Abstract
Rationale and Objectives
5-HT6 receptors are mainly expressed in brain areas associated with learning and memory. Several studies have reported procognitive effects of both 5-HT6 agonist and antagonists. However, the exact mechanism 5-HT6 receptor modulation has not been properly studied especially in the context of cholinergic functions, cerebral blood flow (CBF), brain-derived neural factor (BDNF), oxidative stress, and behavioral changes.
Methods
In the present study, memory impairment was induced in albino Wistar rats by two doses of intracerebroventricular (ICV) injection of streptozotocin (STZ, 3 mg/kg) on first and third day. These rats were evaluated in a battery of behavioral tasks after 14 days from the first day of ICV-STZ.
Results
Significant memory impairment was seen when ICV-STZ induced rats are assessed by Morris water maze, novel object recognition, social recognition, and passive avoidance tests. There was a significant reduction in CBF, increased oxidative stress (MDA, GSH, and ROS), acetylcholinesterase (AChE) activity, and a decrease in BDNF. Treatment with selective 5-HT6 agonist EMD-386088 (5 mg/kg) and antagonist SB-399885 (10 mg/kg) prevented ICV-STZ-induced memory impairment when assessed by behavioral tests. Treatment with 5-HT6 ligands significantly prevented the change in CBF and BDNF. Further, protected from MDA and ROS and decreasing GSH in the brain compared to ICV-STZ rats. The rice in brain AChE activity was normalized by both ligands. The changes in locomotor activity by EMD-386088 and SB-399885 treatment were negligible.
Conclusion
The findings in this study support the therapeutic potential of 5-HT6 receptor ligands in the treatment of cognitive dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
5-Hydroxytryptamine (5-HT, serotonin) is an important neurotransmitter in the central and peripheral nervous system modulating variety of physiological processes such as cognition, mood, perception, pain, and feeding behavior (Hoyer and Martin 1996; Meneses 2015). These effects are mediated through seven families of 5-HT receptors and all these receptors belong to the G-protein coupled receptor superfamily except 5-HT3 which signals through ligand-gated ion channels (Hoyer et al. 1994; Liu and Robichaud 2010). The 5-HT6 receptors are exclusively expressed in brain especially at areas like frontal cortex and hippocampus which are associated with learning and memory (Kohen et al. 1996). The observation of antipsychotics ability to bind to 5HT-receptors triggered the interest in role of 5-HT6 receptors in CNS-functions (Ramirez 2013). Studies in the Alzheimer’s disease (AD) patients showed a significant reduction in 5-HT6 receptor density in cortical areas (Lorke et al. 2006). Further, it has been reported that dysregulation of 5-HT6 receptor activation by 5-HT in the temporal cortex may be related to behavioral symptoms in AD (Marcos et al. 2008).
Preclinical studies have shown that modulation of 5-HT6 receptor function exert beneficial effects on learning and memory in rodent models of amnesia. Administration of 5-HT6 receptor antagonist enhanced learning and memory in several preclinical behavioral tasks like Novel Object Recognition Test (NORT), Social Recognition Task (SRT), and Morris Water Maze (Hirst et al. 2006; King et al. 2004; King et al. 2009; Woods et al. 2012). SB-271046 and other 5-HT6 receptor antagonist prevented memory impairment induced by scopolamine or phencyclidine or MK-801 (Arnt et al. 2010; Da Silva Costa-Aze et al. 2012; de Bruin et al. 2011; Fone 2008). Similarly, the 5-HT6 receptor agonists are also reported for procognitive or antiamnesic effects. Meneses (2015) have reported that selective 5-HT6 receptor agonists restore memory impairments in the novel object discrimination paradigm (Meneses 2015). 5-HT6 receptor agonist E-6801 enhanced recognition memory through the facilitation of cholinergic and glutamatergic neurotransmission in animal models. Subtherapeutic dose of E-6801 has shown synergistic effect with non-active doses of cholinesterase inhibitor donepezil and NMDA receptor antagonist memantine (Kendall et al. 2011). Another 5-HT6 agonist EMD-386088 reversed cholinergic- and glutamatergic-induced deficits in associative learning in conditioned emotion response task (Woods et al. 2012). However, in the social recognition paradigm, the 5-HT6 receptor agonist impaired the memory (Loiseau et al. 2008). The uncertainty about the role of 5-HT6 receptor in neurodegenerative disorders reinforced by the clinical report on selective 5-HT6 receptor antagonist such as idalopirdine which improved cognitive function in donepezil-treated patients with moderate AD (Wilkinson et al. 2014).
In our earlier report, we have shown that 5-HT6 receptor agonist EMD-386088 and antagonist SB-399885 protected PC12 cells from neurotoxicity induced by Aβ25–35 in vitro suggesting the role of this receptor in neurodegenerative conditions. We have seen that the neuroprotection is through prevention of impairment in neurite outgrowth in PC12 cells and by reducing intracellular reactive oxygen species (ROS) (Bokare et al. 2017). Hence, the present study was designed to evaluate the effect of 5-HT6 receptor agonist EMD-386088 and antagonist SB-399885 on memory impairment, oxidative stress, cholinergic dysfunction, reduced BDNF, and impaired cerebral circulation in intracerebroventricular (ICV) streptozotocin (STZ)-induced memory impaired rat model. The ICV STZ-induced memory impairment is a well-established model which is associated with oxidative stress, cholinergic dysfunction, impairment in brain energy metabolism, and reduced cerebral circulation (Kamat et al. 2016; Saxena et al. 2010; Tota et al. 2010; Tota et al. 2009; Tota et al. 2012; Tota et al. 2011).
Materials and methods
Animals
Young male albino Wistar (WH) rats (225–250 g, 8–9 weeks old, n = 170) and Juvenile- male albino Wistar rats (50–60 g, 3–4 weeks old) were procured from in-house, AAALAC accredited, Research Animal Facility, Lupin Research Park, Pune, India. They were kept in polycarbonate cages (22.5 × 37.5 cm) and maintained under standard housing conditions (room temperature 22–26 °C and humidity 50–70%) with a 12 h light and dark cycle. Food and water were available ad libitum. Animals were assigned randomly to treatment groups. All behavioral experiments were carried out between 9 AM and 4 PM on separate sets of animal groups. All animals were used only once for behavioral paradigms. Experiments were performed according to internationally followed ethical standards and approved by the research ethics committee of Lupin Ltd. (Lupin Research Park) framed as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
Materials
Streptozotocin (STZ), chloral hydrate, sodium chloride (NaCl), dichlorodihydrofluorescein diacetate (DCF-DA), MDA assay kit, GSH assay kit, bicinchoninic acid protein assay kit, and TRIzol reagent were purchased from Sigma-Aldrich, USA. Acetylcholinesterase assay kit was procured from BioAssay Systems, USA. BDNF assay kit was obtained from Boster Biological Technology, CA. Artificial CSF (aCSF) purchased from Harvard Apparatus, USA. SB-399885 and EMD-386088 were synthesized in-house at Lupin Research Park, Lupin Limited, Pune, India.
Intracerebroventricular injection of streptozotocin in rats
Rats were anesthetized with chloral hydrate (300 mg/kg, i.p.). STZ (3 mg/kg) was freshly dissolved in aCSF and injected slowly in a volume of 10 μl into each lateral cerebral ventricle (ICV) on days 1 and 3 using the coordinates: 0.8 mm posterior to bregma, 1.5 mm lateral to sagittal suture, 3.6 mm ventral by Hamilton microsyringe using motorized integrated stereotaxic injector system (Stoelting Co, USA) (Tota et al. 2012). To the vehicle group rats, ICV aCSF was injected in the same volume (10 μl) as STZ on days 1 and 3.
Experimental protocol and administration of 5-HT6 ligands
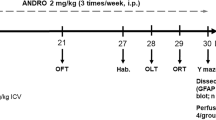
The study was done with seven to eight rats per group. EMD-386088 was dissolved in a normal saline and administered by i.p. route, starting with the day of first dose ICV-STZ and given 1 h before STZ injection on day 1 and day 3. The control rats of aCSF and STZ groups received normal saline. The effective dose of EMD-386088 was derived by a dose response study at 1, 2.5, and 5 mg/kg, i.p., in Social Recognition Test (SRT; as discussed below, 2.5.1.). Administration of EMD-386088 at 5 mg/kg significantly prevented STZ-induced memory impairment in SRT, while lower doses were found ineffective (data not shown). Therefore, the effective dose 5 mg/kg was used in assessing memory tasks. The 5-HT6 receptor antagonist SB-399885 was administered at 10 mg/kg orally twice daily as reported earlier (Hirst et al. 2006). The antioxidant, melatonin (10 mg/kg, i.p.), which is reported to prevent ICV-STZ-induced memory impairment, was used as reference standard (Saxena et al. 2010). Separate set of all animal groups was used to perform different behavioral studies. Figure 1 shows a diagrammatic representation of experimental protocol for test compound administration, behavioral studies, CBF measurement, and biochemical estimations.
Experimental protocol and drug administration for evaluation of 5-HT6 receptor agonist and antagonist in ICV-STZ-induced memory impairment model. *Compounds or vehicle administration was started from first dose of ICV-STZ and continued till the completion of behavioral studies depending on the task. Locomotor activity was evaluated in all the animal groups before initiation of behavioral studies. CBF was measured on day 15. Brains were collected for biochemical estimations on days 14, 15, and 21 after the completion of NORT, passive avoidance, and MWM tasks
Spontaneous locomotor activity
Spontaneous locomotor activity was assessed for 10 min by placing rats in home-cage of Columbus Instruments (Opto-varimex, Model-M4, USA), prior to the trial on memory tasks. A group of rats (n = 6) treated with d-amphetamine HCl (2 mg/kg, i.p.) was used as reference standard (McKinzie et al. 2002).
Assessment of learning and memory in rats
Social recognition test to assess the efficacy of 5-HT6 ligands
Male young rats (225–250 g) were used to assess effect of 5-HT6 receptor agonist and antagonist on ICV STZ-induced social recognition memory impairment. The experiment was performed in 42 rats divided in to six groups (n = 7 per group). Rats were housed individually and testing was carried out in their home cages on 14th day after the 1st dose of ICV-STZ injection. An unfamiliar juvenile rat (50–60 g) was used as social stimuli. This juvenile rat was introduced to the home cage. The time spent by young rat in overall social interaction with juvenile was recorded for 5-min interval (SIT1). The juvenile rat was then separated from the home cage. After 2 h, the same juvenile rat was reintroduced and overall investigation duration was recorded again during a second 5-min trial (SIT2). A statistically significant reduction in social interaction time in SIT2 in comparison to SIT1 using paired t test was considered as successful social recognition by young rat (Griebel et al. 2012). Recognition index (SIT2/SIT1) and % reduction in social interaction time (% RSIT) were calculated.
The recognition index and % RSIT were compared between the treatment groups by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test.
Novel object recognition test to assess the efficacy of 5-HT6 ligands
The NORT was assessed from 14th day after the administering first dose of ICV-STZ. The experiment was conducted in 40 rats that were divided in to five groups (n = 8 per group). On day 13, rats were acclimatized for 10 min to their testing arenas and then returned to the respective home cages. On day 14, the rats were subjected to familiarization phase. During which, rats could explore for 5 min the arenas having two similar objects. The time spent by rats with each object was noted. After 2 h, the rats were subjected to choose trial for a period of 5 min. Here, the rats could explore the arena in which a copy of the familiar object is used along with a novel object. A statistically significant increase (P < 0.05) in time spent with the novel object in comparison familiar object during choice (testing) by paired t test was considered as successful recognition (Hirst et al. 2006; Liu et al. 2014). The discrimination index (DI) as an index of recognition memory for each animal calculated by using the formula:
where TNovis the duration of exploration of the novel object in trial 2, TFam is the duration of exploration of familiar object in trial 2, and e2 is the total exploration (TNov + TFam) in the trial 2.
The DI for the animals in different treatment group was compared using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test.
Passive avoidance test to assess the efficacy of 5-HT6 ligands
Passive avoidance (PA) apparatus (TSE, Germany) consisted of two identical light and dark square boxes separated by a guillotine door. The test was performed from 14th day after first dose ICV-STZ. The experiment was performed in 40 rats that were divided in to five groups (n = 8 per group). During acquisition trial, rats were initially placed in the illuminated compartment. After an acclimatization period of 50 s, the guillotine door was opened and closed automatically after entry of the rat into the dark compartment. The animal received a low-intensity foot shock (0.8 mA, 3 s, once) in the dark compartment. Infrared sensors monitored the transfer of the animal from one compartment to another, which was recorded as transfer latency time (TLT) in seconds. Then, the rat was removed and transferred to home cages. The retention trial was given 24 h after the acquisition trial. The duration of a trial was 300 s. A significant increase in TLT in retention trial in comparison to that of acquisition trial was considered as successful learning (Awasthi et al. 2010; Tota et al. 2009). The TLT of retention trial was compared to acquisition trial using paired t test. The TLT between different treatment groups was compared using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test.
Morris water maze test to assess the efficacy of 5-HT6 ligands
Rats were subjected to Morris water maze (MWM) test from 14th day onward after the administration of first dose of ICV-STZ. The experiment was performed in 42 rats that were divided in to six groups (n = 7 per group). The MWM consists of a large circular black pool of 200 cm diameter, 60 cm height, filled to a depth of 45 cm with water at 26 ± 2 °C (Panlab, Spain). A round platform of 16 cm diameter was placed 1 cm below the surface of water in a constant position. The pool was divided into four hypothetical quadrants. The animals could climb on the platform to escape from the necessity of swimming. Data was acquired through a video camera connected to the computerized tracking system (VideoMot2, TSE, Germany) fixed above the center of the pool. Trials were given on 14th, 15th, and 21st day after first ICV-STZ injection. Each rat was given a total three sessions of four trials each. The rats were given a maximum time of 60 s, to find the hidden platform and could stay on it for 20 s. Rats that failed to locate the platform were put on it manually during first session only (acquisition trial). Latency time to reach the platform was recorded in each trial. A significant decrease in latency time of retention trials in comparison to that of acquisition trial indicates spatial learning (Tota et al. 2012). The latency time for rats within the group and between the groups was compared using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Also, data was analyzed by two-way ANOVA followed by Bonferroni’s multiple comparison test for sessions and groups as two factors (variables), respectively.
Measurement of cerebral blood flow to assess the efficacy of 5-HT6 ligands
The CBF was measured by using laser Doppler flowmetry (moor VMS-LDF, USA) on 15th day in the animals after being tested for memory assessment in SRT. LDF qualitatively measures CBF in arbitrary blood perfusion units (BPU) (Tonnesen et al. 2005). One hour after administration of vehicle or drugs (on 15th day), rats were anesthetized with chloral hydrate (300 mg/kg, i.p.) and skull bone was exposed and cleaned, and a 0.5-mm diameter micro-fiber laser doppler probe was fixed on the skull (6 mm lateral and 1 mm posterior of bregma) using brain ATLAS and Stoelting stereotaxic apparatus. CBF was monitored within cortical region (Awasthi et al. 2010; Tota et al. 2012). The results were expressed as BPU. The BPU between different treated groups was compared statistically using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test.
Biochemical estimation in brain tissues
Tissue collection and sample preparation for biochemical estimations
Biochemical studies were done in rat brain regions (cortex and hippocampus) after the completion of behavioral studies. Brain regions of two rats from each group of animals subjected to NORT, passive avoidance, and MWM tasks were utilized to estimate biochemical parameters. The brains were collected on days 14, 15, and 21 after completion of respective behavioral paradigms and stored at − 80 °C until estimation of biochemical parameters. Rats were sacrificed with ether anesthesia and brain was quickly isolated, kept on ice-cold plate, and then dissected into cerebral cortex and hippocampus as described previously (Tota et al. 2012). A 10% w/v homogenate of rat brain regions was prepared in sodium phosphate buffer (0.03 M, pH 7.4) using Precellys Homogenizer (Bertin Technologies, Germany).
Estimation of acetylcholinesterase activity in the brain
A 10% w/v homogenate of brain samples in 0.1 M sodium phosphate buffer (pH 7.5) and centrifuged at 14,000 rpm at 4 °C in a centrifuge (Eppendorf, USA) for 5 min. Supernatant was collected and used for AChE estimation by Ellkman’s method (Ellman et al. 1961). The kinetic profile of enzyme activity was measured at 412 nm in ELISA plate reader (Biotek, USA) using commercially available assay kit (BioAssay Systems, USA). The specific activity of AChE was expressed in μmoles/min/mg protein (Awasthi et al. 2010; Tota et al. 2012).
Estimation of oxidative stress markers (MDA, GSH, and ROS)
Malondialdehyde (MDA), a lipid peroxidation marker, was estimated in the brain tissues according to the method reported earlier. Rat brain homogenate was mixed with 30% trichloroacetic acid (TCA), 5 N HCl, and 2% thiobarbituric acid (TBA) in 0.5 N NaOH. The mixture was heated at 90 °C for 15 min and centrifuged (Eppendorf, USA) at 10,000 rpm for 10 min. The pink color of the supernatant was eluted in 96-well plate and absorbance was measured at 532 nm using ELISA plate reader (Biotek, USA). MDA concentration was expressed as nmol/mg protein.
Glutathione (GSH), an endogenous antioxidant, was estimated by its reaction with 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) to yield a yellow chromophore which was measured at 412 nm by using ELISA plate reader. The brain homogenate was mixed with an equal amount of 10% TCA and centrifuged at 10,000 rpm for 10 min at 4 °C. To 0.1 ml of supernatant, 2 ml of phosphate buffer (pH 8.4), 0.5 ml of DTNB (0.2%), and 0.4 ml of distilled water were added and the mixture was shaken vigorously on vortex. GSH concentration was expressed as μg/mg protein (Awasthi et al. 2010; Pachauri et al. 2013).
Amount of ROS in rat brain regions was measured using a fluorogenic dye 2′,7′-dichlorofluorescin–diacetate (DCF-DA), which is deacetylated by cellular esterases to a non-fluorescent compound, which is later oxidized by ROS into highly fluorescent 2′,7′-dichlorofluorescein (DCF). In brief, brain was homogenized in ice-cold 40 mM Tris-HCl buffer (pH 7.4). The resulting brain homogenate was incubated with DCF-DA (1.25 mM) for 15 min in a 37 °C water bath. The formation of the fluorescent product DCF was monitored by a fluorescence spectrometer (Biotek, USA) with excitation wave length of 488 nm and emission wave length of 530 nm.
Estimation of brain-derived neurotrophic factor
Brain tissue (10%) homogenized in 0.5 ml of lysis buffer (100 mM HEPES pH 7, 500 mM NaCl, 0.2%, 0.1% Triton X-100, 2 mM EDTA, 200 μM PMSF, and protease inhibitor cocktail 0.1%) and sonicated at power level 4 and pulses at 1 s intervals for 15 s. The homogenate was then centrifuged at 14,000 rpm for 30 min at 4 °C. The resulting supernatant was used for BDNF estimation by using BDNF Elisa kit (Boster Biological Technology, CA) according to manufacturer instructions.
Protein estimation
Protein was measured in all brain samples (data not shown) by using Bicinchoninic acid protein assay kit (#B9643, Sigma, USA) with bovine serum albumin (BSA) as standard plot (Lowry et al. 1951; Wang and Smith 1975).
Statistical analysis
The results were expressed as mean ± SEM. Statistical comparison between two trial of same group in SRT, NORT, and passive avoidance tests was done by paired t test while one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test was employed to compare the data between groups. Analysis of MWM data within each group (between sessions) was carried out by one-way ANOVA followed by Tukey’s multiple comparisons and, within and between the groups by two-way ANOVA followed by Bonferroni’s multiple comparison test considering session and group as two factors (variables). Cerebral blood flow and biochemical parameter data was compared by one-way ANOVA followed by Tukey’s multiple comparison test. The data were checked by extreme studentized deviate method using GraphPad software to determine whether the data set contains any significant outlier, which were removed. All statistical comparisons were performed using GraphPad Prism version 5.0, GraphPad Software, USA.
Results
Effect of EMD-386088 and SB-399885 on spontaneous locomotor activity
Spontaneous locomotor activity was assessed in all the animal groups before the initiation of behavioral studies. No significant change in ambulatory counts and total counts were observed in treated groups in comparison to control indicating no change in animal locomotion. On the other hand, d-amphetamine HCl caused significant increase in locomotor counts confirming the validity of the procedure (data not shown).
Effect of EMD-386088 and SB-399885 on ICV-STZ-induced memory impairment in social recognition test (SRT)
The memory function in SRT was evaluated on 14th day after 1st ICV injection of STZ in rats. The social investigation time during trial 2 (SIT2) was significantly (P < 0.05) reduced in comparison to that of trial 1 (SIT1) in control and vehicle (aCSF, ICV) treated rats indicating successful learning of task. Administration of ICV-STZ caused memory impairment as shown by no significant (P > 0.05) change in the SIT2 in comparison to SIT1. Treatment with EMD-386088 at 5 mg/kg dose prevented STZ-induced memory deficit in SRT as indicated by significant decrease (P < 0.01) in SIT2 when compared with SIT1. However, lower doses (1 and 2.5 mg/kg) of EMD-386088 failed to prevent STZ-induced memory impairment in rats (data not shown). Further, SB-399885 significantly (P < 0.001) decreased SIT2 in comparison to SIT1 indicating amelioration of ICV-STZ-induced memory deficit. The reference standard melatonin prevented STZ-induced memory impairment as shown by significant (P < 0.01) decrease in SIT2 (Fig. 2a). Further, there was a significant difference in the %RSIT [F (5, 36) = 6.86, P < 0.001] and recognition index [F (5, 36) = 6.88, P < 0.001] of control, vehicle, EMD-386088, SB-399885, and melatonin-treated groups in comparison to ICV-STZ group (Fig. 2b, c).
Effect of 5-HT6 receptor agonist and antagonist on ICV-STZ-induced memory impairment in rats using social recognition test. a Social investigation time in trial 1 and trial 2. b % reduction in social interaction time (%RSIT). c Recognition index. Results were expressed as mean ± SEM (n = 7). Data was analyzed by paired t test and one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. ***P < 0.001, **P < 0.01, and *P < 0.05 compared to SIT1. a P < 0.001 compared to the normal control; b P < 0.001as compared to vehicle control, c P < 0.01 compared to ICV-STZ group
Effect of EMD-386088 and SB-399885 on ICV-STZ-induced memory impairment in novel object recognition test
ICV-STZ impaired responding in the NOR test as indicated by failure to discriminate between a familiar and a novel object during choice trial done 2 h after familiarization phase. In vehicle-treated rats, a significant increase (P < 0.01) in exploration time towards novel object was seen in comparison to that of familiar object. Administration of EMD-386088 prevented ICV-STZ-induced memory deficit as shown by significant increase (P < 0.01) in exploration time of novel object. Similarly, SB-399885 caused significant increase (P < 0.01) in exploration time of novel object in comparison to that of familiar object. Melatonin improved memory in ICV-STZ-treated rats significantly (P < 0.01) in exploration time for novel object in comparison to the familiar one (Fig. 3a). Similar results were obtained when results were expressed as discrimination index [F (4, 35) = 4.69, P < 0.01; Fig. 3b].
Effect of 5-HT6 receptor agonist and antagonist on ICV-STZ-induced memory impairment in rats using novel object recognition test. Results were expressed as mean ± SEM (n = 8). a Exploration time during familiarization and testing phase. Data was analyzed by paired t test. Discrimination index. Data was one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. **P < 0.01 as compared to exploration time of familiar object during testing phase of respective groups. a P < 0.01as compared to vehicle control, b P < 0.01 as compared to ICV-STZ group
Effect of EMD-386088 and SB-399885 on ICV-STZ-induced memory impairment in passive avoidance test
The transfer latency time (TLT) was significantly (P < 0.01) increased during retention trial as compared to the acquisition trial in vehicle-treated rats. ICV-STZ caused memory impairment as shown by no significant (P > 0.05) change in the TLT of the retention trial as compared to the acquisition trial. Administration of EMD-386088 and SB-399885 for 14 days improved memory function in ICV-STZ rats. As shown in Fig. 4, there was a significant increase (P < 0.01) in TLT of retention trial in EMD-386088 and SB-399885-treated groups. Further, melatonin also improved memory in ICV-STZ-treated rats. The latency time in retention trial of vehicle control, EMD-386088, SB-399885, and melatonin groups was significantly higher [F (4, 34) = 6.85, P < 0.01] than ICV-STZ-treated group indicating improvement of memory (Fig. 4).
Effect of 5-HT6 receptor agonist and antagonist on ICV-STZ-induced memory impairment in rats using passive avoidance test. Results were expressed as mean ± SEM (n = 7–8). Data was analyzed by paired t test and one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. ***P < 0.001 as compared to retention trial of STZ group; a P < 0.05 as compared to retention trial of vehicle control; b P < 0.01 as compared to transfer latency time of acquisition trial of respective group
Effect of EMD-386088 and SB-399885on ICV-STZ-induced memory impairment in Morris water maze test
As shown in Fig. 5, normal control [F (2, 81) = 6.75, P < 0.01] and vehicle control [F (2, 81) = 6.43, P < 0.01] groups showed a significant decrease in latency time during 2nd and 3rd sessions in comparison to session 1. However, no significant decrease in latency time [F (2, 81) = 0.06, P > 0.05] was observed throughout all the sessions in ICV-STZ-treated animals. EMD-386088 administration has prevented STZ-induced memory impairment in MWM task as shown by significant reduction in latency to reach hidden platform from session 2 onwards in comparison to session 1 [F (2, 81) = 6.70, P < 0.01]. Similarly, SB-399885 significantly decreased [F (2, 81) = 5.67, P < 0.01] latency to reach platform in STZ-treated rats from session 2 onwards. Melatonin also significantly decreased [F (2, 81) = 6.36, P < 0.01] latency time in ICV-STZ-injected animals indicating prevention of memory deficit. Data was analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test.
Effect of 5-HT6 receptor agonist and antagonist on ICV-STZ-induced memory impairment in rats using Morris water maze test. Results were expressed as mean ± SEM (n = 7). Data was analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. **P < 0.01 and *P < 0.05 as compared to session 1 of respective group rats. a P < 0.05 as compared to respective session of normal control; b P < 0.01 as compared to respective session of vehicle control; c P < 0.01 compared to respective sessions of ICV-STZ group. Data was also analyzed by two-way ANOVA followed by Bonferroni’s multiple comparison test considering session and group as two factors (variables). ***P < 0.001 as compared to respective sessions of ICV-STZ group and ***P < 0.001 as compared to ICV-STZ group
Also, data was analyzed by two-way ANOVA followed by Bonferroni’s multiple comparison test showed [F (2, 108) = 27.87, P < 0.001] and [F (5, 108), P < 0.001] for sessions and groups as two factors (variables), respectively.
Effect of EMD-386088 and SB-399885 on cerebral blood flow in rats
Cerebral blood flow (CBF) was measured on 15th day after the first dose of ICV-STZ administration in the rats subjected to SRT. ICV-STZ caused a significant reduction (p < 0.01) in CBF in comparison to vehicle group. Chronic treatment with EMD-386088, SB-399885, and melatonin [F (4, 30) = 16.03, P < 0.001] attenuated STZ-induced reduction in CBF in rats (Fig. 6).
Effect of 5-HT6 receptor agonist and antagonist on cerebral blood flow in ICV-STZ-treated rats. Results were expressed as mean blood perfusion units (BPU) ± SEM (n = 7). Data was analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. a P < 0.001 in comparison to vehicle control and b P < 0.001 as compared to ICV-STZ group
Changes in the biochemical parameters
Effect of 5-HT6 ligands on acetylcholinesterase activity in brains of ICV-STZ rats
Acetylcholinesterase (AChE) activity was measured in cortex and hippocampus after the completion of behavioral studies. As shown in Fig. 7, AChE activity was significantly increased in cortex (P < 0.05) and hippocampus (P < 0.05) of ICV-STZ-treated rat in comparison to vehicle group. Administration of EMD-386088 and SB-399885 caused numerically lower AChE activity in ICV-STZ-treated rat brain regions while melatonin significantly prevented ICV-STZ-induced rise in AChE activity in both cortex [F (4, 25) = 3.63, P < 0.05] and hippocampus [F (4, 25) = 4.21, P < 0.05].
Effect of 5-HT6 receptor agonist and antagonist on AChE activity in ICV-STZ-treated rats. Results were expressed as mean AChE activity ± SEM (n = 6). Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. #P < 0.05, in comparison to vehicle control group and *P < 0.05 in comparison to ICV-STZ group
Effect of 5-HT6 ligands on oxidative stress (MDA, GSH, and ROS) in the brains of ICV-STZ rats
ICV-STZ caused oxidative stress in cortex and hippocampus of rats. There was significant rise of MDA and ROS, and significant reduction in GSH (P < 0.05) in comparison to vehicle control group. Administration of EMD-386088, SB-399885, and melatonin prevented STZ-induced oxidative stress. Both compounds are equipotent in alleviating oxidative stress and they are comparable to that of melatonin (Tables 1 and 2).
Effect of 5-HT6 ligands on brain-derived neurotrophic factor in the brains of ICV-STZ rats
BDNF level was significantly reduced in cortex and hippocampus of ICV-STZ rats as compared to vehicle control group. Administration of EMD-386088, SB-399885, and melatonin significantly prevented ICV-STZ-induced reduction in BDNF (Tables 1 and 2).
Discussion
The role of 5-HT6 receptors in cognitive functions has been well documented. Several preclinical studies have shown the beneficial effects of 5-HT6 receptor antagonists in models of memory impairment using different behavioral tasks (de Bruin et al. 2011; Hirst et al. 2006; King et al. 2004; Lieben et al. 2005; Rogers and Hagan 2001; Schaffhauser et al. 2009; Schreiber et al. 2007; Woolley et al. 2001). Accordingly, it was hypothesized that 5-HT6 receptor agonists would have opposing effects to the antagonists and impair learning and memory (Fone 2008; King et al. 2008). However, this assumption was disproved by initial preclinical studies (Burnham et al. 2010; Kendall et al. 2011; Loiseau et al. 2008; Meneses et al. 2008). Therefore, the present study investigated effect of chronic treatment with 5-HT6 receptor agonist, EMD-386088, and antagonist, SB-399885, on memory impairment in rats induced by ICV-STZ. Upon ICV administration of STZ, rats exhibited memory impairment when they are tested for their behavioral paradigms by Morris water maze (MWM), novel object recognition (NOR), passive avoidance (PA), and social recognition tests (SRT).
In SRT, control and aCSF (vehicle of STZ)-treated rats showed significant reduction in social investigation time in trial 2 indicating successful learning of the task. However, ICV-STZ-treated rats showed higher recognition index (SIT2/SIT1) indicating impairment of memory. In NOR test, vehicle control rats showed higher preference towards novel object while ICV-STZ rats had poor discrimination index (Liu et al. 2014; Shoham et al. 2007). Confirming previous studies elsewhere, ICV-STZ-treated rats showed impairment in memory as indicated by no significant changes in transfer latency time and escape latency time in PA and MWM tests, respectively (Awasthi et al. 2010; Pachauri et al. 2013; Tota et al. 2010; Tota et al. 2009; Tota et al. 2012; Tota et al. 2011). An antioxidant melatonin was used as a reference standard, and it was found that melatonin prevented ICV-STZ-induced memory impairment in all the behavioral paradigms. It was also confirmed that the anti-dementia effects of EMD-386088 and SB-399885 were not associated with any change in locomotor activity (data not shown).
The results obtained for 5-HT6 ligands EMD-386088 and SB-399885 matches with the reported preclinical studies on modulation of 5-HT6 receptor function restore drug-induced, neurodevelopmental-, or age-related memory impairment or time-dependent natural forgetting in rodents (Arnt et al. 2010; Da Silva Costa-Aze et al. 2012; de Bruin et al. 2011; Fone 2008; Hirst et al. 2006; King et al. 2004; King et al. 2009; Schreiber et al. 2007; Woods et al. 2012; Woolley et al. 2001). The ICV injection of STZ caused disruption in social behavior and social recognition as tested in SRT which takes advantage of the spontaneous behavior of rats to investigate conspecifics (Kaidanovich-Beilin et al. 2011). EMD-386088 at 5-mg/kg dose caused significant reduction in social interaction time in trial 2 in comparison to trial 1 indicating prevention of ICV-STZ-induced memory deficit. Similarly, SB-399885 was administered at 10 mg/kg, p.o., b.i.d. prevented ICV-STZ-induced memory deficit in SRT as reported elsewhere (Hirst et al. 2006). Hence, in the present study, all the other behavioral, biochemical, and CBF studies were conducted using EMD-386088 at 5 mg/kg and SB-399885 at 10 mg/kg. This result with EMD-386088 is inconsistent with a previous report, showing impaired social recognition in normal adult rats by 5-HT6 receptor agonist WAY 181187 (Loiseau et al. 2008). The discrepancy between these results might be because of difference in the experimental designs. Loiseau et al. (2008) injected WAY 181187 directly into frontal cortex and studied the effect in normal adult rats whereas we administered EMD-386088 for longer duration and evaluated the effect in memory deficit rats. The effect of SB-399885 in SRT greatly confirms and extends previous observations that 5-HT6 receptor antagonist reversed social recognition deficits in rodent models (de Bruin and Kruse 2015). In passive avoidance test, which can also be explained as fear-motivated behavioral paradigm, both EMD-386088 and SB-399885 attenuated ICV-STZ-induced memory impairment. These results are in agreement with earlier studies reported with 5-HT6 receptor ligands in scopolamine model (Pereira et al. 2015; Woods et al. 2012).
In MWM, EMD-386088 and SB-399885 significantly decreased time to reach hidden platform in ICV-STZ rats suggesting improvement in spatial visual learning and memory. This observation for SB-399885 is consistent with the previous studies of 5-HT6 receptor antagonists in scopolamine model of amnesia (Hirst et al. 2006; Rogers and Hagan 2001; Woolley et al. 2001). Previous studies reported that 5-HT6 receptor agonist (Kendall et al. 2011) and antagonists (de Bruin et al. 2011; Hirst et al. 2006; King et al. 2004; Lieben et al. 2005) elicit pro-cognitive effects in novel object recognition, a non-spatial visual learning, and memory test. This type of finding was also reported by Woods et al. (2012), using conditioned emotion response paradigm in rodents. The author reported that 5-HT6 receptor agonist and antagonists enhance memory through modulation of cholinergic and glutamatergic mechanisms (Woods et al. 2012). The exact mechanism for these paradoxically similar effects of agonist and antagonist is not completely understood so far. However, it was hypothesized that agonists and antagonists act differentially in different neuronal populations and may enhance glutamate function and cholinergic neurotransmission in the cortex and/or hippocampus producing similar beneficial effects on learning and memory (Woods et al. 2012).
Another mechanism for paradoxically similar effects of agonist/antagonists could be related to the presence of alternative biochemical pathways activated by 5-HT6 receptors. Selective binding of 5-HT to Gs-protein coupled 5-HT4, 5-HT6, and 5-HT7 receptors activates protein kinase A (PKA), which in turn activates extracellular signal-regulated kinases (ERK) and MEK (MAPkinase-ERK kinase) signaling, the process is said to be important in the physiological protection of memory loss (Fisher et al. 2016). In addition to Gαs coupling, 5-HT6 receptors are also coupled to Gαi/o or Gαq subunits and to Ca2+ signaling (Ramirez 2013). Similar protective effects were also observed in our recent study where both ligands protected PC-12 cell lines from amyloid-β induced toxicity (Bokare et al. 2017).
There was a significant reduction in cerebral blood flow (CBF) in ICV-STZ-rats as measured by Laser doppler flowmetry (Tota et al. 2010). This finding is substantiated by many clinical studies which suggest alteration in cerebral microcirculation in patients with AD, probably due to vascular amyloidosis, oxidative stress, and endothelial dysfunction causing restriction of blood flow to the brain (Crawford 1996). In the present study, administration of ICV-STZ produced oxidative stress as shown by significant increase in cortical and hippocampal MDA and ROS, and decrease in GSH. This is a well-established fact that ICV-STZ cause oxidative stress in brain due to impaired glucose utilization and altered brain energy metabolism (Tota et al. 2011). This increase in oxidative stress leads to cerebral endothelial dysfunction causing reduced cerebral circulation which in turn collectively responsible for memory impairment (Cai and Harrison 2000; Massaad 2011; Nash and Fillit 2006). Chronic treatment with 5-HT6 receptor agonist and antagonist decrease oxidative stress in rat brain cortex and hippocampus. Further, both the compounds significantly prevented ICV-STZ-induced impairment in CBF. However, per se administration of 5-HT6 receptor agonist and antagonist had no significant effect on CBF in comparison to control animals (data not shown).
Acetylcholine is an important neurotransmitter which is degraded by the enzyme AChE and AChE inhibitors are the most effective pharmacological approach for the management of AD. In the present study, STZ caused increase in AChE-activity in cortex and hippocampus of rats indicating cholinergic dysfunction. These observations are consistent with number of previous studies (Awasthi et al. 2010; Pachauri et al. 2013; Tota et al. 2010; Tota et al. 2009; Tota et al. 2011). 5-HT6 receptor ligands prevented STZ-induced elevation in AChE activity. The brain-derived neurotrophic factor (BDNF) is involved in regulation of the survival and differentiation of neuronal populations during development (Huang and Reichardt 2001). BDNF regulates the structure and functions of different neuronal circuits throughout life and is one of the most important molecules involved in learning and memory processes. The selective 5-HT6 receptor agonist LY-586713 was reported to increase expression of frontal cortical BDNF and these effects were not antagonized by 5-HT6 receptor antagonist (de Foubert et al. 2007). In the present study, EMD-386088 and SB-399885 prevented the reduction of BDNF in both cortex and hippocampus. All the biochemical measurements were expressed as units/mg protein, and we did not find any difference in the mean protein content between groups (data not shown). Thus, the 5-HT6 ligands are beneficial in improving memory by their wholistic properties such as modulating BDNF, AChE, oxidative stress, and alleviated cerebral circulation apart from signaling through the receptors.
Conclusion
The 5-HT6 receptor modulation prevents ICV-STZ-induced memory impairment in rats through reducing oxidative stress, cholinergic dysfunction, impairment in cerebral circulation, and BDNF. These findings corroborated earlier reports showing pro-cognitive effects of both 5-HT6 receptor agonists and antagonist in rodent models.
References
Arnt J, Bang-Andersen B, Grayson B, Bymaster FP, Cohen MP, DeLapp NW, Giethlen B, Kreilgaard M, McKinzie DL, Neill JC, Nelson DL, Nielsen SM, Poulsen MN, Schaus JM, Witten LM (2010) Lu AE58054, a 5-HT6 antagonist, reverses cognitive impairment induced by subchronic phencyclidine in a novel object recognition test in rats. Int J Neuropsychopharmacol 13:1021–1033. https://doi.org/10.1017/S1461145710000659
Awasthi H, Tota S, Hanif K, Nath C, Shukla R (2010) Protective effect of curcumin against intracerebral streptozotocin induced impairment in memory and cerebral blood flow. Life Sci 86:87–94. https://doi.org/10.1016/j.lfs.2009.11.007
Bokare AM, Praveenkumar AK, Bhonde M, Nayak Y, Pal R, Goel R (2017) 5-HT6 receptor agonist and antagonist against beta-amyloid-peptide-induced neurotoxicity in PC-12 cells. Neurochem Res 42:1571–1579. https://doi.org/10.1007/s11064-017-2217-9
de Bruin NM, Kruse CG (2015) 5-HT6 receptor antagonists: potential efficacy for the treatment of cognitive impairment in schizophrenia. Curr Pharm Des 21:3739–3759
de Bruin NM et al (2011) Two novel 5-HT6 receptor antagonists ameliorate scopolamine-induced memory deficits in the object recognition and object location tasks in Wistar rats. Neurobiol Learn Mem 96:392–402. https://doi.org/10.1016/j.nlm.2011.06.015
Burnham KE, Baxter MG, Bainton JR, Southam E, Dawson LA, Bannerman DM, Sharp T (2010) Activation of 5-HT(6) receptors facilitates attentional set shifting. Psychopharmacology 208:13–21. https://doi.org/10.1007/s00213-009-1701-6
Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844
Crawford JG (1996) Alzheimer's disease risk factors as related to cerebral blood flow. Med Hypotheses 46:367–377
Da Silva Costa-Aze V, Quiedeville A, Boulouard M, Dauphin F (2012) 5-HT6 receptor blockade differentially affects scopolamine-induced deficits of working memory, recognition memory and aversive learning in mice. Psychopharmacology 222:99–115. https://doi.org/10.1007/s00213-011-2627-3
Ellman GL, Courtney KD, Andres Jr V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–90 IN81-IN82,91-95
Fisher JR, Wallace CE, Tripoli DL, Sheline YI, Cirrito JR (2016) Redundant Gs-coupled serotonin receptors regulate amyloid-beta metabolism in vivo. Mol Neurodegener 11:45. https://doi.org/10.1186/s13024-016-0112-5
Fone KC (2008) An update on the role of the 5-hydroxytryptamine6 receptor in cognitive function. Neuropharmacology 55:1015–1022. https://doi.org/10.1016/j.neuropharm.2008.06.061
de Foubert G, O'Neill MJ, Zetterstrom TS (2007) Acute onset by 5-HT(6)-receptor activation on rat brain brain-derived neurotrophic factor and activity-regulated cytoskeletal-associated protein mRNA expression. Neuroscience 147:778–785. https://doi.org/10.1016/j.neuroscience.2007.04.045
Griebel G, Pichat P, Pruniaux MP, Beeské S, Lopez-Grancha M, Genet E, Terranova JP, Castro A, Sánchez JA, Black M, Varty GB, Weiner I, Arad M, Barak S, de Levie A, Guillot E (2012) SAR110894, a potent histamine H(3)-receptor antagonist, displays procognitive effects in rodents. Pharmacol Biochem Behav 102:203–214. https://doi.org/10.1016/j.pbb.2012.04.004
Hirst WD, Stean TO, Rogers DC, Sunter D, Pugh P, Moss SF, Bromidge SM, Riley G, Smith DR, Bartlett S, Heidbreder CA, Atkins AR, Lacroix LP, Dawson LA, Foley AG, Regan CM, Upton N (2006) SB-399885 is a potent, selective 5-HT6 receptor antagonist with cognitive enhancing properties in aged rat water maze and novel object recognition models. Eur J Pharmacol 553:109–119. https://doi.org/10.1016/j.ejphar.2006.09.049
Hoyer D, Martin GR (1996) Classification and nomenclature of 5-HT receptors: a comment on current issues. Behav Brain Res 73:263–268
Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP (1994) International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev 46:157–203
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736. https://doi.org/10.1146/annurev.neuro.24.1.677
Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR (2011) Assessment of social interaction behaviors. J Vis Exp. https://doi.org/10.3791/2473
Kamat PK, Kalani A, Rai S, Tota SK, Kumar A, Ahmad AS (2016) Streptozotocin Intracerebroventricular-induced neurotoxicity and brain insulin resistance: a therapeutic intervention for treatment of sporadic Alzheimer's disease (sAD)-like pathology. Mol Neurobiol 53:4548–4562. https://doi.org/10.1007/s12035-015-9384-y
Kendall I, Slotten HA, Codony X, Burgueno J, Pauwels PJ, Vela JM, Fone KC (2011) E-6801, a 5-HT6 receptor agonist, improves recognition memory by combined modulation of cholinergic and glutamatergic neurotransmission in the rat. Psychopharmacology 213:413–430. https://doi.org/10.1007/s00213-010-1854-3
King MV, Sleight AJ, Woolley ML, Topham IA, Marsden CA, Fone KC (2004) 5-HT6 receptor antagonists reverse delay-dependent deficits in novel object discrimination by enhancing consolidation--an effect sensitive to NMDA receptor antagonism. Neuropharmacology 47:195–204. https://doi.org/10.1016/j.neuropharm.2004.03.012
King MV, Marsden CA, Fone KC (2008) A role for the 5-HT(1A), 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol Sci 29:482–492
King MV, Spicer CH, Sleight AJ, Marsden CA, Fone KC (2009) Impact of regional 5-HT depletion on the cognitive enhancing effects of a typical 5-ht(6) receptor antagonist, Ro 04-6790, in the novel object discrimination task. Psychopharmacology 202:111–123. https://doi.org/10.1007/s00213-008-1334-1
Kohen R, Metcalf MA, Khan N, Druck T, Huebner K, Lachowicz JE, Meltzer HY, Sibley DR, Roth BL, Hamblin MW (1996) Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J Neurochem 66:47–56
Lieben CK, Blokland A, Sik A, Sung E, van Nieuwenhuizen P, Schreiber R (2005) The selective 5-HT6 receptor antagonist Ro4368554 restores memory performance in cholinergic and serotonergic models of memory deficiency in the rat. Neuropsychopharmacology 30:2169–2179. https://doi.org/10.1038/sj.npp.1300777
Liu KG, Robichaud AJ (2010) 5-HT6 medicinal chemistry. Int Rev Neurobiol 94:1–34. https://doi.org/10.1016/B978-0-12-384976-2.00001-0
Liu P, Zou LB, Wang LH, Jiao Q, Chi TY, Ji XF, Jin G (2014) Xanthoceraside attenuates tau hyperphosphorylation and cognitive deficits in intracerebroventricular-streptozotocin injected rats. Psychopharmacology 231:345–356. https://doi.org/10.1007/s00213-013-3240-4
Loiseau F, Dekeyne A, Millan MJ (2008) Pro-cognitive effects of 5-HT6 receptor antagonists in the social recognition procedure in rats: implication of the frontal cortex. Psychopharmacology 196:93–104. https://doi.org/10.1007/s00213-007-0934-5
Lorke DE, Lu G, Cho E, Yew DT (2006) Serotonin 5-HT2A and 5-HT6 receptors in the prefrontal cortex of Alzheimer and normal aging patients. BMC Neurosci 7:36. https://doi.org/10.1186/1471-2202-7-36
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marcos B, García-Alloza M, Gil-Bea FJ, Chuang TT, Francis PT, Chen CP, Tsang SWTY, Lai MKP, Ramirez MJ (2008) Involvement of an altered 5-HT -{6} receptor function in behavioral symptoms of Alzheimer's disease. J Alzheimers Dis 14:43–50
Massaad CA (2011) Neuronal and vascular oxidative stress in Alzheimer's disease. Curr Neuropharmacol 9:662–673. https://doi.org/10.2174/157015911798376244
McKinzie DL, McBride WJ, Murphy JM, Lumeng L, Li TK (2002) Effects of amphetamine on locomotor activity in adult and juvenile alcohol-preferring and -nonpreferring rats. Pharmacol Biochem Behav 71:29–36
Meneses A (2015) Serotonin, neural markers, and memory. Front Pharmacol 6:143. https://doi.org/10.3389/fphar.2015.00143
Meneses A, Perez-Garcia G, Liy-Salmeron G, Flores-Galvez D, Castillo C, Castillo E (2008) The effects of the 5-HT(6) receptor agonist EMD and the 5-HT(7) receptor agonist AS19 on memory formation. Behav Brain Res 195:112–119. https://doi.org/10.1016/j.bbr.2007.11.023
Nash DT, Fillit H (2006) Cardiovascular disease risk factors and cognitive impairment. Am J Cardiol 97:1262–1265. https://doi.org/10.1016/j.amjcard.2005.12.031
Pachauri SD, Verma PR, Dwivedi AK, Tota S, Khandelwal K, Saxena JK, Nath C (2013) Ameliorative effect of noni fruit extract on streptozotocin-induced memory impairment in mice. Behav Pharmacol 24:307–319. https://doi.org/10.1097/FBP.0b013e3283637a51
Pereira M, Martynhak BJ, Andreatini R, Svenningsson P (2015) 5-HT6 receptor agonism facilitates emotional learning. Front Pharmacol 6:200. https://doi.org/10.3389/fphar.2015.00200
Ramirez MJ (2013) 5-HT6 receptors and Alzheimer's disease. Alzheimers Res Ther 5:15. https://doi.org/10.1186/alzrt169
Rogers DC, Hagan JJ (2001) 5-HT6 receptor antagonists enhance retention of a water maze task in the rat. Psychopharmacology 158:114–119. https://doi.org/10.1007/s002130100840
Saxena G, Bharti S, Kamat PK, Sharma S, Nath C (2010) Melatonin alleviates memory deficits and neuronal degeneration induced by intracerebroventricular administration of streptozotocin in rats. Pharmacol Biochem Behav 94:397–403. https://doi.org/10.1016/j.pbb.2009.09.022
Schaffhauser H, Mathiasen JR, DiCamillo A, Huffman MJ, Lu LD, McKenna BA, Qian J, Marino MJ (2009) Dimebolin is a 5-HT6 antagonist with acute cognition enhancing activities. Biochem Pharmacol 78:1035–1042. https://doi.org/10.1016/j.bcp.2009.06.021
Schreiber R, Vivian J, Hedley L, Szczepanski K, Secchi RL, Zuzow M, van Laarhoven S, Moreau JL, Martin JR, Sik A, Blokland A (2007) Effects of the novel 5-HT(6) receptor antagonist RO4368554 in rat models for cognition and sensorimotor gating. Eur Neuropsychopharmacol 17:277–288. https://doi.org/10.1016/j.euroneuro.2006.06.009
Shoham S, Bejar C, Kovalev E, Schorer-Apelbaum D, Weinstock M (2007) Ladostigil prevents gliosis, oxidative-nitrative stress and memory deficits induced by intracerebroventricular injection of streptozotocin in rats. Neuropharmacology 52:836–843. https://doi.org/10.1016/j.neuropharm.2006.10.005
Tonnesen J, Pryds A, Larsen EH, Paulson OB, Hauerberg J, Knudsen GM (2005) Laser Doppler flowmetry is valid for measurement of cerebral blood flow autoregulation lower limit in rats. Exp Physiol 90:349–355
Tota S, Kamat PK, Awasthi H, Singh N, Raghubir R, Nath C, Hanif K (2009) Candesartan improves memory decline in mice: involvement of AT1 receptors in memory deficit induced by intracerebral streptozotocin. Behav Brain Res 199:235–240. https://doi.org/10.1016/j.bbr.2008.11.044
Tota S, Awasthi H, Kamat PK, Nath C, Hanif K (2010) Protective effect of quercetin against intracerebral streptozotocin induced reduction in cerebral blood flow and impairment of memory in mice. Behav Brain Res 209:73–79. https://doi.org/10.1016/j.bbr.2010.01.017
Tota S, Kamat PK, Shukla R, Nath C (2011) Improvement of brain energy metabolism and cholinergic functions contributes to the beneficial effects of silibinin against streptozotocin induced memory impairment. Behav Brain Res 221:207–215. https://doi.org/10.1016/j.bbr.2011.02.041
Tota S, Kamat PK, Saxena G, Hanif K, Najmi AK, Nath C (2012) Central angiotensin converting enzyme facilitates memory impairment in intracerebroventricular streptozotocin treated rats. Behav Brain Res 226:317–330. https://doi.org/10.1016/j.bbr.2011.07.047
Wang C, Smith RL (1975) Lowry determination of protein in the presence of triton X-100. Anal Biochem 63:414–417
Wilkinson D, Windfeld K, Colding-Jorgensen E (2014) Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer's disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 13:1092–1099. https://doi.org/10.1016/S1474-4422(14)70198-X
Woods S, Clarke NN, Layfield R, Fone KC (2012) 5-HT(6) receptor agonists and antagonists enhance learning and memory in a conditioned emotion response paradigm by modulation of cholinergic and glutamatergic mechanisms. Br J Pharmacol 167:436–449. https://doi.org/10.1111/j.1476-5381.2012.02022.x
Woolley ML, Bentley JC, Sleight AJ, Marsden CA, Fone KC (2001) A role for 5-ht6 receptors in retention of spatial learning in the Morris water maze. Neuropharmacology 41:210–219
Acknowledgements
The authors express their gratitude to Lupin Ltd., Lupin Research Park, Pune and Manipal University, Manipal for their support and help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Experiments were performed according to internationally followed ethical standards and approved by the research ethics committee of Lupin Ltd. (Lupin Research Park) framed as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bokare, A.M., Bhonde, M., Goel, R. et al. 5-HT6 receptor agonist and antagonist modulates ICV-STZ-induced memory impairment in rats. Psychopharmacology 235, 1557–1570 (2018). https://doi.org/10.1007/s00213-018-4866-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-4866-z