Abstract
Rationale
The low self-administration (LS)/Kgras (LS) and high self-administration (HS)/Kgras (HS) rat lines were generated by selective breeding for low- and high-intravenous cocaine self-administration, respectively, from a common outbred Wistar stock (Crl:WI). This trait has remained stable after 13 generations of breeding.
Objective
The objective of the present study is to compare cocaine preference, neurotransmitter release, and dopamine receptor activation in LS and HS rats.
Methods
Levels of dopamine, acetylcholine, and cocaine were measured in the nucleus accumbens (NA) shell of HS and LS rats by tandem mass spectrometry of microdialysates. Cocaine-induced locomotor activity and conditioned-place preference were compared between LS and HS rats.
Results
HS rats displayed greater conditioned-place preference scores compared to LS and reduced basal extracellular concentrations of dopamine and acetylcholine. However, patterns of neurotransmitter release did not differ between strains. Low-dose cocaine increased locomotor activity in LS rats, but not in HS animals, while high-dose cocaine augmented activity only in HS rats. Either dose of cocaine increased immunoreactivity for c-Fos in the NA shell of both strains, with greater elevations observed in HS rats. Activation identified by cells expressing both c-Fos and dopamine receptors was generally greater in the HS strain, with a similar pattern for both D1 and D2 dopamine receptors.
Conclusions
Diminished levels of dopamine and acetylcholine in the NA shell, with enhanced cocaine-induced expression of D1 and D2 receptors, are associated with greater rewarding effects of cocaine in HS rats and an altered dose-effect relationship for cocaine-induced locomotor activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although family, twin, and adoption studies in humans indicate that genetic factors play a significant role in the etiology of substance abuse disorders, it has been difficult to identify the specific genes involved (Faraone et al. 2008). Knowledge of the genetic elements that influence substance abuse would likely contribute to the diagnosis and treatment of these conditions. Breeding of model organisms under selective pressure allows for the unbiased enrichment of genetic variants influencing a given biologic trait, independent of any knowledge of the underlying mechanisms (Weiss et al. 2008), and therefore has the potential to provide new insights into the neurobiology of addictive behaviors that are not based on initial preconceptions.
Intravenous drug self-administration remains one of the preferred animal models of drug-reinforced behavior. Substances abused by humans are reliably self-administered by mice, rats, and monkeys (Collins et al. 1984; Haney and Spealman 2008). Animals are typically trained to respond at high operant levels to obtain drug infusions, using appropriate controls for non-drug reinforcers. The low self-administration (LS) and high self-administration (HS) rat strains were developed in our laboratory through selective breeding of Wistar rats for low and high levels of intravenous drug self-administration (He et al. 2008). Self-administration of low-dose (0.1 mg/kg-injection) cocaine exhibited the largest change after selective breeding, with HS rats self-administering approximately fivefold more than their LS counterparts. Importantly, LS and HS do not differ in food-reinforced behavior. Although a number of rodent strains have been selected for differential responding to drugs of abuse based on oral intake (Bell et al. 2006), to our knowledge, LS and HS are the only lines based on intravenous cocaine self-administration.
The neurotransmitter dopamine plays a key role as a mediator of drug reward (Ikemoto et al. 2015). Cocaine and other drugs of abuse act by rapidly increasing dopamine release beyond its normal physiologic range. Cell bodies of dopaminergic neurons reside in the ventral tegmental area (VTA), and project to various limbic brain structures, including the striatum and its ventral projection, the nucleus accumbens (NA) (Sesack and Grace 2010). The majority of neurons in the striatum are medium spiny neurons that release GABA, making up its main output (Yager et al. 2015). Based on their anatomic projections and functional roles, medium spiny neurons can be divided into two different phenotypes. These include direct pathway neurons that express dopamine D1 receptors (D1Rs) and have primarily excitatory influences on movement and reward and indirect pathway medium spiny neurons expressing D2 receptors (D2Rs) that most often have inhibitory effects (Gardoni and Bellone 2015; Volkow and Morales 2015). Given their very different effects on reward, altered function of direct and indirect pathway neurons could underlie genetic influences on the propensity for drug use.
In addition to the well-known actions of dopamine, both the NA and VTA are richly innervated with neurons that release acetylcholine, which can similarly modulate reward behavior in a complex manner (Lester et al. 2010). Based on this neuropharmacology, genetic influences on the function of dopamine and acetylcholine have the potential to modify an individual’s response to cocaine. Individual differences in the absorption and metabolism of abused substances are yet another means through which vulnerability to drugs of abuse differs with genotype. Although only limited data are available, one study found that brain concentrations of cocaine and its major metabolites varied significantly across different mouse strains (Wiltshire et al. 2015). Therefore, genetic factors that augment tissue levels of cocaine are likely to influence the responsiveness of different individuals.
This study was undertaken to make an initial assessment of the broad phenotypic differences underlying reward behavior in LS and HS rats to more fully understand how this selected model compares to human models of addiction. Firstly, we estimated genetic heterogeneity in both strains. Secondly, in vivo microdialysis was used to compare concentrations of dopamine, acetylcholine, and cocaine in the NA shell. Finally, we compared cocaine-induced conditioned-place preference and locomotor activity in LS and HS rats. In these animals, cocaine-induced activation of immediate early genes and activation of direct and indirect pathways were also determined in brain reward regions. Our general hypothesis was that LS and HS animals would differ in one or more of these broad areas.
Materials and methods
Animal care and maintenance
Rats were evaluated according to standards outlined in the Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011 ), with procedures approved by the Kansas City VA Animal Care and Use Committee. The LS/Kgras (LS, strain 536) and HS/Kgras (HS, strain 537) lines were resuscitated from frozen embryos stored at the Rat Resource and Research Center, Columbia Missouri (http://www.rrrc.us/).
For all experiments, male and female rats were initially allowed to develop with ad libitum food and water under a standard light-dark cycle. Food restriction enhances animal health (McShane and Wise 1996) and drug-reinforced behavior (Bell et al. 1997; Cabeza de Vaca and Carr 1998). Starting at 10 weeks of age, animals were single-housed with food restricted to 13 or 14 g of standard rat chow daily, for female and male rats, respectively. Drinking water continued to be available ad libitum. To facilitate operant responding, rats were maintained under a reversed light-dark cycle (12 h of darkness beginning at 4:00 a.m., followed by 12 lighted hours).
Microdialysis for determination of brain levels of dopamine, acetylcholine, and cocaine
CMA 12 Elite microdialysis probes with 2 mm polyarylethersulphone membranes were purchased through Harvard Apparatus (Holliston, MA). After being anesthetized with 50 mg/kg of intraperitoneal pentobarbital, rats were implanted with a unilateral guide cannula aimed at the right NA shell (1.7 mm anterior, 1.0 mm lateral, and 6.0 mm ventral to bregma). Following at least 3 days of recovery from surgery, rats were connected to tethers (M115TS, Instech Laboratories, Plymouth Meeting, PA) and allowed 3-day acclimation. Approximately 16 h prior to initial injections, microdialysis probes were inserted through guide cannulas and perfused with artificial cerebrospinal fluid (147 mM NACl, 2.7 mM KCl, 1.0 mM NaH2PO4, 1.4 mM Na2HPO4, 2.1 mM MgCl2, and 0.1 μM neostigmine, pH 7.4). Neostigmine at this concentration is recommended as part of a standardized protocol to detect acetylcholine in the brain through microdialysis (Noori et al. 2012). After overnight flow at 0.1 μL/min, the rate was increased to 0.75 μL/min at the onset of the lighted phase (4:00 a.m.). Beginning 2 h later, dialysate samples were collected at 20-min intervals into vials that contained 1.0 μL of antioxidant (20 mM oxalic acid and 2.0 M acetic acid). After collection of three baseline samples, rats received single, intraperitoneal injections of saline (time 0), low-dose cocaine (3.2 mg/kg, 1.33 h), and high-dose cocaine (16.0 mg/kg, 3.33 h). Samples were stored at −80 °C until analysis, with brain tissue fixed as outlined in the following. To verify probe locations, brains were sectioned at 40 μM using a cryostat (Leica Biosystems, Buffalo Grove, IL).
Determinations of dopamine, acetylcholine, and cocaine were made by tandem mass spectrometry using a TSQ Quantum Access MAX triple quadrupole mass spectrometer (Thermo Fisher Scientific, West Palm Beach, Florida). Acid-stabilized 10 μL aliquots of dialysate were injected by a refrigerated autosampler (5 °C) onto a Waters column (model HSS T3, 2.1 × 150 mm, particle size 2.5 μM, part number 186 00 6739). Mobile phase consisted of 5 mM formic acid and 5.0% acetonitrile (aqueous component, solution A) and 5 mM formic acid in acetonitrile (organic component, solution B). Sample injections were performed as solution A was running at a flow rate of 0.6 mL/min. Continuing at the same flow rate, solution B was increased from 0 to 30% between 0.5 and 2.5 min after sample injection, 30 to 90% between 2.5 and 4.3 min, and finally from 90 to 0% between 4.8 and 5.0 min. Chromatographic peaks were identified using mass transitions of dopamine, 154 > 91 m/z; acetylcholine, 146 > 87 m/z; and cocaine, 304 > 182 m/z.
Conditioned-place preference with activation of c-Fos and dopamine receptors
Conditioning was performed with three-chamber polycarbonate shuttle boxes using an unbiased procedure. These consisted of three distinct compartments: two at either end for conditioning (black or white in color, both measuring 22.5 cm long, 30.2 cm wide, and 26.8 cm tall) and a central area (gray, 13.8 cm long, 30.2 cm wide, and 26.8 cm tall). The floor of black compartment was constructed of metal bars 5 mm in diameter spaced 1.5 cm apart (Med Associates part number ENV-013WR), the floor of the white compartment was made of a wire-mesh grid (ENV-013BM), and the gray compartment had a smooth polycarbonate floor. Shuttle boxes were enclosed within larger sound-attenuating chambers each equipped with two ventilation fans that supplied white noise. Three soft-white incandescent bulbs (4 W) on the inside of these chambers were mounted above each of the compartments to eliminate shadows and provide dim illumination (4.0 lx). For conditioning, partitions were inserted to constrain animals to either the black or white compartments. Test sessions were conducted by giving rats free access to all three compartments over 20 min. During test sessions, different colored compartments were separated by openings that were 2.5 cm off the floor, 9.0 cm wide, and 11.5 cm high, with a rounded top. Infrared beams passed through eight holes along the sides of shuttle boxes, allowing computer monitoring of locomotor activity and animal location using Med Associates, Inc. software and instrumentation (ENV-253SD, ENV-258-8, and ENV-256C). We recorded both consecutive activations of the same infrared detector (showing repeated activity in one place, which may reflect stereotypy) and activation of two different detectors (reflecting linear activity across the cage).
Table 1 shows the timing of cocaine doses, behavioral measures, and tissue collection. To allow habituation, rats were handled over 2 days, allowed free access to shuttle boxes for 10 min, and given an initial test session prior to cocaine injections. Conditioning sessions were conducted on four consecutive days during two 25-min sessions, separated by at least 4 h. Rats of either strain were randomly assigned to three treatments: saline (vehicle) twice daily, saline in the morning with afternoon low-dose ascending doses of cocaine, and saline in the morning with afternoon high-dose ascending doses of cocaine.
Shuttle boxes were washed with detergent and warm water after each use. To conceal their orientation, rats were transported under an opaque cover. Because it can enhance the magnitude of conditioned-place preference in mice (Itzhak and Anderson 2012; Conrad et al. 2013), we utilized an ascending schedule of cocaine dose. Cocaine injections were paired with the side of shuttle boxes that animals spent less time in during their first test session. Both saline and cocaine were administered intraperitoneally, and chambers were cleaned with detergent following each session. Infrared beam interruptions were used to quantify locomotor activity during conditioning sessions and time spent in different compartments for test sessions. A second test session was conducted 72 h after the final conditioning session to determine cocaine preference without injecting rats.
Immunofluorescence staining
One day following the final conditioned-place preference test session, rats received a second injection of their final treatment (vehicle or cocaine, 5.3 or 16.0 mg/kg). Three hours later, they were anesthetized by intraperitoneal injection of 50 mg/kg of sodium pentobarbital. Rats were then perfused through the ascending aorta with ice-cold 0.1 M phosphate-buffered saline (PBS) pH 7.4 followed by 0.1 M PBS containing 4% (w/v) ice-cold paraformaldehyde using a peristaltic pump. Perfused brains were stored in 4% paraformaldehyde overnight at 4 °C. Brains were then cryoprotected in 0.1 M PBS with 20% sucrose at 4 °C until the tissue sank, followed by in 0.1 M PBS with 30% sucrose at 4 °C, again until sinking. They were then cut into 2-mm sections containing the NA shell and core, caudate putamen (CPu), VTA, and dentate gyrus (DG), according to the brain atlas of Paxinos and Watson (1986). Values from the hippocampal DG served as a control region not typically associated with drug-reinforced behavior. Blocks were mounted on weight boats using tissue embedding medium (Sakura Finetek USA, Torrance, USA) and stored at −80 °C.
Brains were cut into 15-μm coronal sections on a cryostat microtome (Leica Biosystems, Buffalo Grove, IL). The sections were again fixed in 4% paraformaldehyde in 0.1 M PBS for 10 min at room temperature (RT), washed three times with PBS, permeabilized with 0.5% Triton X-100 in PBS for 15 min, and blocked with 10% bovine serum album in PBS for 1 h at RT. The sections were then incubated with sheep anti-c-Fos (1:500, CBL440, Millipore, Temecula, USA), combined with either mouse anti-dopamine D1R (1:1000, NB110, Novus Biologicals, Littleton, USA) or rabbit anti-dopamine D2R (1:500, AB5084P, Millipore, Temecula, USA) antibodies over 48 h in a humidified chamber at 4 °C. When different dopamine receptors were expressed S9 cells, the Novus NB110 antibody identified dopamine D1 but not D2 or D3 receptor subtypes in immunoblot experiments (Luedtke et al. 1999). For the D2R antibody, Western blot data provided by the manufacturer comparing lysate from rat, mouse, and human brain showed no obvious activity against D1, D3, or D4 receptors.
Next, the sections were incubated with Alexa Fluor 488 Donkey Anti-Sheep IgG (1:1000, A11015, Molecular Probes, Grand Island, USA). This was combined with either Alexa Fluor 555 Goat Anti-Mouse IgG (1:1000, A21424, Molecular Probes) or Alexa Fluor 647 Goat Anti-Rabbit IgG (1:1000, A21245, Molecular Probes) secondary antibodies for 2 h at RT. Coverslips were mounted onto microscope slides using fluorescence mounting medium containing DAPI (H-5000, Vector Laboratories, Burlingame, USA). The sections were imaged with a Nikon Eclipse fluorescence microscope (Show Me Optical, Kansas City, MO). Counts of c-Fos-immunoreactive nuclei were performed by an observer who was blind to treatment group, using NIS-Elements imaging software. Immunoreactivity was defined as the number of stained cells per field, with brain regions again verified according to the atlas of Paxinos and Watson (1986).
Whole-genome sequencing
Genetic heterogeneity was quantitatively determined in sequences from LS, HS, and Wistar rats. Size-selected fragment libraries were prepared using LS and HS genomic DNA extracted from liver tissue. Genomic fragment libraries were prepared from one LS and one HS individual from generation 13 using the Ion Xpress Plus Fragment Library kit (Thermo Fisher Scientific, USA) with size selection performed using Pippin Prep (Sage Bioscience). Libraries were templated using the Ion OneTouch 2 system and sequenced on the Ion Proton system (Thermo Fisher Scientific, USA). Sequences were mapped to UCSC Genome Browser assembly rn5 using tmap-f3 and genotypes called using samtools mpileup (v.1.2) and bcftools (v.1.2). Data for three sequencing runs per animal were combined to produce coverage of 9.2× (LS) and 11.54× (HS). Heterozygosity was calculated as the number of heterozygous genotypes per 10,000 bp sequenced. Inbreeding was estimated by comparison against average observed heterozygosity in exome sequencing of stock Wistar rats (n = 8).
Statistical analysis
Comparisons were made by t tests between pairs of measures or analysis of variance (ANOVA) for three or more measures using Systat software (version 13), with strain, dose, and time as factors. Data are presented as group means and standard error. According to the correction by Sidak (Sankoh et al. 1997) for a 40% correlation between interrelated areas, an alpha (type I error) value of 0.026 was used post hoc comparisons.
To estimate change in neurotransmitter concentrations after treatment with vehicle or cocaine, baseline levels were averaged over an initial three 20-min intervals as each individual animal was left undisturbed. Drug effects were then estimated as the average of four posttreatment samples following intraperitoneal injections of vehicle, 3.2 mg/kg of cocaine, and 16.0 mg/kg of cocaine, using the following formula where “|” indicates absolute value:
Pharmacokinetic measures were determined using PCNONLIN 4.2 (SCI Software, Apex, NC). Non-compartmental models were derived from changes in dialysate concentrations of cocaine over time, following treatment with different doses of cocaine. Area under the brain concentration versus time curve (AUC) and area under the first moment curve (AUMC) were estimated from these models. Maximum dialysate concentration (C max) and time to reach C max (T max) were determined by data inspection. Mean residence time (MRT) was estimated as the quotient (AUMC/AUC). Half-life (t½) was calculated from the quotient of natural logarithm(2)/k e, where k e is the derived elimination rate constant. Because drug was administered extravascularly, neither clearance nor volume of distribution of the central compartment is reported.
Cocaine-induced conditioned-place preference score was calculated as the difference between times spent in the drug-paired compartment after conditioning (test session 2) and before conditioning (prior to cocaine injections, test session 1).
Results
Inbreeding levels of LS and HS rats
The LS and HS were selectively bred from outbred Wistar rats (Crl:WI) for altered drug self-administration over six generations. Subsequently, both strains have been inbred for an additional six generations without further selection, with the current studies conducted on generation 12 and 13 animals. Preliminary studies of generation 14 and 15 have shown self-administration by HS rats under FR-5 that was increased by factors of 1.89, 2.58, and 2.73 above LS rats and for 0.1, 0.2, and 0.4 mg/kg-injection of cocaine available, respectively. When compared to outbred Wistar animals from which they were derived, 12th- and 13th-generation LS and HS rats used in the present studies exhibited no obvious differences in appearance or behavior. Rates of heterozygous genotypes per 10,000 sequenced and genotyped nucleotide positions were 6.9 × 104, 5.58 × 104, and 3.8 × 104 for outbred Wistar and 13th-generation LS and HS strains, respectively. These values correspond to approximately 19% inbreeding for LS and 45% inbreeding for HS rats. Despite the relatively low degrees of inbreeding observed for these lines, the drug self-administration trait remains stable.
Accumbal levels of cocaine, dopamine, and acetylcholine
Exposure to different classes of abused drugs preferentially increases dopamine in the NA shell (Pontieri et al. 1995). As shown in Fig. 1, active probe areas were localized to this brain region. Intraperitoneal administration of cocaine produced a large increase in cocaine concentration in the NA shell, with similar temporal profiles in both strains (Fig. 2a). However, maximum concentrations of cocaine were significantly lower in HS rats following high-dose but not low-dose cocaine [t(12) = 2.71, p < 0.02]. Otherwise, additional pharmacokinetic measures derived by non-compartmental modeling did not differ in LS and HS animals (Table 2).
Microdialysis probe placement. A photomicrograph showing placement of a probe in the nucleus accumbens shell is shown above. Line drawings adapted from Paxinos and Watson (1986) are shown below. Black bars show the location of the active membranes of individual probes from six LS and eight HS rats, with numbers indicating distance from bregma
Simultaneous determinations of cocaine, dopamine, and acetylcholine in the NA shell of LS and HS rats. In the legend, self-administration is abbreviated as SFA. Concentration corrected for probe efficiency is plotted on the left-hand side of the figure (a, b, d), with change in neurotransmitter concentration shown on the right (c, e). Time points for single, intraperitoneal injections are shown by vertical dashed lines which are colored light gray for vehicle, dark gray for 3.2 mg/kg of cocaine, and black for 16.0 mg/kg of cocaine. Group means and standard error are shown for six LS (one male and five female) and eight HS rats (one male and six female; open and filled symbols, respectively). Number signs over broad horizontal lines correspond to p < 0.001, for comparison between LS and HS rats, for results combined across all time points. Asterisks over smaller horizontal lines indicate significant differences with vehicle treatment for results of both strains combined, with one and two symbols corresponding to p < 0.026 and p < 0.001 respectively
For dopamine concentrations in dialysate from the NA shell (Fig. 2b), ANOVA showed significant main effects of strain [F (1,257) = 36.2; p < 0.001 and time [F (21,257) = 8.10; p < 0.001], but not the interaction of strain and time [F (21,257) = 1.53; p not significant]. When averaged across all time points, dopamine concentration was 59.1% lower in HS rats than in LS animals (mean and standard error of 0.213 ± 0.050 and 0.522 ± 0.102, respectively). Similarly, basal dopamine concentrations were diminished in HS rats (means and standard error of 0.0980 ± 0.0244 and 0.254 ± 0.0422, [t(12) = 3.14, p < 0.01]). Analysis by percent change from baseline (Fig. 2c) showed a significant effect of cocaine dose [F (2,36) = 25.9; p < 0.001], but not strain [F (1,36) = 0.138; p not significant] or the interaction of strain and dose [F (2,36) = 0.138; p not significant]. When corrected for baseline levels, low- or high-dose cocaine increased dialysate concentrations of dopamine to a similar degree in either strain.
Dialysate concentrations of acetylcholine (Fig. 2d) varied significantly with strain [F (1,260) = 126; p < 0.001], but not time [F (21,260) = 0.150; p not significant] nor the interaction of strain and time [F (21,260) = 0.132; p not significant]. Across all time points, acetylcholine in the NA shell was 70.0% lower in HS rats than in LS animals (5.3 ± 1.0 and 17.5 ± 4.8 nM, respectively). Basal acetylcholine values were also diminished in HS rats (4.8 ± 0.9 and 16.3 ± 4.2 nM, respectively, [t(12) = 2.83, p < 0.02]). Analysis by percent change from baseline (Fig. 2e) failed to show significant effects of strain [F (1,36) = 2.40; p not significant], cocaine dose [F (2,36) = 1.34; p not significant], or the interaction of strain and dose [F (2,36) = 0.89; p not significant]. Neither neurotransmitter concentrations nor pharmacokinetic measures varied significantly with sex.
Cocaine-induced conditioned-place preference
Cocaine produced preferences for the drug-paired side of shuttle boxes in rats conditioned with either dose of cocaine, but not in vehicle-treated animals (Fig. 3). For preference score, ANOVA showed significant main effects of cocaine dose (F (2,63) = 11.1; p < 0.0001) and strain (LS or HS, F (1,63) = 4.78; p < 0.05), but not the interaction of strain and dose (F(2,63) = 0.501; p not significant). Preference scores were 91.8 and 58.4% greater in HS rats, for low- and high-dose cocaine, respectively. After either low- or high-dose cocaine, HS rats had greater preference scores than LS animals (t (51) = 2.68; p < 0.01). There was no significant effect of sex (F (1,57) = 0.262; p not significant) or significant interaction of sex with other factors.
Strain effects on cocaine-induced conditioned-place preference. Preference score and cocaine dose are shown on the vertical and horizontal axes, respectively. Values for cocaine dose are the final dose administered on the fourth day of ascending-dose injections. Data are shown for 8 to 14 animals per condition (3 to 10 female and 4 to 7 male). Open and filled symbols correspond to LS and HS animals. Asterisks over smaller horizontal lines indicate significant differences compared with saline treatment for both strains combined; number sign over broad horizontal line shows significant differences compared with the LS strain after treatments with either dose of cocaine are combined. One and two symbols correspond to p < 0.026 and p < 0.001, respectively
Cocaine-induced locomotor activity
For repeated activity, ANOVA demonstrated significant main effects of cocaine dose (Fig. 4, F (2,215) = 3.05; p < 0.05), session number (1 to 4, F (3,215) = 5.37; p < 0.02), and sex (F (1,215) = 11.3; p < 0.001), but not strain (F (1,215) = 0.004; p not significant). There were significant interactions between strain and cocaine dose (F (2,215) = 7.27; p < 0.001), as well as strain and sex (F (2,215) = 11.5; p < 0.001). Further interactions, such as strain, dose, and session; sex and dose; or sex and session number, were all negative. When collapsed across session number and sex, low-dose cocaine increased repeated activity in LS but not HS rats. Considering only sex and strain, male HS rats had lower repeated activity than HS females or LS rats of either sex.
Repeated and linear locomotor activity during conditioning with ascending doses of cocaine. Injections of vehicle (saline) were given prior to the morning (a.m.) conditioning session, with cocaine or vehicle injections given before the second (p.m.) conditioning session. Mean values with standard error are shown for eight vehicle-treated rats from each strain (4 female) and 11 to 14 animals (7 to 9 female) from either stain that received either dose of cocaine. Data for LS and HS strains are shown by open and filled symbols, respectively. The two columns of graphs on the left show results of repeated locomotor activity (plotted against the left axis), with the two columns on the right showing results of linear activity (center axes). Asterisks over horizontal lines indicate significant differences compared to rats receiving twice-daily saline (lower panels) for results from either strain collapsed across session number (time), p < 0.026; with number sign denoting comparison with LS males, LS females, or HS females; collapsed across dose and session number; p < 0.02
For linear activity, ANOVA showed significant main effects of cocaine dose (F (2,218) = 4.24; p < 0.02) and session number (F (3,218) = 6.53; p < 0.001), but not strain (F (1,218) = 1.54; p not significant) or sex (F (1,254) = 1.49; p not significant). There was a significant interaction between strain and cocaine dose (F (2,218) = 7.46; p < 0.001), but not strain, cocaine dose, and session number (F (6,218) = 1.10; p not significant). Interactions of sex with strain, dose, and session were also negative. When collapsed across session number, linear activity was increased by low-dose cocaine in LS rats or high-dose cocaine in HS rats.
Expression and activation of c-Fos, D1R, and D2R in brain reward regions
Counts of cells expressing c-Fos, D1R, and D2R are shown in Table 3. For c-Fos-positive cells, ANOVA showed significant interactions of rat strain and cocaine dose for all of the brain regions examined (F (2,24) > 3.73; p < 0.05 for all regions). Considering D1R-positive cells, strain and dose exhibited a positive interaction for only the DG (F (2,24) = 6.98; p < 0.01). For D2R-positive cells, significant interactions of rat strain and cocaine dose were shown for NA shell, CPu, and DG (F (2,24) > 7.06; p < 0.01), but not for the NAc or VTA (F (2,24) < 1.97; p not significant).
Following saline treatment, post hoc comparisons did not show an effect of strain on expression of c-Fos, D1R, or D2R in any brain region. In only the DG, treatment with either dose of cocaine increased independent expression of D1R in HS but not LS rats. Treatment with either dose of cocaine increased independent expression of c-Fos for most brain regions in both LS and HS animals, often with greater effects in HS rats and sometimes with a larger effect following low-dose cocaine. In several regions, independent expression of D2R was also greater in HS rats that received low-dose cocaine. We did not observe significant effects of sex or its interaction with strain or dose.
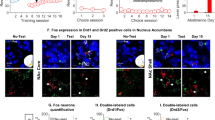
Combined activation of c-Fos with either D1R or D2R is shown in Fig. 5. For c-Fos and D1R, ANOVA showed significant interactions of rat strain and cocaine dose for NA shell, VTA, and DG (F (2,24) > 6.34; p < 0.01), but not the NAc and CPu (F (2,24) < 1.84; p not significant). Analysis of colocalized c-Fos and D2R expression revealed significant interactions between strain and cocaine dose for all brain regions evaluated (F (2,24) > 5.86; p < 0.01).
Counts of cells expressing both c-Fos and either type 1 or 2 dopamine receptors. Group means and standard error are shown for five animals for each condition (1 to 4 female), with open and filled symbols corresponding to LS and HS animals. Asterisks indicate significant differences compared with saline treatment, number signs show significant differences compared with the LS strain, for the same treatment, and caret symbols indicate significant differences for the same strain receiving low-dose cocaine. One and two symbols correspond to p < 0.026 and p < 0.01, respectively
Cocaine treatment increased combined c-Fos and D1R or D2R expression to a similar degree in each of the brain regions evaluated. Following saline treatment, there was no effect of strain in any brain region. For most of the regions evaluated, greater drug-induced activation of c-Fos with either D1R or D2R was observed in HS rats, relative to LS animals, and after low-dose cocaine, relative to high dose. In only the NA shell and DG, either dose of cocaine increased combined expression of c-Fos with either D1R or D2R. In the VTA, high-dose cocaine increased combined activation of c-Fos and D1R or D2R in HS but not LS rats. Again, there were no significant effects of sex, and its interaction with strain and dose was also negative.
Discussion
In summary, inbred rat lines originally developed by selective breeding for intravenous cocaine self-administration have been characterized for neurotransmitter release, cocaine-induced place preference, and activation of neurons expressing dopamine D1 and D2 receptors. Despite a modest degree of inbreeding, HS (high reward) rats exhibit greater drug preferences but have diminished cocaine-induced locomotor activity after low-dose cocaine. HS rats have lower maximum concentrations of cocaine in the NA shell. Baseline and postcocaine administration acetylcholine and dopamine levels in this brain region are also lower in HS rats. These differences may contribute to greater drug taking. Cocaine-induced activation of c-Fos and dopamine receptors was generally greater in HS animals, with a similar pattern of increases for both D1 and D2 receptors, for the NA shell and core and several other limbic regions.
HS rats have lower C max (maximum concentration) values after receiving 16.0 but not 3.2 mg/kg of cocaine. Because cocaine was administered by intraperitoneal rather than the intravenous route used for selective breeding, this finding is most relevant to conditioned-place preferences shown in the present study. In a previous experiment, sixth-generation animals self-administered mean values of 4.4 and 17.7 mg/kg-day during 2-h sessions, for LS and HS strains, respectively (He et al. 2008). Although further study is needed to determine how selective breeding has influenced the pharmacokinetics of intravenously administered cocaine, self-administered doses of cocaine for HS rats roughly correspond to those for which differences in C max values would be expected between strains. Lower C max (peak) levels of brain cocaine could motivate animals to take more drug to achieve a similar pharmacologic effect.
Reinforcing effects of cocaine occur through rapid increases in dopamine caused by inhibition of the dopamine transporter (Rocha 2003). Cocaine and other drugs of abuse may cause dependence by producing short-term increases in dopamine release, which are followed by delayed reductions in dopamine transmission (Mateo et al. 2005). Accordingly, basal dopamine levels in the NA shell are reduced by about one half relative to values in drug-naive rats prior to cocaine or heroin self-administration sessions (Gerrits et al. 2002). Abstinent cocaine-dependent individuals have diminished release of dopamine estimated by positron emission tomography following oral treatment with either methylphenidate (Volkow et al. 1997) or amphetamine (Martinez et al. 2007). In the latter case, decreased dopamine transmission in the ventral striatum was predictive of choosing cocaine over money. Cocaine-dependent patients with lower methylphenidate-induced dopamine release in the limbic striatum estimated by positron emission tomography are also less likely to have a positive response to treatment (Martinez et al. 2011).
In the current study, HS and LS rats exhibited a similar pattern of augmented dopamine release in the NA shell following treatment with low- and high-dose cocaine. However, the magnitude of dopamine values was 59.1% lower in HS animals. If augmented dopamine underlies the acute reinforcing effects of cocaine, lower values in HS rats would be expected to decrease reinforcement in this strain, possibly resulting in augmented lever pressing during drug self-administration sessions. Taken together, the findings support the concept that blunted dopamine release in the ventral striatum is a biomarker for an increased risk of substance abuse disorders (Trifilieff and Martinez 2014).
Although they are not as well characterized as effects on dopamine and other monoamines, drugs of abuse also acutely augment acetylcholine in brain reward regions (Williams and Adinoff 2008). For both the NA (Mark et al. 1999) and VTA (You et al. 2008), these increases are greater following contingent rather than experimenter-initiated cocaine injections. Increases in acetylcholine transmission in the NA may also limit the duration of natural and drug rewards (Avena and Rada 2012). Basal acetylcholine in the nucleus accumbens shell of HS rats was lower than values for LS rats or those reported for other rat strains evaluated using similar concentrations of neostigmine (Noori et al. 2012). The magnitude of acetylcholine release in the NA shell was also 70% lower in HS rats. As was hypothesized for dopamine, lower values may increase the motivation for lever pressing by HS rats that is required to achieve intermediate, rewarding levels of acetylcholine transmission. This is analogous to observations that rats increase responding for cocaine after blockade of muscarinic input to the VTA, preventing decreases in dopamine levels that would otherwise occur after muscarinic blockade (You et al. 2008). Genetic factors that attenuate cocaine-induced increases in acetylcholine may also oppose appetitive limits associated with higher levels of acetylcholine (Grasing 2016).
We found that HS rats exhibit greater cocaine-induced conditioned-place preferences than LS animals. The LS and HS rat lines were primarily derived from selective breeding for intravenous self-administration of cocaine (He et al. 2008), which has been interpreted as an example of operant or instrumentally conditioned behavior (Jones and Comer 2013). In contrast, conditioned-place preference relies on a combination of operant and classical (Pavlovian) conditioning (Sanchis-Segura and Spanagel 2006; Huston et al. 2013). Presumably, genetic elements that modify positive and negative drug reinforcement and cause altered cocaine self-administration also lead to differences in conditioning of place preferences, mediated by shared mechanisms.
In the present study, cocaine produced complex effects on locomotor activity in LS and HS rats. Low-dose cocaine increased locomotor activity in LS rats, but not in HS animals, while high-dose cocaine augmented locomotor activity only in HS rats. Mice selected for increased methamphetamine-induced locomotor activity have lower oral intake of methamphetamine solutions (Kamens et al. 2005; Scibelli et al. 2011). Similarly, cocaine-injected outbred Sprague-Dawley rats exhibit a wide range of locomotor activity, which is not explained by cocaine pharmacokinetics but negatively correlated with the degree to which individual rats self-administer low-dose cocaine under a progressive-ratio schedule (Yamamoto et al. 2013). However, individual rats with an increased locomotor response to novelty self-administer greater amounts of intravenous amphetamine (Piazza et al. 1989) or cocaine (Piazza et al. 2000). We conclude that there is not a consistent relationship between drug-induced locomotor activity and stimulant reward, which varies across different animal models.
Previous studies have shown that chronic self-administration of cocaine acutely increases expression of c-Fos in the nucleus accumbens shell and core, with larger elevations in yoked animals that passively receive injections (Larson et al. 2010). This investigation also found that c-Fos immunoreactivity was inversely related to drug intake during cocaine self-administration sessions for both the NA shell and core. Our results show that cocaine-induced c-Fos immunoreactivity is greater in HS rats that passively receive cocaine, for both regions, at both of the doses of evaluated. Although seemingly at odds with findings of an inverse relationship with drug intake during cocaine self-administration, this may be based on the use of non-contingent injections for the conditioned-place preference procedure. Larson et al. (2010) did find a positive correlation for the amount of self-administered cocaine by individual rats and levels of c-Fos immunoreactivity in the caudate putamen, in parallel with our findings of greater c-Fos immunoreactivity in HS rats.
We found that chronic treatment with cocaine increased coexpression of c-Fos with D1 and D2 receptors in the nucleus accumbens shell and VTA of either LS or HS animals, with greater activation of both receptors in the HS strain. Although cocaine induces c-Fos expression in striatal medium spiny neurons predominantly through D1 receptors, combined c-Fos and D2 receptor expression occurs in other cells that include cholinergic interneurons (Bertran-Gonzalez et al. 2008). D2 receptor signaling in medium spiny neurons that make intrastriatal connections is required for full expression of cocaine-induced increases in locomotor activity (Kharkwal et al. 2016). Activation of the D2 receptor observed in the present study likely reflects these other cell types.
Genetic manipulations that activate striatal direct and indirect pathways can enhance or attenuate cocaine-induced conditioned-place preference (Lobo et al. 2010). Medium spiny neurons in these two pathways rely on D1 and D2 dopaminergic transmission, respectively. If similar genetic variants were to occur naturally, these could explain individual differences in susceptibility to substance abuse disorders. At least in the case of the LS and HS lines, we found no evidence to support this hypothesis. HS rats carrying a genetic predisposition for increased cocaine self-administration have greater cocaine-induced D2 immunoreactivity in the NA shell, caudate putamen, and dentate gyrus, opposite of its hypothesized inhibitory influence on drug reward.
An alternative explanation of increased dopamine D2 receptor immunoreactivity in HS rats is that it reflects an adaptation to diminished dopamine levels. Parkinson’s disease models have shown augmented D2 receptor binding after removal of dopaminergic projections to the striatum in rodents (Pellegrino et al. 2007) and non-human primates (Bezard et al. 2003). This has been interpreted as a functional hypersensitivity of dopamine receptors at postsynaptic terminals. In addition to lower levels of stimulant-induced dopamine release discussed earlier, reduced dopamine D2 receptor binding is an alternative biomarker for substance abuse disorders (Trifilieff and Martinez 2014; Bough et al. 2014). As is suggested by findings in Parkinson’s disease and HS rats, diminished dopamine release may be associated with heightened dopamine D2 receptor binding, causing these two attributes to functionally oppose each other. This could lead to dissimilar neurochemical mechanisms underlying enhanced motivation in different individuals who present with the same predisposition for increased cocaine use. The common thread may be lower dopamine transmission that motivates increased drug taking.
A recent review concluded that vulnerability to drug addiction involves an interaction between many brain systems (Ouzir and Errami 2016). Our initial evaluation of LS and HS rat lines echoes this conclusion, identifying differences between strains in neurotransmitter concentration, receptor activation, and pharmacokinetic measures. During selection, Wistar stock alleles that cause greater drug taking segregated to the HS line, while those with opposite effects were accumulated in the LS line. Further genetic characterization of these lines will likely identify the specific alleles that promote the observed behavioral responses and neurotransmitter differences, providing new insights into the pathways that promote drug use.
References
Avena NM, Rada PV (2012) Cholinergic modulation of food and drug satiety and withdrawal. Physiol Behav 106:332–336
Bell SM, Stewart RB, Thompson SC, Meisch RA (1997) Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology 131:1–8
Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ (2006) The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol 11:270–288
Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA (2008) Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 28:5671–5685
Bezard E, Gross CE, Brotchie JM (2003) Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends Neurosci 26:215–221
Bough KJ, Amur S, Lao G, Hemby SE, Tannu NS, Kampman KM, Schmitz JM, Martinez D, Merchant KM, Green C, Sharma J, Dougherty AH, Moeller FG (2014) Biomarkers for the development of new medications for cocaine dependence. Neuropsychopharmacology 39:202–219
Cabeza de Vaca S, Carr KD (1998) Food restriction enhances the central rewarding effect of abused drugs. J Neurosci 18:7502–7510
Collins RJ, Weeks JR, Cooper MM, Good PI, Russell RR (1984) Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology 82:6–13
Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for care and use of laboratory animals, Eighth edn. The National Academies Press, Washington, D.C.
Conrad KL, Louderback KM, Milano EJ, Winder DG (2013) Assessment of the impact of pattern of cocaine dosing schedule during conditioning and reconditioning on magnitude of cocaine CPP, extinction, and reinstatement. Psychopharmacology 227:109–116
Faraone SV, Adamson JJ, Wilens TE, Monuteaux MC, Biederman J (2008) Familial transmission of derived phenotypes for molecular genetic studies of substance use disorders. Drug Alcohol Depend 92:100–107
Gardoni F, Bellone C (2015) Modulation of the glutamatergic transmission by dopamine: a focus on Parkinson, Huntington and addiction diseases. Front Cell Neurosci 9:25
Gerrits MA, Petromilli P, Westenberg HG, Di Chiara G, Van Ree JM (2002) Decrease in basal dopamine levels in the nucleus accumbens shell during daily drug-seeking behaviour in rats. Brain Res 924:141–150
Grasing K (2016) A threshold model for opposing actions of acetylcholine on reward behavior: molecular mechanisms and implications for treatment of substance abuse disorders. Behav Brain Res 312:148–162
Haney M, Spealman R (2008) Controversies in translational research: drug self-administration. Psychopharmacology 199:403–419
He S, Yang Y, Mathur D, Grasing K (2008) Selective breeding for intravenous drug self-administration in rats: a pilot study. Behav Pharmacol 19:751–764
Huston JP, Silva MA, Topic B, Muller CP (2013) What’s conditioned in conditioned place preference? Trends Pharmacol Sci 34:162–166
Ikemoto S, Yang C, Tan A (2015) Basal ganglia circuit loops, dopamine and motivation: a review and enquiry. Behav Brain Res 290:17–31
Itzhak Y, Anderson KL (2012) Changes in the magnitude of drug-unconditioned stimulus during conditioning modulate cocaine-induced place preference in mice. Addict Biol 17:706–716
Jones JD, Comer SD (2013) A review of human drug self-administration procedures. Behav Pharmacol 24:384–395
Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ (2005) Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav 4:110–125
Kharkwal G, Radl D, Lewis R, Borrelli E (2016) Dopamine D2 receptors in striatal output neurons enable the psychomotor effects of cocaine. Proc Natl Acad Sci U S A 113:11609–11614
Larson EB, Akkentli F, Edwards S, Graham DL, Simmons DL, Alibhai IN, Nestler EJ, Self DW (2010) Striatal regulation of DeltaFosB, FosB, and cFos during cocaine self-administration and withdrawal. J Neurochem 115:112–122
Lester DB, Rogers TD, Blaha CD (2010) Acetylcholine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 16:137–162
Lobo MK, Covington HE III, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ (2010) Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330:385–390
Luedtke RR, Griffin SA, Conroy SS, Jin X, Pinto A, Sesack SR (1999) Immunoblot and immunohistochemical comparison of murine monoclonal antibodies specific for the rat D1a and D1b dopamine receptor subtypes. J Neuroimmunol 101:170–187
Mark GP, Hajnal A, Kinney AE, Keys AS (1999) Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology 143:47–53
Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M (2007) Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry 164:622–629
Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van HR, Kleber HD, Nunes E (2011) Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry 168:634–641
Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR (2005) Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology 30:1455–1463
McShane TM, Wise PM (1996) Life-long moderate caloric restriction prolongs reproductive life span in rats without interrupting estrous cyclicity: effects on the gonadotropin-releasing hormone/luteinizing hormone axis. Biol Reprod 54:70–75
Noori HR, Fliegel S, Brand I, Spanagel R (2012) The impact of acetylcholinesterase inhibitors on the extracellular acetylcholine concentrations in the adult rat brain: a meta-analysis. Synapse 66:893–901
Ouzir M, Errami M (2016) Etiological theories of addiction: a comprehensive update on neurobiological, genetic and behavioural vulnerability. Pharmacol Biochem Behav 148:59–68
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, Second edn. Academic Press, New York
Pellegrino D, Cicchetti F, Wang X, Zhu A, Yu M, Saint-Pierre M, Brownell AL (2007) Modulation of dopaminergic and glutamatergic brain function: PET studies on parkinsonian rats. J Nucl Med 48:1147–1153
Piazza PV, Dominiére JM, LeMoal M, Simon H (1989) Factors that effect individual vulnerability to amphetamine self-administration. Science 245:1511–1513
Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le MM (2000) Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci 20:4226–4232
Pontieri FE, Tanda G, Di Chiara G (1995) Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A 92:12304–12308
Rocha BA (2003) Stimulant and reinforcing effects of cocaine in monoamine transporter knockout mice. Eur J Pharmacol 479:107–115
Sanchis-Segura C, Spanagel R (2006) Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol 11:2–38
Sankoh AJ, Huque MF, Dubey SD (1997) Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med 16:2529–2542
Scibelli AC, McKinnon CS, Reed C, Burkhart-Kasch S, Li N, Baba H, Wheeler JM, Phillips TJ (2011) Selective breeding for magnitude of methamphetamine-induced sensitization alters methamphetamine consumption. Psychopharmacology 214:791–804
Sesack SR, Grace AA (2010) Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35:27–47
Trifilieff P, Martinez D (2014) Blunted dopamine release as a biomarker for vulnerability for substance use disorders. Biol Psychiatry 76:4–5
Volkow ND, Morales M (2015) The brain on drugs: from reward to addiction. Cell 162:712–725
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N (1997) Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386:830–833
Weiss JM, West CH, Emery MS, Bonsall RW, Moore JP, Boss-Williams KA (2008) Rats selectively-bred for behavior related to affective disorders: proclivity for intake of alcohol and drugs of abuse, and measures of brain monoamines. Biochem Pharmacol 75:134–159
Williams MJ, Adinoff B (2008) The role of acetylcholine in cocaine addiction. Neuropsychopharmacology 33:1779–1797
Wiltshire T, Ervin RB, Duan H, Bogue MA, Zamboni WC, Cook S, Chung W, Zou F, Tarantino LM (2015) Initial locomotor sensitivity to cocaine varies widely among inbred mouse strains. Genes Brain Behav 14:271–280
Yager LM, Garcia AF, Wunsch AM, Ferguson SM (2015) The ins and outs of the striatum: role in drug addiction. Neuroscience 301:529–541
Yamamoto DJ, Nelson AM, Mandt BH, Larson GA, Rorabaugh JM, Ng CM, Barcomb KM, Richards TL, Allen RM, Zahniser NR (2013) Rats classified as low or high cocaine locomotor responders: a unique model involving striatal dopamine transporters that predicts cocaine addiction-like behaviors. Neurosci Biobehav Rev 37:1738–1753
You ZB, Wang B, Zitzman D, Wise RA (2008) Acetylcholine release in the mesocorticolimbic dopamine system during cocaine seeking: conditioned and unconditioned contributions to reward and motivation. J Neurosci 28:9021–9029
Acknowledgements
This study is supported by grants R21-DA029787 and R21-DA037556 issued to KG from the National Institutes of Health, National Institute on Drug Abuse, and grant 589-KG-0012 from the Medical Research Service, Department of Veterans Affairs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xu, H., Das, S., Sturgill, M. et al. Extracellular dopamine, acetylcholine, and activation of dopamine D1 and D2 receptors after selective breeding for cocaine self-administration in rats. Psychopharmacology 234, 2475–2487 (2017). https://doi.org/10.1007/s00213-017-4640-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4640-7