Abstract
Introduction
Postoperative adhesions pose a continued healthcare problem. We previously demonstrated that intraperitoneal (IP) administration of a neurokinin-1 receptor antagonist (NK-1RA) at surgery reduces intraabdominal adhesions in rats. The NK-1RA aprepitant (Emend™, Merck) is clinically approved for preventing postoperative nausea and vomiting; however, its effects on adhesion formation are unknown. Thus, we determined the effects of IP and oral administration of aprepitant on adhesion formation in a rat model.
Methods
Adhesions were surgically induced in rats that were randomized to receive either one or five oral preoperative doses or a single intraoperative IP dose of aprepitant (50 mg/kg). Adhesions were scored at 7 days. In similar experiments using IP dosing, animals were sacrificed at 24 h and peritoneal fluid, and tissue were collected to assess fibrinolytic activity and tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1) mRNA levels, respectively.
Results
IP aprepitant reduced adhesion formation by 33% (p < 0.05) compared with controls while oral aprepitant had no effect. Compared to controls IP aprepitant reduced tPA activity by 55% (p < 0.05), increased PAI-1 mRNA levels by 140% (p < 0.05), and had no affect on tPA mRNA levels.
Conclusion
These data suggest that aprepitant maybe a useful pharmacologic agent for reducing adhesion formation clinically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative adhesions comprise a source of morbidity for patients undergoing intraabdominal surgery. Some of the complications caused by intraabdominal adhesions include pain, infertility in women,1 small bowel obstruction,2,3 bowel ischemia, and possibly death. The end result is increased hospital visits and increased cost of care for the postsurgical patient.4–6
Postoperative adhesions result from the injury and inflammation to the peritoneal lining of the abdominal cavity. Examples of peritoneal injury caused by surgery include cutting, crush, cautery, infection, ischemia, and rough handling of the peritoneal surfaces.7 Areas of subperitoneal extracellular matrix are denuded followed by platelet adhesion and degranulation.8 Inflammatory mediators such as transforming growth factor-β-1, prostaglandins, leukocyte chemotaxins, and fibrinogen are released and aggregate in the wound,8 leading to the formation of fibrin bands. When these fibrin bands form between two opposing surfaces, an adhesion may form. The fibrin bands can act as scaffolding for inflammatory cell in growth.7 The wound is first populated by neutrophils, followed by macrophages, angioblasts, and fibroblasts. Fibroblasts deposit collagen into the fibrin band leading to permanent adhesion formation by 7 days following surgery.7,8
Under normal circumstances, the fibrin bands are degraded by plasmin prior to recruitment of inflammatory cells. However, under postoperative conditions, plasminogen activator activity is decreased and the fibrin bands persist and progress to adhesion maturation.7 Peritoneal fibrinolytic activity is closely associated with levels of tissue plasminogen activator (tPA) and plasminogen activator inhibitor-l (PAI-1).9 tPA functions by activating plasmin while PAI-1 acts by directly inhibiting tPA. Previous studies have demonstrated that modulation of tPA activity and PAI-1 levels can affect adhesion formation; downregulation of the fibrinolytic system results in increased adhesions; while upregulation results in decreased adhesions.10–12
Many methods for preventing postoperative intraabdominal adhesions have been investigated in the past, such as antibiotics, anti-inflammatory medications, and physical barriers.13–16 To date, no pharmacologic compounds have been approved for use in preventing adhesions. Currently approved anti-adhesion methods are limited to physical barriers only. By physically separating two inflamed surfaces, adhesion formation can be reduced. However, the anti-adhesion activity of barrier-based methods is likely limited to where they are placed, potentially leaving adhesiogenic surfaces. Placement of a solid barrier is also limited to open abdominal procedures. A pharmacologic method may allow for treatment of all adhesiogenic surfaces as well as allow adhesion prevention during laparoscopic operations.
One class of pharmacologic compounds previously investigated by this laboratory is the neurokinin-1 receptor antagonists (NK-1RA). The neurokinin-1 receptor (NK-1R) is a high affinity receptor that is activated by binding tachykinins of which the proinflammatory neuropeptide substance-P is the best studied. Our laboratory has demonstrated that both substance-P and NK-1R are upregulated during peritoneal injury.17 We also showed that intraperitoneal administration of an NK-1RA increases peritoneal tPA activity and decreases adhesion formation.18 This drug family shows promise for decreasing intraabdominal adhesions.
Currently, the only FDA-approved NK-1RA is aprepitant (Emend™, Merck, Rahway, NJ, USA), which is in use for postoperative and chemotherapy-related nausea and vomiting. The perioperative usage of this drug and its pharmacologic activity create a unique opportunity for study of its potential anti-adhesion effects. Our goal was to evaluate the effect of orally or intraperitoneally administered aprepitant on the reduction of abdominal adhesion formation in a rat model.
Methods
Materials
All chemicals were obtained from Sigma (St. Louis, MO, USA) unless otherwise noted. The highly specific, nonpeptide NK-1RA 5-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl] methyl]-1,2-dihydro-3H-1,2,4-triazol-3-one (aprepitant) was used in this study (Merck, Rahway, NJ, USA). This antagonist is highly specific for the NK-1R and has no affinity for the NK-2 or NK-3 receptors.19 Sterile 100% dimethyl sulfoxide (DMSO) was used to dissolve the pure aprepitant for intraperitoneal delivery while a solution of 2% carboxymethylcellulose (CMC) was used for delivery of the oral formulation (Emend™) as slurry.
Animals
Male Wistar rats weighing 175–200 g (Charles River Labs, Wilmington, MA, USA) were used for all experiments. Rats were housed at a constant room temperature of 25°C, with 12-h light and dark cycles, and were provided standard rodent chow (Purina, No. 5001) and water ad libitum. The Institutional Animal Care and Use Committee at Boston University School of Medicine approved these studies, and all procedures and animal care were performed in accordance with recommendations outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Sialogogic assay
A sialogogic assay was performed as described by Leeman et al. to determine whether oral and intraperitoneal administration of aprepitant effectively blocks NK-1R activity in rats.20 Rats were gavaged or intraperitoneally injected with aprepitant (50 mg/kg) or vehicle alone (N = 3 per group). Animals were anesthetized and intravenously injected with SP (0.1 μg/100g) 3 h after oral gavage or 30 min after intraperitoneal injection. Saliva from the sublingual salivary gland was collected immediately with a Pasteur pipette and weighed to determine volume.
Induction of intraabdominal adhesions
Intraabdominal adhesions were induced in rats using our previously described model.18 Briefly, animals were anesthetized using continuous isoflurane 2–4% in 100% oxygen. A midline laparotomy was performed, and three ischemic buttons were created along the paracolic gutters bilaterally, spaced 1 cm apart. The abdomen and skin were closed using braided absorbable sutures and clips, respectively. Animals received a subcutaneous dose of buprenorphine (0.1 mg/kg body weight) at the time of operation, and then every 12 h as needed for up to 72 h postoperation.
Experimental design
The first group of rats received an intraoperative 1 ml peritoneal lavage with either aprepitant (50 mg/kg, N = 14), or vehicle control (100% DMSO, N = 12), followed by gentle abdominal massage to distribute the solutions throughout the peritoneal cavity. A second group received oral gavages of 1 ml (50 mg/kg) of aprepitant suspended in 2% CMC or the vehicle alone. Two dosing schedules were used: one dose given 3 h preoperatively (aprepitant N = 8, vehicle N = 7), or five doses given preoperatively every 12 h over 60 h (aprepitant N = 13, vehicle N = 14). Since a single oral dose of aprepitant effectively blocked the sialogogic response suggesting that this dose may be globally effective in the rat, we chose to determine if one oral dose of aprepitant was effective in reducing adhesion formation. However, given the fact that the oral formulation of aprepitant is less than 60% bioavailable, we decided to significantly increase the number of doses of aprepitant the rats received to five preoperative oral doses. To determine the effect of aprepitant on the intraperitoneal fibrinolytic system and gene expression, the experimental design described above for intraperitoneal administration was used with the addition of a nonoperative group receiving only an intraperitoneal injection of aprepitant or DMSO. For this second set of experiments, the rats were anesthetized with a ketamine–xylazine solution (75 and 5 mg/ml) at 24 h after surgery at which time a second laparotomy was performed to collect peritoneal fluid and ischemic button tissue for analysis of tPA activity and mRNA expression levels, respectively. Rats were then euthanized by a combination of pneumothorax and cardiac puncture. The peritoneal fluid was collected by rinsing the peritoneal cavity with 2 ml of 37°C phosphate buffer containing heparin (1 IU/ml). Approximately 1 ml of the fluid was recovered and added to an equal volume of 0.2 M sodium acetate, pH 3.9, and cellular debris was removed by microcentrifugation (2,000 rpm × 1 min). The resulting supernatants were immediately frozen in liquid nitrogen and stored at −80°C until assayed for tPA activity. Ischemic button tissue was removed within a 1-mm rim of surrounding tissue and frozen in liquid nitrogen and stored at –80°C until ready for reverse transcription polymerase chain reaction (RT-PCR) analysis.
Evaluation of fibrinolytic activity in peritoneal fluid
Acetate-treated peritoneal fluid samples were acidified with 0.2 volumes 0.375 N HCl and then diluted tenfold with distilled water. The fibrinolytic activity due to tPA in each sample was assayed in duplicate by adding 50 μl of the diluted sample to wells of a 96-well microtiter plate containing 50 μl of tPA stimulator (0.6 mg/ml cyanogen bromide digested fibrinogen, American Diagnostica, Stamford, CT, USA). Next, 150 μl of assay buffer [16.7 μg/ml human plasminogen (Athens Research and Technologies, Athens, GA, USA), 667 μM S-2251 substrate (American Diagnostica, Inc.), and 20 mM Tris, pH 8.3] was added to each well and gently mixed. Cleavage of the S-2251 substrate by tPA-activated plasmin produces a yellow color that absorbs at 405 and 490 nm (calibration blank). The change in absorbance was measured at 37°C over a 6-h period with a Spectra Max 250 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). The activity of tPA in each sample was determined by extrapolation from a tPA (human; Calbiochem, San Diego, CA, USA) standard curve. In a separate control experiment designed to determine if the aprepitant or DMSO interfered with tPA activity assays, the above protocol was followed with the addition of a known concentration of pure tPA (2.5 U/ml) to peritoneal fluid samples before the addition of the S-2251 substrate.
RNA isolation and quantitative RT-PCR
Ischemic buttons were homogenized by pulverization at –70°C on a liquid nitrogen-cooled plate. Total RNA was isolated from peritoneal ischemic button tissue (50 mg) with the SV Total RNA Isolation System (Promega), and RT-PCR was conducted with the Gene-AMP RNA PCR system (Applied Biosystems), as described by Reed.17 The following primer sets were used to amplify tPA and PAI-1 (28 cycles of 95°C, 60°C, and 70°C for 30 s each): tPA, 5′TCTGACTTCGTCTGCCAGTG-3′ (sense) and 5′-GAGGCCTTGGAT-GTGGTAAA-3′ (antisense); PAI-1, 5′-ATCAACGACTGGGTGGAGAG-3′ (sense) and 5′-AGCCTGGTCATGTTGCTCTT-3′ (antisense). Real time PCR was performed on an Applied Biosystems 7000 Sequence Detection System machine using SYBR green. Levels of mRNA were normalized to glyceraldehyde 3-phosphate dehydrogenase, a constitutively expressed gene that did not vary among treatment groups.
Statistical analysis
Data were analyzed with the Sigma Stat program (SPSS, Inc., Chicago, IL, USA) with one-way analysis of variance (ANOVA). When significant effects were detected (p < 0.05), the difference between specific means was determined by the Student–Newman–Keuls test. If a test of normality failed, Dunn’s test of ANOVA by ranks was used. Differences were considered to be statistically significant if p < 0.05. All data are expressed as mean ± SEM.
Results
Intraperitoneal administration of aprepitant during surgery reduced intraabdominal adhesion formation
The sialogogic assay demonstrated that aprepitant effectively blocked the NK-1R by both enteral and intraperitoneal routes. Rats administered oral aprepitant showed a significant decrease in saliva production (p < 0.05) compared to the vehicle (0.06 ± 0.01 vs. 0.0003 ± 0.0002 ml/200 gm body weight, respectively). Rats that received intraperitoneal aprepitant also showed a significant decrease in saliva production (p < 0.05) compared to vehicle controls (0.11 ± 0.03 vs. 0.02 ± 0.001 ml/200 gm body weight, respectively).
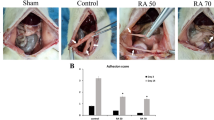
To test the anti-adhesion effects of aprepitant, the drug was first delivered locally (IP) at the time of surgery. Intraperitoneal administration of aprepitant decreased intraabdominal adhesion formation significantly (p < 0.05) at postoperative day 7. Rats given the NK-1RA had a mean intraabdominal adhesion formation score of 38.1 ± 5.1% compared to 56.9 ± 5.6% in the vehicle control group (Fig. 1). Neither schedule of oral aprepitant showed a significant effect on adhesion formation at postoperative day 7. Rats receiving a single dose 3 h prior to surgery had mean adhesion scores of 72 ± 7% (aprepitant) and 68 ± 9% (vehicle control), while rats receiving five doses preoperatively, once every 12 h over 60 h, had mean adhesion scores of 59% ± 11 (aprepitant) and 75 ± 9% (vehicle control; Fig. 1).
Intraperitoneal (IP) administration of aprepitant (50 mg/kg, N = 14) at the time of operation shows decreased adhesion formation 7 days later compared with controls (N = 12). Oral dosing with a single 3-h preoperative dose of aprepitant (50mg/kg; N = 8) shows no significant difference in adhesion formation at 7 days postoperation when compared to vehicle control (2% CMC; N = 7). Five oral doses of aprepitant (50 mg/kg; N = 13), starting 2 days preoperation given every 12 h, shows no significant difference at postoperative day 7 when compared to vehicle control (2% CMC; N = 14). Data are shown as mean ± SEM.
Intraperitoneal administration of aprepitant during surgery decreased tPA activity
Total tPA activity at 24 h postoperation was significantly decreased (p < 0.05) in peritoneal fluid from rats treated with a single intraoperative dose of aprepitant compared to vehicle controls. There was no effect on tPA activity when aprepitant was injected into non-operated animals 24 h prior to sacrifice (Fig. 2). In order to evaluate for potential interference of the aprepitant or DMSO with the tPA activity assay, 2.5 U/ml of tPA was added to peritoneal fluid from non-operated, aprepitant, and DMSO-treated animals. The assays were performed as described above. The addition of 2.5 U/ml increased tPA activity as expected, suggesting that aprepitant and DMSO do not inhibit the tPA assay (Table 1).
Twenty-four-hour postoperative (postop) tPA activity decreases in rats receiving IP aprepitant compared with vehicle controls (DMSO; N = 6 per group). Non-operative (nonop) rats receiving IP injections of aprepitant or vehicle DMSO (N = 4 per group) showed no difference between the two groups in tPA activity at 24 h post injection. Data are shown as mean ± SEM.
Intraperitoneal administration of aprepitant increased PAI-1 mRNA expression but and had no effect on tPA
PAI-1 mRNA expression levels were 133% greater (p < 0.05) in peritoneal adhesion tissue from rats treated with a single intraoperative dose of aprepitant compared to vehicle controls at 24 h postoperation. There was no difference in tPA mRNA expression levels in these same tissue samples (Fig. 3).
Discussion
This study demonstrates that intraperitoneal administration of aprepitant is effective in reducing adhesion formation while oral administration is not. One possible explanation for this observation is that despite the fact that orally administered aprepitant antagonizes the NK-1R, as demonstrated by the sialogogic assay, adequate peritoneal concentrations of aprepitant may not have been achieved at a critical time to prevent adhesion formation. Thus, the continuation of oral aprepitant administration after surgery would be of no further benefit to adhesion reduction. Our previous data suggest that there is a temporal window in which the NK-1RA is effective in reducing adhesion formation. We determined that the NK-1RA, CJ-12,255, must be available in the peritoneum no later than 5–6 h postoperation in order to reduce adhesion formation.21 Other studies have also failed to show anti-adhesion effects of orally administered drugs that were effective by intraperitoneal administration. Oral doses of atorvastatin did not reduce adhesion formation in rats while intraoperative doses did.22 Data presented here further support that the intraperitoneal administration of a pharmacologic agent during surgery may be the best method for adhesion prevention due to direct delivery to target tissues. This may be beneficial since a single intraperitoneal dose of a drug may limit the potential side effects associated with longer oral dosing regimens.
The tPA activity assays yielded unexpected results. Our previous work with the NK-1RA, CJ-12,255, showed increased postoperative tPA activity and increased tPA gene transcription compared to saline controls at 24 h.18 However, intraperitoneally administered aprepitant decreased tPA activity compared to the DMSO vehicle. This correlated with the PCR results that showed tPA mRNA expression remained unchanged while PAI-1 mRNA expression increased, resulting in an overall downregulation of the fibrinolytic system. The tPA assays performed with an added known concentration of tPA demonstrate that neither aprepitant nor DMSO interfere with the tPA activity assay, eliminating this as a possible reason for the decreased measurement. Thus, the observed decrease in tPA activity is likely due to increased expression of PAI-1, not to changes in tPA expression.
In contrast to our current observations, several studies suggest that agents which elevate postoperative peritoneal fibrinolytic activity have the ability to reduce adhesion formation. In animal models, pharmacologic upregulation of peritoneal fibrinolysis, across a broad range of drug types, is associated with decreased adhesion formation. Such drugs include the NK-1RA CJ-12,255, methylene blue, atorvastatin, pentoxifylline, and octreotide.18,21–30 Studies in both humans and animals demonstrate the ability of the peritoneal fibrinolytic system to regulate adhesion formation. Patients with the most severe adhesions have decreased postoperative tPA activity as well as increased levels of PAI-1.31 In animals, increasing postoperative peritoneal fibrinolytic activity by either inhibition of PAI-1 or IP administration of tPA reduces adhesion formation.32,33 Furthermore, Sulaiman et al. demonstrated that tPA-null mice have increased postoperative adhesion formation compared to their wild-type counterparts.12
While the fibrinolytic system has been demonstrated to have a strong relationship with adhesion formation, the current data suggest that aprepitant may reduce adhesion formation via other mechanisms. Possible alternative mechanisms include modulation of proinflammatory mediators such as cyclooxygenase-2 (COX-2) and transforming growth factor (TGF)-β. The COX-2 enzyme has been demonstrated to have proinflammatory, proangiogenic, and pro-fibroblastic effects, all of which may promote adhesion formation, and inhibition of the COX-2 enzyme, which is expressed by adhesion fibroblasts,34 has been shown to reduce adhesion formation in rodents.14,35,36 Overproduction of TGF-β, a multifunctional growth factor, correlates with adhesion formation in both humans and animals,37–39 and in animal models, blocking TGF-β activity has been shown to reduce adhesion formation.40,41 In support of a possible link between aprepitant and COX-2 and/or TGF-β, the NK-1R ligand substance P has been shown to upregulate COX-2 expression in cultured human umbilical vein endothelial cells and colonic epithelial cells,42,43 and substance P has been shown to increase TGF-β expression in rat fibroblasts.44 IP administration of aprepitant at surgery may also directly affect wound healing processes. Substance P released at sites of tissue injury, in addition to promoting inflammation, is thought to stimulate proliferation of epithelial, vascular, and connective tissue cells as part of the wound healing process.45,46 Substance P may induce tissue fibrosis via augmentation of cytokine-induced fibroblast proliferation, effects on collagen organization, and regulation of matrix metalloproteinase expression.47–50 We have previously reported that administration of the NK-1RA CJ-12,255, in addition to reducing adhesion formation, increases matrix metalloproteinase expression and activity in the postoperative peritoneum.51 In the current study, aprepitant may reduce adhesion formation by modulating the inflammatory and wound-healing processes that begin with the onset of surgery well before the changes in peritoneal fibrinolytic activity occur. A possible scenario is that administration of aprepitant reduces postoperative fibrin band formation, thereby diminishing the need for fibrinolysis. Further investigation is needed to delineate the mechanisms by which aprepitant, as well as other NK-1RAs, reduce adhesion formation.
Conclusion
Intraperitoneal administration of the FDA approved NK-1RA, aprepitant, is effective in reducing intraabdominal adhesions while oral administration is not. The peritoneum may act as a selective barrier preventing orally administered or systemic drugs from significantly affecting processes in the peritoneum including adhesion formation. Thus, intraoperative peritoneal administration of a pharmacologic agent appears to be the most effective method for drug delivery to target adhesiogenesis. The results also suggest that the anti-adhesion effects of aprepitant may be via an alternative pathway other than the peritoneal fibrinolytic system. Although further investigation into the mechanism(s) of N K-1RA effects on adhesion formation is warranted, these studies support the use of an NK-1RA in adhesion prevention.
References
Stovall TG, Elder RF, Ling FW. Predictors of pelvic adhesions. J Reprod Med 1989;34:345–348.
Ivarsson ML, Holmdahl L, Franzen G, Risberg B. Cost of bowel obstruction resulting from adhesions. Eur J Surg 1997;163:679–684.
Menzies D, Ellis H. Intestinal obstruction from adhesions–how big is the problem? Ann R Coll Surg Engl 1990;72:60–63.
Ellis H, Moran BJ, Thompson JN, Parker MC, Wilson MS, Menzies D, et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet 1999;353:1476–1480. doi:10.1016/S0140-6736(98)09337-4.
Grant HW, Parker MC, Wilson MS, Menzies D, Sunderland G, Thompson JN, et al. Population-based analysis of the risk of adhesion-related readmissions after abdominal surgery in children. J Pediatr Surg 2006;41:1453–1456. doi:10.1016/j.jpedsurg.2006.04.023.
Thompson J, Ellis H, Parker MC, Menzies D, Moran BJ, Wilson MS, et al. Surgical impact of adhesions following surgery in the upper abdomen. Br J Surg 1999;86:418–419. doi:10.1046/j.1365-2168.1999.1062d.x.
Tingstedt B, Isaksson K, Andersson E, Andersson R. Prevention of abdominal adhesions–present state and what’s beyond the horizon? Eur Surg Res 2007;39:259–268. doi:10.1159/000102591.
Boland GM, Weigel RJ. Formation and prevention of postoperative abdominal adhesions. J Surg Res 2006;132:3–12. doi:10.1016/j.jss.2005.12.002.
Scott-Coombes D, Whawell S, Vipond MN, Thompson J. Human intraperitoneal fibrinolytic response to elective surgery. Br J Surg 1995;82:414–417. doi:10.1002/bjs.1800820346.
Menzies D, Ellis H. The role of plasminogen activator in adhesion prevention. Surg Gynecol Obstet 1991;172:362–366.
Montz FJ, Fowler JM, Wolff AJ, Lacey SM, Mohler M. The ability of recombinant tissue plasminogen activator to inhibit post-radical pelvic surgery adhesions in the dog model. Am J Obstet Gynecol 1991;165:1539–1542.
Sulaiman H, Dawson L, Laurent GJ, Bellingan GJ, Herrick SE. Role of plasminogen activators in peritoneal adhesion formation. Biochem Soc Trans 2002;30:126–131. doi:10.1042/BST0300126.
Bothin C, Midtvedt T, Perbeck L. Orally delivered antibiotics which lower bacterial numbers decrease experimental intraabdominal adhesions. Langenbecks Arch Surg 2003;388:112–115.
Greene AK, Alwayn IP, Nose V, Flynn E, Sampson D, Zurakowski D, et al. Prevention of intraabdominal adhesions using the antiangiogenic COX-2 inhibitor celecoxib. Ann Surg 2005;242:140–146. doi:10.1097/01.sla.0000167847.53159.c1.
Reijnen MM, Bleichrodt RP, van Goor H. Pathophysiology of intraabdominal adhesion and abscess formation, and the effect of hyaluronan. Br J Surg. 2003;90:533–541. doi:10.1002/bjs.4141.
Verco SJ, Peers EM, Brown CB, Rodgers KE, Roda N, diZerega G. Development of a novel glucose polymer solution (icodextrin) for adhesion prevention: pre-clinical studies. Hum Reprod 2000;15:1764–1772. doi:10.1093/humrep/15.8.1764.
Reed KL, Fruin AB, Bishop-Bartolomei KK, Gower AC, Nicolaou M, Stucchi AF, et al. Neurokinin-1 receptor and substance P messenger RNA levels increase during intraabdominal adhesion formation. J Surg Res 2002;108:165–172. doi:10.1006/jsre.2002.6533.
Reed KL, Fruin AB, Gower AC, Stucchi AF, Leeman SE, Becker JM. A neurokinin 1 receptor antagonist decreases postoperative peritoneal adhesion formation and increases peritoneal fibrinolytic activity. Proc Natl Acad Sci USA 2004;101:9115–9120. doi:10.1073/pnas.0403210101.
Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek JJ, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 1998;281:1640–1645. doi:10.1126/science.281.5383.1640.
Leeman SE, Hammerschlag R. Stimulation of salivary secretion by a factor extracted from hypothalamic tissue. Endocrinology 1967;81:803–810.
Cohen PA, Aarons CB, Gower AC, Stucchi AF, Leeman SE, Becker JM, et al. The effectiveness of a single intraperitoneal infusion of a neurokinin-1 receptor antagonist in reducing postoperative adhesion formation is time dependent. Surgery 2007;141:368–375. doi:10.1016/j.surg.2006.09.007.
Aarons CB, Cohen PA, Gower A, Reed KL, Leeman SE, Stucchi AF, et al. Statins (HMG-CoA reductase inhibitors) decrease postoperative adhesions by increasing peritoneal fibrinolytic activity. Ann Surg 2007;245:176–184. doi:10.1097/01.sla.0000236627.07927.7c.
Baykal A, Ozdemir A, Renda N, Korkmaz A, Sayek I. The effect of octreotide on postoperative adhesion formation. Can J Surg 2000;43:43–47.
Cetin M, Ak D, Duran B, Cetin A, Guvenal T, Yanar O. Use of methylene blue and N,O-carboxymethylchitosan to prevent postoperative adhesions in a rat uterine horn model. Fertil Steril 2003;80(Suppl 2):698–701. doi:10.1016/S0015-0282(03)00777-5.
Heydrick SJ, Reed KL, Cohen PA, Aarons CB, Gower AC, Becker JM, et al. Intraperitoneal administration of methylene blue attenuates oxidative stress, increases peritoneal fibrinolysis, and inhibits intraabdominal adhesion formation. J Surg Res 2007;143:311–319. doi:10.1016/j.jss.2006.11.012.
Kaleli B, Ozden A, Aybek Z, Bostanci B. The effect of L-arginine and pentoxifylline on postoperative adhesion formation. Acta Obstet Gynecol Scand 1998;77:377–380. doi:10.1034/j.1600-0412.1998.770403.x.
Kucuk HF, Kaptanoglu L, Kurt N, Uzun H, Eser M, Bingul S, et al. The role of simvastatin on postoperative peritoneal adhesion formation in an animal model. Eur Surg Res 2007;39:98–102. doi:10.1159/000099156.
Lai HS, Chen Y. Effect of octreotide on postoperative intraperitoneal adhesions in rats. Scand J Gastroenterol 1996;31:678–681. doi:10.3109/00365529609009149.
Lai HS, Chu SY, Chen Y, Wu CH, Lin LT. Effect of pentoxifylline on intraperitoneal adhesions after intestinal resection in rats. J Formos Med Assoc 1994;93:911–915.
Tarhan OR, Barut I, Sutcu R, Akdeniz Y, Akturk O. Pentoxifylline, a methyl xanthine derivative, reduces peritoneal adhesions and increases peritoneal fibrinolysis in rats. Tohoku J Exp Med 2006;209:249–255. doi:10.1620/tjem.209.249.
Holmdahl L, Eriksson E, Eriksson BI, Risberg B. Depression of peritoneal fibrinolysis during operation is a local response to trauma. Surgery 1998;123:539–544. doi:10.1067/msy.1998.86984.
Dorr PJ, Vemer HM, Brommer EJ, Willemsen WN, Veldhuizen RW, Rolland R. Prevention of postoperative adhesions by tissue-type plasminogen activator (t-PA) in the rabbit. Eur J Obstet Gynecol Reprod Biol 1990;37:287–291. doi:10.1016/0028-2243(90)90037-2.
Falk K, Bjorquist P, Stromqvist M, Holmdahl L. Reduction of experimental adhesion formation by inhibition of plasminogen activator inhibitor type 1. Br J Surg 2001;88:286–289. doi:10.1046/j.1365-2168.2001.01647.x.
Saed GM, Munkarah AR, Diamond MP. Cyclooxygenase-2 is expressed in human fibroblasts isolated from intraperitoneal adhesions but not from normal peritoneal tissues. Fertil Steril 2003;79:1404–1408. doi:10.1016/S0015-0282(03)00257-7.
Guvenal T, Cetin A, Ozdemir H, Yanar O, Kaya T. Prevention of postoperative adhesion formation in rat uterine horn model by nimesulide: a selective COX-2 inhibitor. Hum Reprod 2001;16:1732–1735. doi:10.1093/humrep/16.8.1732.
Katada J, Saito H, Ohashi A. Significance of cyclooxygenase-2 induced via p38 mitogen-activated protein kinase in mechanical stimulus-induced peritoneal adhesion in mice. J Pharmacol Exp Ther 2005;313:286–292. doi:10.1124/jpet.104.078717.
Ghellai AM, Stucchi AF, Chegini N, Ma C, Andry CD, Kaseta JM, et al. Role of transforming growth factor beta-1 in peritonitis-induced adhesions. J Gastrointest Surg 2000;4:316–323. doi:10.1016/S1091-255X(00)80082-7.
Holmdahl L, Kotseos K, Bergstrom M, Falk P, Ivarsson ML, Chegini N. Overproduction of transforming growth factor-beta1 (TGF-beta1) is associated with adhesion formation and peritoneal fibrinolytic impairment. Surgery 2001;129:626–632. doi:10.1067/msy.2001.113039.
Saed GM, Collins KL, Diamond MP. Transforming growth factors beta1, beta2 and beta3 and their receptors are differentially expressed in human peritoneal fibroblasts in response to hypoxia. Am J Reprod Immunol 2002;48:387–393. doi:10.1034/j.1600-0897.2002.01090.x.
Fukui N, Tashiro T, Hiraoka H, Oda H, Nakamura K. Adhesion formation can be reduced by the suppression of transforming growth factor-beta1 activity. J Orthop Res 2000;18:212–219. doi:10.1002/jor.1100180208.
Gorvy DA, Herrick SE, Shah M, Ferguson MW. Experimental manipulation of transforming growth factor-beta isoforms significantly affects adhesion formation in a murine surgical model. Am J Pathol 2005;167:1005–1019.
Gallicchio M, Rosa AC, Benetti E, Collino M, Dianzani C, Fantozzi R. Substance P-induced cyclooxygenase-2 expression in human umbilical vein endothelial cells. Br J Pharmacol 2006;147:681–689. doi:10.1038/sj.bjp.0706660.
Koon HW, Zhao D, Zhan Y, Rhee SH, Moyer MP, Pothoulakis C. Substance P stimulates cyclooxygenase-2 and prostaglandin E2 expression through JAK-STAT activation in human colonic epithelial cells. J Immunol 2006;176:5050–5059.
Lai XN, Wang ZG, Zhu JM, Wang LL. Effect of substance P on gene expression of transforming growth factor beta-1 and its receptors in rat’s fibroblasts. Chin J Traumatol. 2003;6:350–354.
Felderbauer P, Bulut K, Hoeck K, Deters S, Schmidt WE, Hoffmann P. Substance P induces intestinal wound healing via fibroblasts–evidence for a TGF-beta-dependent effect. Int J Colorectal Dis 2007;22:1475–1480. doi:10.1007/s00384-007-0321-z.
Schaffer M, Beiter T, Becker HD, Hunt TK. Neuropeptides: mediators of inflammation and tissue repair? Arch Surg 1998;133:1107–1116. doi:10.1001/archsurg.133.10.1107.
Burssens P, Steyaert A, Forsyth R, van Ovost EJ, Depaepe Y, Verdonk R. Exogenously administered substance P and neutral endopeptidase inhibitors stimulate fibroblast proliferation, angiogenesis and collagen organization during Achilles tendon healing. Foot Ankle Int 2005;26:832–839.
Cury PR, Canavez F, de Araujo VC, Furuse C, de Araujo NS. Substance P regulates the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinase in cultured human gingival fibroblasts. J Periodontal Res 2008;43:255–260.
Rameshwar P, Oh HS, Yook C, Gascon P, Chang VT. Substance p-fibronectin-cytokine interactions in myeloproliferative disorders with bone marrow fibrosis. Acta Haematol 2003;109:1–10. doi:10.1159/000067268.
Ramos C, Montano M, Cisneros J, Sommer B, Delgado J, Gonzalez-Avila G. Substance P up-regulates matrix metalloproteinase-1 and down-regulates collagen in human lung fibroblast. Exp Lung Res 2007;33:151–167. doi:10.1080/01902140701364409.
Cohen PA, Gower AC, Stucchi AF, Leeman SE, Becker JM, Reed KL. A neurokinin-1 receptor antagonist that reduces intraabdominal adhesion formation increases peritoneal matrix metalloproteinase activity. Wound Repair Regen 2007;15:800–808. doi:10.1111/j.1524-475X.2007.00291.x.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussion
Margo Shoup, M.D. (Maywood, IL): I would like to congratulate the authors for trying to tackle a difficult problem that we all face with our patients with adhesion formation in a small bowel obstruction, and we really haven't made much headway in this in the last couple of decades. The authors in this paper attempt to study the effects of HA/CMC, or seprafilm, and neurokinin-1 receptor antagonist. The adhesions were measured at seven days postoperatively and placement of the buttons, and the Tpa was measured 24 hours after laparotomy. And we know that the synergistic effects of NK1RA and seprafilm is evident, but we are not really sure what is going on with the Tpa during all that. So I have a few questions for you.
Have you first looked at dose escalation studies with increasing NK1 receptor antagonists to evaluate the effects on Tpa, because this would clarify whether this is truly the mechanisms through which this is working. Also, you checked the Tpa levels 24 hours after surgery and, like I said, the adhesions at seven days. Have you looked at different time frames for both of these to see if there is more of a correlation? And at this point do you have any information on the status of the soluble seprafilm that is available in Europe, and if so, where do you think this may impact your study?
Thank you.
Rizal Lim, M.D. (Boston, MA): In terms of dose escalation of our antagonist, going back to the original parent compound, it is actually based off of a drug called ezlopitant. When we received this compound as a gift, the doses were actually based on the then maximum recommended dose of 25 mg/kg, which we used. But in earlier studies, as we started off with 5 mg/kg and then went to 10 mg/kg, we saw a progressive increase in adhesion prevention from those two doses.
In terms of the different time frames, looking at adhesions with this model specifically, our personal experience and data we have collected in the past have shown that when we look at adhesion formation beyond 7 days, we haven't really seen much of a difference in terms of severity. The same is true for tPA. In fact, what we have seen in previous studies is that tPA immediately post-op, at least within the rat, drops significantly and hits its nadir at approximately 24 hours and following that period of time begins to slowly rise back towards normal levels. So we chose that simply because it gives us a general idea of what the fibrinolytic activity within the abdomen is doing at its worst case scenario. We have also shown that giving the drug at 24 hours, we can alter that fibrinolytic activity.
And the final question, in terms of the soluble and gel forms of various barrier compounds, I am not firmly sure as to how far the various companies have progressed in terms of getting that approved within the U.S. But some of the implications which it may convey are that currently some of the biggest limitations of using HA/CMC barriers involve its actual application. It is a brittle, stiff material. It is difficult to use in certain cases such as laparoscopy, and I think that progressing to more of a gel type of device would improve its utility.
This work was supported in part, by Merck, Rahway, NJ, USA and by the Smithwick Endowment Fund to the Department of Surgery at Boston University School of Medicine.
An erratum to this article can be found at http://dx.doi.org/10.1007/s11605-008-0767-5
Rights and permissions
About this article
Cite this article
Lim, R., Morrill, J.M., Prushik, S.G. et al. An FDA Approved Neurokinin-1 Receptor Antagonist is Effective in Reducing Intraabdominal Adhesions when Administered Intraperitoneally, But Not Orally. J Gastrointest Surg 12, 1754–1761 (2008). https://doi.org/10.1007/s11605-008-0634-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-008-0634-4