Abstract
Rationale
Pharmacological and genetic evidence support antidepressant-like effects elicited by the blockade of the NOP receptor. The learned helplessness (LH) model employs uncontrollable and unpredictable electric footshocks as a stressor stimulus to induce a depressive-like phenotype that can be reversed by classical antidepressants.
Objectives
The present study aimed to evaluate the action of NOP receptor antagonists in helpless mice.
Methods
Male Swiss mice were subjected to the three steps of the LH paradigm (i.e., (1) induction, (2) screening, and (3) test). Only helpless animals were subjected to the test session. During the test session, animals were placed in the electrified chamber and the latency to escape after the footshock and the frequency of escape failures were recorded. The effect of the following treatments administered before the test session were evaluated: nortriptyline (30 mg/kg, ip, 60 min), fluoxetine (30 mg/kg, ip, four consecutive days of treatment), and NOP antagonists SB-612111 (1–10 mg/kg, ip, 30 min) and UFP-101 (1–10 nmol, icv, 5 min). To rule out possible biases, the effects of treatments on controllable stressful and non stressful situations were assessed.
Results
In helpless mice, nortriptyline, fluoxetine, UFP-101 (3–10 nmol), and SB-612111 (3–10 mg/kg) significantly reduced escape latencies and escape failures. No effects of drug treatments were observed in mice subjected to the controllable electric footshocks and non stressful situations.
Conclusions
Acute treatment with NOP antagonists reversed helplessness similarly to the classical antidepressants. These findings support the proposal that NOP receptor antagonists are worthy of development as innovative antidepressant drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder is one of the most common psychiatric disorders in the world, causing psychological, behavioral, and physical symptoms that negatively affect the quality of life of patients. Major depression affects people at any age and is more prevalent in women than in men (Kessler and Bromet 2013).
Current evidence supports the idea of dysfunction in noradrenergic and serotonergic transmission to explain the neurobiology of major depression. In fact, most of the antidepressants available nowadays increase monoamine levels in the synaptic cleft, and this consequently leads to long-term plasticity in neural circuits (Nemeroff and Owens 2002). The onset of the therapeutic effect of antidepressants occurs after weeks of use, and their side effects appear at the beginning of the treatment and sometimes can be enduring. Moreover, only a relative low percentage of patients are fully responsive to current antidepressant drugs (Thase and Denko 2008). Therefore, new and more efficacious therapeutic treatments are mandatory.
Nociceptin/orphanin FQ (N/OFQ) is a heptadecapeptide acting as the endogenous ligand of an inhibitory G protein-coupled receptor named N/OFQ peptide receptor (NOP) (Lambert 2008). Both peptide and its receptor are widely distributed in the limbic structures involved in processing emotional stimuli which support a regulatory role of N/OFQ signaling in stress-related psychiatric disorders, such as depression and anxiety, feeding and homeostasis of body weight, and drug abuse (for reviews, see Gavioli and Calo’ 2013; Witkin et al. 2014).
A growing body of evidence supports the benefits of NOP antagonists on the treatment of mood disorders (Gavioli and Calo’ 2013). In fact, NOP antagonists induce antidepressant-like effect in rodents subjected to the forced swim, tail suspension, and chronic mild stress assays, which were abrogated by previous administration of N/OFQ (Redrobe et al. 2002; Gavioli et al. 2003; Gavioli et al. 2004; Vitale et al. 2009). NOP antagonists also counteracted LPS-induced depressive-like behavior in mice (Medeiros et al. 2015). Importantly, NOP receptor knockout mice and rats display an antidepressant-like phenotype in behavioral despair assays (Gavioli et al. 2003; Rizzi et al. 2011). Recently, these preclinical findings were corroborated by studies performed with the novel NOP receptor antagonist LY2940094 (Toledo et al. 2014). This compound induces antidepressant-like effects in rodent models, and more importantly displayed antidepressant efficacy in patients with major depressive disorder (Post et al. 2015).
Animal models that mimic more specifically the pathophysiologic context of major depression may contribute to extend our knowledge about the role of N/OFQ-NOP receptor system in mood disorders. In the learned helplessness (LH) model, rodents display helpless condition after being subjected to a protocol of uncontrollable and unpredictable electric footshocks (Overmier and Seligman 1967; O’Neil and Moore 2003). The helpless condition characterizes a depressive-like phenotype with changes in animal behavior that simulate the symptomatology of major depression patients (Dess et al. 1988; Greenberg et al. 1989; Adrien et al. 1991). Chronic and acute treatment with several classes of antidepressants prevents or reverses this depressive-like behavior (Telner et al. 1981; Sherman et al. 1982; Martin et al. 1987; Takamori et al. 2001; Chourbaji et al. 2005; Joca et al. 2006; Valentine et al. 2008; Zazpe et al. 2007).
The antidepressant-like effects produced by the NOP receptor blockade has been well established, at least by analyzing the rodents basal behavior in despair tests (Redrobe et al. 2002; Gavioli et al. 2003; Gavioli et al. 2004; Rizzi et al. 2007; Goeldner et al. 2010; Post et al. 2015). However, these tests, despite their wide use to predict the effects of antidepressants, have no direct relationship to depressive symptoms in humans (Holmes 2003; Petit-Demouliere et al. 2005). Thus, considering the face, predictive, and constructive validity of the LH model, the aim of the present study was to evaluate the effects of peptide and non peptide NOP receptor antagonists in helpless mice.
Material and methods
Animals
Experiments were performed using Swiss male mice breaded at the Federal University of Rio Grande do Norte (Natal, Brazil). Despite the higher prevalence of mood disorders in women compared to men, literature data have already reported absence of helpless behavior in female rodents (Dalla et al. 2008); for this reason, only male mice were employed in this study. Mice were 12–16 weeks old (30–35 g) and were housed in groups of 10–16 under standard conditions (22 °C; 12-h light:12-h dark cycle, lights on at 6:00 am) with food and water ad libitum in plastic cages (33 × 40 × 17 cm). A total number of 188 mice were used to develop this study, 104 mice being used for uncontrollable footshock experiments, 40 mice for non stressful situations, and 44 mice for controllable footshock assays. The experiments were performed during the mornings between the period of 7:00 and 11:00 am. When subjected to the inescapable and uncontrollable footshock sessions, approximately 80 % of animals developed the helpless phenotype. All the experimental series were conducted in accordance with the Brazilian law no. 11.794/2008 for care and use of experimental animals and were approved by the Local Ethics Committee for Animal Use of the Federal University of Rio Grande do Norte (License no. 063/2013). This study is reported following the ARRIVE guidelines (Kilkenny et al. 2010).
Drugs and treatments
Nortriptyline (Pamelor®, NOVARTIS Biociências S.A., São Paulo, Brazil), fluoxetine (Medley Pharmaceutical Industry, Campinas, SP, Brazil), UFP-101 ([Nphe1,Arg14,Lys15]N/OFQ-NH2, synthesized by Prof. Guerrini, Department of Pharmaceutical Sciences, University of Ferrara, Italy), and SB-612111 (Tocris Bioscience, Bristol, UK) were used. Both nortriptyline and fluoxetine were dissolved in saline solution while SB-612111 was solubilized in dimethylsulphoxide (DMSO) in a final concentration not exceeding 0.8 %. Stock solutions of UFP-101 (50 mmol) were stored at −20 °C and were diluted to the desired concentrations in saline before experiments. Acute nortriptyline (30 mg/kg) and fluoxetine (30 mg/kg) were intraperitoneally injected 60 min before the test session (session 3). Moreover, fluoxetine (30 mg/kg, ip) was repeatedly injected during four consecutive days, starting after the screening session (session 2), and the fourth dose was injected 60 min before the test session (session 3). Only for the repeated fluoxetine administration, an extension of 72 h in the time frame between the screening session (session 2) and test session (session 3) was assumed. The dosages of nortriptyline and fluoxetine were based on previous studies demonstrating reversal of shock-induced shuttle box escape deficits after acute or repeated injections of these antidepressants (Telner et al. 1981; Zazpe et al. 2007) and decreased immobility in the forced swimming test (De Moura et al. 2014; Da-Rocha et al. 1997).
The non peptide SB-612111 (1, 3, and 10 mg/kg, ip) and the peptide NOP antagonist UFP-101 (1, 3, and 10 nmol, intracerebroventricularly (icv)) were injected 30 and 5 min, respectively, before the test session. The doses, route of administration, and pretreatment time for SB-612111 and UFP-101 were based on previous studies which demonstrated antidepressant-like actions for these compounds in mice (Rizzi et al. 2007; Gavioli et al. 2003). Icv injections were performed at the rate of 2 μl/min through a needle (27 G) protruding 1 mm from the cannula tip. Control groups were treated with their respective vehicles (saline or DMSO 0.8 % solutions) following the same schedule described to treatment groups. All drugs injected ip were given in a volume of 10 ml/kg. All drugs were freshly prepared before experiments.
Cannula implantation
Surgical procedure to implantation the cannula in lateral ventricle was conducted in mice under anesthesia with ketamine and xylazine (100 and 10 mg/kg, ip, respectively) placed in a stereotaxic apparatus. A stainless steel 8-mm guide cannula (25 × 0.7 mm) was placed in the lateral ventricle according to the atlas of Paxinos and Franklin (2008) (coordinates: lateral +1.1 mm, posterior −0.6 mm, and ventral −1.0 mm); cannula was fixed with dental cement. To prevent occlusion, a dummy cannula was inserted inside the guide cannula. After surgery, pain, inflammation, and possible infections were prevented by treating animals with subcutaneous diclofenac sodium administration (10 mg/kg) and tetracycline (20 mg/kg, im). The animals were allowed to recover for at least 4 days before being tested. At the end of experimental procedures, all animals were euthanized with an overdose of sodium thiopental and transcardially perfused with saline solution. The placement of the cannula was verified under a light microscope. Results of animals in which the cannula was not correctly placed were excluded for further analysis (less than 10 %).
Learned helplessness model
The LH model was conducted as previously described (Maeng et al. 2008; Malkesman et al. 2012). The animals were individually placed in a Plexiglas box with a stainless steel grid floor (0.3 × 1 cm) attached to an electric shock generator (AVS Projetos, Ribeirão Preto, SP, Brazil). The apparatus is divided in two compartments (50 × 30 × 30 cm) by a guillotine door (12 × 25 cm). Mice were subjected to electric footshocks during three consecutive days. At the first experimental session (session 1—induction), mice were exposed to 180 cycles of randomically assigned inescapable electric footshocks (0.5 mA, 1–10 s shock duration, 10-s average interval). At the second (session 2—screening) and third (session 3—test) experimental sessions, mice were exposed to 30 cycles of electric footshocks (0.5 mA, 1–10 s shock duration, 20-s average interval); in this case, animals could escape from electric footshocks by moving to the other side of the box. The duration of induction session was 60 min, while the screening and test sessions last about 15 min. Mice that failed in more than 20 attempts to escape at the second session were considered helpless. Those animals that did not reach this criterion were excluded from this study. At the third session, the following parameters were evaluated: escape latency (s) and number of escapes. The assessment of movement between chamber sides was recorded manually by an experienced observer who was blind with respect to the treatment conditions. Helpless mice were tested at the third session, after treatment with nortriptyline, fluoxetine, SB-612111, UFP-101, or vehicle. The detailed information about the LH model is illustrated in Table 1.
Treatment in non stressful and controllable footshock situations
Additional experiments were performed in order to rule out putative drug effects on the behavior of mice under non stressful and controllable footshock situations. Only the active doses were tested under these conditions. To evaluate the effects of drugs on non stressful situations, naïve mice were placed in the chamber with the guillotine door closed at the first day, being opened at the two consecutive days. During these series, mice did not receive any electric footshock. These 3-day sessions (without footshocks) lasted approximately the same time as in the LH model. In contrast, in experiments aimed at putative effects of treatments on controllable stressful situations, electric footshocks were delivered to mice placed in the chamber with the guillotine door open during all the 3 days of experiment. The number of cycles with electric footshocks during each experimental session was the same as in the LH model. On day 3 for both series of experiments (i.e., non stressful and controllable footshocks situations) mice were treated with the drugs and the following parameters were recorded: escape latencies (s) and number of escapes. The detailed information about the effects of drug treatments on non stressful and controllable stressful situations is illustrated in Figure S1 and S2.
Data analysis
Data are presented as mean (for escape latencies) and mean ± sem (for frequency of escape) of n animals. Data sets were initially checked for normality and homogeneity of variance before use of parametric statistical tests. Significant differences between escape latency data were evaluated using two-way ANOVA (independent factors: trials and treatments) followed by Dunnett’s post hoc test. The differences between number of escapes of distinct treatment groups were evaluated through unpaired Student’s t test or one-way ANOVA followed by Dunnett’s post hoc test, when two or more groups were compared, respectively. Differences were considered statistically significant when P < 0.05. For the statistical analysis, the GraphPad Prism software version 5.0 (Graph Pad Software Inc., San Diego, USA) was used.
Results
Effects of standard antidepressants in the learned helplessness model
Under the present experimental conditions, an overall analysis indicated that approximately 80 % of mice subjected to the LH model developed the helpless phenotype. As shown in Fig. 1, helpless mice displayed average escape latency in the range of 20–25 s and their average of successful escapes after footshock was around 5 (within 30 trials). The acute treatment with nortriptyline prior to the test session significantly reduced escape latencies and increased the number of escapes (Fig. 1a, treatment factor: F(1,360) = 461.8, P < 0.05, two-way ANOVA, Dunnett’s test; Fig. 1b, t (12) = 15.0, P < 0.05, unpaired Student’s t test).
Effects of acute nortriptyline (30 mg/kg, ip) administration on the escape latencies (a) and number of escapes (b) in helpless mice. Data are the mean (escape latency) and mean ± sem (escapes) of 5–9 mice/group. *P < 0.05 vs. saline, according to two-way ANOVA followed by Dunnett’s test (a) and unpaired Student’s t test (b)
In order to further validate experimental conditions, the effects of fluoxetine were also tested. As shown in Fig. 2, the acute administration of fluoxetine did not modify escape behavior deficits (Fig. 2a, treatment factor: F(1,270) = 1.8, P > 0.05, two-way ANOVA, Dunnett’s test; Fig. 2b, t (9) = 1.4, P > 0.05, unpaired Student’s t test). On the contrary, the repeated treatment (4 days) with this drug promoted a significant improvement on the behavioral deficits induced by the LH model (Fig. 2c, treatment factor: F(1,360) = 119.4, P < 0.05, two-way ANOVA, Dunnett’s test; Fig. 2d, t (12) = 3.4, P < 0.05, unpaired Student’s t test).
Effects of acute (a, b) and four consecutive days of administration (c, d) of fluoxetine (30 mg/kg, ip) on the escape latencies and number of escapes in helpless mice. Data are the mean (escape latency) and mean ± sem (escapes) of 5–6 (a, b) and 7 (c, d) mice/group. *P < 0.05 vs. saline, according to two-way ANOVA followed by Dunnett’s test (a, c) and unpaired Student’s t test (b, d)
Effects of NOP receptor antagonists in the mouse learned helplessness
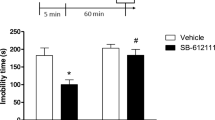
Two chemically different NOP receptor antagonists, the peptide UFP-101 and the non peptide SB-612111, were tested in the LH model aiming to evaluate the influence of NOP receptor blockade in the mouse behavior. Three different doses of SB-612111 and UFP-101 were given to mice on day 3 of the LH procedure. As illustrated in Fig. 3, the administration of the two higher doses of SB-612111 (3 and 10 mg kg, ip) were able to significantly reduce escape latencies and increase the number of successful escapes compared to vehicle-treated mice (Fig. 3a, treatment factor: F(3,630) = 125.8, P < 0,05, two-way ANOVA, Dunnett’s test; Fig. 3b, F (3,21) = 71.0, P < 0.05, one-way ANOVA, Dunnett’s test). Similar antidepressant-like effects were observed in helpless mice treated icv with UFP-101 at 3 and 10 nmol (Fig. 4a, treatment factor: F(3,1080) = 225.9, P < 0.05, two-way ANOVA, Dunnett’s test; Fig. 4b, F (3,36) = 150.1, P < 0.05, one-way ANOVA, Dunnett’s test). The lower doses of SB-612111 and UFP-101 did not evoke any significant change in the behavior of helpless mice.
Effects of acute SB-612111 (1, 3, and 10 mg/kg, ip), a non peptide NOP antagonist, on the escape latencies (a) and number of escapes (b) in helpless mice. Data are the mean (escape latency) and mean ± sem (escapes) of 6–8 mice/group. *P < 0.05 vs. vehicle, according to two-way ANOVA followed by Dunnett’s test (a) and one-way ANOVA followed by Dunnett’s test (b)
Effects of acute UFP-101 (1, 3, and 10 nmol, icv), a peptide NOP antagonist, on the escape latencies (a) and number of escapes (b) in helpless mice. Data are the mean (escape latency) and mean ± sem (escapes) of 10 mice/group. *P < 0.05 vs. saline, according to two-way ANOVA followed by Dunnett’s test (a) and one-way ANOVA followed by Dunnett’s test (b)
Effects treatments on controllable electric footshock and non stressful situations
This series of data was performed to investigate putative drug effects on the behavior of animals subjected to non stressful and controllable footshock situations. The active doses of nortriptyline (30 mg/kg, ip), fluoxetine (30 mg/kg, four consecutive days of treatment, ip), SB-61211 (10 mg/kg, ip), and UFP-101 (10 nmol, icv) were administered in mice. No changes in animal behavior were detected in response to these treatments as assessed by the number of escapes and the latencies to escape from one chamber to other (Figure S1 and S2, P > 0.05).
Discussion
In this study, we showed that the administration of nortriptyline and fluoxetine completely reversed the mouse helpless phenotype. Similar to standard antidepressants, the treatment with the NOP receptor antagonists UFP-101 and SB-612111 restored in a dose-dependent manner the behavior of mice subjected to the LH model.
Learned helplessness was one of the earliest paradigms used to model depression (Seligman et al. 1968). Together with the chronic mild stress (Wiborg 2013) and chronic psychosocial stress models (Fuchs et al. 2004), it belongs to the environmental stress models of depression. The exposure to unavoidable and unpredictable electric footshocks generates a coping deficit in aversive but avoidable situations—i.e., session 3 of LH paradigm (Vollmayr and Gass 2013). Additionally, others changes such increasing stereotyping, decreasing appetitive discrimination, and modification of eating habits besides increased ulceration and heart rate were found in helpless rodents (Pryce et al. 2011). The LH model has translational validity according to the following proposed criteria for animal models of depression (Vollmayr and Gass 2013): (i) construct validity, since the experience in facing aversive and uncontrolled events appears to be the basis of physiological, emotional, cognitive, and motivational changes related with depression in both rodents and humans; (ii) face validity, since there is phenomenological similarity (i.e., motivational deficit, passive behavior) between the animal behavior after exposure to the LH model and depressed patients; and (iii) predictive validity, since the animal helpless behavior is sensitive to drugs currently used for treating depressed patients (for a review see Vollmayr and Gass 2013).
With the experimental conditions adopted in the present study, the above mentioned criteria were met; in fact, a high percentage of mice subjected to inescapable footshock sessions acquired the helpless phenotype. The treatment with two different classes of antidepressants restored the behavioral deficits generated by the exposure to the LH paradigm. In fact, the acute administration of the tricyclic antidepressant nortriptyline decreased escape latencies and escapes failure; similar results have been already described in the literature with nortriptyline and other tricyclic antidepressants (Telner et al. 1981; Kametani et al. 1983). Interestingly enough, acute treatment with the serotonin selective reuptake inhibitor fluoxetine was ineffective. On the other hand, when the drug was given during four consecutive days, it completely reversed the helpless phenotype. Our data are in line with the literature since some studies have demonstrated SSRI effectiveness in the LH model only with repeated administrations (Valentine et al. 2008; Zazpe et al. 2007). Subchronic treatment with different doses of fluoxetine decreases the freezing response and increases the avoidance responsiveness of rats against electric footshocks (Ferguson et al. 2000; Zazpe et al. 2007).
In the present study, we demonstrated that two chemically different selective NOP receptor antagonists elicited robust antidepressive-like effects in helpless mice. Importantly, the antagonists were effective in dose ranges that have been previously shown to counteract the actions induced by exogenous N/OFQ (Calo et al. 2002; Rizzi et al. 2007). Thus, these findings further support a large body of evidence indicating that the blockade of NOP receptors promotes antidepressant-like effects. In fact, the actions of distinct NOP antagonists (i.e., [Nphe1]N/OFQ(1-13)-NH2, UFP-101, SB-612111, J-113397, LY2940094) has been well established in behavioral despair tests (Gavioli and Calo’ 2013; Post et al. 2015). Moreover, it is worth highlighting the studies of Vitale et al. (2009) and Medeiros et al. (2015) which have demonstrated that the blockade of NOP receptors reversed depressive-like behaviors induced by chronic exposure to an unpredictable sequence of mild stressful stimuli in rats and by the transient inflammatory process elicited by a single administration of lipopolysaccharide in mice. Very recently, a study performed with LY2940094 provided the first clinical evidence for an antidepressant effect of a NOP antagonist in depressed patients (Post et al. 2015). Thus, there is now in literature robust evidence that NOP antagonists represent promising candidates for the treatment of major depression.
The effects of drug treatment on rodent locomotion and cognition can bias the outcome of the LH model. To deal with this issue, a triadic protocol design with two control groups has been used to unambiguously interpret the drug effects in the LH model (Drugan et al. 1997). One group of animals is exposed to footshocks that can be controlled by escaping. Animals of the second group are not exposed to stress. Thus, the effects of drugs in animals exposed to uncontrollable shocks (helpless mice) can be compared with animals exposed to controllable shocks (cognitive assessment) and to unstressed controls (locomotor assessment). In this study, standard antidepressants nortriptyline and fluoxetine, as well as the NOP antagonists UFP-101 and SB-612111 at the higher doses tested, did not evoke locomotor alterations, since treated animals had equivalent transitions compared to control mice. Similarly, all treatments did not modify the animal behavior under a controllable stressful situation. Thus, the effects of NOP antagonists in the helpless animals are not biased by the actions of drug treatments on controllable and non stressful situations and can be interpreted as genuine antidepressant-like action.
Evidence from microdialysis studies support a role for monoamines underlying the behavioral changes evoked by inescapable footshocks (Vollmayr and Gass 2013). In fact, saline-treated helpless mice had significantly lower frontal cortex 5-HT levels compared to animals treated with antidepressants (Petty et al. 1992). Moreover, resilient animals in the LH model exhibited higher levels of hippocampal noradrenaline compared to the helpless rats (Petty et al. 1993). Therefore, uncontrollable footshock promotes a deficit of monoamine neurotransmission that can be reversed by antidepressants. Interesting enough, literature findings suggest that the monoaminergic system plays also a central role in the mechanism of action of NOP antagonists as antidepressant (for a review, see Gavioli and Calo’ 2013). Briefly, it has been hypothesized that NOP antagonists could evoke antidepressant-like actions by blocking the pre- and/or postsynaptic inhibitory effects exerted by endogenous N/OFQ on monoaminergic cortical terminals and dorsal raphe/locus coeruleus neurons. This seems to be particularly relevant during stressful situations (Nazzaro et al. 2010), in which NOP antagonists might act by restoring monoamine levels in the brain circuitry controlling mood behaviors.
In conclusion, the acute administration of NOP antagonists dose dependently reversed the mouse helpless behavior. This action of NOP antagonists must be interpreted as a genuine antidepressant-like effect since no effects controllable stressful and non stressful situations were observed. The present findings thus corroborate the proposal that blocking NOP receptor signaling is a rather promising strategy for the development of innovative antidepressant.
References
Adrien J, Dugovic C, Martin P (1991) Sleep-wakefulness patterns in the helpless rat. Physiol Behav 49:257–262
Calo G, Rizzi A, Rizzi D, Bigoni R, Guerrini R, Marzola G, Marti M, McDonald J, Morari M, Lambert DG, Salvadori S, Regoli D (2002) [Nphe1, Arg14, Lys15]nociceptin-NH2, a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. Br J Pharmacol 136(2):303–311
Chourbaji S, Zacher C, Sanchis-Segura C, Dormann C, Vollmayr B, Gass P (2005) Learned helplessness: validity and reliability of depressive-like states in mice. Brain Res Protocol 16:70–78
Dalla C, Edgecomb C, Whetstone AS, Shors TJ (2008) Females do not express learned helplessness like males do. Neuropsychopharmacol 33(7):1559–1569
Da-Rocha MA Jr, Puech AJ, Thiébot MH (1997) Influence of anxiolytic drugs on the effects of specific serotonin reuptake inhibitors in the forced swimming test in mice. J Psychopharmacol 11(3):211–218
De Moura JC, Noroes MM, Rachetti Vde P, Soares BL, Preti D, Nassini R, Materazzi S, Marone IM, Minocci D, Geppetti P, Gavioli EC, André E (2014) The blockade of transient receptor potential ankirin 1 (TRPA1) signalling mediates antidepressant- and anxiolytic-like actions in mice. Br J Pharmacol 171(18):4289–4299
Dess NK, Raizer J, Chapman CD, Garcia J (1988) Stressors in the learned helplessness paradigm: effects on body weight and conditioned taste aversion in rats. Physiol Behav 44:483–490
Drugan RC, Basile AS, Ha JH, Healy D, Ferland RJ (1997) Analysis of the importance of controllable versus uncontrollable stress on subsequent behavioral and physiological functioning. Brain Res Brain Res Protocol 2(1):69–74
Ferguson SM, Brodkin JD, Lloyd GK, Menzaghi F (2000) Antidepressant-like effects of the subtype-selective nicotinic acetylcholine receptor agonist, SIB-1508Y, in the learned helplessness rat model of depression. Psychopharmacol (Berl) 152(3):295–303
Fuchs E, Czéh B, Flügge G (2004) Examining novel concepts of the pathophysiology of depression in the chronic psychosocial stress paradigm in tree shrews. Behav Pharmacol 15(5-6):315–325
Gavioli EC, Calo’ G (2013) Nociceptin/orphanin FQ receptor antagonists as innovative antidepressant drugs. Pharmacol Ther 140:10–25
Gavioli EC, Marzola G, Guerrini R, Bertorelli R, Zucchini S, De Lima TC (2003) Blockade of nociceptin/orphanin FQ-NOP receptor signaling produces antidepressant-like effects: pharmacological and genetic evidences from the mouse forced swimming test. Eur J Neurosci 17:1987–1990
Gavioli EC, Vaughan CW, Marzola G, Guerrini R, Mitchell VA, Zucchini S (2004) Antidepressant-like effects of the nociceptin/orphanin FQ receptor antagonist UFP-101: new evidence from rats and mice. Naunyn Schmiedebergs Arch Pharmacol 369:547–553
Goeldner C, Reiss D, Kieffer BL, Ouagazzal AM (2010) Endogenous nociceptin/orphanin-FQ in the dorsal hippocampus facilitates despair-related behavior. Hippocampus 20(8):911–916
Greenberg L, Edwards E, Henn FA (1989) Dexamethasone suppression test in helpless rats. Biol Psychiatry 26:530–532
Holmes PV (2003) Rodent models of depression: reexamining validity without anthropomorphic inference. Trends Pharmacol Sci 15:143–174
Joca S, Zanelati T, Guimaraes F (2006) Post-stress facilitation of serotonergic, but not noradrenergic, neurotransmission in the dorsal hippocampus prevents learned helplessness development in rats. Brain Res 1087:67–74
Kametani H, Nomura S, Shimizu J (1983) The reversal effect of antidepressants on the escape deficit induced by inescapable shock in rats. Psychopharmacol (Berl) 80(3):206–208
Kessler RC, Bromet EJ (2013) The epidemiology of depression across cultures. Annu Rev Public Health 34:119–138
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010) NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160(7):1577–1579
Lambert DG (2008) The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov 7(8):694–710
Maeng S, Hunsberger JG, Pearson B, Yuan P (2008) BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proc Natl Acad Sci U S A 105:8766–8771
Malkesman O, Austin DR, Tragon T, Wang G, Rompala G, Hamidi AB, Cui Z, Young WS, Nakazawa K, Zarate CA Jr, Manji HK, Chen G (2012) Acute D-serine treatment produces antidepressant-like effects in rodents. I. J Neuropsychopharmacol 15(8):1135–1148
Martin P, Soubrie P, Simon P (1987) The effect of monoamine oxidase inhibitors compared with classical tricyclic antidepressants on learned helplessness paradigm. Prog Neuropsychopharmacol Biol Psychiatry 11(1):1–7
Medeiros IU, Ruzza C, Asth L, Guerrini R, Romão PR, Gavioli EC, Calo G (2015) Blockade of nociceptin/orphanin FQ receptor signaling reverses LPS-induced depressive-like behavior in mice. Peptides 72:95–103
Nazzaro C, Barbieri M, Varani K, Beani L, Valentino RJ, Siniscalchi A (2010) Swim stress enhances nociceptin/orphanin FQ induced inhibition of rat dorsal raphe nucleus activity in vivo and in vitro: role of corticotrophin releasing factor. Neuropharmacology 58(2):457–464
Nemeroff CB, Owens MJ (2002) Treatment of mood disorders. Nat Neurosci 5:1068-1070 (Suppl.)
O’Neil MF, Moore NA (2003) Animal models of depression: are there any? Hum Psychopharmacol 8(4):239–254
Overmier JB, Seligman ME (1967) Effects of inescapable shock upon subsequent escape and avoidance responding. J Comp Physiol Psychol 63:28–33
Paxinos G, Franklin KBJ (2008) The mouse brain in stereotaxic coordinates, 3rd edn. Academic Press, San Diego
Petit-Demouliere B, Chenu F, Bourin M (2005) Forced swimming test in mice: a review of antidepressant activity. Psychopharmacol (Berl) 177(3):245–255
Petty F, Kramer G, Wilson L (1992) Prevention of learned helplessness: in vivo correlation with cortical serotonin. Pharmacol Biochem Behav 43(2):361–367
Petty F, Kramer G, Wilson L, Chae Y (1993) Learned helplessness and in vivo hippocampal norepinephrine release. Pharmacol Biochem Behav 46(1):231–235
Post A, Smart TS, Krikke-Workel J, Dawson GR, Harmer CJ, Browning M, Jackson K, Kakar R, Mohs R, Statnick M, Wafford K, McCarthy A, Barth V, Witkin JM (2015) A selective nociceptin receptor antagonist to treat depression: evidence from preclinical and clinical studies. Neuropsychopharmacol. doi:10.1038/npp.2015.348
Pryce CR, Azzinnari D, Spinelli S, Seifritz E, Tegethoff M, Meinlschmidt G (2011) Helplessness: a systematic translational review of theory and evidence for its relevance to understanding and treating depression. Pharmacol Ther 132(3):242–267
Redrobe JP, Calo’ G, Regoli D, Quirion R (2002) Nociceptin receptor antagonists display antidepressant-like properties in the mouse forced swimming test. Naunyn Schmiedebergs Arch Pharmacol 365:164–167
Rizzi A, Gavioli EC, Marzola G, Spagnolo B, Zucchini S, Ciccocioppo R, Trapella C, Regoli D, Calò G (2007) Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol]: in vivo studies. J Pharmacol Exp Ther 321(3):968–974
Rizzi A, Molinari S, Marti M, Marzola G, Calo’ G (2011) Nociceptin/orphanin FQ receptor knockout rats: in vitro and in vivo studies. Neuropharmacol 60(4):572–579
Seligman ME, Maier SF, Geer JH (1968) Alleviation of learned helplessness in the dog. J Abnorm Psychol 73(3):256–262
Sherman AD, Sacquitne JL, Petty F (1982) Specificity of the learned helplessness model of depression. Pharmacol Biochem Behav 16:449–454
Takamori K, Yoshida S, Okuyama S (2001) Availability of learned helplessness test as a model of depression compared to a forced swimming test in rats. Pharmacology 63:147–153
Telner JI, Singhal RL, Lapierre YD (1981) Reversal of learned helplessness by nortriptyline. Prog Neuropsychopharmacol 5(5-6):587–590
Thase ME, Denko T (2008) Pharmacotherapy of mood disorders. Annu Rev Clin Psychol 4:53–91
Toledo MA, Pedregal C, Lafuente C, Diaz N, Martinez-Grau MA, Jiménez A, Benito A, Torrado A, Mateos C, Joshi EM, Kahl SD, Rash KS, Mudra DR, Barth VN, Shaw DB, McKinzie D, Witkin JM, Statnick MA (2014) Discovery of a novel series of orally active nociceptin/orphanin FQ (NOP) receptor antagonists based on a dihydrospiro(piperidine-4,7'-thieno[2,3-c]pyran) scaffold. J Med Chem 57(8):3418–3429
Valentine G, Dow A, Banasr M, Pittman B, Duman R (2008) Differential effects of chronic antidepressant treatment on shuttle box escape deficits induced by uncontrollable stress. Psychopharmacol (Berl) 200:585–596
Vitale G, Ruggieri V, Filaferro M, Frigeri C, Alboni S, Tascedda F, Brunello N, Guerrini R, Cifani C, Massi M (2009) Chronic treatment with the selective NOP receptor antagonist [Nphe 1, Arg 14, Lys 15]N/OFQ-NH 2 (UFP-101) reverses the behavioural and biochemical effects of unpredictable chronic mild stress in rats. Psychopharmacol (Berl) 207(2):173–189
Vollmayr B, Gass P (2013) Learned helplessness: unique features and translational value of a cognitive depression model. Cell Tissue Res 354(1):171–178
Wiborg O (2013) Chronic mild stress for modeling anhedonia. Cell Tissue Res 354(1):155–169
Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, Tucker RC, Ciccocioppo R (2014) The biology of nociceptin/orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol Ther 141(3):283–299
Zazpe A, Artaiz I, Labeaga L, Lucero ML, Orjales A (2007) Reversal of learned helplessness by selective serotonin reuptake inhibitors in rats is not dependent on 5-HT availability. Neuropharmacology 52:975–984
Acknowledgments
This work was supported by funds from the Brazilian National Council Research (CNPq Grant Nos. 304453/2012-9 and PVE401289/2014-1) and PRONEM/FAPERN/CNPq. VADH and LA held fellowships founded by CAPES/Brazil. IUM held a PDJ fellowship by CNPq/Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the experimental series were conducted in accordance with the Brazilian law no. 11.794/2008 for care and use of experimental animals and were approved by the Local Ethics Committee for Animal Use of the Federal University of Rio Grande do Norte (License no. 063/2013). This study is reported following the ARRIVE guidelines (Kilkenny et al. 2010).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Effects of nortriptyline (30 mg/kg, ip, acute treatment) (A,B), fluoxetine (30 mg/kg, ip, 4 consecutive days of administration) (C,D), SB-612111 (10 mg/kg, ip, acute treatment) (E,F) and UFP-101 (10 nmol, icv, acute treatment) (G,H) on the transitions latencies and frequency of transitions into the chamber; no electric footshocks were delivered during these sessions. Data are the mean (transitions latencies) and mean ± SEM (transitions) of n = 5 mice/group. (DOC 415 kb)
Figure S2
Effects of nortriptyline (30 mg/kg, ip, acute treatment) (A,B), fluoxetine (30 mg/kg, ip, 4 consecutive days of administration)(C,D), SB-612111 (10 mg/kg, ip, acute treatment) (E,F) and UFP-101 (10 nmol, icv, acute treatment) (G,H) on the escape latencies and frequency of escapes from the electrified chamber. Electric footshocks were delivered with the guilhotine door open during all the sessions. Data are the mean (escape latencies) and mean ± SEM (escapes) of n = 5 mice/group. (DOC 416 kb)
Rights and permissions
About this article
Cite this article
Holanda, V.A.D., Medeiros, I.U., Asth, L. et al. Antidepressant activity of nociceptin/orphanin FQ receptor antagonists in the mouse learned helplessness. Psychopharmacology 233, 2525–2532 (2016). https://doi.org/10.1007/s00213-016-4310-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4310-1