Abstract
Rationale
Selective attention toward emotional cues and emotion recognition of facial expressions are important aspects of social cognition. Stress modulates social cognition through cortisol, which acts on glucocorticoid (GR) and mineralocorticoid receptors (MR) in the brain.
Objectives
We examined the role of MR activation on attentional bias toward emotional cues and on emotion recognition.
Methods
We included 40 healthy young women and 40 healthy young men (mean age 23.9 ± 3.3), who either received 0.4 mg of the MR agonist fludrocortisone or placebo. A dot-probe paradigm was used to test for attentional biases toward emotional cues (happy and sad faces). Moreover, we used a facial emotion recognition task to investigate the ability to recognize emotional valence (anger and sadness) from facial expression in four graded categories of emotional intensity (20, 30, 40, and 80 %).
Results
In the emotional dot-probe task, we found a main effect of treatment and a treatment × valence interaction. Post hoc analyses revealed an attentional bias away from sad faces after placebo intake and a shift in selective attention toward sad faces compared to placebo. We found no attentional bias toward happy faces after fludrocortisone or placebo intake. In the facial emotion recognition task, there was no main effect of treatment.
Conclusions
MR stimulation seems to be important in modulating quick, automatic emotional processing, i.e., a shift in selective attention toward negative emotional cues. Our results confirm and extend previous findings of MR function. However, we did not find an effect of MR stimulation on emotion recognition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social cognition is an important aspect of human emotional competence and is crucial for adequate social interaction. Social cognition is altered in stressful situations through activation of the hypothalamic–pituitary–adrenal (HPA) axis and consecutive increased cortisol secretion (Sandi and Haller 2015).
One important aspect of social cognition is selective attention after emotional priming (attentional bias), favoring the cognitive processing of emotional information (MacLeod et al. 1986). Some studies have focused on the role of cortisol on attentional bias toward emotional cues suggesting that cortisol is associated with an attentional bias toward negative stimuli (Putman and Roelofs 2011; Roelofs et al. 2007; Tsumura and Shimada 2012; van Honk et al. 1998). However, McHugh et al. (2010) found an attenuated attentional bias toward threatening stimuli after stress induction.
The ability to recognize emotions in facial expressions is another important subdomain of social cognition. Deckers et al. (2015) found improved emotion recognition after psychosocial stress (Trier Social Stress Test) in patients with borderline personality disorder and in healthy participants. Duesenberg et al. (2016) have investigated the role of the glucocorticoid receptor agonist hydrocortisone on emotion recognition in healthy subjects but did not find a hydrocortisone effect on emotion recognition.
In the brain, cortisol binds at two receptors, the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR). Whereas the GR is abundant throughout the brain, the MR is mainly distributed within limbic structures, especially in the hippocampus, the lateral septum, and the amygdala (de Kloet 2014). Previously, GR function attracted most research attention, but more recently, studies have also begun to emphasize the role of the MR (Joëls et al. 2008). For example, animal studies have shown that the MR is important for behavioral regulation in emotionally charged situations. The MR has been linked to fear memory (Brinks et al. 2009), to retrieval of emotional information and fear response selection (Zhou et al. 2011), to reactivity in unfamiliar situations (de Kloet et al. 1999), to the extinction of fear-motivated behavior (Ter Horst et al. 2012), and to reduced anxiety (Lai et al. 2007; Mitra et al. 2009; Rozeboom et al. 2007). In humans, emotional memory is impaired by MR blockage (Rimmele et al. 2013) and by MR depletion via metyprapone (Rimmele et al. 2015). In addition, Wingenfeld et al. (2014) have shown that the MR agonist fludrocortisone improves emotional empathy in patients with borderline personality disorder and in healthy women. However, no study up to now has examined the role of MR stimulation on selective attention toward emotional cues and on emotion recognition.
Given the above findings, we decided to examine the effects of fludrocortisone on attentional bias and emotion recognition. As cortisol—endogenously released through psychosocial stress induction or exogenously by hydrocortisone administration—stimulates both receptors, the GR and the MR, the aim of the current study is to investigate the effects of selective MR stimulation on important aspects of social cognition, namely selective attention and emotion recognition. In accordance with research stressing the role of the MR for “fight, fright or flight” behavioral adaptation with regard to selective attention (de Kloet 2014), we hypothesize an attentional bias toward negative stimuli after fludrocortisone intake. This hypothesis is supported by the work of Tsumura and Shimada (2012) and Roelofs et al. (2007) who found an increased attentional bias toward negative stimuli in the early phase of stress response, when MR activity is most pronounced. We do not hypothesize an attentional bias toward positive stimuli, since happy facial expression does not imply hazard, threat, peril, or distress.
Since, in a previous study, we found improved emotional empathy after fludrocortisone administration in healthy participants and controls (Wingenfeld et al. 2014), it was promising to examine whether the general ability to recognize the emotions from facial expressions is improved after fludrocortisone intake. The recognition of emotions from facial expression is a necessary prerequisite for empathy. Given the results on the positive effects of MR stimulation on emotional empathy (Wingenfeld et al. 2014), we further hypothesize that fludrocortisone administration will facilitate emotion recognition.
Since there are heterogeneous results on sex differences with regard to selective attention (Breitberg et al. 2013; Carr et al. 2016; McHugh et al. 2010) and emotion recognition (Duesenberg et al. 2016; Hoffmann et al. 2010; Montagne et al. 2005; Thompson and Voyer 2014), it was promising to examine potential sex differences in an explorative manner. There are previous findings showing that women are more reactive to emotional stimuli than men (Bradley et al. 2001; Cahill 2006). Moreover, there is increasing evidence for sex-dependent effects of the MR (ter Heegde et al. 2015). Therefore, we hypothesize that there are sex differences and that they have an influence as moderator between the association of MR stimulation and selective attention as well as emotion recognition.
Methods and materials
Participants
We recruited 40 healthy women and 40 healthy men. All participants had a university-entrance diploma (German Abitur), were currently enrolled at university, and between 18 and 30 years of age. The mean age was 23.9 years (SD = 3.3). They were recruited through local advertisements such as postings at hospitals, universities, other public places, and on Web sites. Past research has indicated that the sample size of 80 participants is of adequate power for our purpose (Duesenberg et al. 2016). We have conducted post hoc power analysis to confirm this assumption and underpin our stopping rule of data collection.
All participants were medication-free. Exclusion criteria comprised central nervous system diseases, severe somatic diseases, previous traumatic brain injury, malignant tumors, HIV infection, symptomatic cardiac arrhythmias as well as myocardial infarction, hypertension, autoimmune diseases, metabolic or endocrine disease like type I and type II diabetes mellitus, ingestion of oral and inhalative glucocorticoids (all cortisol containing compounds), current infections, allergies, and pregnancy. All potential participants were screened for previous or current axis I disorders according to the Diagnostic and Statistical Manual of Mental Disorders—DSM-IV (Wittchen and Fydrich 1997), and were excluded if any psychiatric disorder was present. Due to the influence of the female cycle on the HPA-axis, all women not using oral contraceptives were tested in the luteal phase (Gaffey et al. 2014; Kirschbaum et al. 1999). The Ethics Committee of the German Psychology Association (DGPs) approved this study, and it is in line with the World Medical Association Declaration of Helsinki. Participants signed a written informed consent before participation.
Procedure
This is a double-blind, placebo-controlled randomized controlled study. In this between-group design, participants received either 4 pills of 0.1 mg fludrocortisone (Florinef®) or 4 identical looking placebo pills. Due to the circadian rhythm of cortisol (Edwards et al. 2001; Kirschbaum and Hellhammer 1989), fludrocortisone or placebo was administered in the afternoon at 1 p.m. The testing started 2 h after drug intake (3 p.m.) at maximum plasma concentrations of fludrocortisone (Ribot et al. 2013). The testing was conducted in a quiet surrounding. The participants followed rules of conduct that prompted participants not to do sports, not to smoke, eat or drink alcoholic or caffeinated beverages within 1 h prior to testing (12 p.m.–1 p.m.). During the 2 h after drug intake until the testing, the participants were sitting in a quite room, where they could only drink water and read.
Salivary cortisol was collected five times using Salivettes® (blue cap, Sarstedt, Germany): 0 (baseline—immediately before fludrocortisone or placebo intake), 90, 120, 180, and 210 min. Salivary cortisol samples were retained at −80 °C (−112 °F) until biochemical analysis. Systolic and diastolic blood pressure as well as heart rate were measured at the same time points. Furthermore, the current psychological state of participants was quantified immediately before testing with the short version of the Multidimensional Mood State Questionnaire (MDBF), i.e., Mehrdimensionaler Befindlichkeitsfragebogen (Steyer et al. 1997). The MDBF is divided into three subscales: mood, vigilance-fatigue, and composure-restlessness. The total score for each subscale of the MDBF ranges between 4 and 20 and every subscale has 4 items. Moreover, we used the Beck Depression Inventory (BDI-II) to measure potential depressive symptoms during the past 2 weeks (Beck et al. 1996).
Hormonal assessment
Cortisol levels were analyzed using an adapted homogenous time-resolved fluorescence resonance energy transfer (HTR-FRET)-based competitive immunoassay. The analysis was conducted in the Neurobiology Laboratory of the Department of Psychiatry, Charité, Universitätsmedizin Berlin. Intra-assay coefficients of variation were below 8 %; inter-assay coefficients of variation were below 10 %. All samples and standards were measured in duplicate, and the limit of detection was 0.2 nmol/L. For a detailed description of the method that was used for hormonal assessment, see Duesenberg et al. (2016).
Assessment of social cognition
We confined the assessment of social cognition to its emotional aspects as captured by the emotional dot-probe task and the facial emotion recognition task.
Emotional dot-probe
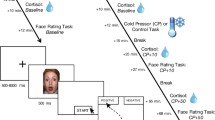
The emotional dot-probe is a computer-based, emotional version of the visual dot-probe paradigm. It is a measure of selective attention toward emotional cues and takes for about 15 min (MacLeod et al. 1986). We used a set of human faces from the FACES database (Ebner et al. 2010), comprising 20 persons (10 female and 10 male) illustrating happy, sad, and neutral facial expressions. As stimuli, two pictures of human faces are quickly presented on a computer screen (for 500 ms). One picture set always consists of two facial expressions of the same person, either paired as neutral-sad, neutral-happy, or neutral-neutral. Afterward, either the left or the right picture is replaced by a vertical bar as the cue (for 1100 ms). The participants are instructed to press one of two keys (left vs right) as quickly as possible in reaction to the position of the cue. The attentional capture is reflected in the response latency. It is assumed that participants react quicker when the cue replaces the picture at which they antecedently concentrated their attention on. The condition in which the cue replaces the location on the screen where the emotional stimulus was placed is called “congruence” (Fig. 1). The condition in which the probe replaces the picture on the opposite side (neutral facial expression) is called “incongruence” (Fig. 1). The neutral-neutral condition was used as control condition to measure the baseline reaction time.
Schematic representation of the emotional dot-probe task—on the left side: procedure of a congruent trial, i.e., the probe follows at the location of the emotional facial expression (happy face). On the right side: procedure of an incongruent trial, i.e., the probe follows at the opposite side (neutral face) of the emotional facial expression
Overall, each participant completes 200 trials, i.e., 40 trials in the neutral condition, 40 trials in the positive congruent condition, 40 trials in the negative congruent condition, 40 trials in the positive incongruent condition, and 40 trials in the negative incongruent condition. The position of the facial pictures is counterbalanced between left and right position, and the order between all trials is quasi-randomized.
The “attentional bias index” can be determined by calculating the average reaction time for incongruence minus congruence (MacLeod and Mathews 1988; Tsumura and Shimada 2012):
A positive attentional bias index score can be interpreted as an attentional bias toward the emotional stimulus (i.e., orientation toward the happy or sad faces), and a negative score as an attentional bias toward the neutral stimulus (i.e., avoiding the sad or happy faces).
Facial emotion recognition task
The facial emotion recognition task is a computer-based test, which measures the ability to recognize facially expressed emotions. Pictures of human faces were presented for 1 s, expressing an emotion (sad, neutral, or angry) followed by a gray screen (for 4 s). The participants are asked to classify the emotion by selecting one of three possible answers (sadness, neutral, or anger) by pressing the respective key on the keyboard (arrow keys). The two basic emotions, anger and sadness, were used as emotion recognition task, and the neutral stimuli were used as control trial. Facial expressions of the emotion anger and sadness were morphed into four emotional intensities (20, 30, 40, and 80 %) based on the full (100 %) facial expression of the emotion (Fig. 2). For each emotional valence (sadness and anger), 24 trials per intensity (20, 30, 40, and 80 %) and 24 control trials (neutral–0 % emotion) were shown. In sum, each participant was asked to classify 216 pictures of facial expressions in randomized order. Both correct answers and errors (false classification of emotion) were recorded. The facial expressions (6 male and 6 female faces) were taken from the NIMSTIM scale (Tottenham et al. 2009). The facial emotion recognition task lasted for about 15 min.
Statistical analyses
SPSS version 22.0 was used for all statistical analyses. χ 2 tests were used for categorical data and Student’s t tests for continuous data in order to analyze demographic differences between the treatment groups (fludrocortisone vs placebo).
Moreover, we used a 2 × 2 ANOVA to analyze the emotional dot-probe with valence (attentional bias index for sad faces and for happy faces) as within-subject factor and treatment (fludrocortisone vs placebo) as between-subject factor.
We used a 2 × 2 × 4 ANOVA to analyze the facial emotion recognition task with intensity (20, 30, 40, and 80 %) and valence (anger vs sadness) as within-subject factors and treatment (fludrocortisone vs placebo) as between-subject factor.
RmANOVAs with time (0, 90, 120, 180, and 210 min after drug intake) as within-subject factor and treatment (fludrocortisone vs. placebo) as between-subject factor were used to analyze salivary cortisol, blood pressure, and heart rate.
Greenhouse-Geisser corrections were applied when indicated. Post hoc tests (ANOVA and t tests) were performed in event of a significant interaction. For ANOVA, partial η 2 was used as effect size and for t tests, Cohen’s d, respectively.
Results
Demographic characteristics
There were no significant differences in the treatment groups (fludrocortisone vs placebo) regarding age, body mass index, smoking, and psychological state immediately prior to testing. Female participants who took fludrocortisone and female participants who took placebo did not differ with respect to the intake of oral contraceptives. Demographic characteristics of our sample are summarized in Table 1.
Salivary cortisol secretion during testing
In order to minimize the influence of potential outliers, we excluded participant’s salivary cortisol values from statistical analyses if they deviated ±2 SD from the mean for more than 50 % of the time (i.e., at least in 3 of 5 salivary cortisol values). Therefore, salivary cortisol values from 4 participants (3 from the fludrocortisone group and 1 from the placebo group) were excluded. Due to significant baseline differences between the fludrocortisone and placebo group (t(74) = −2.55, p = .013; d = 0.6), we performed a baseline correction by subtracting the baseline value at 0 min from the subsequent values at 90, 120, 180, and 210 min after drug/placebo intake. RmANOVA with time (0, 90, 120, 180, and 210 min after drug intake) as within-subject factor and treatment as between-subject factor revealed no significant main effect of treatment. There was a significant main effect of time (F(4, 71) = 28.31, p < .001; η p 2 = .29) and a significant time × treatment interaction (F(4, 71) = 9.71, p < .001; η p 2 = .12). Post hoc tests revealed significant differences between the cortisol values of the placebo and the fludrocortisone group at 180 min (t(71) = 2.03, p = .047) and 210 min (t(72) = 2.95, p = .004) after drug intake. Cortisol delta values (i.e., baseline value at 0 min minus the last value 210 min after drug intake) were calculated, and we found significant differences between the placebo and the fludrocortisone group (t(73) = −2.96, p = .004; d = 0.7), indicating greater cortisol suppression after fludrocortisone intake compared to placebo (see Fig. 3).
The course of salivary cortisol levels—shown are salivary cortisol values (nmol/l) after fludrocortisone and placebo intake after baseline correction. Error bars represent standard errors. The duration of the facial emotion recognition task (FER) and the emotional dot-probe task (EDP) are marked. Both lasted for about 15 min
Blood pressure and heart rate
We found no significant treatment effect on systolic and diastolic blood pressure. There was a significant main effect of time on systolic (F(4, 78) = 13.70, p < .001; η p 2 = .15) and diastolic blood pressure (F(4, 78) = 5.68, p < .001; η p 2 = .07), indicating a decrease over time. However, we found no significant time × treatment interaction in systolic and diastolic blood pressure.
Regarding the heart rate, there was no significant main effect of treatment. We found a significant effect of time (F(4, 78) = 21.54, p < .001, η p 2 = .22), indicating a decrease in heart rate over time. We found no significant time × treatment interaction.
The data of systolic and diastolic blood pressure as well as heart rate were summarized in Table 2.
Emotional dot-probe task—attentional bias toward emotional cues
We excluded reaction times from statistical analyses that were less than 100 ms (anticipation error) and greater than 1500 ms (lack of concentration) in order to minimize the influence of outliers. We only analyzed response latencies from correct responses. Loss of data was less than 2 %.
2 × 2 ANOVA revealed a significant treatment effect (F(1, 78) = 4.18, p = .04, η p 2 = .05). There was no significant main effect of valence. However, we found a significant interaction of treatment × valence (F(1, 78) = 6.20, p = .02, η p 2 = .07).
To disentangle the interaction of valence × treatment, we performed separate ANOVAs for attentional bias indices for sad and happy faces, respectively. Univariate ANOVA revealed a significant treatment effect (F(1, 76) = 9.39, p = .003; η p 2 = .11) for sad faces, indicating an attentional bias away from sad faces after placebo intake and a shift in selective attention toward sad faces compared to placebo (see Fig. 4). There was no significant treatment effect for facial expressions of happiness. To examine whether the attentional bias scores differ significantly from zero, we used one sample t test and found an attentional bias away from sad faces in the placebo group (t(39) = −2.9, p = .006), but not toward sad faces in the fludrocortisone group (t(39) = 1.579, p = .12). Respectively, for happy faces, there were no significant differences after placebo intake (t(39) = .267, p = .791), nor after fludrocortisone intake (t(39) = .443, p = .660).
Attentional bias index for the valences sadness and happiness—the attentional bias away from sad faces in the placebo group and the shift in selective attention toward sad faces compared to placebo is shown on the left. The lack of a significant attentional bias toward happy faces is shown on the right. Error bars represent standard errors
Furthermore, we examined whether the attentional bias index differs between sad and happy faces in both groups and found significant differences between the attentional bias index of sad and happy faces in the placebo group (t(39) = −2.38, p = .022), but not in the fludrocortisone group (t(39) = 1.11, p = .27).
There was no significant difference between the placebo and the fludrocortisone group regarding the baseline reaction time, measured by the neutral-neutral condition (t(78) = −.244, p = .808).
There were no differences between women and men in the emotional dot-probe task.
Correlation between salivary cortisol and attentional bias to emotional cues
There was no significant correlation between cortisol suppression after fludrocortisone intake and attentional bias index for sad faces (r = −.117, p = .472) or for happy faces (r = .027, p = .869).
Moreover, there were no significant correlations between the raw data for cortisol at each time point and the attentional bias index for sad faces. The same was true for the attentional bias index for happy faces.
Facial emotion recognition task
The 2 × 2 × 4 ANOVA revealed no significant treatment effect. There was a significant main effect of valence (F(1, 78) = 55.82, p < .001; η p 2 = .42), i.e., the number of correct answers was lower in anger than in sadness. As expected, there was a significant main effect of intensity (F(3, 234) = 1501.12, p < .001; η p 2 = .95); the higher the intensity of emotional expression, the higher the accuracy of recognition.
There was a significant interaction of treatment × intensity (F(3, 234) = 3.15, p = .025; η p 2 = .04). However, post hoc t test revealed no significant differences between the placebo and the fludrocortisone group.
Furthermore, the 2 × 2 × 4 ANOVA revealed a significant valence × intensity interaction (F(3, 234) = 12.20, p < .001; η p 2 = .14). Post hoc t test revealed significant differences between anger and sadness, i.e., the number of correct answers was lower in anger than in sadness when the intensity was 20 % (t(79) = −.7.58, p < .001; d = 1.69), 30 % (t(79) = −6.72, p < .001; d = 1.50), or 40 % (t(79) = −6.23, p < .001; d = 1.39), but not when the intensity was 80 %. All other interactions were not significant (p > .05).
Women and men did not differ in the facial emotion recognition task.
Discussion
We examined the effect of the MR agonist fludrocortisone on attentional bias toward emotional cues and on emotion recognition in young healthy subjects. As predicted, fludrocortisone administration increased selective attention toward negative emotional cues. By contrast, fludrocortisone did not affect attentional bias toward happy faces and did not improve overall emotion recognition accuracy in our healthy, young, high-educated sample.
Emotional dot-probe task
Our results of significantly increased selective attention toward negative emotional cues after MR stimulation are in line with Tsumura and Shimada (2012), who found evidence for an attentional bias toward sadness- and depression-related words immediately after a psychosocial stressor, which may well reflect the early and rapid effects of MR activation (Joëls et al. 2012; Oitzl et al. 2010; Tsumura and Shimada 2012). Moreover, Roelofs et al. (2007) have found that psychosocial stress-induced cortisol release is associated with an increased attentional bias toward angry faces, but not toward happy faces by using the masked emotional Stroop task. Again, this effect was measured shortly after stress induction and may, therefore, also be linked to the rapid non-genomic effects of MR occupation (Joëls et al. 2012; Oitzl et al. 2010). In contrast, McHugh et al. (2010) report decreased attentional bias toward threatening stimuli 30 min following a psychosocial stressor. This may further indicate that the attentional bias is to be found in the early phase (within seconds to minutes) of stress response, where the activation of the noradrenergic system and the rapid (selective) MR effects play a crucial role. Since the MR agonist fludrocortisone leads to a shift in selective attention toward sad faces compared to placebo, our findings substantiate the interpretation of Tsumura and Shimada (2012) that rapid MR stimulation may play a critical role on increased selective attention toward negative emotional cues. These results are consistent with the well-established hypothesis that emotional information processing is improved in the early phase (within seconds to minutes) of cortisol response (de Kloet 2014; Oitzl et al. 2010).
The MR seems to modulate selective attention in a valence-specific way. Especially those studies investigating the early effects of stress revealed an enhanced selective attention to negative stimuli, which was also the case in the current study. The emotional valence of sadness, unlike happiness, is closely linked to fear, threat, and anger as being an expression of aversive and negative, psychosocial stress-associated stimuli. This is consistent with evidence that the MR is crucial for stress-associated emotional arousal and adaptive behavior (Brinks et al. 2007), as well as for the appraisal of new, unknown situations (De Kloet 2013) and for the rapid selection of response strategies (Vogel et al. 2016). This is biologically plausible, because the hippocampus, the lateral septum, and the amygdala, where the MR is highly expressed (de Kloet 2014), are closely related to the processing of emotional information (Groeneweg et al. 2012; Joëls et al. 2011), and because the lateral septum and the amygdala are associated with effective stress coping (Singewald et al. 2011) and emotion (Rolls 2015; Sheehan et al. 2004). The attentional bias away from sad faces, which was found in our placebo group, is in line with previous studies on healthy subjects (e.g., Joormann and Gotlib 2007).
Facial emotion recognition task
Fludrocortisone did not improve overall emotion recognition accuracy in our study. A post hoc power analysis with α = 0.05 and η p 2 = 0.04 revealed a sufficient power of 0.99, i.e., only 54 participants were needed to detect the interaction of treatment × intensity that we found in our study (Faul et al. 2007). Therefore, the hypothesis that an inadequate test power is the reason of the non-significant treatment effect appears unlikely.
However, we did find that it was more difficult to recognize anger correctly than sadness, if the emotion was not very salient and task difficulty was high, i.e., for emotional intensities of 20, 30, and 40 %, but not for 80 %. As one would expect, the recognition accuracy was better when the emotion was vivid and well-marked, i.e., task difficulty was low (80 % emotional intensity) and the recognition accuracy was worse when task difficulty was high (20, 30, and 40 % emotional intensity).
In a recent study, Deckers et al. (2015) found that healthy controls were significantly better in emotion recognition following the Trier Social Stress Test compared to baseline recognition accuracy before psychosocial stress. In contrast, fludrocortisone intake did not have this effect in our sample of young healthy subjects. Therefore, it seems unlikely that MR activation alone explains the findings of Deckers et al. (2015). Since the results of Duesenberg et al. (2016) suggest that GR is also not directly modulating emotion recognition, it is necessary to examine the role of other hormones, for example adrenaline or noradrenaline, that are released in response to stress and might alter emotion recognition. However, Deckers et al. (2015) had no non-stress control group, and they used a repeated measurement design. Therefore, a practice effect cannot be excluded as an explanation of their results.
Sex differences
Contrary to our hypothesis, we failed to find a significant main effect of sex for the emotional dot-probe task and the facial emotion recognition task. These results do not fit to previous studies that found differences between men and women in the facial emotion recognition task (Duesenberg et al. 2016; Hoffmann et al. 2010; Montagne et al. 2005; Thompson and Voyer 2014). Regarding selective attention, there are only few and more heterogeneous results. Most of the studies did not report any sex effects (Roelofs et al. 2007; Tsumura and Shimada 2012), or examined only men (Putman et al. 2007, 2010; van Honk et al. 2000). In contrast to our results, Breitberg et al. (2013) found a dose-dependent reduction of an attentional bias toward happy faces in women, but not in men. Furthermore, McHugh et al. (2010) found a higher association between acute changes in cortisol levels after a psychological stressor and changes in a negative attentional bias in women than in men. However, comparable with Taylor et al. (2011) and van Honk et al. (1998), we found no significant sex differences.
Salivary cortisol secretion
We used fludrocortisone, because it is known to activate the MR even better than physiological aldosterone and because the affinity of the MR to fludrocortisone is about 15 times higher than the affinity of the GR (Agarwal et al. 1977; Otte et al. 2003). As expected, we found a decrease of salivary cortisol levels over time after fludrocortisone intake compared to placebo intake in our sample (baseline corrected salivary cortisol values). This fits very well to the known inhibitory effect of MR stimulation on the HPA-axis, even in non-stress conditions (Buckley et al. 2007; Karamouzis et al. 2013; Otte et al. 2003). Moreover, there were significantly higher absolute cortisol levels (raw data) in the fludrocortisone group compared to the placebo group at 0, +90, and +120 min, but not at +180 and +210 min. In spite of this drop of salivary cortisol levels taking place between +120 and +180 min, there is a limitation of our study in this regard. Since testing took place between +150 to +180 min, it would have been best to measure salivary cortisol also at +150 min. This would have made it even clearer that absolute cortisol levels had no influence on our outcome variables with regard to the time frame between +150 to +180 min. Nonetheless, we found no significant correlations between the absolute data of cortisol at each time point and the attentional bias index.
Strength and weaknesses
Our study has several strengths: it is a well-controlled study and it is the first study that examines the effects of fludrocortisone on attentional bias and emotion recognition. Moreover, we balanced female and male participants at the outset in order to analyze potential sex effects and we had a relatively large sample size. Inclusion and exclusion criteria were clearly stated, and since the participants had to pursue rules of conduct, potential confounding effects could be minimized.
One limitation of our study is the restriction on anger and sadness as the sole emotional valences in the facial emotion recognition task and likewise happiness and sadness in the emotional dot-probe. It might have been revealing to include different emotions. Moreover, we did not measure plasma concentration of fludrocortisone.
Since we only included young and well-educated participants, our results cannot be generalized to other populations. Furthermore, fludrocortisone has at least some GR potency, although its affinity to the MR is about 15 times higher than its affinity to the GR (Agarwal et al. 1977). Therefore, a potential influence of GR activity cannot be completely excluded.
Future studies may further examine the role of mineralocorticoid receptor stimulation on attentional bias toward emotional cues and may also include additional emotions like fear and anger. Findings have demonstrated that genetic variances, haplotypes, and polymorphisms of the MR gene (NR3C2) moderate aspects of affective processing like amygdala reactivity after emotional childhood neglect (Bogdan et al. 2012) and elevated optimism as a protective factor for depression in women (Klok et al. 2011). Even more relevant, Vogel et al. (2014) have linked variances of NR3C2 with memory bias for negative emotion. Therefore, it might be very interesting to systematically explore the differential role of NR3C2 variances on affective processing, especially in reactivity-, withdrawal-, and social threat-related emotions like sadness, anger, fear, or disgust and to examine attentional bias in particular.
Due to our homogeneous sample characteristics, it would be enlightening to compare the results of these young, healthy, and high-educated subjects with other samples as well as in clinical samples, e.g., in depression. It is also important to investigate whether the observed fludrocortisone-related effect on attentional bias toward sad emotional cues is dose-dependent and to assess its exact timing.
Conclusion
To our knowledge, this is the first study examining the effects of MR stimulation on the emotional dot-probe task and the facial emotion recognition task. We found a significant shift in selective attention toward sad faces compared to placebo, but no selective attention toward happy faces after fludrocortisone intake. This extends the existing literature that MR function plays a crucial role in quick and automatic behavioral adaption in response to emotional-charged situations that typically arise from stressful social interaction.
References
Agarwal M, Coupry F, Philippe M (1977) Physiological activity and receptor binding of 9α fluorohydrocortisone. Biochem Biophys Res Commun 78:747–753
Beck AT, Steer RA, Brown GK (1996) Manual for the beck depression inventory-II. San Antonio, TX: Psychological Corporation, 1, 82
Bogdan R, Williamson DE, Hariri AR (2012) Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am J Psychiatr
Bradley MM, Codispoti M, Sabatinelli D, Lang PJ (2001) Emotion and motivation II: sex differences in picture processing. Emotion 1:300
Breitberg A, Drevets WC, Wood SE, Mah L, Schulkin J, Sahakian BJ, Erickson K (2013) Hydrocortisone infusion exerts dose-and sex-dependent effects on attention to emotional stimuli. Brain Cogn 81:247–255
Brinks V, van der Mark MH, de Kloet ER, Oitzl MS (2007) Differential MR/GR activation in mice results in emotional states beneficial or impairing for cognition. Neural plasticity 2007
Brinks V, Berger S, Gass P, De Kloet E, Oitzl M (2009) Mineralocorticoid receptors in control of emotional arousal and fear memory. Horm Behav 56:232–238
Buckley TM, Mullen BC, Schatzberg AF (2007) The acute effects of a mineralocorticoid receptor (MR) agonist on nocturnal hypothalamic–adrenal–pituitary (HPA) axis activity in healthy controls. Psychoneuroendocrinology 32:859–864
Cahill L (2006) Why sex matters for neuroscience. Nat Rev Neurosci 7:477–484
Carr AR, Scully A, Webb M, Felmingham KL (2016) Gender differences in salivary alpha-amylase and attentional bias towards negative facial expressions following acute stress induction. Cognit Emot 30:315–324
De Kloet E (2013) Functional profile of the binary brain corticosteroid receptor system: mediating, multitasking, coordinating, integrating. Eur J Pharmacol 719:53–62
de Kloet RE (2014) From receptor balance to rational glucocorticoid therapy. Endocrinology 155(8):2754–2769
de Kloet ER, Oitzl MS, Joëls M (1999) Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci 22:422–426
Deckers JW, Lobbestael J, van Wingen GA, Kessels RP, Arntz A, Egger JI (2015) The influence of stress on social cognition in patients with borderline personality disorder. Psychoneuroendocrinology 52:119–129
Duesenberg M, Weber J, Schulze L, Schaeuffele C, Roepke S, Hellmann-Regen J, Otte C, Wingenfeld K (2016) Does cortisol modulate emotion recognition and empathy? Psychoneuroendocrinology
Ebner NC, Riediger M, Lindenberger U (2010) FACES—a database of facial expressions in young, middle-aged, and older women and men: development and validation. Behav Res Methods 42:351–362
Edwards S, Clow A, Evans P, Hucklebridge F (2001) Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci 68:2093–2103
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Gaffey AE, Wirth MM, Hoks RM, Jahn AL, Abercrombie HC (2014) Circulating cortisol levels after exogenous cortisol administration are higher in women using hormonal contraceptives: data from two preliminary studies. Stress 17:314–320
Groeneweg FL, Karst H, de Kloet ER, Joëls M (2012) Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Mol Cell Endocrinol 350:299–309
Hoffmann H, Kessler H, Eppel T, Rukavina S, Traue HC (2010) Expression intensity, gender and facial emotion recognition: women recognize only subtle facial emotions better than men. Acta Psychol 135:278–283
Joëls M, Karst H, DeRijk R, De Kloet ER (2008) The coming out of the brain mineralocorticoid receptor. Trends Neurosci 31:1–7
Joëls M, Fernandez G, Roozendaal B (2011) Stress and emotional memory: a matter of timing. Trends Cogn Sci 15:280–288
Joëls M, Sarabdjitsingh RA, Karst H (2012) Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev 64:901–938
Joormann J, Gotlib IH (2007) Selective attention to emotional faces following recovery from depression. J Abnorm Psychol 116:80
Karamouzis I, Berardelli R, Marinazzo E, D’Angelo V, Zinnà D, Minetto MA, Zichi C, Fussotto B, Giordano R, Ghigo E (2013) The acute effect of fludrocortisone on basal and hCRH-stimulated hypothalamic–pituitary–adrenal (HPA) axis in humans. Pituitary 16:378–385
Kirschbaum C, Hellhammer DH (1989) Salivary cortisol in psychobiological research: an overview. Neuropsychobiology 22:150–169
Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH (1999) Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 61:154–162
Klok MD, Alt SR, Irurzun Lafitte AJ, Turner JD, Lakke EA, Huitinga I, Muller CP, Zitman FG, de Kloet ER, Derijk RH (2011) Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. J Psychiatr Res 45(7):871–878
Lai M, Horsburgh K, Bae SE, Carter RN, Stenvers DJ, Fowler JH, Yau JL, Gomez‐Sanchez CE, Holmes MC, Kenyon CJ (2007) Forebrain mineralocorticoid receptor overexpression enhances memory, reduces anxiety and attenuates neuronal loss in cerebral ischaemia. Eur J Neurosci 25:1832–1842
MacLeod C, Mathews A (1988) Anxiety and the allocation of attention to threat. Q J Exp Psychol 40:653–670
MacLeod C, Mathews A, Tata P (1986) Attentional bias in emotional disorders. J Abnorm Psychol 95:15
McHugh RK, Behar E, Gutner CA, Geem D, Otto MW (2010) Cortisol, stress, and attentional bias toward threat. Anxiety Stress Coping 23:529–545
Mitra R, Ferguson D, Sapolsky RM (2009) Mineralocorticoid receptor overexpression in basolateral amygdala reduces corticosterone secretion and anxiety. Biol Psychiatry 66:686–690
Montagne B, Kessels RP, Frigerio E, de Haan EH, Perrett DI (2005) Sex differences in the perception of affective facial expressions: do men really lack emotional sensitivity? Cogn Process 6:136–141
Oitzl MS, Champagne DL, van der Veen R, de Kloet ER (2010) Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev 34:853–866
Otte C, Yassouridis A, Jahn H, Maass P, Stober N, Wiedemann K, Kellner M (2003) Mineralocorticoid receptor-mediated inhibition of the hypothalamic-pituitary-adrenal axis in aged humans. J Gerontol A Biol Sci Med Sci 58:B900–B905
Putman P, Roelofs K (2011) Effects of single cortisol administrations on human affect reviewed: Coping with stress through adaptive regulation of automatic cognitive processing. Psychoneuroendocrinology 36:439–448
Putman P, Hermans EJ, Koppeschaar H, Van Schijndel A, Van Honk J (2007) A single administration of cortisol acutely reduces preconscious attention for fear in anxious young men. Psychoneuroendocrinology 32:793–802
Putman P, Hermans EJ, van Honk J (2010) Cortisol administration acutely reduces threat-selective spatial attention in healthy young men. Physiol Behav 99:294–300
Ribot M, Polito A, Grassin-Delyle S, Annane D, Alvarez J-C (2013) Human plasma quantification of fludrocortisone using liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometry after low-dosage administration. Clin Chim Acta 420:109–113
Rimmele U, Besedovsky L, Lange T, Born J (2013) Blocking mineralocorticoid receptors impairs, blocking glucocorticoid receptors enhances memory retrieval in humans. Neuropsychopharmacology 38:884–894
Rimmele U, Besedovsky L, Lange T, Born J (2015) Emotional memory can be persistently weakened by suppressing cortisol during retrieval. Neurobiol Learn Mem 119:102–107
Roelofs K, Bakvis P, Hermans EJ, van Pelt J, van Honk J (2007) The effects of social stress and cortisol responses on the preconscious selective attention to social threat. Biol Psychol 75:1–7
Rolls ET (2015) Limbic systems for emotion and for memory, but no single limbic system. Cortex 62:119–157
Rozeboom AM, Akil H, Seasholtz AF (2007) Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci 104:4688–4693
Sandi C, Haller J (2015) Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci 16:290–304
Sheehan TP, Chambers RA, Russell DS (2004) Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Rev 46:71–117
Singewald GM, Rjabokon A, Singewald N, Ebner K (2011) The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology 36:793–804
Steyer R, Schwenkmezger P, Notz P, Eid M (1997) MDBF–Mehrdimensionaler Befindlichkeitsfragebogen. Hogrefe, Göttingen
Taylor VA, Ellenbogen MA, Washburn D, Joober R (2011) The effects of glucocorticoids on the inhibition of emotional information: a dose–response study. Biol Psychol 86:17–25
ter Heegde F, De Rijk RH, Vinkers CH (2015) The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology 52:92–110
Ter Horst J, Carobrez A, Van Der Mark M, De Kloet E, Oitzl M (2012) Sex differences in fear memory and extinction of mice with forebrain‐specific disruption of the mineralocorticoid receptor. Eur J Neurosci 36:3096–3102
Thompson AE, Voyer D (2014) Sex differences in the ability to recognise non-verbal displays of emotion: a meta-analysis. Cognit Emot 28:1164–1195
Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C (2009) The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 168:242–249
Tsumura H, Shimada H (2012) Acutely elevated cortisol in response to stressor is associated with attentional bias toward depression-related stimuli but is not associated with attentional function. Appl Psychophysiol Biofeedback 37:19–29
van Honk J, Tuiten A, van den Hout M, Koppeschaar H, Thijssen J, de Haan E, Verbaten R (1998) Baseline salivary cortisol levels and preconscious selective attention for threat: a pilot study. Psychoneuroendocrinology 23:741–747
van Honk J, Tuiten A, van den Hout M, Koppeschaar H, Thijssen J, de Haan E, Verbaten R (2000) Conscious and preconscious selective attention to social threat: different neuroendocrine response patterns. Psychoneuroendocrinology 25:577–591
Vogel S, Fernández G, Joëls M, Schwabe L (2016) Cognitive adaptation under stress: a case for the Mineralocorticoid Receptor. Trends Cogn Sci
Vogel S, Gerritsen L, van Oostrom I, Arias-Vásquez A, Rijpkema M, Joëls M, Franke B, Tendolkar I, Fernández G (2014) Linking genetic variants of the mineralocorticoid receptor and negative memory bias: interaction with prior life adversity. Psychoneuroendocrinology, 40, 181-190
Wingenfeld K, Kuehl LK, Janke K, Hinkelmann K, Dziobek I, Fleischer J, Otte C, Roepke S (2014) Enhanced emotional empathy after mineralocorticoid receptor stimulation in women with borderline personality disorder and healthy women. Neuropsychopharmacology 39:1799–1804
Wittchen H-U, Fydrich T (1997) Strukturiertes Klinisches Interview für DSM-IV (SKID-I und SKID-II). Hogrefe
Zhou M, Kindt M, Joëls M, Krugers HJ (2011) Blocking mineralocorticoid receptors prior to retrieval reduces contextual fear memory in mice. PLoS One 6, e26220
Acknowledgments
We would like to thank all subjects for their participation in the present study.
Author contributions
Katharina Schultebraucks, Katja Wingenfeld, and Christian Otte developed study concept.
All authors contributed to the study design. Testing and data collection were performed by Katharina Schultebraucks, Antonia Domke and Lisa Lockenvitz. Katharina Schultebraucks performed data analysis and interpretation under the supervision of Katja Wingenfeld and Christian Otte. Katharina Schultebraucks drafted the manuscripts, and Katja Wingenfeld and Christian Otte provided critical revisions. Lars Schulze coded the facial emotion recognition task and Linn K. Kuehl and Christian E. Deuter coded the emotional dot-probe task. Moritz Duesenberg implemented the facial emotion recognition task. Julian Hellmann-Regen analyzed and interpreted the salivary cortisol data. All authors approved the final version of the manuscript for submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared no conflicts of interest with respect to the authorship or the publication of this article.
Funding
No external funding.
Rights and permissions
About this article
Cite this article
Schultebraucks, K., Deuter, C.E., Duesenberg, M. et al. Selective attention to emotional cues and emotion recognition in healthy subjects: the role of mineralocorticoid receptor stimulation. Psychopharmacology 233, 3405–3415 (2016). https://doi.org/10.1007/s00213-016-4380-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4380-0