Abstract
Objective
Cannabinoid receptor agonists such as delta-9-tetrahydrocannabinol (Δ9-THC) enhance the antinociceptive potency of mu opioid receptor agonists such as morphine, indicating that opioid/cannabinoid mixtures might be effective for treating pain. However, such enhancement will be beneficial only if cannabinoids do not also enhance adverse effects of opioids, including those related to abuse. In rhesus monkeys, cannabinoids fail to enhance and often decrease self-administration of the mu opioid receptor agonist heroin, suggesting that opioid/cannabinoid mixtures do not have greater reinforcing effects (abuse potential) compared with opioids alone. Previous studies on the self-administration of opioid/cannabinoid mixtures used single-response procedures, which do not easily differentiate changes in reinforcing effects from other effects (e.g., rate decreasing).
Methods
In this study, rhesus monkeys (n = 4) responded under a choice procedure wherein responding on one lever delivered sucrose pellets and responding on the other lever delivered intravenous infusions of the mu opioid receptor agonist remifentanil (0.032–1.0 μg/kg/infusion) alone or in combination with either Δ9-THC (10–100 μg/kg/infusion) or the synthetically derived cannabinoid receptor agonist CP55940 (3.2–10 μg/kg/infusion).
Results
Remifentanil dose-dependently increased choice of drug over food, whether available alone or in combination with a cannabinoid, and the potency of remifentanil was not significantly altered by coadministration with a cannabinoid. Mixtures containing the largest doses of cannabinoids decreased response rates in most subjects, confirming that behaviorally active doses were studied.
Conclusion
Overall, these results extend previous studies to include choice behavior and show that cannabinoids do not substantially enhance the reinforcing effects of mu opioid receptor agonists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain continues to be a significant clinical problem and mu opioid receptor agonists such as morphine and oxycodone are the gold standard for treating moderate to severe pain. However, numerous adverse effects (e.g., tolerance, dependence, abuse, and overdose) limit the legitimate medical use of opioids. Lowering the dose of opioid required to treat pain adequately could limit the risks associated with larger doses (Dowell et al. 2016). One strategy for reducing the dose of the opioid required for adequate pain treatment while maintaining effectiveness is to combine an opioid with another (non-opioid) drug such that the combination produces the same therapeutic effect as a larger dose of the opioid alone.
Cannabinoid receptor agonists such as delta-9-tetrahydrocannabinol (Δ9-THC), the primary psychoactive constituent of cannabis, as well as synthetically derived agonists such as CP55940 increase the antinociceptive potency of mu opioid receptor agonists such as morphine in non-human primates as measured with the warm water tail withdrawal procedure (Li et al. 2008; Maguire et al. 2013b; Maguire and France 2014). Moreover, cannabinoids have been shown to enhance analgesic effects of opioids in humans (e.g., Cooper et al. 2018) including in pain patients (see Nielson et al. 2017 for a review). However, the therapeutic utility of opioid/cannabinoid mixtures depends upon whether cannabinoids also enhance the potency of opioids to produce other unwanted effects, particularly those contributing to abuse. In rhesus monkeys, response-contingent and/or non-contingent administration of Δ9-THC or CP55940 fails to enhance and often decreases self-administration of the mu opioid receptor agonist heroin (Li et al. 2012; Maguire et al. 2013b; Maguire and France 2016b), suggesting that opioid/cannabinoid mixtures do not have greater reinforcing effects compared with opioids alone. However, previous studies used a single-response, fixed-ratio schedule that does not easily differentiate changes in reinforcing effects from other (e.g., generalized rate suppression) effects (e.g., Katz 1989); thus, the interaction between opioids and cannabinoids with regard to reinforcing effects remains unclear.
Several procedures (e.g., progressive ratio, demand, and second-order schedules) were developed, in part, to address the interpretational challenges associated with the use of single-response, fixed-ratio schedules to characterize the reinforcing effectiveness of drugs. Although each approach has strengths and weaknesses, the current study used a choice procedure to compare the reinforcing effects of an opioid alone to those of an opioid/cannabinoid mixture, with food as the non-drug alterative. Under choice procedures, subjects can choose between two or more alternatives (Catania 1966) with the primary measure of reinforcing effects based on allocation of behavior among the alternatives rather than overall response output (i.e., response rate), which can be influenced by many factors not directly related to reinforcing effectiveness. Moreover, many of the adverse outcomes associated with drug abuse reflect disproportionate allocation of behavior to drug seeking and taking (e.g., Lamb and Ginsburg 2018), and choice procedures have been useful for assessing factors that impact drug taking (e.g., Banks and Negus 2017; Lamb et al. 2016; Perkins and Freeman 2018).
In the current study, responding on one lever delivered food and responding on the other lever delivered an intravenous (i.v.) infusion of remifentanil alone or remifentanil combined with a dose of Δ9-THC or CP55940. Like other mu opioid receptor agonists, such as heroin, remifentanil is self-administered readily by non-human subjects. The faster onset and shorter duration of action of remifentanil, compared with other opioids (e.g., Ko et al., 2002), are preferable under experimental conditions in which subjects make repeated choices because accumulation of the opioid is limited or avoided (Maguire et al. 2013a; Maguire et al. 2013c; Maguire et al. 2016). Δ9-THC and CP55940 were studied because both drugs increase the potency of some mu opioid receptor agonists to produce antinociceptive effects; however, the magnitude of enhancement appears to differ between them possibly related to their different intrinsic efficacies (Maguire and France 2014). If cannabinoids attenuate the reinforcing effects of remifentanil per se, then its potency should be diminished when in a mixture with a cannabinoid, compared with remifentanil alone. On the other hand, if cannabinoids do not attenuate the reinforcing effects of remifentanil, then its potency should not be altered up to unit doses of the cannabinoid that suppress overall response output.
Materials and methods
Animals
Four adult rhesus monkeys (3 males [WI, GI, MO] and 1 female [RI]), weighing between 8.7 and 10.8 kg during this study, were housed individually in stainless steel cages with interior space measuring 81 cm tall by 81 cm wide by 72 cm deep; the home cage also served as the experimental chamber (see below). The colony room was maintained under a 14/10-h light/dark cycle (lights on at 0600 h). Monkeys were fed chow (Harlan Teklad, High Protein Monkey Diet, Madison, WI, USA), fresh fruit, peanuts, and other edible treats daily at approximately 0730 h with water available continuously. Studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the United States National Institutes of Health (National Research Council 2011) and were approved by the University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee.
Surgery and equipment
Monkeys were sedated with 10 mg/kg (i.m.) of ketamine (Henry Schein Animal Health, Dublin, OH, USA), intubated, and then maintained on 2 l/min oxygen and isoflurane anesthesia (Butler Animal Health Supply, Grand Prairie, TX, USA). A 5-french polyurethane catheter (Access Technologies, Skokie, IL, USA) was inserted into a jugular or femoral vein and tunneled subcutaneously to an exit point in the mid-scapular region of the back. Penicillin B&G (35,000 IU/kg) and meloxicam (0.2 mg/kg) were given postoperatively.
The catheter was passed through a flexible stainless steel tether (Lomir Biomedical, Quebec, Canada) and connected to an 18-g single-channel fluid swivel (Lomir Biomedical) that was secured to the rear wall of the cage. Outside of the cage, the swivel was attached to a 30-ml polypropylene syringe mounted in a syringe pump (Razel Scientific Instruments, Fairfax, VT, USA) that infused at a rate of 2.3 ml/min. Monkeys wore a jacket (Lomir Biomedical) that protected the catheter and secured the tether. A stainless steel instrument panel (20 cm high by 28 cm wide) was mounted on one wall of the cage that contained two 4.5-cm wide response levers, positioned 23 cm above the cage floor, and spaced 15 cm apart center to center; two metal partitions attached to the instrument panel between the levers prevented responding on both levers simultaneously with the same limb. Three stimulus lights were horizontally aligned 5 cm above the levers. Directly above the instrument panel was a 6 cm high by 5 cm wide aperture through which 300-mg raspberry flavored sucrose pellets (5TUT, Test Diet, Richmond, IN, USA) were delivered via activation of a pellet dispenser (Med Associates, Inc., Fairfax, VT, USA).
Choice procedure
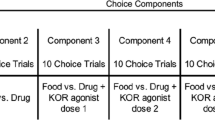
Prior to the start of the session, catheter lines were flushed with 3 ml of heparinized saline (100 U/ml; Hospira Inc., Lake Forest, IL, USA), and a syringe containing the solution available for self-administration that day was connected to the catheter. The pump was then activated to fill the catheter with the new solution. At the beginning of the session (1200 h), one of the side lights was illuminated green. Thirty consecutive responses on the lever directly below the green light turned that light off, turned the white center light on for 2 s, and delivered the reinforcer associated with that lever for that day, either 3 sucrose pellets or an i.v. infusion. Food pellets were delivered at a rate of one every 0.5 s. Infusion durations ranged from 22 to 28 s, with each infusion delivering 1 ml of solution per 10 kg of body weight. Completion of the response requirement also initiated a 10-min timeout, during which all lights were off (except for illumination of the center light for 2 s) and responses were recorded but had no programmed consequence. After completion of the first post-reinforcer timeout, the other side light was illuminated green, and 30 consecutive responses on the lever located below that light delivered the associated reinforcer followed by a timeout. Each session began with four of these sample trials (2 per side), the order of which varied quasi-randomly across trials and sessions. Choice trials began once all sample trials were completed. During choice trials both side lights were illuminated green, and 30 consecutive responses on either lever delivered the reinforcer associated with that lever. The consequences of responding on each lever during choice trials were identical to those presented during the sample trials. For all trials, responses on one lever reset the response counter for the other lever. There was no limited hold for individual trials, and sessions ended after completion of 20 choice trials or 8 h, whichever occurred first.
Experimental design
Dose-effect curves were determined for remifentanil alone and in combination with varying unit doses of Δ9-THC or CP55940 using a within-subjects design wherein data for each monkey served as his or her own control. All monkeys had experience lever pressing for food and/or i.v. drug infusions under various schedules of reinforcement (e.g., Maguire et al. 2016), so no preliminary training was required. Infusions were always available for responding on one lever and food was always available for responding on another lever; however, the lever designations switched during the experiment as indicated below. Dose-effect curves were determined by varying the solution available across conditions. Each solution and lever designation (e.g., food, left; infusion, right) was in effect within and across sessions until responding was stable, as defined by three consecutive sessions in which the percentage of choices for the infusion across sessions did not vary by more than 20% or for up to 8 sessions, whichever occurred first. Once responding was stable, the same solution continued to be available, but the lever designations were switched, confirming that behavior allocation reflected sensitivity to the consequences of responding on each lever rather than other factors (e.g., side bias). After responding was stable once again, the solution changed while the lever designations remained the same.
For each determination of a dose-effect curve, the dose of remifentanil (0.032–1.0 μg/kg/infusion) available alone or in combination with a fixed unit dose of Δ9-THC (10.0–100.0 μg/kg/infusion) or CP55940 (3.2–10.0 μg/kg/infusion) varied in half-log unit increments until the range of remifentanil doses tested included at least one dose that maintained not more than 30% of drug choice and at least one dose that maintained at least 70% of drug choice (see “Data and statistical analyses” section for further details about calculating the percentage of drug choice). Each unit dose of a cannabinoid was tested once per monkey in combination with a range of remifentanil doses. Remifentanil dose-effect curves determined in combination with different doses of a cannabinoid or different cannabinoids were separated by re-determination of a dose-effect curve for remifentanil alone, which required that at least 12 sessions intervened between drug mixture tests. Saline was substituted for drug at the beginning and at the end of the study. During the course of the study, monkeys were sedated every 2 weeks with 10 mg/kg of ketamine to obtain an updated body weight, confirm catheter patency, perform routine health checks, and inspect the equipment.
Drugs
Remifentanil hydrochloride and delta-9-tetrahydrocannabinol (Δ9-THC) were provided by the National Institute on Drug Abuse (NIDA) Drug Supply Program (Bethesda, MD). 2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol (CP55940) was provided by NIDA or purchased (Sigma, St. Louis, MO, USA). Δ9-THC and CP55940 were stored at − 20 °C in absolute ethanol, and dilutions were made by mixing the ethanol solution with an equivalent volume of Alkamuls EL-620 (Rhodia, Cranbury, NJ, USA) and by adding sterile 0.9% saline to obtain the required concentration of drug. Saline made up at least 94% of the total volume for all solutions. Remifentanil was dissolved in saline when administered alone and in the cannabinoid vehicle when administered in combination with a cannabinoid.
Data and statistical analyses
Percentage of drug choice for each session was calculated by dividing the total number of ratios completed on the drug lever during choice trials by the total number of choice trials completed and multiplying by 100. Percentage of drug choice for each condition was obtained by calculating the mean percentage across six sessions, comprising the last three sessions under the first lever designation and the last three sessions following a lever designation switch. Response rate for each session was calculated by dividing the total number of responses on both levers, excluding timeout responses, by the total time that at least one green light was illuminated. Response rate for each condition was obtained by calculating the mean response rate across the six sessions indicated above.
Effects of the cannabinoids were analyzed by comparing the potency of remifentanil to increase drug choice when available alone with the potency of remifentanil in combination with each unit dose of cannabinoid. Percentage of drug choice was plotted as a function of the log-transformed (base 10) unit dose of remifentanil. Dose-effect data for individual curve determinations were fit with a straight line using linear regression and only data comprising the linear portion of the curve, ranging from the largest dose that produced not more than 30% of drug choice to the smallest dose that produced at least 70% drug choice. The slope and the y-intercept were then used to estimate the dose of remifentanil producing 50% drug choice (ED50). The ED50 values for all dose-effect curves for remifentanil alone (7 or 8 determinations across monkeys) were averaged for individual monkeys to determine the control ED50. Potency ratios for remifentanil in combination with each unit dose of a cannabinoid were calculated for individual monkeys by dividing the control ED50 by the ED50 for each drug mixture; potency ratios were calculated using anti-log transformed (i.e., arithmetic) ED50 values. Potency ratios greater than 1.0 indicate a leftward shift in the remifentanil dose-effect curve (increased potency) whereas ratios less than 1.0 indicate a rightward shift in the dose-effect curve (decreased potency). For each unit dose of a cannabinoid, 95% confidence limits around the mean potency ratio for the group were calculated, and changes in the potency of remifentanil were considered significant if the confidence limits did not include 1.0. All curve fitting and data analyses were conducted using Microsoft Excel® 2016 (Redmond, WA, USA).
Results
When given a choice between 3 sucrose pellets and saline, monkeys responded predominantly for food (not more than 8% choice of infusions) at rates ranging from 0.8 to 3.0 responses per second, completing all choice trials (Fig. 1, circles above “S”). When available alone, remifentanil dose-dependently increased choice of drug, with 0.32 or 1.0 μg/kg/infusion maintaining near exclusive choice of drug over food (Fig. 1, top row, circles); in 3 of 4 monkeys, response rates also increased as a function of remifentanil dose, ranging from 1.8 to 3.2 responses per second at the largest doses tested (Fig. 1, middle row, circles). Control ED50 values for choice of remifentanil available alone ranged from 0.13 to 0.25 μg/kg/infusion across monkeys, with a group mean of 0.17 μg/kg/infusion (Table 1; Fig. 1 rightmost column).
Dose-effect curves for remifentanil alone (circles) or in combination with varying unit doses of Δ9-THC (squares, upright triangles, and inverted triangles). Percentage choice of drug during choice trials (top row), overall response rate in responses per second (middle row), and total number choice trials completed (bottom row) are plotted as a function of unit dose of remifentanil (μg/kg/infusion) for individual monkeys as well as for the grouped data (rightmost panels). The shaded region for the individual plots indicates ± 1 standard deviation of the mean of multiple (7 or 8) determinations of the dose-effect curve for remifentanil alone; error bars for the group plots indicate ± 1 standard error of the group. The asterisk next to the group dose-effect curve for remifentanil in combination with 32 μg/kg/infusion of Δ9-THC for percentage of drug choice (upright triangles, top-right panel) indicates that this unit dose of Δ9-THC significantly decreased the potency of remifentanil (see Table 1 for quantification of this effect)
When available in a mixture with Δ9-THC or CP55940 (Figs. 1 and 2, respectively), remifentanil increased drug choice in a dose-dependent manner. In some cases, responding increased for particular doses of remifentanil, compared with remifentanil alone, whereas in other cases responding for remifentanil decreased. For most drug mixtures, the potency of remifentanil to increase drug choice was not significantly altered by the addition of a cannabinoid (Table 1). However, a mixture with 32 μg/kg/infusion of Δ9-THC significantly decreased the potency of remifentanil, as indicated by a nearly 2-fold shift rightward in the remifentanil dose-effect curve (Fig. 1, top row, upright triangles). In some cases, mixtures of remifentanil with the largest doses of Δ9-THC (100 μg/kg/infusion) or CP55940 (10 μg/kg/infusion) decreased response rates compared with remifentanil alone (middle row, Figs. 1 and 2, respectively); however, monkeys continued to complete most choice trials (bottom row, Figs. 1 and 2).
Discussion
Cannabinoid receptor agonists such as Δ9-THC and CP55940 increase the antinociceptive potency of some mu opioid receptor agonists in non-human primates without enhancing their reinforcing or discriminative stimulus effects (Li et al. 2008; Li et al. 2012; Maguire et al. 2013b; Maguire and France 2016a,b), suggesting opioid/cannabinoid mixtures could be used to treat pain without increasing, and possibly decreasing, abuse. In previous studies, cannabinoids reduced self-administration of heroin; those results could be interpreted to suggest that cannabinoids decrease the reinforcing effects of opioids and, thus, potential for abuse. However, those studies used a single-response, fixed-ratio schedule of self-administration that does not easily differentiate changes in reinforcing effects from other (e.g., generalized rate decreasing) effects, so the interaction between opioids and cannabinoids with regard to reinforcing effects remains unclear. The current study used a choice procedure to compare reinforcing effects of an opioid alone with those of an opioid/cannabinoid mixture. One advantage of choice procedures is that the primary measure of reinforcing effects is allocation of behavior among alternatives, rather than overall response output, which can be sensitive to many factors other than reinforcing effectiveness.
Monkeys chose food over saline and over small doses of remifentanil and increasingly chose remifentanil as the unit dose of remifentanil increased. Dose-effect curves for remifentanil alone were very stable over the course of the study, with potency not varying by more than a half a log unit for individual monkeys, which is critical for studies using within-subject experimental designs. The potency of remifentanil to increase drug choice over three 300-mg sucrose pellets in the current study, with 0.32–1.0 μg/kg/infusion producing exclusive drug choice, was comparable to previous studies using choice procedures wherein the alternative reinforcer was a grain-based food pellet (Maguire et al. 2013a) or an infusion of cocaine (Wade-Galuska et al. 2007; Koffarnus and Woods 2008; Wade-Galuska et al. 2011; Freeman and Woolverton 2011). These doses are also within the range of doses that maintained self-administration under a variety of single-response procedures (Woods and Winger 2002; Ko et al. 2002; Winger et al. 2006; Woolverton et al. 2008; Podlesnik et al. 2011; Koffarnus et al. 2012; Lagorio and Winger 2014). Remifentanil was used in the current study to minimize accumulation of opioid during the session that can occur with longer acting opioids such as heroin, which can reduce response rates (e.g., Stevenson et al. 2005; Negus 2005a, 2006). Rate-decreasing effects of the opioid in combination with those of a second drug in a mixture could limit the range of doses studied. In the current study, when remifentanil was available alone, response rates remained high despite high levels of drug choice, likely due to the combination of a relatively long post-infusion timeout (10 min) and the short duration of action of remifentanil (< 10 min; for example, see Ko et al. 2002).
Across multiple unit doses, Δ9-THC and CP55940 did not significantly enhance the potency of remifentanil to increase drug choice, and the absence of enhancement was evident in grouped data as well as at the individual-subject level (Figs. 1 and 2), indicating that group data were representative of individual subjects. These data are consistent with previous results showing that cannabinoid receptor agonists fail to enhance self-administration of heroin (e.g., Li et al. 2012; Maguire et al. 2013b; Maguire and France 2016b) and extend these studies to include another opioid receptor agonist (remifentanil) and procedure (choice). One unit dose of Δ9-THC (32 μg/kg/infusion) decreased the potency of remifentanil, although the magnitude of effect was modest, producing less than a 2-fold shift on average across monkeys, and was not clearly dose related as the larger unit dose of Δ9-THC was ineffective with regard to choice.
In previous studies, co-administration of a cannabinoid receptor agonist dose-dependently decreased heroin self-administration, as indicated by a flattening of the self-administration dose-effect curve (e.g., Li et al. 2012). The absence of marked, dose-related rightward shifts in the remifentanil dose-effect under the choice procedure suggests that effects of cannabinoids reported in previous studies were likely due to general rate-suppressant effects of the cannabinoid (possibly combined with those of heroin) rather than a specific attenuation of reinforcing effects. Likewise, in a recent study with healthy human participants, smoked cannabis enhanced the antinociceptive effects of oxycodone with experimental cold pain at doses that did not substantially increase or decrease abuse-related effects of oxycodone (Cooper et al. 2018). Recent reports suggest that the use of opioids for pain (e.g., Bradford et al. 2018; Boehnke et al. 2016; Wen and Hockenberry 2018) as well as fatalities from opioid overdose (Bachhuber et al. 2014) have decreased concomitantly with the increased legalization of cannabis for medicinal and/or recreational use. However, the extent to which those associations are causally related and whether increased access to cannabis alters rates opioid abuse and dependence remain open questions (e.g., see discussions by Hall et al. 2018; Hayes and Brown 2014).
Monkeys responded for mixtures of remifentanil with Δ9-THC or CP55940 despite significant reductions in response rate, exemplifying the dissociation of response allocation and response rate in this choice procedure. Δ9-THC or CP55940 have aversive effects under some conditions (e.g., Elsmore and Fletcher 1972; McGregor et al. 1996), and decreased rates of drug self-administration have been interpreted as reflecting aversive effects of drugs (e.g., Riley 2011). However, in the current study, it is unlikely that aversive effects contributed to lowered rates of responding. Otherwise, monkeys would be expected to reallocate behavior toward the food alternative and avoid taking infusions of the drug mixture (e.g., Negus 2005b). Rather, monkeys self-administered nearly all of the available drug on a daily basis, albeit at longer inter-injection intervals. On the other hand, cannabinoids also have well-documented hyperphagic effects (e.g., Abel 1975; Kirkham and Williams 2001; Bellocchino et al. 2010), which could increase the reinforcing effectiveness of food and decrease the relative reinforcing effectiveness of an alternative drug reinforcer (e.g., Nader and Woolverton 1991, 1992). In such a case, choice of remifentanil would decrease when combined with a cannabinoid; however, that was not the case in the current study.
It is possible that the cannabinoids were not studied at doses large enough to alter significantly choice for remifentanil; however, this is unlikely for several reasons. First, mixtures of remifentanil and larger unit doses of each cannabinoid decreased response rates in some cases, indicating that monkeys received behaviorally active doses. Second, the largest dose of Δ9-THC (100 μg/kg/infusion) tested in the current study was 3-fold larger than the dose (32 μg/kg/infusion) that markedly decreased responding for heroin in monkeys responding under a single-response procedure (Li et al. 2012). Third, the largest unit doses of Δ9-THC and CP55940 tested in the current study have discriminative stimulus effects and decrease rates of operant behavior in rhesus monkeys when given as a single bolus injection by the same route (i.v.) of administration (e.g., McMahon 2006; McMahon 2011; Hruba and McMahon 2014). In fact, in the current study, when cannabinoids were studied in combination with larger doses of remifentanil, monkeys completed most trials, resulting in cumulative intake of up to 2200 μg/kg of Δ9-THC or 220 μg/kg of CP55940 per session. These total doses are at least 20-fold larger than discriminable doses of these drugs in rhesus monkeys trained to discriminate 100 μg/kg of Δ9-THC (e.g., McMahon 2006). Moreover, the total cumulative dose of Δ9-THC achieved in the currently study was at least twice as large as a dose that enhanced the antinociceptive effects of some opioids when given subcutaneously as a bolus injection (Li et al. 2008; Maguire and France 2014).
The cannabinoid receptor agonists Δ9-THC and CP55940 have a rapid onset of action in rhesus monkeys (e.g., Hruba and McMahon 2014) and man (e.g., Ohlsson et al. 1980; Grotenhermen 2003) following i.v. administration; however, behavioral effects can last longer than an hour following a single infusion (e.g., Hruba and McMahon 2014; Ohlsson et al. 1980). For studies in which subjects make repeated choices with drug as an alternative, long-lasting drug effects could interfere with choices on subsequent trials. For tests with remifentanil alone, carryover effects are less of a concern due to its very short duration of action (less than 10 min) and the 10-min inter-trial interval (discussed above; see Ko et al. 2002). However, it is possible, in fact likely, that effects of cannabinoid infusions early in the session persisted during subsequent choices trials and that the cannabinoid accumulated during the session with repeated choices of the drug mixture. Persistent effects of the cannabinoid could influence the ability to discriminate between the sources of each reinforcer later in the session. For example, it might decrease the ability to determine whether delivery of the previous cannabinoid infusion was the result of responding on the left or right lever, or whether it was delivered with food or remifentanil. However, this seems unlikely for several reasons. First, drug was never administered concurrent with food delivery. Given the quick onset of action of remifentanil and both cannabinoids following i.v. administration, the onset of drug action would not have overlapped significantly with food delivery in subsequent trials. Second, each dose of remifentanil alone or in a mixture was studied under steady state conditions wherein a single drug or drug mixture was available for at least six sessions (with a minimum of three sessions under each lever designation) and until choice was stable. There were multiple opportunities across sessions to sample either option at the beginning of the session while in a “drug-free” state, and switching lever designations tested whether responding was sensitive to the reinforcement contingencies in effect at that time. Third, failure to discriminate between the two options would likely result in a flattening of the remifentanil dose-effect curve and/or a lever bias (i.e., responding only or predominantly on the left or right lever). However, the slope of the remifentanil dose-effect curve did not differ across conditions, and it spanned the entire range of the ordinate. Moreover, responding typically adjusted quickly following a change in dose or lever designation, suggesting adequate discriminability between the food and drug alternatives.
In summary, this study found that the cannabinoid receptor agonists Δ9-THC and CP55940 failed to alter reliably the potency of remifentanil in rhesus monkeys responding under a food/drug choice procedure. These data confirm and extend previous results showing that, in non-human primates, cannabinoid receptor agonists do not appear to enhance the reinforcing effects of mu opioid receptor agonists. Moreover, this study is relevant to interpretation of prior results with the current study suggesting that decreased opioid self-administration reported previously (e.g., Li et al. 2012) was the result of a generalized rate-suppressant effect of the cannabinoid or the mixture of a cannabinoid and heroin, rather than reduced reinforcing effects.
References
Abel EL (1975) Cannabis: effects on hunger and thirst. Behav Bio 15:255–281. https://doi.org/10.1016/S0091-6773(75)91684-3
Bachhuber MA, Saloner B, Cunningham CO, Barry CL (2014) Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999-2010. JAMA Intern Med 174:1668–1673. https://doi.org/10.1001/jamainternmed.2014.4005
Banks ML, Negus SS (2017) Insights from preclinical choice models on treating drug addiction. Trends Pharmacol Sci 38:181–194. https://doi.org/10.1016/j.tips.2016.11.002
Bellocchio L, Lafenêtre P, Cannich A, Cota D, Puente N, Grandes P, Chaouloff F, Piazza PV, Marsicano G (2010) Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci 13:281–283. https://doi.org/10.1038/nn.2494
Boehnke KF, Litinas E, Clauw DJ (2016) Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain 17:739–774. https://doi.org/10.1016/j.jpain.2016.03.002
Bradford AC, Bradford WD, Abraham A, Bagwell Adams G (2018) Association between US state medical cannabis laws and opioid prescribing in the Medicare part D population. JAMA Intern Med. 178:667-672. https://doi.org/10.1001/jamainternmed.2018.0266
Catania AC (1966) Concurrent operants. In: Honig WK (ed) Operant behavior: areas of research and application, Appleton-Century-Crofts, New York, pp 213–270
Cooper ZD, Bedi G, Ramesh D, Balter R, Comer SD, Haney M (2018) Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology. https://doi.org/10.1038/s41386-018-0011-2 [Epub ahead of print]
Dowell D, Haegerich TM, Chou R (2016) CDC guideline for prescribing opioids for chronic pain—United States. JAMA 315:1624–1645. https://doi.org/10.1001/jama.2016.1464
Elsmore TF, Fletcher GV (1972) Δ9-tetrahydrocannabinol: aversive effects in rat at high doses. Science 175:911–912. https://doi.org/10.1126/science.175.4024.911
Freeman KB, Woolverton WL (2011) Self-administration of cocaine and remifentanil by monkeys: choice between single drugs and mixtures. Psychopharmacology 215:281–290. https://doi.org/10.1007/s00213-010-2131-1
Grotenhermen F (2003) Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42:327–360. https://doi.org/10.2165/00003088-200342040-00003
Hall W, West R, Marsden J, Humphreys K, Neale J, Petry N (2018) It is premature to expand access to medicinal cannabis in hopes of solving the US opioid crisis. Addiction 113:987–988. https://doi.org/10.1111/add.14139
Hayes MJ, Brown MS (2014) Legalization of medical marijuana and incidence of opioid mortality. JAMA Intern Med 174:1673–1674. https://doi.org/10.1001/jamainternmed.2014.2716
Hruba L, McMahon LR (2014) The cannabinoid agonist HU-210: Pseudo-irreversible discriminative stimulus effects in rhesus monkeys. Eur J Pharmacol 727:35-42. https://doi.org/10.1016/j.ejphar.2014.01.041
Katz JL (1989) Drugs as reinforcers: pharmacological and behavioural factors. In: JM Liebman JM, Cooper SJ (eds) The Neuropharmacological basis of reward. Oxford University Press, Oxford, pp 164–213
Kirkham TC, Williams CM (2001) Endogenous cannabinoids and appetite. Nutr Res Rev 14:65–86. https://doi.org/10.1079/NRR200118
Ko MC, Terner J, Hursh S, Woods JH, Winger G (2002) Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther 301:698–704. https://doi.org/10.1124/jpet.301.2.698
Koffarnus MN, Woods JH (2008) Quantification of drug choice with the generalized matching law in rhesus monkeys. J Exp Anal Behav 89:209–224. https://doi.org/10.1901/jeab.2008.89-209
Koffarnus MN, Hall A, Winger G (2012) Individual differences in rhesus monkeys’ demand for drugs of abuse. Addict Biol 17:887–896. https://doi.org/10.1111/j.1369-1600.2011.00335.x
Lagorio CH, Winger G (2014) Random-ratio schedules produce greater demand for iv drug administration than fixed-ratio schedules in rhesus monkeys. Psychopharmacology 231:2981–2988. https://doi.org/10.1007/s00213-014-3477-6
Lamb RJ, Ginsburg BC (2018) Addiction as a BAD, a behavioral allocation disorder. Pharmacol Biochem Behav 164:62–70. https://doi.org/10.1016/j.pbb.2017.05.002
Lamb RJ, Maguire DR, Ginsburg BC, Pinkston JW, France CP (2016) Determinants of choice, and vulnerability and recovery in addiction. Behav Process 127:35–42. https://doi.org/10.1016/j.beproc.2016.04.001
Li JX, McMahon LR, Gerak LR, Becker GL, France CP (2008) Interactions between Δ 9-tetrahydrocannabinol and μ opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology 199:199–208. https://doi.org/10.1007/s00213-008-1157-0
Li JX, Koek W, France CP (2012) Interactions between delta9-tetrahydrocannabinol and heroin: self-administration in rhesus monkeys. Behav Pharmacol 23:754–761. https://doi.org/10.1097/FBP.0b013e32835a3907
Maguire DR, France CP (2014) Impact of efficacy at the μ-opioid receptor on antinociceptive effects of combinations of μ-opioid receptor agonists and cannabinoid receptor agonists. J Pharmacol Exp Ther 351:383–389. https://doi.org/10.1124/jpet.114.216648
Maguire DR, France CP (2016a) Interactions between cannabinoid receptor agonists and mu opioid receptor agonists in rhesus monkeys discriminating fentanyl. Eur J Pharmacol 784:199–206. https://doi.org/10.1016/j.ejphar.2016.05.018
Maguire DR, France CP (2016b) Effects of daily delta-9-tetrahydrocannabinol treatment on heroin self-administration in rhesus monkeys. Behav Pharmacol 27:249–257. https://doi.org/10.1097/FBP.0000000000000192
Maguire DR, Gerak LR, France CP (2013a) Delay discounting of food and remifentanil in rhesus monkeys. Psychopharmacology 229:323–330. https://doi.org/10.1007/s00213-013-3121-x
Maguire DR, Yang W, France CP (2013b) Interactions between μ-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. J Pharmacol Exp Ther 345:354–362. https://doi.org/10.1124/jpet.113.204099
Maguire DR, Gerak LR, France CP (2013c) Effect of delay on self-administration of remifentanil under a drug versus drug choice procedure in rhesus monkeys. J Pharmacol Exp Ther 347:557–563. https://doi.org/10.1124/jpet.113.208355
Maguire DR, Gerak LR, France CP (2016) Delay discounting of the mu opioid receptor agonist remifentanil in rhesus monkeys. Behav Pharmacol 27:148–154. https://doi.org/10.1097/FBP.0000000000000193
McGregor IS, Issakidis CN, Prior G (1996) Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol Biochem Behav 53:657–664. https://doi.org/10.1016/0091-3057(95)02066-7
McMahon LR (2006) Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther 319:1211–1218. https://doi.org/10.1124/jpet.106.107110
McMahon LR (2011) Chronic Δ9-tetrahydrocannabinol treatment in rhesus monkeys: differential tolerance and cross-tolerance among cannabinoids. Br J Pharmacol 162:1060–1073. https://doi.org/10.1111/j.1476-5381.2010.01116.x
Nader MA, Woolverton WL (1991) Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology 105:169–174. https://doi.org/10.1007/BF02244304
Nader MA, Woolverton WL (1992) Choice between cocaine and food by rhesus monkeys: effects of conditions of food availability. Behav Pharmacol 3:635-638. https://doi.org/10.1097/00008877-199212000-00010
National Research Council (2011). Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, D.C.
Negus SS (2005a) Interactions between the reinforcing effects of cocaine and heroin in a drug-vs-food choice procedure in rhesus monkeys: a dose-addition analysis. Psychopharmacology 180:115–124. https://doi.org/10.1007/s00213-004-2133-y
Negus SS (2005b) Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharmacology 181:244–252. https://doi.org/10.1007/s00213-005-2266-7
Negus SS (2006) Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther 317:711–723. https://doi.org/10.1124/jpet.105.095380
Nielsen S, Sabioni P, Trigo JM, Ware MA, Betz-Stablein BD, Murnion B, Lintzeris N, Khor KE, Farrell M, Smith A, Le Foll B (2017) Opioid-sparing effect of cannabinoids: a systematic review and meta-analysis. Neuropsychopharmacology 42:1752–1765. https://doi.org/10.1038/npp.2017.51
Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK (1980) Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther 28:409–416. https://doi.org/10.1038/clpt.1980.181
Perkins FN, Freeman KB (2018) Pharmacotherapies for decreasing maladaptive choice in drug addiction: targeting the behavior and the drug. Pharmacol Biochem Behav 164:40–49. https://doi.org/10.1016/j.pbb.2017.06.015
Podlesnik CA, Ko MC, Winger G, Wichmann J, Prinssen EP, Woods JH (2011) The effects of nociceptin/orphanin FQ receptor agonist Ro 64-6198 and diazepam on antinociception and remifentanil self-administration in rhesus monkeys. Psychopharmacology 213:53–60. https://doi.org/10.1007/s00213-010-2012-7
Riley AL (2011) The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav 103:69–78. https://doi.org/10.1016/j.physbeh.2010.11.021
Stevenson GW, Folk JE, Rice KC, Negus SS (2005) Interactions between δ and μ opioid agonists in assays of schedule-controlled responding, thermal nociception, drug self-administration, and drug versus food choice in rhesus monkeys: studies with SNC80 [(+)-4-[(αR)-α-((2S, 5R)-4-allyl-2, 5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N, N-diethylbenzamide] and heroin. J Pharmacol Exp Ther 314:221–231. https://doi.org/10.1124/jpet.104.082685
Wade-Galuska T, Winger G, Woods JH (2007) A behavioral economic analysis of cocaine and remifentanil self-administration in rhesus monkeys. Psychopharmacology 194:563–572. https://doi.org/10.1007/s00213-007-0858-0
Wade-Galuska T, Galuska CM, Winger G (2011) Effects of daily morphine administration and deprivation on choice and demand for remifentanil and cocaine in rhesus monkeys. J Exp Anal Behav 95:75–89. https://doi.org/10.1901/jeab.2011.95-75
Wen H, Hockenberry JM (2018) Association of medical and adult-use marijuana laws with opioid prescribing for medicaid enrollees. JAMA Intern Med 178:673-679. https://doi.org/10.1001/jamainternmed.2018.1007
Winger G, Galuska CM, Hursh SR, Woods JH (2006) Relative reinforcing effects of cocaine, remifentanil, and their combination in rhesus monkeys. J Pharmacol Exp Ther 318:223–229. https://doi.org/10.1124/jpet.105.100461
Woods JH, Winger G (2002) Observing responses maintained by stimuli associated with cocaine or remifentanil reinforcement in rhesus monkeys. Psychopharmacology 163:345–351. https://doi.org/10.1007/s00213-002-1201-4
Woolverton WL, Wang Z, Vasterling T, Tallarida R (2008) Self-administration of cocaine–remifentanil mixtures by monkeys: an isobolographic analysis. Psychopharmacology 198:387–394. https://doi.org/10.1007/s00213-008-1152-5
Acknowledgements
The authors thank Eli Desarno, Steven Garza, Sarah Howard, Jade Juarez, Krissian Martinez, Emily Spolarich, and Samuel Womack for excellent technical assistance. Special thanks to Drs. Gail Winger and Yonggong Shi for technical assistance. This work was supported by the National Institutes of Health (R01DA005018) and the Welch Foundation (AQ-0039). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute on Drug Abuse.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Maguire, D.R., France, C.P. Reinforcing effects of opioid/cannabinoid mixtures in rhesus monkeys responding under a food/drug choice procedure. Psychopharmacology 235, 2357–2365 (2018). https://doi.org/10.1007/s00213-018-4932-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-4932-6