Abstract
Rationale
Genetically determined differences in susceptibility to drug-induced sensitization could be related to risk for drug consumption.
Objectives
Studies were performed to determine whether selective breeding could be used to create lines of mice with different magnitudes of locomotor sensitization to methamphetamine (MA). MA sensitization (MASENS) lines were also examined for genetically correlated responses to MA.
Methods
Beginning with the F2 cross of C57BL/6J and DBA/2J strains, mice were tested for locomotor sensitization to repeated injections of 1 mg/kg MA and bred based on magnitude of sensitization. Five selected offspring generations were tested. All generations were also tested for MA consumption, and some were tested for dose-dependent locomotor-stimulant responses to MA, consumption of saccharin, quinine, and potassium chloride as a measure of taste sensitivity, and MA clearance after acute and repeated MA.
Results
Selective breeding resulted in creation of two lines [MA high sensitization (MAHSENS) and MA low sensitization (MALSENS)] that differed in magnitude of MA-induced sensitization. Initially, greater MA consumption in MAHSENS mice reversed over the course of selection so that MALSENS mice consumed more MA. MAHSENS mice exhibited greater sensitivity to the acute stimulant effects of MA, but there were no significant differences between the lines in MA clearance from blood.
Conclusions
Genetic factors influence magnitude of MA-induced locomotor sensitization and some of the genes involved in magnitude of this response also influence MA sensitivity and consumption. Genetic factors leading to greater MA-induced sensitization may serve a protective role against high levels of MA consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behavioral sensitization reflects underlying neural changes that occur as a result of repeated exposure to drugs of abuse. Sensitization can be remarkably persistent (Paulson et al. 1991; Lessov and Phillips 1998; De Vries et al. 1998; Boileau et al. 2006; Boehm et al. 2008). It is reflected in behaviors like increased stereotypy (Ellinwood and Balster 1974; Camp et al. 1994; Ferrario et al. 2005) and locomotor stimulation (Post and Rose 1976; Kuribara 1998; Meyer and Phillips 2007; Kelly et al. 2008), but can also be measured as an escalation in a neurochemical response (Pierce et al. 1996; Vezina 2004). Changes in physical attributes of neurons with repeated drug exposure have also been seen (Ferrario et al. 2005). Sensitization has been often studied in rodents, but also demonstrated in nonhuman primates and humans (Paulson et al. 1991; Phillips et al. 1998; Strakowski and Sax 1998; Boileau et al. 2006; Castner and Williams 2007; Leyton 2007). All reinforcing drugs have been demonstrated to induce sensitization, but this phenomenon has been most commonly studied for psychostimulants, such as methamphetamine (MA) and cocaine (Post and Rose 1976; Phillips et al. 1994, 1998; Janowsky et al. 2001; Kelly et al. 2008). Sensitization appears to be a significant factor in treatment resistance and thus relapse to drug use (Kalivas et al. 1998; Heilig et al. 2009; Post 2010).
There is overlap of some neural substrates that influence sensitivity to drug-induced locomotor activation/sensitization and sensitivity to reinforcing drug effects (Kazahaya et al. 1989; Benwell and Balfour 1992; Kalivas and Duffy 1993; Phillips and Shen 1996; Pierce and Kalivas 1997; Zapata et al. 2003; Vezina 2004). Facilitated acquisition and escalation of drug self-administration have been seen in sensitized animals (Deminiere et al. 1989; Mendrek et al. 1998; Vezina 2004; Ferrario and Robinson 2007). Sensitized animals show increased responding for drug-paired conditioned reinforcers (Di Ciano 2007) and nonpaired natural reinforcers (Mendez et al. 2009; Pastor et al. 2010), and enhanced preference for drug-paired stimuli (Lett 1989). Individual differences in the degree to which reward-related cues are imbued with salience are related to susceptibililty to locomotor sensitization to drugs (Flagel et al. 2008). However, research on specific genetic factors that influence psychostimulant-related behaviors like sensitization has mainly focused on single-gene effects related to neurobiological actions (for a comprehensive review, see Phillips et al. 2008). Data are lacking on genetic factors that may impact susceptibility to sensitization.
Described herein are the results of a bidirectional short-term selective breeding project for MA-induced locomotor sensitization. This selection compliments our previous selective breeding projects for acute locomotor response to MA (Kamens et al. 2005) and for MA consumption (Wheeler et al. 2009). One goal of this selection project was to determine whether selection for sensitization would reveal shared genetic influence with acute stimulation and MA consumption; that is, whether it would reveal that these traits are genetically correlated. Mice from the high acute MA sensitivity line displayed significantly greater sensitization to MA than did the low acute sensitivity line (Kamens et al. 2005). We therefore predicted that the MA high sensitization (MAHSENS) line would be more sensitive to the acute stimulant effects of MA, compared to the MA low sensitization (MALSENS) line. However, data for MA consumption in the acute MA sensitivity lines suggested that higher sensitivity to MA may be aversive (Kamens et al. 2005); thus, we predicted a negative genetic correlation between degree of sensitization to MA and consumption of MA. Based on the absence of differences in the consumption of distinctively flavored solutions between pairs of lines selected for high and low levels of MA consumption or selected for acute MA stimulation (Kamens et al. 2005; Wheeler et al. 2009), we predicted that the sensitization lines would not differ in the amounts of quinine-, saccharin-, or potassium chloride-flavored water they would consume. Finally, we did not expect to see differences between the lines in MA clearance, based on previous work showing a lack of genetic correlation between brain MA levels and MA-induced locomotor activation (Grisel et al. 1997).
Materials and methods
Animals
These experiments were performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals. All procedures were approved by the Portland Veterans Affairs Medical Center (PVAMC) Institutional Animal Care and Use Committee. Reciprocal F1 and then F2 crosses were generated between C57BL/6J (B6) and DBA/2J (D2) inbred strain mice that were obtained from The Jackson Laboratory West (Sacramento, CA). The B6D2F2 cross served as the founding population for selective breeding. This cross was chosen to complement other work utilizing the same cross (Kamens et al. 2005; Wheeler et al. 2009). Mice used for testing were weaned at 21 + 2 days into same-sex groups, 2–5 per cage (28.5 × 17.5 × 12 cm, lined with Bed-o’-cobs® bedding; Animal Specialties, Hubbard, OR), and maintained on a 12:12 h light:dark cycle, with lights on at 0600 h, and ambient temperature of 21 ± 2°C. Standard rodent diet food (Purina 5001™; Animal Specialties) and tap water were available ad libitum. All behavioral testing was conducted during the light phase between 0800 and 1600 h, except for the drinking studies, as detailed below.
Drugs
(+)-MA, saccharin sodium salt, quinine hemisulfate salt, and potassium chloride salt were obtained from Sigma (St. Louis, MO). For injections, MA was mixed in physiological saline (0.9% NaCl; Baxter Healthcare, Deerfield, IL) and delivered intraperitoneally (i.p.) in a volume of 10 ml/kg body weight. For drinking solutions, drugs and tastants were constituted in tap water to the concentrations given below.
Apparatus
Activity was measured in clear acrylic boxes (40 × 40 × 30 cm) placed inside of automated activity monitors encompassed by environmental control chambers designed to exclude external light and sound (Accuscan Instruments, Columbus, OH). Eight evenly spaced photocell beams and detectors, located 2 cm above the chamber floor, recorded beam breaks, which were translated into distance traveled (cm). Each chamber was equipped with a 3.3 W incandescent bulb to provide illumination, and a fan provided ventilation and additional noise masking.
Testing and selective breeding for MA-induced locomotor sensitization
B6D2F2 mice were tested for magnitude of locomotor sensitization to MA, using a 12-day procedure (Table 1) that has been previously used in our laboratory (Phillips et al. 1994; Kamens et al. 2005). On test days, mice were moved to the testing room 45–60 min prior to testing to permit acclimation, weighed, and then locomotor activity was monitored for 15 min in 5-min periods. Testing began immediately after i.p. injection. Day 1 saline data provided a measure of activity in a novel environment. Day 2 saline data provided a measure of baseline activity in the now familiar environment. On days 3, 5, 7, 9, and 11, response to 1 mg/kg MA was measured. This dose was chosen based on data that indicated it was not subject to ceiling effects in the measurement of sensitization (Kamens et al. 2005); with higher doses, acute stimulation can be profound so that behavioral sensitization may not be expressed due to a ceiling effect. The every other day MA treatment interval was chosen because others have shown a more intermittent treatment schedule to be more efficacious in producing sensitization than a daily schedule (e.g., Hirabayashi and Alam 1981). Mice were left undisturbed in the colony room on days 4, 6, 8, and 10. Selection for magnitude of sensitization was based on a difference score for each animal, created by subtracting the day 3 initial MA response from the day 11 final MA response (day 11–day 3). Day 12 saline data provided a measure of conditioned locomotion associated with prior repeated drug exposure in the test environment.
To establish the high (MAHSENS) and low (MALSENS) lines, the 26 highest and 26 lowest scoring mice (half of each sex) were selected for breeding of the S1 generation. Mass selection was used, consistent with other similar short-term selective breeding projects (Belknap et al. 1997; Kamens et al. 2005; Phillips et al. 2005; Wheeler et al. 2009); the mating of siblings or mice with common grandparents was avoided to reduce inbreeding. In each subsequent generation, the 13 male and 13 female mice, within each line, with the smallest (MALSENS) or largest (MAHSENS) day 11–day 3 sensitization scores were paired to perpetuate the lines. Acute sensitivity to MA was also examined by correcting the day 3 response to MA for saline baseline (day 3–day 2 score). A sixth generation of mice, S5G6 (where G stands for a nonselected generation), was produced by randomly breeding S5 animals within each selected line to generate additional offspring for the testing of additional traits.
Dose–response analysis of the acute locomotor response to MA
Experimentally naive male and female, second or later litter mice from the S5 generation were tested for their locomotor responses to several doses of MA. The testing procedure was identical to that used during the first 3 days of sensitization testing. The MA doses used were 1, 2, 4, and 8 mg/kg.
Two-bottle choice MA drinking
In each selection generation, experimentally naive male and female, second litter mice were tested in a two-bottle choice procedure for MA preference drinking. Testing procedures were identical to those used for the phenotyping of mice selectively bred for high and low levels of MA consumption (Wheeler et al. 2009). Mice were singly housed and then presented for 2 days with two, tap water-filled 25 ml graduated cylinders fitted with sipper tubes, to familiarize them with the drinking apparatus. Mice were then offered a choice between tap water and a water solution containing MA in increasing concentrations (20 mg/l MA during days 1–4, then 40 mg/l MA during days 5–8). Mice had 18-h access to the MA solution to prevent anorexia associated with MA consumption when available for 24 h (unpublished data). Food was distributed around both drinking tubes, and tube positions were alternated every 2 days to account for drinking tube location biases. Consumption was measured daily in ml (accuracy–0.2 ml) and converted to mg/kg based on body weight measured every 2 days. To account for possible leakage and evaporation, similarly filled tubes were placed on empty cages on the same racks, and the average volume lost from these tubes was subtracted each day from the individual drinking values. The second and fourth day mg/kg/18 h consumption values were averaged for each drug concentration, and used as the individual consumption values. This method provides an adequate period for the animals to identify the new location of the MA-containing solution and to stabilize their intake. Preference ratios (ml MA solution consumed/total ml consumed from both drinking tubes) were also determined.
Tastant consumption and preference
Following testing for MA consumption in the S2 generation, animals were returned to plain tap water for a 4-day period and were then tested for tastant preference. Experimentally naive S5G6 mice were also tested for tastant preference. The tastants were bitter quinine (0.015 followed by 0.03 mM), which is likely the most similar in taste to MA, sweet saccharin (0.033 followed by 0.066%), and salty potassium chloride (100 followed by 200 mM). Each tastant was offered for 8 days, with 4-day access to the lower concentration followed by 4-day access to the higher concentration. The order of tastant presentation was counterbalanced to control for order effects. All other methods and dependent measures were the same as for measurement of MA consumption and preference.
MA clearance
Experimentally naive male and female, second or later litter mice from the S5 generation were examined for rate of MA clearance from blood. Mice were treated five times, once every other day, with 1 mg/kg MA, using the same treatment schedule used for sensitization testing. They were returned to their home cages after MA injection, rather than being tested for locomotor behavior. Blood samples were taken after the first and fifth MA injections to assess possible differences in MA clearance rate after single and repeated treatments. Mice were placed in plastic restrainers, a small segment of tail was removed with scissors, and blood samples (80 μl) were taken from the lateral tail vein at six time points (7.5, 15, 30, 60, 120, and 180 min following MA injection), using heparinized microcapillary tubes. Blood was placed in microcentrifuge tubes on ice. Each animal contributed only two of the six possible blood samples to conform with guidelines requiring that no more than 1% of a mouse’s body weight in blood be removed in a single 24-h period. Individual mice contributed samples at 7.5 and 60 min, at 15 and 120 min, or at 30 and 180 min. Blood samples were diluted 1:5 with enzyme immunoassay buffer and processed according to manufacturer’s instructions (Neogen, Lexington, KY), using a MA enzyme-linked immunosorbent assay (ELISA). The lower limit of detection was 9.5 ng MA/ml. Each sample was analyzed in duplicate, and MA concentrations were interpolated from a standard curve constructed from samples ranging from 10 to 100 ng MA/ml. To perform time-dependent analyses, it was necessary to consider the samples as independent, since samples at all time points were not obtained from each animal.
Analyses
Data were analyzed by factorial ANOVA, with repeated measures when appropriate, using Statistica software (StatSoft Version 6.1, Tulsa, OK). Complex multiple-factor interactions were deconstructed to two-way interactions, and two-way interactions were considered using simple effect analysis and Newman–Keuls post hoc mean comparisons. In selected lines, when a line difference for a trait other than the selection trait is found, this trait is said to be genetically correlated with the selection trait. This means that some of the same genes influence the magnitude of both the selection and the other measured trait (Crabbe et al. 1990). Realized heritability (h 2) of the bidirectional selection trait was calculated with MathCad 8 Professional software (MathSoft, Cambridge, MA), using methods consistent with Falconer and Mackay (1996). Because h 2 calculated from all selection generations may not reflect h 2 in the base population, particularly when there is an immediate response to selection that does not change or changes little with additional selection pressure, h 2 was calculated for both the first selection generation and from data for all generations. Results are expressed as mean ± SEM. Figures were created using SigmaPlot 2002 for Windows, Version 8.0 (SPSS, Chicago, IL).
Results
Response to selection for sensitivity to MA-induced sensitization
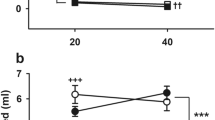
Figure 1 shows selection response data and mean values for the parents selected to generate offspring in each generation. Across all generations, MAHSENS mice displayed 43–78% greater sensitization than MALSENS mice. Because data for each generation were generated in separate experiments, factorial ANOVAs were performed separately for each generation. In all generations, there was a main effect of line (see Fig. 1). In the S3 through S5 generations, there were also significant interactions of line and sex; S3: [F(1,62) = 4.6, p < 0.05]; S4: [F(1,124) = 13.2, p < 0.001]; S5: [F(1,113) = 4.2, p < 0.05], which were associated with larger differences in the magnitude of sensitization between male MALSENS and MAHSENS mice in the S3 generation, but between female MALSENS and MAHSENS mice in the S4 and S5 generations. Thus, the impact of sex was unsystematic across generations.
Locomotor sensitization to MA is a heritable trait. Shown are the sensitization score (day 11–day 3) means (±SEM) for the F2 population (n = 57–60 per sex; total N = 117; age = 64 ± 1 days), for the parents (n = 13 per sex and line) selected to produce offspring for each selection generation and for the offspring of each generation (n = 66–128 mice per generation; age = 63 ± 1 days old on the first day of testing). The dose of MA administered on all test days was 1 mg/kg. *Main effect of line in S1 [F(1,100) = 6.1, p < 0.02]; S2 [F(1,65) = 12.9, p < 0.001], S3 [F(1,62) = 15.2, p < 0.001], S4 [F(1,124) = 8.6, p < 0.01], and S5 [F(1,113) = 11.9, p < 0.001]
Magnitude of sensitization was altered by selection in only the downward direction. Two-way ANOVAs (generation × sex) were performed that included sensitization score data from the F2 and MALSENS line or F2 and MAHSENS line. For the F2 comparison with MALSENS, a main effect of generation [F(5,364) = 6.4, p < 0.001] was associated with a significantly larger sensitization score in F2, compared to MALSENS mice in all selection generations; there was no interaction with sex. There were no differences between pairs of MALSENS S1 through S5 means, indicating that all response to selection occurred in the first selection generation. There were no differences in mean response between F2 and MAHSENS mice or between means of any MAHSENS generations.
A statistic that indicates the amount of variance accounted for by genetic factors, h 2, was calculated from the slope of the regression of R (selection response based on offspring means) on cumulative S (selection differential based on population and selected parent means). h 2 after the first generation of selection was 0.17, and after five generations was 0.08. This reduction can be explained by the fact that virtually all of the response to selection occurred in the first generation of selection.
Sensitivity to the acute stimulant effects of MA during the course of selection
Mean values for the acute stimulant score (day 3–day 2) for the F2 population and each generation of MA sensitization (MASENS) line mice are shown in Fig. 2. Factorial ANOVAs were performed with data grouped on line and sex for each generation. There were statistical trends or significant differences between the lines in all generations. MAHSENS mice were more stimulated, compared to MALSENS mice. The parent means are shown in the inset of the figure. Note that the MAHSENS and MALSENS parents chosen for their high or low sensitization scores (see Fig. 1) did not differ in most generations with regard to their acute stimulant scores. Therefore, the difference in sensitivity of the MASENS lines to the acute stimulant effects of MA cannot be explained by differential sensitivity of their parents to the acute stimulant effects of MA. However, the magnitude of the difference between the MAHSENS and MALSENS offspring was small, not reaching typically accepted levels of statistical significance in some generations. There were no significant main effects or interactions with sex on magnitude of acute stimulant response for any generation.
Acute locomotor stimulation to MA is genetically correlated with magnitude of MA-induced sensitization. Main figure: Shown are the acute stimulant score (day 3–day 2) means (±SEM) for the same F2 population and selected line offspring whose sensitization scores are shown in Fig. 1. The dose of MA given on day 3 was 1 mg/kg. †Statistical trend for a main effect of line in S1 [F(1,100) = 2.8, p = 0.09], S2 [F(1,65) = 3.0, p = 0.09], and S5 [F(1,113) = 2.9, p = 0.09]. *Main effect of line in S3 [F(1,62) = 5.5, p < 0.05] and S4 [F(1,124) = 5.2, p < 0.05]. Inset: Mean (±SEM) acute stimulant scores for the parents shown in Fig. 1
Baseline activity and conditioned activation
Data from saline test days 2 and 12 were analyzed to determine whether selection for magnitude of sensitization had an impact on basal activity levels (day 1) or on conditioned activation (day 12). A repeated measures ANOVA (grouped on line and sex), with saline test day as the repeated measure, was performed for each selection generation. Sex, genotype and day interacted significantly in only the S4 generation (F(1,1240) = 5.2, p < 0.05). Because there were no sex or sex interaction effects for any other generation, we summarized the data and statistical results in Fig. 3 for the sexes combined. In the S1–S3 generations, there were no line differences or sex effects, but a main effect of day reflected heightened activity levels on day 12 in all three generations (S1: F(1,100) = 119.8, p < 0.001; S2: F(1,65) = 37.1, p < 0.001; S3: F(1,62) = 82.6, p < 0.001). S4 MALSENS mice of both sexes displayed significantly higher levels of locomotor activity on day 2, compared to MAHSENS mice; however, only S4 male MALSENS mice were more active than male MAHSENS mice on day 12. In S5, this sex difference was absent, and MALSENS mice also exhibited higher levels of activity on both days 2 and 12, compared to MAHSENS mice. Evidence for conditioned activation was present in both lines of these generations. These results suggest that selection for magnitude of MA-induced sensitization also altered basal activity levels.
Activity levels on saline treatment days of MAHSENS and MALSENS mice across selection generations. Shown is mean (±SEM) distance traveled in cm for the same F2 population and selected line offspring whose sensitization and acute MA scores are shown in Figs. 1 and 2. * p < 0.05 for the comparison of MAHSENS and MALSENS mice of that selection generation
Dose–response analysis of the acute locomotor response to MA
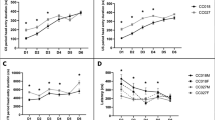
MAHSENS mice exhibited significantly larger stimulant responses than MALSENS mice to some doses of MA. Data for the acute stimulant response (day 3–day 2) were analyzed by factorial ANOVA (line × dose × sex), and a significant three-way interaction was found [F(4,178) = 3.8, p < 0.01]. The line by dose interaction was significant for both females [F(4,96) = 3.7, p < 0.01] and males [F(4,82) = 13.7, p < 0.001]. The source of the sex interaction was the large difference in response between male and female MAHSENS mice after treatment with the highest dose of MA (see Fig. 4). The lines did not differ in amount of change in activity after saline treatment from day 2 to day 3 (0 mg/kg data), and did not differ significantly in response to the 1 mg/kg dose of MA. Significant mean differences are indicated by symbols in Fig. 4.
MAHSENS mice exhibit greater sensitivity to the acute stimulant effects of MA compared to MALSENS mice. Shown are mean (±SEM) acute stimulant scores for S5 MAHSENS and MALSENS mice (n = 7–12 per selected line, sex, and dose; total N = 198; age = 84 ± 1 days) that were tested after saline on 2 consecutive days and then after saline or several doses of MA on day 3. * p < 0.05 for the comparison of MAHSENS and MALSENS mice of the same sex. ** p < 0.001 for the comparison of MAHSENS and MALSENS mice of the same sex. a p < 0.05 for the comparison of the effect of that MA dose to the saline group (0 mg/kg) of the same line and sex
MA consumption and preference
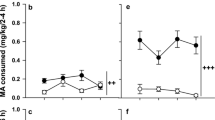
MA and water drinking data are shown in Fig. 5. Initially greater MA consumption and preference in S1 MAHSENS mice compared to S1 MALSENS mice reversed across generations. In every generation, there was an effect of concentration, such that higher amounts of MA were consumed when MA was offered in a higher concentration (all p < 0.02), although the line difference was largely independent of concentration. Data were analyzed separately for each generation by repeated measures ANOVA, with concentration of MA as the repeated measure. S1 generation MAHSENS mice consumed more MA (Fig. 5a, b), compared to MALSENS mice [F(1,31) = 5.0, p < 0.05]; there were no significant effects involving sex. Further examination of a nearly significant interaction of line and concentration [F(1,31) = 3.5, p = 0.07], indicated that the line difference was larger for consumption of the 40 mg/l MA concentration. S2 MALSENS mice consumed more of both concentrations of MA, compared to MAHSENS mice [F(1,38) = 4.3, p < 0.05 for the line by concentration interaction]. Examination of a significant line by sex interaction [F(1,38) = 4.8, p < 0.05] indicated that the line difference was larger in male than in female mice (data not shown). Although there was no significant difference in MA consumption between the lines in the S3 generation, in both the S4 and S5 generations, MALSENS mice consumed significantly more MA than MAHSENS mice [S4: F(1,37) = 5.7, p < 0.05; S5: F(1,41) = 5.2, p < 0.05 for the main effect of line]. There were no significant interactions with sex or concentration, but, for consistency with analyses for other generations, we examined the line difference at each concentration and found higher MA consumption by MALSENS mice at both concentrations.
Line differences in the amount of MA consumed reverse over the course of selection for magnitude of MA-induced sensitization. Shown are mean (±SEM) MA consumption (a, b) and preference (c, d) values for a 20 and 40 mg/l solution of MA offered vs. tap water for MAHSENS and MALSENS mice tested in each selection generation (n = 8–12 per line and sex in each generation; 75 ± 1 days old). Panels e and f show total volume of fluid consumed from both the MA- and water-containing tubes during the 18-h period when the 20 and 40 mg/l MA solutions were offered, respectively. Mice had ad libitum access to water during the remaining 6-h period. * p < 0.05 for the main effect of line
Preference ratio data indicated that pure water was generally preferred over solutions containing MA, as can be seen in Fig. 5c, d; a preference ratio of 0.5 would indicate that mice were consuming equal amounts from the two bottles, whereas a preference ratio below 0.5 indicates that more fluid was consumed from the water bottle. The line differences in magnitude of the preference ratio largely mimicked the results for mg/kg MA consumption. In the S1 generation, MAHSENS mice exhibited a larger preference ratio compared to MALSENS mice [F(1,31) = 4.4, p < 0.05]. In the S2 generation, the line difference reversed [F(1,38) = 7.0, p < 0.02], and a significant line by sex interaction [F(1,38) = 4.7, p < 0.05] again indicated a larger line difference for male mice (data not shown). There was no significant line difference for preference ratio in the S3 generation, but S4 and S5 generation MALSENS mice had larger preference ratios compared to MAHSENS mice [S4: F(1,37) = 7.3, p < 0.02; S5: F(1,41) = 6.2, p < 0.02], and there were no interactions with sex.
There were no differences between the lines in volume of water consumed on the 2 days prior to MA presentation in any selection generation (data not shown). A line difference in total fluid consumed during measurement of MA consumption (Fig. 5e, f) was found only in the S2 generation [F(1,38) = 9.6, p < 0.01]; S2 generation MALSENS mice consumed more total fluid, compared to MAHSENS mice.
Tastant consumption and preference
Tastant consumption (Fig. 6) and preference were measured to inform interpretation of the MA drinking results. Repeated measures ANOVA (line × sex × concentration as the repeated measure) was used to analyze data for each tastant, within each generation. For generation S2, MALSENS mice consumed more [F(1,38) = 8.2, p < 0.01] and showed a greater preference for [F(1,38) = 10.8, p < 0.01; data not shown] saccharin than MAHSENS mice. Females consumed more saccharin than males [F(1,38) = 15.6, p < 0.001], but there was no interaction of sex with line. S2 MAHSENS mice consumed more quinine than MALSENS mice [F(1,38) = 11.2, p < 0.01], and showed a greater preference [F(1,38) = 17.2, p < 0.001; data not shown]. There were no significant effects of sex. For potassium chloride in S2 mice, there were no significant line or sex effects. For generation S5G6, a sex-specific line difference, opposite to that found in S2, for saccharin consumption and preference was found. Examination of the significant line by sex interaction [F(1,48) = 11.2, p < 0.01) revealed greater saccharin consumption only in female MAHSENS, compared to female MALSENS, mice, as shown in Fig. 6b. A similar interaction [F(1,48) = 9.7, p < 0.01] and difference only in female mice was found for saccharin preference (data not shown). There were no significant line differences in S5G6 mice for quinine or potassium chloride consumption or preference.
Consumption of novel tastants in S2 and S5G6 MASENS mice. Shown are mean (±SEM) saccharin (a, b), quinine (c, d), and potassium chloride (e, f) consumed in generation S2 (n = 10–11 per line and sex) and S5G6 (n = 7–18 per line and sex) MALSENS and MAHSENS mice, when each tastant solution was offered vs. tap water. ** p < 0.01 for the main effect of line in panels a and c and for the effect of line within females only in panel b
MA clearance
Results are presented in Table 2. First and fifth treatment data were included in a repeated measures ANOVA, with line and time as grouping factors; sex was excluded as we were not able to include an adequate sample size of each sex (4–5 per sex, line and time period), and selection experiment results indicated line differences in magnitude of sensitization for both sexes. Data from two of the 60 animals were lost for one of the days, due to incomplete MA injections. The ANOVA for ng/ml blood MA identified a main effect of time [F(5,104) = 82.6, p < 0.001]. Whether the injection was the first or fifth did not impact blood MA level at the various time points, and the lines did not differ significantly. Although there was a statistical trend for a day by line interaction [F(1,104) = 3.7, p = 0.06], there was no three-way interaction with time, indicating that the lines were similar with regard to MA clearance rate.
Discussion
Bidirectional selective breeding for high and low sensitivity to the locomotor sensitizing effects of MA resulted in divergence in magnitude of sensitization. However, selection was successful only in the direction of a reduction in mean level of sensitization. The difference between the selected lines, as well as heritability calculations, indicated that the majority of selection response occurred in the first selection generation. Despite modest heritability, several genetically correlated responses were identified and conformed to predictions based on previous literature. MAHSENS mice exhibited greater sensitivity to the acute stimulant effects of MA and consumed less MA than MALSENS mice at the end of selection. The lines exhibited differences in consumption of saccharin and quinine in an early selection generation, but only a sex-specific difference in saccharin consumption at the end of selection, and were similar in their rate of MA clearance from blood.
We are aware of no reported selection projects for magnitude of drug-induced behavioral sensitization. Studies using a panel of recombinant inbred strains suggested that the genetic component contributing to individual variation in magnitude of drug-induced sensitization is relatively low (Phillips et al. 1995, 1998), compared to other drug phenotypes, such as ethanol drinking (Belknap et al. 1997; Phillips et al. 2005) and acute sensitivity to MA, cocaine and ethanol (Phillips et al. 1995; 1998; Grisel et al. 1997; Kamens et al. 2005). The current data support this. In the presence of varying amounts of selection pressure, as indicated by mean values of parents chosen to contribute offspring each generation, there was little variation in the magnitude of the line difference across the course of selection. Even with added selection pressure in the S5 generation, the difference in mean value between the MALSENS and MAHSENS offspring remained about the same.
Advantages of short-term selected lines are that they can be rapidly produced and replicated in series. This provides an opportunity to confirm significant findings from an initial set of lines, or to extend initial findings in studies designed to examine mechanism. For example, initial quantitative trait locus (QTL) mapping results from a set of short-term selected lines for ethanol preference drinking (Belknap et al. 1997), were subsequently followed in a second set of similar lines by studies of genetically correlated responses to selection (Metten et al. 1998; Phillips et al. 2005). A negative genetic correlation between ethanol consumption and withdrawal severity found in these lines was followed up at the level of QTL mapping, and QTLs were identified that appear to influence the inverse genetic relationship; nitric oxide signaling was proposed as one mechanism involved (Hitzemann et al. 2009). Using genetic samples from MASENS mice, work is underway in our lab, directed at identifying the alleles that differ in frequency and the genes that differ in expression, to develop hypotheses about genetic mechanisms underlying the difference in sensitization and in genetically correlated responses. This information could be carried forward to a set of replicate lines to explore mechanistic hypotheses based on genetic results. We have recently taken this approach to examine genetic factors that influence MA consumption (Wheeler et al. 2009).
We have not yet produced a replicate set of sensitization lines; however, genetic relationships between traits studied in other selected lines provide a means for verification of some of the genetic correlations reported here. We previously found that mice bred for high vs. low sensitivity to the acute stimulant effects of MA also differed in magnitude of MA-induced sensitization and in amount of MA consumed (Kamens et al. 2005); the low acute stimulation line exhibited reduced sensitization and consumed more MA, compared to the high acute stimulation line. The same relationship was found in the MASENS lines; MAHSENS mice exhibited greater sensitivity to the stimulant effects of MA and consumed less MA, compared to MALSENS mice, at the end of selection. We previously speculated that genes that confer high sensitivity to stimulation and sensitization may protect against higher levels of MA consumption (Kamens et al. 2005). We have not studied the mechanisms underlying this relationship, but it is possible that there is a certain range of sensitivity to the stimulant/sensitizing effects of MA that is rewarding, and that higher levels are aversive.
We measured MA consumption and preference in every selected generation of the MASENS lines. In S1, MAHSENS mice consumed more MA than MALSENS mice, but this line difference reversed in subsequent generations. However, there was not increasing divergence between the lines in magnitude of the measured sensitization trait. It is possible that selection resulted in changes in allele frequencies that were not revealed in changes in sensitization, but were responsible for the line reversal in MA consumption and preference. Sensitization studies underway in lines of mice selected for high and low MA drinking (Wheeler et al. 2009) may help to clarify the genetic relationship between these traits. Differences in taste sensitivity do not appear to account for differences in MA consumption and preference either in the MASENS lines or in the MA drinking lines (Wheeler et al. 2009). Chance fixation of selection trait-irrelevant alleles is always a possibility in selected line results; however, it is difficult to explain the reversal in the line difference for MA consumption using this explanation. In addition, we do not yet know what blood or brain MA levels are achieved with this drinking model, something we are currently working toward evaluating by first examining patterns of drinking to determine the best time for blood sampling. The i.p. injections used for the measurement of sensitization, likely resulted in faster absorption and higher peak brain MA levels, compared to drinking. How such pharmacodynamic and pharmacokinetic factors may be involved in differences for MA-related traits between the selected lines is currently unknown.
It has been suggested that relative insensitivity to bitter tastes might reduce oral-sensory hindrances for alcohol consumption (Duffy et al. 2004). The bitter taste qualities of MA (http://www.nida.nih.gov/Infofacts/methamphetamine.html) could influence MA intake, independently of sensitivity to the rewarding effects of MA. Quinine is most relevant for determining whether differences in preference/aversion for bitter taste might have been responsible for the difference in MA consumption seen between the MALSENS and MAHSENS lines. Although we did find a line difference in quinine consumption in the S2 generation, the line that consumed more MA consumed less quinine (opposite to expectation) and there was no line difference in S5G6 mice. Furthermore, quinine avoidance did not correspond with differences in MA consumption in lines selectively bred for high and low MA drinking, whereas differences in sensitivity to the positive and negative motivational effects of MA did (Wheeler et al. 2009). Therefore, we do not think that individual differences in aversion to the taste of MA provide a likely explanation for differences in MA intake in the MASENS lines.
MAHSENS mice exhibited heightened acute activation to MA at some doses. However, at the 8 mg/kg dose, male MAHSENS mice were less activated than male MALSENS mice. It is possible that MAHSENS mice are more sensitive to the stereotypic effects of MA, which begin to occur in mice at about this dose, and that stereotypy competed with locomotor stimulation (Atkins et al. 2001). When a similar result was found in lines selected for high and low acute MA activation, we examined locomotor behavior during the first 5 min after MA treatment, a time when stereotypic effects were less likely to have developed prior to full absorption of the drug dose. The high acute stimulation line mice were more activated than the low acute stimulation line mice (Kamens et al. 2005). We examined the current data for the first 5-min period, and obtained a similar outcome; the MAHSENS mice were twice as stimulated by the 8 mg/kg dose of MA during this time period, compared to the MALSENS mice, regardless of sex (mean ± SEM = 1,043 ± 221 and 635 ± 256 cm for MALSENS female and male mice, respectively; mean ± SEM = 2,207 ± 231 and 1,313 ± 256 cm for MAHSENS female and male mice, respectively).
Basal activity levels were examined for correspondence with MA-induced sensitization and for conditioned activation. Day 12 activity levels were higher than levels on day 2, suggesting that prior drug exposure in the test environment may have led to conditioned activation. Over the course of selection, a line difference in activity level on saline treatment days emerged between the lines, with MALSENS mice exhibiting higher activity than MAHSENS mice. However, as shown in Fig. 1, the size of the difference in magnitude of sensitization between the lines remained relatively consistent across generations, suggesting little impact of the change in basal activity level on this measure. In our prior selection for acute response to MA, a similar result was found; lower activity levels after saline were found in the line selected for higher sensitivity to the acute stimulant effect of MA (Kamens et al. 2005). However, in lines selected for cocaine-induced stimulation, the opposite relationship was found (Marley et al. 1998). When the genetic relationship between acute stimulation and sensitization was studied using recombinant inbred strains for ethanol and for cocaine, no relationships were found (Shuster et al. 1977; Phillips et al. 1995, 1998). Furthermore, in D2 dopamine receptor knockout mice, which have markedly reduced basal activity levels, both MA- and cocaine-induced stimulation were also reduced (Welter et al. 2007; Kelly et al. 2008). The D2 receptor mutant mice also exhibited more sustained sensitization to MA (Kelly et al. 2008). Thus, the relationship of basal activity levels with acute drug sensitivity and sensitization is not straightforward.
We have now identified an inverse genetic relationship between locomotor response to MA (acute activation and sensitization) and MA consumption in two selected line models. Expression analyses in the MA acute activation lines identified differentially expressed genes that could contribute to differences in all three traits (Palmer et al. 2005). Differences in the expression of casein kinase 1 epsilon (Csnkle), GABA B1 receptor (Gabbr1), and dopamine- and cAMP-regulated phosphoprotein of 32 kDa (Darpp-32) are compelling, because they can be integrated into a signaling pathway that is implicated in the modulation of locomotor response to MA (Palmer et al. 2005). In a translational study, a relationship was identified in humans between CSNK1E and sensitivity to the stimulant effects of amphetamine (Veenstra-VanderWeele et al. 2006). In addition, among the many sequences and genes represented on the Affymetrix gene array that were differentially expressed in the high and low MA sensitivity lines was the glutamate receptor, ionotropic, AMPA1 (GluR1). These expression differences were found using nucleus accumbens tissue samples from drug-naive mice of the high and low MA sensitivity lines. However, the difference in GluR1 expression is compelling because a role for this receptor subtype has been considered in the actions of psychostimulant drugs. For example, cocaine has been shown to increase the expression of GluR1 protein levels in the dopamine-rich ventral tegmental area (VTA; Fitzgerald et al. 1996), an effect that may be dependent upon the time that tissue is taken after repeated cocaine treatment (Lu et al. 2002). Recently, cocaine was found to alter the subcellular distribution of GluR1 subunit protein in VTA neurons (Lane et al. 2010). However, there is some question as to whether MA would have a similar effect, since no relationship was found between behavioral sensitization to amphetamine and changes in GluR1 surface expression in the nucleus accumbens of the rat (Nelson et al. 2009). However, data from the acute MA sensitivity lines suggest that a difference in GluR1 expression is related to genetic susceptibility to stimulation by MA, which is different from the question of how MA may affect glutamate systems. A role for preexisting differences in GluR1 distribution remains an interesting question with regard to genetic susceptibility to the stimulant and sensitizing effects of MA and level of MA consumption.
Expression data from MA drinking line mice are relevant. Nucleus accumbens tissue from drug-naive or MA-treated mice was examined using a qPCR array on which genes were represented that are relevant to addiction-related processes (Wheeler et al. 2009). In drug naive mice, the serotonin (Slc6a4 or Sert) and noradrenaline (Slc6a2 or Net) transporter genes, as well as the serotonin 3a receptor gene (Htr3a), were differentially expressed. The metabotropic 4 glutamate receptor gene was also differentially expressed (Grm4). Examination of differentially expressed genes between the high and low MA drinking line mice after MA treatment suggested the involvement of signaling pathways that include MAP kinase signaling, cFos, and nuclear factor κB (NfκB). The qPCR array had only 384 genes represented, so this analysis provided a much smaller snapshot of differential gene regulation compared to Affymetrix arrays such as those used by Palmer et al. (2005). We are currently evaluating expression differences in the drinking line mice for several brain regions using Affymetrix arrays and will be examining the same tissues in the MASENS lines. These data will allow us to identify gene expression pathway differences that may be shared in common and thus have a role in MA drinking and sensitization, as well as acute sensitivity to MA.
Heightened alcohol-induced locomotor stimulation and sensitization were associated with reduced alcohol consumption in some genetic mouse models (Phillips et al. 1995, 2005), similar to the results obtained here for MA. Furthermore, lines of mice selectively bred for high sensitivity to acute MA-induced stimulation were also less stimulated by alcohol, but consumed more alcohol (Kamens et al. 2006), supporting a negative genetic relationship between sensitivity to drug stimulation and drug intake. Relationships between sensitivity to the intoxicating and stimulant effects of alcohol and risk for high alcohol intake or the development of an alcohol use disorder have also been suggested by the results of multiple human studies (Schuckit 1994; Holdstock et al. 2000; King et al. 2002; Hinckers et al. 2006). However, we are not aware of investigations in human populations to ascertain whether sensitivity to the psychomotor stimulant effects of MA might predict risk for addiction. Our data suggest that there is a genetic relationship between these factors that could have predictive value and should be further explored.
References
Atkins AL, Helms ML, O’Toole LA, Belknap JK (2001) Stereotypic behaviors in mice selectively bred for high and low methamphetamine-induced stereotypic chewing. Psychopharmacology (Berl) 157:96–104
Belknap JK, Richards SP, O’Toole LA, Helms ML, Phillips TJ (1997) Short-term selective breeding as a tool for QTL mapping: ethanol preference drinking in mice. Behav Genet 27:55–66
Benwell ME, Balfour DJ (1992) The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol 105:849–856
Boehm SL II, Goldfarb KJ, Serio KM, Moore EM, Linsenbardt DN (2008) Does context influence the duration of locomotor sensitization to ethanol in female DBA/2 J mice? Psychopharmacology (Berl) 197:191–201
Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C (2006) Modeling sensitization to stimulants in humans: an [11 C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry 63:1386–1395
Camp DM, Browman KE, Robinson TE (1994) The effects of methamphetamine and cocaine on motor behavior and extracellular dopamine in the ventral striatum of Lewis versus Fischer 344 rats. Brain Res 668:180–193
Castner SA, Williams GV (2007) From vice to virtue: insights from sensitization in the nonhuman primate. Prog Neuropsychopharmacol Biol Psychiatry 31:1572–1592
Crabbe JC, Phillips TJ, Kosobud A, Belknap JK (1990) Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res 14:141–151
Deminiere JM, Piazza PV, Le Moal M, Simon H (1989) Experimental approach to individual vulnerability to psychostimulant addiction. Neurosci Biobehav Rev 13:141–147
De Vries TJ, Schoffelmeer ANM, Binnekade R, Mulder AH, Vanderschuren LJMJ (1998) Drug-induced reinstatement of heroin- and cocaine-seeking behavior following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci 10:3565–3571
Di Ciano P (2007) Facilitated acquisition but not persistence of responding for a cocaine-paired conditioned reinforcer following sensitization with cocaine. Neuropsychopharmacology 33:1426–1431
Duffy VB, Peterson JM, Bartoshuk LM (2004) Associations between taste genetics, oral sensation and alcohol intake. Physiol Behav 82:435–445
Ellinwood EH, Balster RL (1974) Rating the behavioral effects of amphetamine. Eur J Pharmacol 28:35–41
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Longman, Harlow
Ferrario CR, Robinson TE (2007) Amphetamine pretreatment accelerates the subsequent escalation of cocaine self-administration behavior. Eur Neuropsychopharmacol 17:352–357
Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE (2005) Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry 58:751–759
Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ (1996) Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci 16:274–282
Flagel SB, Watson SJ, Akil H, Robinson TE (2008) Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res 186:48–56
Grisel JE, Belknap JK, O’Toole LA, Helms ML, Wenger CD, Crabbe JC (1997) Quantitative trait loci affecting methamphetamine responses in BXD recombinant inbred mouse strains. J Neurosci 17:745–754
Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, Hommer D, Barr CS (2009) Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neurosci Biobehav Rev. doi:10.1016/j.neubiorev.2009.11.018
Hinckers AS, Laucht M, Schmidt MH, Mann KF, Schumann G, Schuckit MA, Heinz A (2006) Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biol Psychiatry 60:282–287
Hirabayashi M, Alam MR (1981) Enhancing effect of methamphetamine on ambulatory activity produced by repeated administration in mice. Pharmacol Biochem Behav 15:925–932
Hitzemann R, Edmunds S, Wu W, Malmanger B, Walter N, Belknap J, Darakjian P, McWeeney S (2009) Detection of reciprocal quantitative trait loci for acute ethanol withdrawal and ethanol consumption in heterogeneous stock mice. Psychopharmacology (Berl) 203:713–772
Holdstock L, King AC, de Wit H (2000) Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res 24:789–794
Janowsky A, Mah C, Johnson RA, Cunningham CL, Phillips TJ, Eshleman CJC, AJ BJK (2001) Mapping genes that regulate density of dopamine transporters and correlated behaviors in recombinant inbred mice. J Pharmacol Exp Ther 298:634–643
Kalivas PW, Duffy P (1993) Time course of extracellular dopamine and behavioral sensitization to cocaine: I. Dopamine axon terminals. J Neurosci 13:266–275
Kalivas PW, Pierce RC, Cornish J, Sorg BA (1998) A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol 12:49–53
Kamens HM, Burkhart-Kasch S, McKinnon CS, Reed C, Phillips TJ (2005) Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav 4:110–125
Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ (2006) Ethanol-related traits in mice selectively bred for differential sensitivity to methamphetamine-induced activation. Behav Neurosci 120:1356–1366
Kazahaya Y, Akimoto K, Otsuki S (1989) Subchronic methamphetamine treatment enhances methamphetamine- or cocaine-induced dopamine efflux in vivo. Biol Psychatry 25:903–912
Kelly MA, Low MJ, Rubinstein M, Phillips TJ (2008) Role of dopamine D1-like receptors in methamphetamine locomotor responses of D2 receptor knockout mice. Genes Brain Behav 7:568–577
King AC, Houle T, de Wit H, Holdstock L, Schuster A (2002) Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res 26:827–835
Kuribara H (1998) Importance of initial environments in the development of ambulatory sensitization to methamphetamine and cocaine in mice. J Pharm Pharmacol 50:303–309
Lane DA, Jaferi A, Kreek MJ, Pickel VM (2010) Acute and chronic cocaine differentially alter the subcellular distribution of AMPA GluR1 subunits in region-specific neurons within the mouse ventral tegmental area. Neuroscience 169:559–573
Lessov CN, Phillips TJ (1998) Duration of sensitization to the locomotor stimulant effects of ethanol in mice. Psychopharmacology (Berl) 135:374–382
Lett BT (1989) Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine and cocaine. Psychopharmacology (Berl) 98:357–362
Leyton M (2007) Conditioned and sensitized responses to stimulant drugs in humans. Prog Neuropsychopharmacol Biol Psychiatry 31:1601–1613
Lu W, Monteggia LM, Wolf ME (2002) Repeated administration of amphetamine or cocaine does not alter AMPA receptor subunit expression in the rat midbrain. Neuropsychopharmacology 26:1–13
Marley RJ, Arros DM, Henricks KK, Marley ME, Miner LL (1998) Sensitivity to cocaine and amphetamine among mice selectively bred for differential cocaine sensitivity. Psychopharmacology (Berl) 140:42–51
Mendez IA, Williams MT, Bhavsar A, Lu AP, Bizon JL, Setlow B (2009) Long-lasting sensitization of reward-directed behavior by amphetamine. Behav Brain Res 201:74–79
Mendrek A, Blaha CD, Phillips AG (1998) Pre-exposure of rats to amphetamine sensitized self-administration of this drug under a progressive ratio schedule. Psychopharmacology (Berl) 135:416–422
Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK (1998) High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome 9:983–990
Meyer PJ, Phillips TJ (2007) Behavioral sensitization to ethanol dose not result in cross-sensitization to NMDA receptor angonists. Psychopharmacology (Berl) 195:103–115
Nelson CL, Milovanovic M, Wetter JB, Ford KA, Wolf ME (2009) Behavioral sensitization to amphetamine is not accompanied by changes in glutamate receptor surface expression in the rat nucleus accumbens. J Neurochem 109:35–51
Palmer AA, Verbitsky M, Suresh R, Kamens HM, Reed CL, Li N, Burkhart-Kasch S, McKinnon CS, Belknap JK, Gilliam TC, Phillips TJ (2005) Gene expression differences in mice divergently selected for methamphetamine sensitivity. Mamm Genome 16:291–305
Pastor R, Kamens HM, McKinnon CS, Ford MM, Phillips TJ (2010) Repeated ethanol administration modifies the temporal structure of sucrose intake patterns in mice: effects associated with behavioral sensitization. Addiction Biol 15:324–335
Paulson PE, Camp DM, Robinson TE (1991) The time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 103:480–492
Phillips TJ, Shen EH (1996) Neurochemical bases of locomotion and ethanol stimulant effects. Int Rev Neurobiol 39:243–282
Phillips TJ, Dickinson S, Burkhart-Kasch S (1994) Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci 108:789–803
Phillips TJ, Huson M, Gwiazdon C, Burkhart-Kasch S (1995) Effects of acute and repeated ethanol exposures on locomotor activity of BXD recombinant inbred mice. Alcohol Clin Exp Res 19:269–278
Phillips TJ, Huson MG, McKinnon CS (1998) Localization of genes mediating acute and sensitized locomotor responses to cocaine in BXD/Ty recombinant inbred mice. J Neurosci 18:3023–3034
Phillips TJ, Broadbent J, Burkhart-Kasch S, Henderson C, Wenger CD, McMullin C, McKinnon CS, Cunningham CL (2005) Genetic correlational analysis of ethanol reward and aversion phenotypes in short-term selected mouse lines for ethanol drinking or ethanol-induced conditioned taste aversion. Behav Neurosci 119:892–910
Phillips TJ, Kamens HM, Wheeler JM (2008) Behavioral genetic contributions to the study of addiction-related amphetamine effects. Neurosci Biobehav Rev 32:707–759
Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25:192–216
Pierce RC, Born B, Adams M, Kalivas PW (1996) Repeated intra-ventral tegmental area administration of SKF-38393 induces behavioral and neurochemical sensitization to a subsequent cocaine challenge. Pharmacol Exp Ther 278:384–392
Post RM (2010) Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. doi:10.1007/s12640-010-9182-2
Post RM, Rose H (1976) Increasing effects of repetitive cocaine administration in the rat. Nature 260:731–732
Schuckit MA (1994) Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 151:184–189
Shuster L, Yu G, Bates A (1977) Sensitization to cocaine stimulation in mice. Psychopharmacology (Berl) 52:185–190
Strakowski SM, Sax KW (1998) Progressive behavioral response to repeated d-amphetamine challenge: further evidence for sensitization in humans. Biol Psychiatry 44:1171–1177
Veenstra-VanderWeele J, Qaadir A, Palmer AA, Cook EH Jr, de Wit H (2006) Association between the casein kinase 1 epsilon gene region and subjective response to d-amphetamine. Neuropsychopharmacology 31:1056–1063
Vezina P (2004) Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev 27:827–839
Welter M, Vallone D, Samad TA, Meziane H, Usiello A, Borrelli E (2007) Absence of dopamine D2 receptors unmarsks an inhibitory control over the brain circuitries activated by cocaine. Proc Natl Acad Sci USA 104:6840–6845
Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, Franken FH, Wiren KM, Hashimoto JG, Scibelli AC, Phillips TJ (2009) Genetically correlated effects of selectively breeding for high and low methamphetamine consumption. Genes Brain Behav 8:758–771
Zapata A, Chefer VI, Ator R, Shippenberg TS, Rocha BA (2003) Behavioral sensitization and enhanced dopamine response in the nucleus accumbens after intravenous cocaine self-administration in mice. Eur J Neurosci 17:590–596
Acknowledgements
This work was supported by grants from NIDA (P50 DA018165 and T32 DA07262) and by the Department of Veterans Affairs. We are grateful to John Belknap for providing statistical software for performance of heritability calculations. All experiments were conducted in compliance with current laws in the United States of America.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scibelli, A.C., McKinnon, C.S., Reed, C. et al. Selective breeding for magnitude of methamphetamine-induced sensitization alters methamphetamine consumption. Psychopharmacology 214, 791–804 (2011). https://doi.org/10.1007/s00213-010-2086-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-2086-2