (0.1–3.0 μg/animal; n = 7–8) or vehicle (C, n = 9) on copulation of sexually satiated male rats. The percentages of animals showing mounts and intromissions (M&I), ejaculating (E), and resuming copulation after ejaculation (CR) are shown in the first graph. Fisher F test. *P ≤ 0.05; **P ≤ 0.01 vs. C. The rest of the graphs depict the specific sexual behavior parameters of the satiated males that copulated in response to 0.3 μg/rat quinpirole, which were compared to sexually experienced males infused with vehicle into the mPOA (CEx; n = 8). Mann-Whitney U test *P < 0.05. Mean values are indicated with dashed lines. IL = intromission latency, M = number of mounts, I = number of intromissions, EL = ejaculation latency, PEI = post-ejaculatory interval, Qui = quinpirole
The specific sexual parameters of the males that ejaculated in response to quinpirole were compared to those of the sexually experienced animals infused with vehicle into the mPOA, since control satiated rats essentially do not show sexual activity 24 h after copulation to exhaustion (Rodríguez-Manzo et al. 2011). Although sexually experienced males are not an appropriate control group for sexually exhausted animals, the comparison with this group allows to establish if the sexual performance of the satiated rats that copulate after a drug treatment is deficient or as efficient as that of fully sexually competent animals. No significant differences were found in the copulatory parameters of the sexually satiated rats ejaculating in response to quinpirole as compared to those of sexually experienced control rats, except for the PEI, which was significantly increased [Mann-Whitney U test, P < 0.01]. Location of mPOA cannula tips is shown in supplementary Figs. S1 and S2.
The proportion of sexually satiated males showing sexual behavior after apomorphine infusion into the NAcc, as well as their copulatory parameters, is shown in Fig. 2. All apomorphine doses tested produced a statistically significant increase in the proportion of satiated rats mounting, intromitting, ejaculating [Fisher F test, P < 0.001 each], and resuming copulation after ejaculation [Fisher F test, P < 0.01 for 0.6 and 2 μg/rat and P < 0.001 for 6 μg/rat]. The sexually satiated males infused with 0.6 or 2.0 μg/rat apomorphine showed increased values for IL [Kruskal-Wallis ANOVA H(3) = 11.313, P = 0.01; Dunn’s test, P < 0.05] and PEI [Kruskal-Wallis ANOVA H(3) = 13.488, P = 0.004; Dunn’s test, P < 0.05] and the I number was augmented only after the 2.0 μg/rat dose [Kruskal-Wallis ANOVA H(3) = 8.016, P = 0.046; Dunn’s test, P < 0.05] as compared to intra-NAcc vehicle-infused sexually experienced males. The parameters of the satiated males infused with the 6.0 μg/rat dose were not different from those of sexually experienced control rats.
Effects of intra-NAcc infusion of different doses of apomorphine (0.6–6.0 μg/animal; n = 6–9) or vehicle (C, n = 7) on copulation of sexually satiated male rats. The first graph shows the percentage of sexually satiated rats that showed M&I, E, and CR. Fisher F test. **P ≤ 0.01; ***P ≤ 0.001 vs. C. The rest of the graphs depict the specific sexual behavior parameters of the satiated males that copulated to ejaculation in response to apomorphine, compared to sexually experienced males infused with vehicle into the NAcc (CEx; n = 8). Kruskal-Wallis ANOVA followed by Dunn’s test *P < 0.05. Mean values are indicated with dashed lines. Abbreviations as in Fig. 1; Apo = apomorphine
Figure 3 shows the percentage of sexually satiated rats displaying sexual behavior responses after intra-NAcc quinpirole infusion, together with the specific parameters of those males attaining ejaculation. The proportion of exhausted males copulating to ejaculation was statistically significantly increased by all quinpirole doses tested [Fisher F test, P < 0.001 for 0.3 and 1 μg/rat; P < 0.05 for 3 μg/rat]. The proportion of rats resuming copulation thereafter was only increased by the two lower quinpirole doses [Fisher F test, P < 0.01]. The increase in the proportion of animals resuming copulation after the highest quinpirole dose (3.0 μg/rat) was not statistically significant. The parameters of the satiated rats that copulated were not different from those of the control sexually experienced animals infused with vehicle into the NAcc, except for the PEI, which was significantly increased by the 0.3 and 1.0 μg/rat quinpirole doses [Kruskal-Wallis ANOVA H(2) = 11.12, P = 0.004; Dunn’s test, P < 0.05]. Animals infused with 1.0 μg/rat quinpirole showed also a significant increase in the IL with respect to the experienced vehicle-infused males [Kruskal-Wallis ANOVA H(3) = 17.114, P < 0.001; Dunn’s test, P < 0.05]. No significant changes were found in the copulatory parameters of animals infused into the NAcc with 3.0 μg/rat quinpirole as compared to the vehicle-infused sexually experienced males [Kruskal-Wallis ANOVA, non-significant]. In this case, PEI values were not determined due to insufficient data. Histological analysis showed that effective DA agonists’ infusions were located both in the NAcc core and shell sub-regions (supplementary Figs. S3 and S4).
Effects of intra-NAcc infusion of different doses of quinpirole (0.1–3.0 μg/rat; n = 5–10) or vehicle (C, n = 7) on copulation of sexually satiated male rats. The first graph shows the percentages of sexually satiated rats that showed M&I, E, and CR after intra-NAcc infusion of quinpirole. Fisher F test, *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 vs. C. The rest of the graphs depict the specific sexual behavior parameters of the satiated males that copulated to ejaculation in response to quinpirole, compared to sexually experienced males infused with vehicle into the NAcc (CEx; n = 8). Kruskal-Wallis ANOVA followed by Dunn’s test *P < 0.05. Mean values are indicated with dashed lines. Abbreviations as in Fig. 1
Spontaneous locomotor activity data are shown in Table 2. In the groups of sexually experienced rats, only the highest dose of apomorphine, infused into the mPOA, significantly decreased locomotion as compared to vehicle-treated rats [Kruskal-Wallis ANOVA H(3) = 10.267, P < 0.016; Dunn’s test, P < 0.05]. In sexually satiated animals, a statistically significant decrease in locomotor activity was recorded after intra-NAcc infusion of the highest quinpirole dose [Kruskal-Wallis ANOVA H(3) = 8.218, P = 0.016; Dunn’s test, P < 0.05].
Discussion
There is ample evidence showing that DA transmission plays a key role in the control of rewarding behaviors like copulation (Baik 2013; Berridge 2007). However, the literature related to DA actions on male sexual behavior shows inconsistencies that led even to question if DA plays a physiological role in its control (Paredes and Agmo 2004). Differences among studies in the DAergic drugs, the dose levels, and the administration routes used, as well as in the sexual condition of the animals and the brain regions explored, might have contributed to generate discrepancies. The effects of systemically administered DA receptor agonists on copulation were recently reviewed, controlling aspects such as dose level and sexual condition of the animals (Guadarrama-Bazante et al. 2014). In the present work, we examined the effects of the same doses of DA receptor agonists, infused into the mPOA or the NAcc, on copulatory behavior of fully sexually competent males (sexually experienced) and of sexually competent males, when transiently inhibited, due to sexual exhaustion (sexually satiated).
The results showed that (1) direct activation of DA receptors either in the mPOA or the NAcc does not modify sexual behavior display of fully sexually competent males; (2) activation of NAcc DA receptors reverses the sexual inhibition of sexually satiated rats; and (3) D2-like receptors participate in the induction of sexual behavior expression of sexually satiated males.
Neither the non-specific activation of DA receptors with apomorphine, nor the stimulation of D2-like receptors with quinpirole, in the mPOA, did alter the optimum level of copulation of the sexually experienced males. Intra-mPOA apomorphine was reported to produce sexual facilitative effects in male rats with modest sexual experience (two sexual interactions with a female), which included decreases in the EL and PEI, and an increase in the number of ejaculations displayed (Hull et al. 1986, 1989). A facilitation of the erectile response, in restrained rats, was also described with the infusion of apomorphine into the mPOA (Pehek et al. 1989). On these bases, the authors postulated that in this brain region, apomorphine improved copulation through the facilitation of genital reflexes (Hull et al. 1992). By contrast, intra-mPOA microinjection of a D2-like DA receptor agonist was found to delay the onset of copulation and reduce the I number in rats with moderate sexual experience (Hull et al. 1989). In the present study, intra-mPOA or intra-NAcc administration of either apomorphine or quinpirole did not modify the copulatory behavior of sexually experienced rats. There is only another study examining the effects of intra-NAcc apomorphine infusion, which also reported a lack of effect of this drug on copulation of sexually active male rats (Hull et al. 1986). We believe that the optimal copulatory parameters exhibited by our sexually experienced males precluded a further improvement of copulatory measures by DA receptor activation at either brain region. In addition, differences in the experimental design between the cited works and the present study, such as the testing of several doses of DA receptor agonists in a same animal (allowing a week rest between injections), and its unilateral infusion, versus the use of independent groups and bilateral infusion in our work, may have contributed to the distinct results obtained in the mPOA. Still, the differences in the sexual activity level of the animals appear to be central for the divergent results, since DA receptor activation in neither of the two DAergic brain regions explored modified the copulatory behavior of these fully sexually competent males. In fact, the notion of a facilitative role of DAergic transmission in the control of copulation is importantly supported by studies with DA receptor antagonists, in the mPOA (Hull et al. 1989; Pehek et al. 1988; Pfaus and Phillips 1989, 1991) or the NAcc (Everitt 1990), which evidenced sexual behavior impairments in animals with an optimal sexual performance.
In the sexually satiated animals, intra-NAcc apomorphine or quinpirole administration induced copulation to ejaculation in almost every sexually exhausted male, at all tested doses. The great majority of these animals resumed copulation after ejaculation, an event considered to indicate that the sexual inhibitory state was reversed (Rodríguez-Manzo and Fernández-Guasti 1994). The highest quinpirole dose tested failed to do so, but it also reduced animals’ spontaneous ambulatory activity. This last effect could have contributed to reduce the number of animals resuming copulation after ejaculation. The satiated males infused into the NAcc with the higher apomorphine and quinpirole doses copulated in a very efficient way.
Intra-mPOA apomorphine infusion had no effect on copulation of satiated rats, showing that the unspecific activation of DA receptors in this brain region does not promote sexual behavior expression in these temporarily sexually inhibited animals. Likewise, quinpirole infusion into this brain region essentially lacked effects on copulation. Thus, the low sexual activity level of the sexually satiated males could not evidence a sexual facilitative role of mPOA DA receptor activation. This result suggests that the sexual inhibition that characterizes satiety is not related to DA transmission in the mPOA. Systemic apomorphine has been reported to reverse sexual satiety (Guadarrama-Bazante et al. 2014; Mas et al. 1995; Rodríguez-Manzo 1999a), but systemic quinpirole does not (Guadarrama-Bazante et al. 2014). Altogether, these data indicate on the one side, that the satiety reversal produced by systemic apomorphine is mediated by the activation of DA receptors in the NAcc. On the other side, the satiety reversal produced by quinpirole activation of NAcc D2-like receptors is probably counteracted by D2-like receptor activation in other brain regions, when this agonist is systemically administered.
Intriguingly, quinpirole reversed sexual satiety when infused into the mPOA at a specific low dose, while lower and higher doses did not induce any sexual behavior response in the satiated animals. There is no straight explanation for this result. Quinpirole binds to DA receptors from the D2-like family, with a decreasing affinity for the following subtypes D3 < D2 < D4 (Missale et al. 1998). Low quinpirole doses appear to predominantly activate D2 autoreceptors (Furmidge et al. 1991); therefore, the possibility that at this specific dose quinpirole stimulated D2 inhibitory autoreceptors, located on neurons that project to and modulate the activity of the mesolimbic circuit, could be considered. In line with this hypothesis, DA has been found to activate efferents, within the hypothalamus, that synapse onto VTA neurons and promote DA release in the NAcc (Stolzenberg and Numan 2011). Recently, glutamatergic and GABAergic neural projections, originating in the preoptic hypothalamic region and providing direct input to the VTA, were described and hypothesized to influence reward processes, through the modulation of mesolimbic circuit’s activity (Kalló et al. 2015). Thus, it could be thought that the effective quinpirole dose, in our study, inhibited the activity of mPOA GABAergic neurons projecting to the VTA, through the stimulation of D2 heteroreceptors. This would eliminate the GABAergic input onto VTA DA neurons, disinhibiting them and increasing DA release in the NAcc. Specific experiments should be conducted to test this hypothesis.
Mesolimbic DA is involved in appetitive sexual responses, like sexual excitement and arousal (Pfaus 2009). Since sexually satiated rats have a diminished sexual motivation (Agmo et al. 2004; Rodríguez-Manzo 1999b), present data suggest that NAcc DA receptor activation facilitates sexual behavior expression in animals with a reduced motivation, but that it has no effect in animals with an intact sexual motivation, like sexually experienced rats. The satiety reversal produced by NAcc DA receptor activation strongly indicates that the long-lasting sexual inhibition, characteristic of satiated rats, importantly relies on a decreased DAergic transmission at this brain region, which appears to be central for male sexual behavior expression. Thus, mesolimbic DA seems to play a key role in the capacity of sexually satiated animals to respond to a motivational significant stimulus like the receptive female, since once these animals engage in sexual activity, due to NAcc DA receptor activation, its copulatory performance may be very efficient. In line with this idea, mesolimbic DA neurons have been proposed to be involved in specific aspects of motivation that include behavioral activation, exertion of effort, cue instigated approach, and conditioned learning processes (Salamone and Correa 2012; Salamone et al. 2016). From these aspects, behavioral activation and cue instigated approach seem to be impaired in the satiated rats and rescued by NAcc DA receptor activation, with the clear participation of D2-like receptors.
Our results also indicate that DA transmission in the mPOA does not play a central role in the sexual satiety phenomenon; a result supporting the idea that this brain region plays a less significant role than the NAcc in the control of male sexual motivation (Everitt 1990).
Finally, present data show that activation of DA receptors with the same drug, at the same dose, and in the same brain region, can produce different effects on copulatory behavior of males with a different sexual condition.
Abbreviations
- CR:
-
Copulation resumption
- DA:
-
Dopamine
- DAergic:
-
Dopaminergic
- E:
-
Ejaculation
- EL:
-
Ejaculation latency
- I:
-
Intromission
- IL:
-
Intromission latency
- M:
-
Mount
- mPOA:
-
Medial preoptic area
- NAcc:
-
Nucleus accumbens
- VTA:
-
Ventral tegmental area
References
Agmo A, Turi AL, Ellingsen E, Kaspersen H (2004) Preclinical models of sexual desire: conceptual and behavioral analyses. Pharmacol Biochem Behav 78:379–404. https://doi.org/10.1016/j.pbb.2004.04.013
Ahlenius S, Larsson K (1984) Apomorphine and haloperidol-induced effects on male rat sexual behavior: no evidence for actions due to stimulation of central dopamine autoreceptors. Pharmacol Biochem Behav 21:463–466. https://doi.org/10.1016/S0091-3057(84)80111-2
Baik JH (2013) Dopamine signaling in reward-related behaviors. Front Neural Circuits 7:152. https://doi.org/10.3389/fncir.2013.00152
Balfour ME, Yu L, Coolen LM (2004) Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsycopharmacology 29:718–730. https://doi.org/10.1038/sj.npp.1300350
Beach FA, Jordan L (1956) Sexual exhaustion and recovery in the male rat. Q J Exp Psychol 8:121–133. https://doi.org/10.1080/17470215608416811
Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431. https://doi.org/10.1007/s00213-006-0578-x
Berridge KC, Kringelbach ML (2011) Building a neuroscience of pleasure and well-being. Psychol Well Being 1:1–3. https://doi.org/10.1186/2211-1522-1-3
Bitran D, Hull EM, Holmes GM, Lookingland KJ (1988) Regulation of male rat copulatory behavior by preoptic incertohypothalamic dopamine neurons. Brain Res Bull 20:323–331. https://doi.org/10.1016/0361-9230(88)90062-7
Blackburn JR, Pfaus JG, Phillips AG (1992) Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol 39:247–279. https://doi.org/10.1016/0301-0082(92)90018-A
Everitt B (1990) Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev 14:217–232. https://doi.org/10.1016/S0149-7634(05)80222-2
Foreman M, Hall J (1987) Effects of D2-dopaminergic receptor stimulation on male rat sexual behavior. J Neural Transm 68:153–170. https://doi.org/10.1007/BF02098495
Furmidge L, Tong Z-Y, Petry N, Clark D (1991) Effects of low, autoreceptor selective doses of dopamine agonists on the discriminative cue and locomotor hyperactivity produced by d-amphetamine. J Neural Transm Gen Sect 86:61–70. https://doi.org/10.1007/BF01250376
Guadarrama-Bazante IL, Canseco-Alba A, Rodríguez-Manzo G (2014) Dopamine receptors play distinct roles in sexual behavior expression of rats with a different sexual motivational tone. Behav Pharmacol 25:684–694. https://doi.org/10.1097/FBP.0000000000000086
Hull EM, Rodríguez-Manzo G (2017) Male sexual behavior. In: Pfaff DW, Joëls M (eds) Hormones, brain and behavior, vol 1, chap. 1, 3rd edn. Academic Press, Oxford, pp 1–57
Hull EM, Bitran D, Pehek EA, Warner RK, Band LC, Holmes GM (1986) Dopaminergic control of male sex behavior in rats: effects of an intracerebrally-infused agonist. Brain Res 370:73–81. https://doi.org/10.1016/0006-8993(86)91106-6
Hull EM, Warner RK, Bazzett TJ, Eaton RC, Thompson JT, Scaletta LL (1989) D2/D1 ratio in the medial preoptic area affects copulation of male rats. J Pharmacol Exp Ther 251:422–427
Hull EM, Eaton RC, Markowski VP, Moses J, Lumley LA, Loucks JA (1992) Opposite influence of medial preoptic D1 and D2 receptors on genital reflexes: implications for copulation. Life Sci 51:1705–1713. https://doi.org/10.1016/0024-3205(92)90299-5
Hull EM, Du J, Lorrain D, Matuszewich L (1995) Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci 15:7465–7471. https://doi.org/10.1523/JNEUROSCI.15-11-07465.1995
Kalló I, Molnár CS, Szöke S, Fekete C, Hrabovszky E, Liposits Z (2015) Area-specific analysis of the distribution of hypothalamic neurons projecting to the rat ventral tegmental area, with special reference to the GABAergic and glutamatergic efferents. Front Neuroanat 9:112. https://doi.org/10.3389/fnana.2015.00112
Kelley AE, Berridge KC (2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22:3306–3311. https://doi.org/10.1523/JNEUROSCI.22-09-03306.2002
Kelliher KR, Liu YC, Baum MJ, Sachs BD (1999) Neuronal Fos activation in olfactory bulb and forebrain of male rats having erections in the presence of inaccessible estrous females. Neuroscience 92:1025–1033. https://doi.org/10.1016/S0306-4522(99)00050-0
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2013) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:1–5
Kringelbach ML, Berridge KC (2009) Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn Sci 13:479–487. https://doi.org/10.1016/j.tics.2009.08.006
Malsbury CW (1971) Facilitation of male rat copulatory behavior by electrical stimulation of the medial preoptic area. Physiol Behav 7:797–805. https://doi.org/10.1016/0031-9384(71)90042-4
Markowski VP, Hull EM (1995) Cholecystokinin modulates mesolimbic dopaminergic influence on male rat copulatory behavior. Brain Res 699:266–274. https://doi.org/10.1016/0006-8993(95)00918-G
Mas M, Fumero B, Perez-Rodriguez I (1995) Induction of mating behavior by apomorphine in sexually sated rats. Eur J Pharmacol 280:331–334. https://doi.org/10.1016/0014-2999(95)00270-U
Melis M, Argiolas A (1995) Dopamine and sexual behavior. Neurosci Biobehav Rev 19:19–38. https://doi.org/10.1016/0149-7634(94)00020-2
Merari A, Ginton A (1975) Characteristics of exaggerated sexual behavior induced by electrical stimulation of the medial preoptic area in male rats. Brain Res 86:97–108. https://doi.org/10.1016/0006-8993(75)90641-1
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78:190–212. https://doi.org/10.1152/physrev.1998.78.1.189
Mitchell JB, Gratton A (1994) Involvement of mesolimbic dopamine neurons in sexual behaviors: implications for the neurobiology of motivation. Rev Neurosci 5:324–327. https://doi.org/10.1515/REVNEURO.1994.5.4.317
Moses J, Loucks JA, Watson HL, Matuszewich L, Hull EM (1984) Dopaminergic drugs in the medial preoptic area and nucleus accumbens: effects on motor activity, sexual motivation, and sexual performance. Pharmacol Biochem Behav 51:681–686. https://doi.org/10.1016/0091-3057(94)00437-N
Paglietti E, Pellegrini-Quarantotti B, Mereu G, Gessa G (1978) Apomorphine and L-DOPA lower ejaculation threshold in the male rat. Physiol Behav 20:559–562. https://doi.org/10.1016/0031-9384(78)90247-0
Paredes RG, Agmo A (2004) Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Prog Neurobiol 73:179–226. https://doi.org/10.1016/j.pneurobio.2004.05.001
Paxinos G, Watson C (2009) The rat brain in stereotaxic coordinates, 6th edn. Elsevier, Oxford
Pehek EA, Warner RK, Bazzett TJ, Bitran D, Band LC, Eaton RC, Hull EM (1988) Microinjections of cis-flupenthixol, a dopamine antagonist, into the medial preoptic area impairs sexual behavior of male rats. Brain Res 443:70–76. https://doi.org/10.1016/0006-8993(91)90505-P
Pehek EA, Thompson JT, Hull EM (1989) The effects of intracranial administration of the dopamine agonist apomorphine on penile reflexes and seminal emission in the rat. Brain Res 500:325–332. https://doi.org/10.1016/0006-8993(89)90328-4
Pfaus JG (2009) Pathways of sexual desire. J Sex Med 6:1506–1533. https://doi.org/10.1111/j.1743-6109.2009.01309.x
Pfaus JG, Phillips AG (1989) Differential effects of dopamine receptor antagonists on the sexual behavior of male rats. Psychopharmacology 98:363–368. https://doi.org/10.1007/BF00451688
Pfaus JG, Phillips AG (1991) Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci 105:727–743. https://doi.org/10.1037/0735-7044.105.5.727
Robertson GS, Pfaus JG, Atkinson LJ, Matsumura H, Phillips AG, Fibiger HC (1991) Sexual behavior increases c-fos expression in the forebrain of the male rat. Brain Res 564:352–357. https://doi.org/10.1016/0006-8993(91)91477-I
Rodríguez-Manzo G (1999a) Yohimbine interacts with the dopaminergic system to reverse sexual satiation: further evidence for a role of sexual motivation in sexual exhaustion. Eur J Pharmacol 372(1):1–8. https://doi.org/10.1016/S0014-2999(99)00140-5
Rodríguez-Manzo G (1999b) Blockade of the establishment of the sexual inhibition resulting from sexual exhaustion by the Coolidge effect. Behav Brain Res 100:245–254. https://doi.org/10.1016/S0166-4328(98)00137-5
Rodríguez-Manzo G, Canseco-Alba A (2017) A new role for GABAergic transmission in the control of male rat sexual behavior expression. Behav Brain Res 320:21–29. https://doi.org/10.1016/j.bbr.2016.11.041
Rodríguez-Manzo G, Fernández-Guasti A (1994) Reversal of sexual exhaustion by serotonergic and noradrenergic agents. Behav Brain Res 62:127–134. https://doi.org/10.1016/0166-4328(94)90019-1
Rodríguez-Manzo G, Pellicer F (2007) Electrical stimulation of the ventral tegmental area exerts opposite effects on male rat sexual behaviour expression depending on the stimulated sub region. Behav Brain Res 179:310–313. https://doi.org/10.1016/j.bbr.2007.02.006
Rodríguez-Manzo G, Pellicer F (2010) Electrical stimulation of dorsal and ventral striatum differentially alters the copulatory behavior of male rats. Behav Neurosci 124:686–694. https://doi.org/10.1037/a0020737
Rodríguez-Manzo G, Pellicer F, Larsson K, Fernández-Guasti A (2000) Stimulation of the medial preoptic area facilitates sexual behavior but does not reverse sexual satiation. Behav Neurosci 114:553–560. https://doi.org/10.1037/0735-7044.114.3.553
Rodríguez-Manzo G, Guadarrama-Bazante IL, Morales-Calderón A (2011) Recovery from sexual exhaustion-induced copulatory inhibition and drug hypersensitivity follow a same time course: two expressions of a same process? Behav Brain Res 217:253–260. https://doi.org/10.1016/j.bbr.2010.09.014
Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–485. https://doi.org/10.1016/j.neuron.2012.10.021
Salamone JD, Pardo M, Yohn SE, López-Cruz L, SanMiguel N, Correa M (2016) Mesolimbic dopamine and the regulation of motivated behavior. Curr Top Behav Neurosci 27:231–257. https://doi.org/10.1007/7854_2015_383
Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275(5306):1593–1599. https://doi.org/10.1126/science.275.5306.1593
Stolzenberg DS, Numan M (2011) Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci Biobehav Rev 35:826–847. https://doi.org/10.1016/j.neubiorev.2010.10.003
Tagliamonte A, Fratta W, Del Fiacco M, Gessa GL (1974) Possible stimulatory role of brain dopamine in the copulatory behavior of male rats. Pharmacol Biochem Behav 2:257–260. https://doi.org/10.1016/0091-3057(74)90061-6
Van Dis H, Larsson K (1971) Induction of sexual arousal in the castrated male rat by intracranial stimulation. Physiol Behav 6:85–86. https://doi.org/10.1016/0031-9384(71)90021-7
Acknowledgements
The authors would like to thank Marisol Guerra for the artwork design. The experiments here reported complied with the regulations established in the Mexican official norm for the use and care of laboratory animals NOM-062-ZOO-1999.
Funding
This work was supported by Conacyt Mexico (grant 220772 to G. R-M). The data here reported are part of the PhD thesis of I.L.G-B, who received a fellowship (grant 161083 Conacyt).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
Brain sections showing cannulae placements of the sexually experienced (left column) and sexually satiated animals (right column) infused into the medial preoptic area (mPOA) with different doses of apomorphine. Cannulae tips were in sections corresponding to − 0.0 to − 1.32 mm relative to bregma (Paxinos and Watson 2009). Asterisks indicate cannulae placement of vehicle-treated sexually experienced rats and filled diamonds vehicle-treated sexually satiated rats. Filled circles, triangles, and squares indicate the different apomorphine doses infused (PDF 719 kb)
Fig. S2
Brain sections showing cannulae placements of the sexually experienced (left column) and sexually satiated animals (right column) infused into the medial preoptic area (mPOA) with different doses of quinpirole. Cannulae tips were in sections corresponding to − 0.24 to − 1.32 mm relative to bregma (Paxinos and Watson 2009). Blank hexagons, circles, triangles, and squares indicate the different quinpirole doses infused (PDF 753 kb)
Fig. S3
Brain sections showing cannulae placements of the sexually experienced (left column) and sexually satiated animals (right column) infused into the nucleus accumbens (NAcc) with different doses of apomorphine. Cannulae tips were in sections corresponding to 0.72 to 2.16 mm relative to bregma (Paxinos and Watson 2009). Asterisks indicate cannulae placement of vehicle-treated sexually experienced male rats and filled diamonds those of vehicle-treated sexually satiated rats. Filled circles, triangles, and squares indicate the different apomorphine doses infused (PDF 753 kb)
Fig. S4
Brain sections showing cannulae placements of the sexually experienced (left column) and sexually satiated animals (right column) infused into the nucleus accumbens (NAcc) with different doses of quinpirole. Cannulae tips were in sections corresponding to 0.48 to 1.92 mm relative to bregma (Paxinos and Watson 2009). Blank circles, triangles, and squares indicate the different quinpirole doses infused (PDF 694 kb)
Rights and permissions
About this article
Cite this article
Guadarrama-Bazante, I.L., Rodríguez-Manzo, G. Nucleus accumbens dopamine increases sexual motivation in sexually satiated male rats. Psychopharmacology 236, 1303–1312 (2019). https://doi.org/10.1007/s00213-018-5142-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5142-y