Abstract
Cortisol induces attentional bias toward a negative stimulus and impaired attentional function. Depressed individuals have high levels of cortisol, and exhibit an attentional bias toward a depression-related stimulus and impaired processing speed and executive attention, which are components of attentional function. Therefore, the study tested the hypotheses that an acute increase in cortisol in response to a stressor is associated with attentional bias toward a depression-related stimulus and impaired processing speed and executive attention. Thirty-six participants were administered the dot-probe task for the measurement of attentional bias toward a depression-related stimulus and the Trail Making Test A and B for the measurement of processing speed and executive attention before and after a mental arithmetic task. It was revealed that attentional bias toward a depression-related stimulus following the stressor was observed only among the responders (i.e., participants with cortisol elevation in response to a stressor). On the other hand, no differences in the performance of processing speed and executive attention were noted between the responders and non-responders. The results indicate that acutely elevated cortisol is related to attentional bias, but is not related to processing speed and executive attention. The results have an implication for the etiology of depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cortisol is a steroid hormone secreted from the adrenal cortex in humans and acts on the central nervous system; moreover, with respect to the effects on information processing, it has been reported that cortisol is related to two aspects of dysfunctional attention: attentional bias toward a negative stimulus (Putman and Roelofs 2011; Roelofs et al. 2007; van Honk et al. 1998) and impaired attentional function (Bohnen et al. 1990; Jameison and Dinan 2001; Lupien et al. 1994). Attentional bias toward a negative stimulus refers to preferential attentional allocation toward a negative stimulus relative to a neutral one, and impaired attentional function is general pervasive impairment in ability of voluntarily selecting relevant stimuli or inhibiting irrelevant stimuli. Cortisol affects attentional bias and attentional function under the following biological substrates. Cortisol secreted from the adrenal cortex passes through the brain-blood barrier and accesses the brain. Cortisol activates the neural activity in the amygdala, while it deactivates the neural activity in the prefrontal cortex (McEwen 2005). The amygdala affects attentional bias toward a negative stimulus (Gamer and Büchel 2009; Vuilleumier 2005), and the prefrontal cortex affects attentional function (Fuster 1997), and as a result, excessive cortisol leads to dysfunctional attention.
With respect to the cortisol actions on attentional bias, differential results were reported, and they may be moderated by stimulus type. Most previous studies have investigated the effects of cortisol on attentional bias toward fear, angry, or threatening stimuli. On the basis of the findings of cortisol administration studies, it was suggested that cortisol induces attentional bias toward an angry or a threatening stimulus, and in contrast, attenuates attentional bias toward a fear stimulus (Putman and Roelofs 2011). In addition, previous findings were mostly obtained using facial stimuli displaying emotion expression, and therefore, there may be differences between facial and non-facial stimuli.
It was reported that attentional bias and impaired attentional function become evident especially under stress (Braunstein-bercovitz 2003; Scher et al. 2005). It is assumed that cortisol is the mediator between the stressor and dysfunctional attention (Skosnik et al. 2000), since a stressor activates the hypothalamic–pituitary–adrenal axis (HPA axis). Cortisol is an end product of the HPA axis and is secreted in response to a stressor along with a psychological stress response (Bassett et al. 1987). Stress-induced cortisol acutely increases the amygdala activation and decreases the prefrontal cortex activation, so that dysfunctional attention emerges under stress.
Depressed individuals have high levels of cortisol (Vreeburg et al. 2009), and thus, it is assumed that high cortisol levels may be one of the contributors to the pathophysiology of depression (van Praag 2005). In fact, a longitudinal study revealed that high levels of cortisol are a risk factor for future depression (Halligan et al. 2007), and the drugs that block cortisol actions or inhibit cortisol synthesis alleviate depressive symptoms (Thomson and Craighead 2008). However, the mechanisms through which cortisol develops and exacerbates depression have not yet been revealed.
Depressed individuals exhibit an attentional bias toward a depression-related stimulus such as sad facial pictures or words depicting depressive symptoms (Westra and Kuiper 1997) and impaired attentional function (Landrø et al. 2001) as well as high cortisol levels. Attentional bias toward a depression-related stimulus and impairment in attentional function are cognitive vulnerability to depression. With respect to attentional bias in depression, the association between depression and attentional bias is unclear. In particular, mixed results were reported on the relationship between depression and attentional bias at an early stage attentional bias: it was reported that depressed individuals did not exhibit an early stage attentional bias (e.g., Koster et al. 2005), while it was also reported that an early stage attentional bias were found in depression (e.g., Bradley 1997b). Attentional bias between an early and a late stage is distinguished according to the time course of information processing (Cisler and Koster 2010; Williams et al. 1997). An early stage attentional bias is based on automatic processing, and a late stage attentional bias is based on controlled processing (Cisler and Koster 2010; Williams et al. 1997). However, a meta-analytic study shows that the relationship between depression and attentional bias is stronger in studies using a dot-probe task compared to those using an emotional Stroop task (Peckham et al. 2010). On the basis of the finding that there was no correlation between performances in a dot-probe task and an emotional Stroop task (Mogg et al. 2000), it was suggested that a dot-probe task and an emotional Stroop task may measure different attentional processes (Applehans and Luecken 2006). Regarding an emotional Stroop task, the interpretation of Stroop interference, which is the index of an emotional Stroop task, is unclear; furthermore, it is apprehended that Stroop interference cannot be determined to reflect conflict at the stage of attention allocation or that of response generation (MacLeod 1991). Therefore, the results of the meta-analysis suggest that both early and late stage attentional biases can be detected in depression by using a dot-probe task, which is a more direct measure of attention. Attentional bias toward a depression-related stimulus facilitates detection and elaboration of a depression-related stimulus, and impairment in attentional function leads to difficulty in functioning adequately in employment and social settings. In fact, it was reported that the interventions aimed at modifying attentional bias (Baert et al. 2010; Wells and Beevers 2010) and attentional function (Papageorgiou and Wells 2000) attenuate depressive symptoms. From the findings that both high levels of cortisol and attentional dysfunction are observed in depressed individuals, it is assumed that cortisol may exacerbate depressive symptoms by inducing attentional bias and impairing attentional function.
The cortisol actions on information processing under stress are different from the actions of cortisol elevated by physical exercise or cortisol administration (McHugh et al. 2010). Stress-induced cortisol is accompanied by negative emotional arousal, and it was considered to be the reason for the differences in the cortisol actions (McHugh et al. 2010). Therefore, to elucidate the cortisol actions under stress, it is needed to investigate the effects of stress-induced cortisol on attention through the arousal of negative emotions. However, few researches have investigated the association between stress-induced cortisol and attentional bias toward a depression-related stimulus and attentional function. Some studies investigated the effects of stress-induced cortisol on attentional bias toward an angry (Roelofs et al. 2007) or a threatening stimulus (McHugh et al. 2010), and Ellenbogen et al. (2010) investigated the effects of attentional bias toward a depression-related stimulus on cortisol response to a stressor. However, no study investigated whether stress-induced cortisol influences attentional bias toward a depression-related stimulus. With respect to neurocognitive deficits in depression, inconsistent results were obtained. However, meta-analyses revealed that impairment in processing speed and executive attention is consistently found in depression (McDermott and Ebmeier 2009; Stefanopoulou et al. 2009). Although previous researches investigated the effects of stress-induced cortisol on selective attention and divided attention (Vedhara et al. 2000) and attentional inhibition (Skosnik et al. 2000), only one research investigated the effects of stress-induced cortisol on processing speed and executive attention (Bohnen et al. 1990). However, since Bohnen et al. (1990) compared the attentional performance between individuals who were administered stress tasks and individuals who had control session such as reading popular journals or watching amusement videos without the stress tasks, differential performance cannot be determined due to cortisol or to subjective negative emotions.
In the research on the effects of cortisol on information processing in stress contexts, not all the participants show cortisol elevation following a stressor (i.e., non-responders) (Kirschbaum et al. 1993). Therefore, the data from the non-responders may disturb the true association between cortisol and attention. It is assumed that dysfunctional attention would occur only in individuals with cortisol elevation in response to a stressor (i.e., responders). Thus, to determine whether cortisol is related to attentional bias toward a depression-related stimulus and impairment in processing speed and executive attention, we compared the changes in attentional bias and the attentional functions before and after a stressor among responders and non-responders. In addition, to control the confounding effects of negative emotion on attentional bias and attentional function, both the groups were administered the same stressor, and then, we controlled the influence of negative emotional response. Following hypotheses were tested: responders would show (1) attentional bias toward a depression-related stimulus and (2) impaired processing speed and executive attention after a stressor, but non-responders would not show dysfunctional attention.
Methods
Participants
Thirty-six undergraduate and graduate students (27 females and 9 males) participated in the study. Mean age was 23.0 years (SD = 5.9) ranging from 20 to 40 years of age. The participants were recruited through advertisements on the university campus. All those who reported any medication use on the day of the experiment or had a history of smoking were excluded. Participants received compensation for their participation. The study was approved by the local ethics committee of Waseda university. All the participants provided written informed consent for participation in the study.
Measures
Depressive symptoms. The Japanese version of the Center for Epidemiologic Studies-Depression Scale (CES-D; Shima et al. 1985) was used for measuring depressive symptoms. The CES-D is a 20-item self-rating scale for depressive symptoms, and scores range from 0 to 60. The cut-off score for major depressive disorder is 16, similar to the original version (Radloff 1977). The reliability of the Japanese version of the CES-D was confirmed by demonstrating that the test–retest correlation is r = 0.839 over a 5-day interval, and the split-half reliability corrected by the Spearman–Brown formula is r = 0.794. Validity was confirmed by demonstrating that for depressed individuals, the Japanese version of the CES-D is highly correlated with the Japanese version of the Zung Self-Rating Depression Scale (SDS; Fukuda and Kobayashi 1973; Zung 1965) (r = 0.619) and the Hamilton Rating Scale for Depression (HRSD; Hamilton 1960) (r = 0.846). In the study, CES-D was used to compare the depressive symptom levels between the responder and non-responder groups, as mentioned below in detail.
Negative emotion. Negative emotion was assessed using the negative affect subscale of the Japanese version of Positive and Negative Affect Schedule (PANAS; Sato and Yasuda 2001). PANAS is the self-rating scale composed of two subscales for measurement of positive and negative affect. The negative affect subscale of PANAS (PANAS-N) consists of 8 adjectives such as “distressed” and “nervous.” Two items in the original version (Watson et al. 1988) were excluded from the Japanese version due to low communality in conducting factor analysis (Sato and Yasuda 2001). In the study, current negative affect was rated on a 6-point scale, and the sum of the negative affect ratings generates the negative affect score ranging from 8 to 48. The Japanese version of PANAS was confirmed to be reliable with Cronbach’s alpha coefficients being 0.91, which indicates high internal consistency. The validity was confirmed by demonstrating that the positive and negative affect scores elevate in accordance with positive and negative affect induction, respectively. PANAS has been used to capture the changes in state affect in response to a stressor (e.g., Kuehner et al. 2007). The study used PANAS-N to confirm that negative emotion was elevated in response to the stress task, as mentioned below in detail, and to control the confounding effects of negative emotion on attentional bias and attentional function.
Cortisol levels. Saliva sampling was performed during the time period 13:15–16:15 h because diurnal fluctuation of cortisol is relatively flat in the afternoon (Weitzman et al. 1971). Participants were asked to draw saliva in their mouth for 2 min and drool into a specimen tube through a 4-cm-long straw (i.e., passive drool). Saliva samples were frozen in a freezer at temperatures below −20°C until assay. Salivary cortisol levels were measured by means of enzyme-linked immunoassay using a commercial kit from Salimetrics (State College, PA, USA).
Attentional bias. The dot-probe task (MacLeod et al. 1986) was used for measuring attentional bias toward a depression-related stimulus. The dot-probe task is the computerized cognitive task for measuring attentional bias toward an emotional stimulus. The task presents two stimuli, emotional and neutral ones accompanied by a target, and attentional bias toward an emotional stimulus is measured by the response latency to the target.
Since depressed individuals generally allocate their attention toward a depression-related stimulus (Westra and Kuiper 1997), we used depression-related and neutral personality trait adjectives for the dot-probe task. Most previous studies on the cortisol actions on attentional bias used facial stimuli. However, since depressed individuals exhibits attentional bias toward a verbal stimulus as well as a facial one (Peckham et al. 2010), this study used a verbal stimulus for the dot-probe task. Depression-related and neutral words used in the study were selected from the pool of words that previous studies confirmed were related to depression or had neutral emotional valence. The affective valence of the neutral words was determined by the Japanese population (Aoki 1971). The depression-related words selected from English words were translated into Japanese. To confirm that the depression-related words translated into Japanese were related to depression for Japanese language, and the neutral words selected from the previous researches were not related to depression, two researchers who specialize in clinical psychology independently rated the words on how each was related to depression on a 3-point scale: “0 = not related to depression,” “1 = neither,” and “2 = related to depression.” It was shown that the κ coefficient was 0.79, which indicates substantial agreement (Landis and Koch 1977). Any words that were rated differently by the researchers were discussed and agreed upon by the two researchers. Furthermore, 59 undergraduates (27 males and 32 females; mean age = 20.4 years, SD = 1.1), who were not included in the present study, rated the familiarity of the words on a 3-point scale anchored by “1 = not at all familiar” to “5 = very familiar.” Sets of 24 depression-related and neutral word pairs were randomly determined from the selected words, matching the word length and familiarity (see “Appendix”).
The dot-probe task was controlled by SuperLab Pro (version 4.0, Cedrus, CA, USA), and stimuli were presented on a 17-inch CRT monitor (DV17D2, NEC, Tokyo, Japan) connected to a laptop computer (Loox R70Y, Fujitsu, Tokyo, Japan). At the start of each trial, a fixation cross was presented for 500 ms at the center of the monitor. Participants were instructed to fixate on it. Immediately after the offset of the fixation cross, a depression-related and neutral word pair was presented for 300 ms. It was reported that cortisol is related to an early stage attentional bias rather than a late stage one (Ellenbogen et al. 2006, 2010; van Honk et al. 1998). Many previous studies using a dot-probe task have used a 500-ms stimulus duration for measuring an early stage attentional bias. However, Shane and Peterson (2007) suggested that a dot-probe task with a 500-ms stimulus duration may suffer from contamination of early stage attentional processing by late stage attentional processing. They reached this inference by observing that the initial fixation occurs within 200–300 ms after the stimulus onset (Chun and Wolfe 2001; Duncan et al. 1995). Thus, word pairs were presented for 300 ms in the dot-probe task for measuring an early stage attentional bias without contamination by a late stage attentional processing. One word of the pair was displayed on the left and the other on the right across the center of the monitor, with the words 7.5 cm apart as measured from the center of each word. Both words of the pairs were black on a white background. Immediately after the word pair disappeared, one of the words was replaced by a dot probe. The probe was presented at either the location of the depression-related word or that of the neutral word with 50% probability. Participants were asked to identify the location of the probe as quickly and correctly as possible. Participants responded by pressing one of the two response buttons indicating the location of the probe on the response box (RB-530, Cedrus, CA, USA) using the first or annular fingers of their dominant hand. Response latency was measured between the onset of the probe and the pressing of the button. Upon the participant’s response, the probe disappeared and the next trial started. Participants were seated 65 cm from the monitor; the visual angle was approximately 6.6° between the centers of the word pair. The dot-probe task was administered twice: before and after stress induction. Both tasks consisted of 96 trials, first preceded by 3 practice trials that used a pair of neutral words. Twenty-four word pairs were presented four times in random order; that is, all combinations of the location of the depression-related word (on the left or the right) and that of the probe (on the left or the right).
Attentional function. Attentional function was measured with the Trail Making Test (TMT). TMT is a neuropsychological test composed of two subtests: TMT A and B. TMT A is a measure for processing speed, and TMT B is a measure for executive attention (Sánchez-Cubillo et al. 2009; Strauss et al. 2006). In TMT A, participants draw lines to consecutively connect 25 numbered circles, and in TMT B, 13 numbered and 12 lettered circles by alternating between the two sequences. Time for completion was measured in TMT A and B separately. A shorter time in TMT A and B indicates higher ability of processing speed and executive attention, respectively.
Stress Task
A mental arithmetic task was administered for psychological stress induction. Participants were asked to serially subtract 13 from 2,093 down to zero for 5 min, while speaking out loud, in front of both a video recorder and a judge of the same sex as the participants. The judge was introduced to the participants as an evaluator of their performance in the task. When the participants miscalculated, the judge asked them to restart the calculation from the beginning. A mental arithmetic task has been confirmed to elicit negative emotion and cortisol (Al’Absi et al. 1997).
Procedure
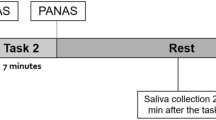
Experiments began at 13:00 h or 15:00 h, and lasted for 90 min. At the beginning of the experiment, written informed consent was provided. Participants were informed that the experiment aimed to investigate the relationship between attention, emotion, and a salivary hormone. Then, they were asked to complete CES-D. Because it is recommended to wait at least 45-min before saliva sampling to control potential confounders (Kudielka et al. 2010), participants remained seated in a quiet room during a 45-min rest period. Immediately after the rest period (T0), they were asked to complete the PANAS-N, and then provide a saliva sample for baseline assessment of negative affect and cortisol. Participants then undertook the dot-probe task and TMT A and B, in that order, for pre-stress assessment of attentional bias and processing speed and executive attention. Then, a judge for the stress task entered the room, and participants were administered the mental arithmetic task for 5 min. After the stress task, the judge left the room. Next, participants had a 10-min rest period, during which they remained seated in the quiet room. Participants were asked to complete PANAS-N and provide a saliva sample two times: at the beginning of (T1) and then immediately after (T2) the rest period. This was followed by the post-stress dot-probe task and TMT A and B, in that order, 15 min after the offset of the stress task, and these tasks lasted within 15 min. This was based on the findings that neural activity starts to be observed approximately 10–20 min after glucocorticoid administration (Makara and Haller 2001), and additionally, cortisol actions on information processing start to attenuate approximately 30 min after the stressor offset (Oitzl et al. 2010). Finally, they were debriefed about the experiment.
Data Analyses
Participants were post-hoc allocated to a responder or a non-responder group. The responder group consisted of the participants whose cortisol elevated from pre-stress cortisol levels (T0) to the maximum value among post-stress cortisol levels (T1 and T2), and the non-responder group consisted of the participants who showed no cortisol elevation following the stress task. To test whether cortisol change influences attention, it is needed to examine whether cortisol elevated in response to the stress task before administering attention tasks. Thus, cortisol levels measured before administering attention tasks were used for grouping. By using a meta-analysis, it was shown that a moderate amount of cortisol elevation is observed 11–20 min after the onset of an acute psychological stressor (Dickerson and Kemeny 2004). Therefore, we speculated that participants can be judged to be responders or non-responders from their cortisol levels until 15 min after the stress task onset, although maximum levels of cortisol response may not be captured. To confirm the responder group showed cortisol elevation, a two-way 2 (Group: responder, non-responder) × 3 (Time: T0, T1, T2) mixed design analysis of variance (ANOVA) on cortisol levels were performed. In addition, we compared the percentage increase from baseline cortisol levels to the maximum cortisol levels after the stress task between the responder and non-responder groups using an unpaired t-test. Because it is reported that males show a larger cortisol response to a stressor (Smyth et al. 1998) and attentional bias is more susceptible to a cortisol change in response to a stressor among females than males (McHugh et al. 2010), sex was compared between these groups by a χ 2 test. In addition, age and depressive symptoms were compared between these groups by unpaired t-tests.
For the dot-probe task, the trials in which the participants responded mistakenly were removed before latency analysis. Among the correct trials, to minimize the influence of outliers, response latencies of more than 2 SDs below each participant’s mean response latency were assumed to be reflected in anticipation errors, and therefore excluded. Similarly, response latencies of more than 2 SDs above each participant’s mean response latencies were considered lapses in concentration, and therefore excluded. The exclusion criteria are in line with previous researches (Bradley 1997a; Donaldson et al. 2007; Mogg et al. 1995). The proportion of lost data was 0.6% due to erroneous responses and 3.9% due to outliers, which are comparable with previous researches using the same task (e.g., Bradley 1997a). In accordance with the methods of MacLeod and Mathews (1988), the attentional bias index was computed using the following equation.
where RpLd indicates mean latency when the probe is presented on the right and a depression-related stimulus is presented on the left, and similarly for others. A positive score for attentional bias index would be interpreted as attentional bias toward a depression-related stimulus, while a negative score for attentional bias index would be interpreted as attentional bias toward a neutral stimulus, and a score of 0 would be interpreted as an absence of attentional bias toward either a depression-related or a neutral stimulus.
For manipulation check of stress induction, a two-way 2 (Group: responder, non-responder) × 3 (Time: T0, T1, T2) mixed design ANOVA on PANAS-N scores was performed. To assess the associations between cortisol and attentional bias and the processing speed and executive attention adjusted for negative emotional response, we performed two-way 2 (Group: responder, non-responder) × 2 (Time: pre-stress, post-stress) mixed-design analyses of covariance (ANCOVAs) with the PANAS-N score increment entered as a covariate on attentional bias index scores and TMT A and B scores. The PANAS-N score increment was computed by subtracting the baseline PANAS-N score (T0) from the maximum value among post-stress PANAS-N scores (T1 and T2). For all statistical analyses, the significance levels were set to 0.05 (two-tailed).
Results
Group Characteristics
Participants were allocated either to a responder (n = 20) or a non-responder (n = 16) group according to the definition described in the “Method” section. Data from one female participant was excluded from all the analyses due to an outlier defined as percentage increase of cortisol response more than 4 SDs from the mean. Therefore, the final sample consisted of 35 participants (the responder group: n = 19; the non-responder group: n = 16). An unpaired t-test with adjusted degree of freedom revealed that the percentage increase of cortisol in the responder group (mean percentage increase = 30.62%, SD = 26.40) was higher than that in the non-responder group (mean percentage increase = −17.78%, SD = 11.72) (t(25.72) = 7.19, p < .01). The means and SDs of age, sex, and depressive symptoms for the responder and the non-responder groups are shown in Table 1. These groups did not differ in age (t(50) = 1.45, n.s.), in sex (χ 2(1) = 0.47, n.s.), or in depressive symptoms (t(50) = 0.47, n.s.).
Cortisol Response
We submitted cortisol levels to a two-way 2 (Group: responder, non-responder) × 3 (Time: T0, T1, T2) mixed design ANOVA. The analysis revealed that the interaction was significant (F(2, 66) = 19.16, p < .01; see Table 2). A post-hoc analysis revealed that among the responder group, cortisol levels at T2 were higher than cortisol levels at T0. In contrast, among the non-responder group, cortisol levels at T1 and T2 were lower than cortisol levels at T0. The results indicate that significant cortisol increase was observed only in the responder group.
Psychological Stress Response
We submitted PANAS-N scores to a two-way 2 (Group: responder, non-responder) × 3 (Time: T0, T1, T2) mixed design ANOVA. The analysis revealed that the main effects of Time were significant in PANAS-N (F(2, 66) = 76.68, p < .01; see Table 2). A post-hoc analysis revealed that PANAS-N score at T1 was higher than PANAS-N scores at any other time points. The results indicate that the stress task successfully elevated negative affect in participants of both groups.
Attentional Bias Toward a Depression-Related Stimulus
We submitted the attentional bias index scores to a two-way 2 (Group: responder, non-responder) × 2 (Time: pre-stress, post-stress) mixed design ANCOVA with the PANAS-N score increment entered as a covariate. The analysis revealed that the interaction was significant (F(1, 32) = 6.59, p < .05; see Table 3). A post-hoc analysis revealed that, for the responders, the post-stress attentional bias index score was higher than the pre-stress attentional bias index score. On the other hand, for the non-responders, neither the main effects nor the interaction were significant. The results indicate that stress-induced cortisol is associated with attentional bias toward a depression-related stimulus.
Attentional Function
We submitted TMT A and B scores to two-way 2 (Group: responder, non-responder) × 2 (Time: pre-stress, post-stress) mixed design ANCOVAs with the PANAS-N score increment entered as a covariate. Analyses revealed that neither the main effects nor the interactions were significant in TMT A and B. The results indicate that cortisol response levels are not related to both processing speed and executive attention.
Discussion
The study tested the hypotheses that an acute increase in cortisol in response to a stressor is related to the following: (1) attentional bias toward a depression-related stimulus and (2) impaired processing speed and executive attention. The results of the study revealed that attentional bias toward a depression-related stimulus following a stressor was observed only in the responders. Thus, the result supports the first hypothesis. On the other hand, no differences in processing speed and executive attention were found between the responders and non-responders. These results do not support the second hypothesis. The results indicate that acutely elevated cortisol is related to attentional bias, but it is not related to processing speed and executive attention.
Based on previous findings that the cortisol actions under psychological stress are different from the actions of cortisol elevated by physical exercise or cortisol administration (McHugh et al. 2010), the study tested the relationship between cortisol and attentional bias and processing speed and executive attention in stress contexts. As a result, it was found that the associations between stress-induced cortisol and attention were selective. Attentional bias and attentional function are related to different brain areas; attentional bias is related to the amygdala, whereas attentional function is related to the prefrontal cortex. The results suggest that the amygdala may be sensitive to stress-induced cortisol, but the prefrontal cortex may not be.
The first hypothesis referred to the relationship between stress-induced cortisol and attentional bias toward a depression-related stimulus. Roelofs et al. (2007) reported that cortisol is related to attentional bias toward an angry stimulus. The present study extends the finding to a depression-related stimulus. However, McHugh et al. (2010) reported a contrary result that stress-induced cortisol attenuates attentional bias toward a threatening stimulus. The inconsistent results may be attributable to the timing of the measurement of attentional bias following a stressor. It is assumed that the effects of cortisol in response to a stressor on emotional information processing change over time: at the early phase of stress response, cortisol facilitates emotional information processing, and at the later phase of stress response, cortisol inhibits emotional information processing (Oitzl et al. 2010). There are two types of cortisol receptors, mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) (de Kloet et al. 2005); the early effects work through membrane-located MRs, while the later effects work through intracellular MRs and GRs (Oitzl et al. 2010). Therefore, when attentional bias is measured in an early of stress response, attentional bias may be induced, and in contrast, when attentional bias is measured in a late phase of stress response, attentional bias may be attenuated. The present study and Roelofs et al. (2007) measured attentional bias within a short time after the offset of stress induction, and therefore cortisol may facilitate attentional bias toward a negative stimulus. On the other hand, McHugh et al. (2010) measured attentional bias more than 30 min after the offset of stress induction, and thus cortisol may attenuate attentional bias toward a negative stimulus.
Another potential moderator may be the processing stage of attentional bias. The present study and McHugh et al. (2010) presented stimuli supraliminal, and in contrast, Roelofs et al. (2007) presented stimuli subliminal. Although supraliminal stimulus presentation may suffer from contamination by late stage attentional processing, it is suggested that a stimulus duration until 300 ms evaluates an early stage attentional bias without contamination by late stage attentional processing (Shane and Peterson 2007). Therefore, the present study and Roelofs et al. (2007) evaluated an early stage attentional bias, while McHugh et al. (2010) evaluated a later-stage attentional bias because of the use of a 500-ms stimulus duration. Since positive correlation between cortisol and attentional bias is found in an early stage attentional bias rather than a late stage attentional bias (Ellenbogen et al. 2006, 2010; van Honk et al. 1998), the inconsistent results in cortisol actions on attentional bias may be attributable to the difference in its processing stage. In addition, it is possible that differential results are attributable to stimulus valence. It was suggested that exogenous cortisol may impact attentional bias differently depending on the stimulus valence (Putman and Roelofs 2011). Therefore, further study is needed to investigate the valence-specific effects of stress-induced cortisol on attentional bias. Furthermore, the present study and many previous studies regarding cortisol actions on attentional bias are methodologically different in that this study employed a verbal stimulus, whereas many previous studies employed a facial stimulus. The present study and that by Roelofs et al. (2007), which also employed a facial stimulus, generated a similar result. Thus, the present result suggests that stress-induced cortisol equally impacts attentional bias toward a facial and a non-facial stimulus, which is consistent with the meta-analytic study that reported no difference in effect size between a facial and a non-facial stimulus on attentional bias in depression (Peckham et al. 2010). However, because facial stimuli are more salient than verbal stimuli, it is necessary to investigate whether the same result is obtained using a facial stimulus for a dot-probe task. The population studied may be another potential moderator between cortisol and attentional bias. It is revealed that cortisol influences attentional bias toward a fear stimulus only for high-anxiety participants (Putman et al. 2007). To investigate the moderating influence of depressive symptom levels, future studies should investigate whether stress-induced cortisol actions on attentional bias toward a depression-related stimulus is found in a depressed population.
The second hypothesis referred to the relationship between stress-induced cortisol and processing speed and executive attention. Contrary to the hypothesis, cortisol is not related to processing speed and executive attention. As with the case of attentional bias, the results may be interpreted in terms of the timing of measurement of attentional function following a stressor. The present study measured processing speed and executive attention within a short time period after stress induction, while Bohnen et al. (1990) measured divided attention more than 4 h after the onset of stress induction. While the prefrontal cortex is dense in GR, low levels of MR are expressed in the prefrontal cortex (Patel et al. 2000). Given that membrane-located MR activation mediates the early effects of cortisol on information processing, cortisol may take longer to impair attentional function because of low expression of MR in the prefrontal cortex. Future studies need to confirm this interpretation to better understand the time-dependent effects of cortisol.
The stressor used in the study may be relatively mild; hence, it may not influence the activity of the prefrontal cortex. Therefore, it is possible that large cortisol increase by a more intense stressor may impair attentional function. Furthermore, although attentional function is not subject to cortisol change elicited by the acute stressor administered in the study, a chronic stressor may influence attentional function. It is revealed in vitro that chronic excessive cortisol exposure to the brain reduces brain-derived neurotrophic factor (BDNF) via GRs (Numakawa et al. 2009). BDNF is essential for neurogenesis, and reduction in BDNF leads to neural shrinking and neural death in the long term. Because GR is densely located in the prefrontal cortex, long-lasting exposure to excessive cortisol may influence the prefrontal cortex, resulting in an attentional function impairment.
Furthermore, it is worth noting that the difference between responders and non-responders showed responders with lower basal cortisol levels than non-responders. Previous studies demonstrated that basal cortisol levels are negatively related to cortisol response to a stressor (Kudielka et al. 2004), and thus this result is in line with the researches, attributing this to ceiling effects due to high basal cortisol levels (Kudielka et al. 2004).
The present results suggest an implication for the mechanism of cortisol actions in the etiology of depression. The study showed that an acute increase in cortisol is related to attentional bias toward a depression-related stimulus. Because attentional bias toward a depression-related stimulus is cognitive vulnerability to depression, cortisol may exacerbate depressive symptoms through facilitating attentional bias. It is assumed that high cortisol levels may be one of the causes of depression (van Praag 2005), and that coping with the cortisol actions by pharmacotherapy or psychotherapy was recommended to alleviate depressive symptoms (Tafet and Smolovich 2004). The present findings are in line with the assumptions. Future studies need to validate the present findings by confirming that, for depressive individuals, blocking the cortisol actions alleviate depressive symptoms, mediating to attenuate attentional bias toward a depression-related stimulus.
The present study has several limitations. First, the data are correlational in nature, and therefore, open to other interpretations on the direction of effects. Based on the findings that cortisol influences the central nervous system, we assume that cortisol induces attentional bias. However, it is possible to interpret the results that another factor such as personality trait (Amin et al. 2004; Hauner et al. 2008) may produce both attentional bias and cortisol elevation during stress exposure. It is needed to determine causal relationship using cortisol administration with negative mood induction or a longitudinal study. Second, as with cortisol, noradrenaline levels increase in response to a stressor, and it also impairs attentional function (Skosnik et al. 2000). It is possible that individual differences in noradrenaline response may disturb the true relationship between attentional function and cortisol. Future studies need to assess the independent contributions of cortisol and noradrenaline to impaired attentional function. Third, because a dot-probe task presents word pairs left and right of the center, laterality may confound the results of the task. To avoid confounding effects of laterality in a dot-probe task, it is usual to present stimulus pairs above and below the center. In this study, however, because a depression-related word was presented as frequently in the left as in the right, the confounding effects of laterality should be balanced out. Fourth, the sample size of the study is small, and thus, the results need to be repeated in larger samples. Last, the small sample size limitation precludes an examination of the gender difference in the cortisol actions on attentional bias toward a depression-related stimulus. Therefore, this needs to be elucidated in future studies.
In conclusion, the study revealed that stress-induced cortisol is associated with attentional bias toward a depression-related stimulus, while it is not associated with processing speed and executive attention. Attentional bias and attentional function are related to different brain areas, attentional bias to the amygdala and attentional function to the prefrontal cortex. Therefore, the results suggest that the amygdala may be sensitive to stress-induced cortisol, but the prefrontal cortex may not be. Furthermore, the results have an implication for the etiology of depression, since the study suggests that cortisol may develop and maintain depressive symptoms by inducing attentional bias toward a depression-related stimulus.
References
Al’Absi, M., Bongard, S., Buchanan, T., Pincomb, G. A., Licinio, J., & Lovallo, W. R. (1997). Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology, 34, 266–275.
Amin, Z., Constable, R. T., & Canli, T. (2004). Attentional bias for valenced stimuli as a function of personality in the dot-probe task. Journal of Research in Personality, 38, 15–23.
Aoki, T. (1971). A psycho-lexical study of personality trait words—selection, classification and desirability ratings of 455 words. Japanese Journal of Psychology, 42, 1–13. (in Japanese with English abstract).
Applehans, B. M., & Luecken, L. J. (2006). Attentional processes, anxiety and the regulation of cortisol reactivity. Anxiety, Stress & Coping: An International Journal, 19, 81–92.
Baert, S., De Raedt, R., Schacht, R., & Koster, E. H. W. (2010). Attentional bias training in depression: Therapeutic effects depend on depressive severity. Journal of Behavior Therapy and Experimental Psychiatry, 41, 265–274.
Bassett, J. R., Marshall, P. M., & Spillane, R. (1987). The physiological measurement of acute stress (public speaking) in bank employees. International Journal of Psychophysiology, 5, 265–273.
Bohnen, N., Houx, P., Nicolson, N., & Jolles, J. (1990). Cortisol reactivity and cognitive performance in a continuous mental task paradigm. Biological Psychology, 31, 107–116.
Bradley, B. P., Mogg, K., & Lee, S. C. (1997a). Attentional bias for negative information in induced and naturally occurring dysphoria. Behaviour Research and Therapy, 35, 911–927.
Bradley, B. P., Mogg, K., Millar, N., Bonham-Carter, C., Fergusson, E., Jenkins, J., et al. (1997b). Attentional biases for emotional faces. Cognition and Emotion, 11, 25–42.
Braunstein-bercovitz, H. (2003). Does stress enhance or impair selective attention? The effects of stress and perceptual load on negative priming. Anxiety, Stress & Coping: An International Journal, 16, 345–357.
Chun, M. M., & Wolfe, J. M. (2001). Visual attention. In B. E. Goldstein (Ed.), Blackwell handbook of perception. Handbook of experimental psychology series (pp. 272–310). Malden, MA: Blackwell.
Cisler, J. M., & Koster, E. H. W. (2010). Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review, 30, 203–216.
de Kloet, E. R., Joëls, M., & Holsboer, F. (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6, 463–475.
Dickerson, S. S., & Kemeny, M. E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130, 355–391.
Donaldson, C., Lam, D., & Mathews, A. (2007). Rumination and attention in major depression. Behaviour Research and Therapy, 45, 2664–2678.
Duncan, J., Ward, R., & Shapiro, K. (1995). Direct measurement of attentional dwell time in human vision. Nature, 369, 313–315.
Ellenbogen, M. A., Carson, R. J., & Pishva, R. (2010). Automatic emotional information processing and the cortisol response to acute psychosocial stress. Cognitive, Affective, & Behavioral Neuroscience, 10, 71–82.
Ellenbogen, M. A., Schwartzman, A. E., Stewart, J., & Walker, C. D. (2006). Automatic and effortful emotional information processing regulates different aspects of the stress response. Psychoneuroendocrinology, 31, 373–387.
Fukuda, K., & Kobayashi, S. (1973). A study on a self-rating depression scale. Seishin shinkeigaku zasshi, 75, 673–679. (in Japanese).
Fuster, J. M. (1997). The prefrontal cortex: Anatomy, physiology, and neuropsychology of the frontal lobe (3rd ed.). Philadelphia: Lippincott-Raven.
Gamer, M., & Büchel, C. (2009). Amygdala activation predicts gaze toward fearful eyes. The Journal of Neuroscience, 29, 9123–9126.
Halligan, S. L., Herbert, J., Goodyer, I., & Murray, L. (2007). Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry, 62, 40–46.
Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry, 23, 56–62.
Hauner, K. K. Y., Adam, E. K., Mineka, S., Doane, L. D., De Santis, A. S., Zinbarg, R., et al. (2008). Neuroticism and introversion are associated with salivary cortisol patterns in adolescents. Psychoneuroendocrinology, 33, 1344–1356.
Jameison, K., & Dinan, T. G. (2001). Glucocorticoid and cognitive function: From physiology to pathophysiology. Human Psychopharmacology: Clinical and Experimental, 16, 293–302.
Kirschbaum, C., Pirke, K. M., & Hellhammer, D. H. (1993). The ‘Trier Social Stress Test’-a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81.
Koster, E. H. W., De Raedt, R., Goeleven, E., Franck, E., & Crombez, G. (2005). Mood-congruent attentional bias in dysphoria: Maintained attention to and impaired disengagement from negative information. Emotion, 5, 446–455.
Kudielka, B. M., Schommer, N. C., Hellhammer, D. H., & Kirschbaum, C. (2004). Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology, 29, 983–992.
Kudielka, B. M., Wüest, S., Kirschbaum, C., & Hellhammer, D. H. (2010). The Trier Social Stress Test (TSST). In G. Fink (Editor-in-Chief), Encyclopedia of stress (2nd ed., Vol. 3, pp. 776–781). Oxford: Academic Press.
Kuehner, C., Holzhauer, S., & Huffziger, S. (2007). Decreased cortisol response to awakening is associated with cognitive vulnerability to depression in a nonclinical sample of young adults. Psychoneuroendocrinology, 32, 199–209.
Landis, J. R., & Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33, 159–174.
Landrø, N. I., Stiles, T. C., & Sletvold, H. (2001). Neuropsychological function in nonpsychotic unipolar major depression. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 14, 223–240.
Lupien, S., Lecours, A. R., Lussier, I., Schwartz, G., Nair, N. P. V., & Meaney, M. J. (1994). Basal cortisol levels and cognitive deficits in human aging. The Journal of Neuroscience, 14, 2893–2903.
MacLeod, C. (1991). Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin, 109, 163–203.
MacLeod, C., & Mathews, A. (1988). Anxiety and the allocation of attention to threat. The Quarterly Journal of Experimental Psychology Section A: Human Experimental Psychology, 40, 653–670.
MacLeod, C., Mathews, A., & Tata, P. (1986). Attentional bias in emotional disorders. Journal of Abnormal Psychology, 95, 15–20.
Makara, G. B., & Haller, J. (2001). Non-genomic effects of glucocorticoids in the neural system: Evidence, mechanisms, and implications. Progress in Neurobiology, 65, 367–390.
McDermott, L. M., & Ebmeier, K. P. (2009). A meta-analysis of depression severity and cognitive function. Journal of Affective Disorders, 119, 1–8.
McEwen, B. S. (2005). Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism: Clinical and Experimental, 54, 20–23.
McHugh, R. K., Behar, E., Gutner, C. A., Geem, D., & Otto, M. W. (2010). Cortisol, stress, and attentional bias toward threat. Anxiety, Stress & Coping: An International Journal, 23, 529–545.
Mogg, K., Bradley, B. P., Dixon, C., Fisher, S., Twelftree, H., & McWilliams, A. (2000). Trait anxiety, defensiveness and selective processing of threat: An investigation uing two measures of attentional bias. Personality and Individual Differences, 28, 1063–1077.
Mogg, K., Bradley, B. P., & Williams, R. (1995). Attentional bias in anxiety and depression: The role of awareness. British Journal of Clinical Psychology, 34, 17–36.
Numakawa, T., Kumamaru, E., Adachi, N., Yagasaki, Y., Izumi, A., & Kunugi, H. (2009). Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-γ signaling for glutamate release via a glutamate transporter. Proceedings of the National Academy of Sciences of the United States of America, 106, 647–652.
Oitzl, M. S., Champagne, D. L., van der Veen, R., & de Kloet, R. E. (2010). Brain development under stress: Hypotheses of glucocorticoid actions revisited. Neuroscience and Biobehavioral Reviews, 34, 853–866.
Papageorgiou, C., & Wells, A. (2000). Treatment of recurrent major depression with attention training. Cognitive and Behavioral Practice, 7, 407–413.
Patel, P. D., Lopez, J. F., Lyons, D. M., Burke, S., Wallace, M., & Schatzberg, A. F. (2000). Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. Journal of Psychiatric Research, 34, 383–392.
Peckham, A. D., McHugh, R. K., & Otto, M. W. (2010). A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety, 27, 1135–1142.
Putman, P., Hermans, E. J., Koppeschaar, H., van Schijndel, A., & van Honk, J. (2007). A single administration of cortisol acutely reduces preconscious attention for fear in anxious young men. Psychoneuroendocrinology, 32, 793–802.
Putman, P., & Roelofs, K. (2011). Effects of single cortisol administrations on human affect reviewed: Coping with stress through adaptive regulation of automatic cognitive processing. Psychoneuroendocrinology, 36, 439–448.
Radloff, L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401.
Roelofs, K., Bakvis, P., Hermans, E. J., van Pelt, J., & van Honk, J. (2007). The effects of social stress and cortisol responses on the preconscious selective attention to social threat. Biological Psychology, 75, 1–7.
Sánchez-Cubillo, I., Periáñez, J. A., Adrover-Roig, D., Rodríguez-Sánchez, J. M., Ríos-Lago, M., Tirapu, J., et al. (2009). Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society, 15, 438–450.
Sato, A., & Yasuda, A. (2001). Development of the Japanese version of Positive and Negative Affect Schedule (PANAS) scales. The Japanese Journal of Personality, 9, 138–139. (in Japanese).
Scher, C. D., Ingram, R. E., & Segal, Z. V. (2005). Cognitive reactivity and vulnerability: Empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review, 25, 487–510.
Shane, M. S., & Peterson, J. B. (2007). An evaluation of early and late stage attentional processing of positive and negative information in dysphoria. Cognition and Emotion, 21, 789–815.
Shima, S., Shikano, T., Kitamura, T., & Asai, M. (1985). New self-rating scales for depression. Seishin-Igaku, 27, 717–723. (in Japanese).
Skosnik, P. D., Chatterton, R. T., Jr., Swisher, T., & Park, S. (2000). Modulation of attentional inhibition by norepinephrine and cortisol after psychological stress. International Journal of Psychophysiology, 36, 59–68.
Smyth, J., Ockenfels, M. C., Porter, L., Kirschbaum, C., Hellhammer, D. H., & Stone, A. A. (1998). Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology, 23, 353–370.
Stefanopoulou, E., Manoharan, A., Landau, S., Geddes, J. R., Goodwin, G., & Frangou, S. (2009). Cognitive functioning in patients with affective disorders and schizophrenia: A meta-analysis. International Review of Psychiatry, 21, 336–356.
Strauss, E., Sherman, E. M. S., & Spreen, O. (2006). A compendium of neuropsychological tests: Administration, norms, and commentary (3rd ed.). New York: Oxford University Press.
Tafet, G. E., & Smolovich, J. (2004). Psychoneuroendocrinological studies on chronic stress and depression. Annals of the New York Academy of Sciences, 1032, 276–278.
Thomson, F., & Craighead, M. (2008). Innovative approaches for the treatment of depression: Targeting the HPA axis. Neurochemical Research, 33, 691–707.
van Honk, J., Tuiten, A., van den Hout, M., Koppeschaar, H., Thijssen, J., de Haan, E., et al. (1998). Baseline salivary cortisol levels and preconscious selective attention for threat: A pilot study. Psychoneuroendocrinology, 23, 741–747.
van Praag, H. M. (2005). Can stress cause depression? The World Journal of Biological Psychiatry, 6(Suppl. 2), 5–22.
Vedhara, K., Hyde, J., Gilchrist, I. D., Tytherleigh, M., & Plummer, S. (2000). Acute stress, memory, attention and cortisol. Psychoneuroendocrinology, 25, 535–549.
Vreeburg, S. A., Hoogendijk, W. J. G., van Pelt, J., Derijk, R. H., Verhagen, J. C. M., van Dyck, R., et al. (2009). Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Archives of General Psychiatry, 66, 617–626.
Vuilleumier, P. (2005). How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9, 585–594.
Watson, D., Clark, L. A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070.
Weitzman, E. D., Fukushima, D., Nogeire, C., Roffwarg, H., Gallagher, T. F., & Hellman, L. (1971). Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. The Journal of Clinical Endocrinology & Metabolism, 33, 14–22.
Wells, T. T., & Beevers, C. G. (2010). Biased attention and dysphoria: Manipulating selective attention reduces subsequent depressive symptoms. Cognition and Emotion, 24, 719–728.
Westra, H. A., & Kuiper, N. A. (1997). Cognitive content specificity in selective attention across four domains of maladjustment. Behaviour Research and Therapy, 35, 349–365.
Williams, J. M. G., Watts, F. N., MacLeod, C., & Mathews, A. (1997). Cognitive psychology and emotional disorders (2nd ed.). West Sussex: Wiley.
Zung, W. W. K. (1965). A self-rating depression scale. Archives of General Psychiatry, 12, 63–70.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 4
Rights and permissions
About this article
Cite this article
Tsumura, H., Shimada, H. Acutely Elevated Cortisol in Response to Stressor Is Associated with Attentional Bias Toward Depression-Related Stimuli but Is Not Associated with Attentional Function. Appl Psychophysiol Biofeedback 37, 19–29 (2012). https://doi.org/10.1007/s10484-011-9172-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10484-011-9172-z