Abstract
Rationale
Although nicotine exposure upregulates the α4β2* subtype of nicotinic acetylcholine receptors (nAChRs), the upregulation of nAChRs in non-human primates voluntarily self-administering nicotine has never been demonstrated.
Objectives
The objective of the study is to determine if short access to nicotine in a non-human primate model of nicotine self-administration is sufficient to induce nAChRs upregulation.
Methods
We combined a nicotine self-administration paradigm with in vivo measure of α4β2* nAChRs using 2-[18F]fluoro-A-85380 (2-FA) and positron emission tomography (PET) in six squirrel monkeys. PET measurement was performed before and after intravenous nicotine self-administration (unit dose 10 μg/kg per injection). Monkeys were trained to self-administer nicotine under a fixed-ratio (FR) schedule of reinforcement. Intermittent access (1 h daily per weekday) to nicotine was allowed for 4 weeks and levels of α4β2* nAChRs were measured 4 days later.
Results
This intermittent access was sufficient to induce upregulation of α4β2* receptors in the whole brain (31 % upregulation) and in specific brain areas (+36 % in amygdala and +62 % in putamen).
Conclusions
These results indicate that intermittent nicotine exposure is sufficient to produce change in nAChRs expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The α4β2* subtype of the nicotinic acetylcholine receptor (nAChR) has been implicated in mediating the reinforcing effects of nicotine (Maskos et al. 2005). Studies in transgenic mice have revealed that β2 subunit deletion decreases sensitivity to nicotine’s reinforcing effects while overexpression of the α4 subunit increases sensitivity to nicotine reinforcement (Picciotto et al. 1998; Tapper et al. 2004). However, the relationship between nAChRs expression and motivation for nicotine intake is complex since lower levels of midbrain nAChRs have been associated with a high motivation to self-administer nicotine (Le Foll et al. 2009).
It is well known that nicotine exposure produces an upregulation of high-affinity nAChRs in the brain (see (Govind et al. 2009) for a review). Based on postmortem brain tissue studies, the density of nAChRs is greater in smokers compared to non-smokers, whereas the density in non-smokers is identical to that in ex-smokers (Benwell et al. 1988; Breese et al. 1997; Perry et al. 1999). This upregulation has been shown in non-human primates (as in (Staley et al. 2006) with oral nicotine administration for example). Nicotine-treated rodents also display enhanced nAChRs density compared to control animals (Besson et al. 2007; Marks et al. 1983; Schwartz and Kellar 1983). Since there are no associated changes of mRNA coding for these receptors, post-transcriptional mechanisms have been proposed to underlie these changes (Govind et al. 2009). Further, the functional role of this receptor upregulation is still unclear (Picciotto et al. 2008; Picciotto and Mineur 2014; Wonnacott 1990).
Although initial experiments to study nAChRs upregulation were performed on rodent brain preparations using binding techniques (Flores et al. 1992, 1997), it is feasible to explore nAChRs expression in vivo using positron emission tomography (PET). Different PET radiotracers have been developed for α4β2* nAChRs quantification (Horti et al. 2013). Among those PET radiotracers, 2-[18F]fluoro-A-85380 (2-FA) has been used in rodents (Vaupel et al. 2007), non-human primates (Chefer et al. 2003; Le Foll et al. 2007a, 2009; Valette et al. 2003; 2005) and human subjects (Mukhin et al. 2008), with an upregulation of nAChRs reported in human smokers (Brody et al. 2013; Mukhin et al. 2008; Wullner et al. 2008). Similar nAChR upregulation in the brains of smokers has been observed using a single-photon emission computed tomography and analog of 2FA, 5-[123I]iodo-A-85380 (Staley et al. 2006).
Due to the ability to tightly control environmental factors and drug exposure history, PET imaging studies in non-human primates have contributed extensively to our understanding of psychostimulant drug addiction (Gould et al. 2014; Howell and Wilcox 2002). However, much less is known about nicotine as compared to cocaine administration. This is likely due to the fact that few research centers have the ability to assess nAChRs in non-human primates with PET imaging along with the ability to study the nicotine dependence process using a drug self-administration paradigm (Le Foll et al. 2007b). Combining these two approaches, an inverse relationship has been found between the baseline midbrain expression of α4β2* nAChRs and the motivation to self-administer nicotine in squirrel monkeys (Le Foll et al. 2009). It is not clear if intermittent access to nicotine, as currently used in a well-developed non-human primate model of nicotine self-administration (Le Foll et al. 2007b), is sufficient to upregulate nAChRs.
In order to test this hypothesis, we trained squirrel monkeys to self-administer nicotine under a fixed ratio schedule of reinforcement with 1-h daily sessions. Pre-exposure baseline and post-exposure levels of nAChRs were measured using the binding potential (BPND) of 2-[18F]fluoro-A-85380 (2-FA), a selective α4β2* nAChR PET ligand. BPND is proportional to the density of receptors available for radioligand binding (Bavail) in vivo. BPND = f ND•Bavail/KD, where KD is the dissociation constant and f ND is a free fraction of radioligand in nondisplaceable compartment (Innis et al. 2007).Nicotine upregulates nAChRs in both an intracellular compartment and on cell surface (Kuryatov et al. 2005; Lester et al. 2009; Lomazzo et al. 2011; Zambrano et al. 2012, 2015).
Therefore, it is reasonable to expect the presence of nicotine-induced upregulation of nAChRs in practically all brain regions expressing nicotinic receptors. Indeed, it was shown that in comparison with non-smokers, smokers have significant increases of nAChRs across almost the entire brain (Mukhin et al. 2008). Therefore, our primary hypothesis was that post-exposure levels of nAChRs in total monkey brain will be higher as compared to pre-exposure baseline levels. As an exploratory analysis and for the purpose of comparison with receptor upregulation in smokers, we also have evaluated pre- and post-exposure levels of nAChRs in a few regions of reasonable size in the squirrel monkey brain and/or were previously assessed for nAChR upregulation in smoker’s brain with 2FA (Mukhin et al. 2008).
Material and methods
Subjects
Six adult drug-naive male squirrel monkeys (Saimiri sciureus), weighing 710 to 950 g, were housed individually in a temperature- and humidity-controlled room and were maintained on a 12-h light/dark cycle; the lights were on from 6:45 AM to 6:45 PM. Experiments were conducted during the light phase. Monkeys were maintained in facilities fully accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC), and all experimentation was conducted in accordance with the guidelines of the Institutional Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, and the 2003 Guide for Care and Use of Laboratory Animals from the National Research Council.
PET imaging studies
Radiochemistry: [18F]Fluoride was produced using an RDS111 negative ion cyclotron, and 2-FA was synthesized using a modified semiautomated method (Horti et al. 1998). The final product was formulated as a sterile and pyrogen-free isotonic solution. Radiochemical purity product was greater than 98 %, and specific activity was in the range from 106 to 648 GBq/μmol (313 ± 166 GBq/μmol, average ± SD).
PET and MRI scanning procedures
Data were acquired on a Siemens Exact ECAT HR+ tomograph (63 slices, center to center spacing of 2.4 mm, with an in-plane reconstructed resolution, full width at half maximum (FWHM), of 4.7 mm at the center of the field of view and reconstructed axial spatial resolution of 4.2 mm in 3D mode). Before each radioligand administration, transmission scans were obtained with three rotating 68Ge-68Ga sources and used to correct for photon attenuation by tissue and facemask. PET images were reconstructed from the raw data with a standard filtered-back projection algorithm and a RAMP filter.
For the PET scans, monkeys were initially anesthetized with 1.5-mg/kg alfadolone and alfaxolone acetate (Saffan®, Arnolds Veterinary Products, Shropshire, UK), given intramuscularly. Anesthesia was then maintained by 1.5–2.5 % isoflurane. An individually molded thermoplastic facemask was secured to a custom-made monkey head-holder attached to a backboard.
Acquisition of dynamic PET scans started with the injection of 2-FA as a bolus (39 ± 11 MBq/kg injected intravenously in approximately 1 ml of saline over 20 s) and continued for 5 h.
Anatomical MRI brain images were acquired on a 3.0 Tesla Siemens Magnetom Allegra MRI unit (Siemens Medical Solutions) using continuous intravenous infusion of 8–11-mg/kg/h Saffan to maintain anesthesia.
Vital signs, including heart rate, ECG (during PET studies), respiration rate, ETCO2, and blood oxygen saturation (always maintained above 95 %), were continuously monitored during the PET and MRI imaging sessions.
PET data analysis
Regions of interest (ROIs) for the whole brain, cerebellum, thalamus, pons, amygdala, putamen, and temporal cortex were defined on the individual T1 MRI images co-registered to PET images, with reference to a stereotaxic atlas (Gergen and MacLean 1962). ROIs for muscle were placed at the back of the neck in the area of the semispinalis cervicis, splenius capitis, and obliquus capitis muscles. BPND values were calculated using a simplified reference tissue model (PMOD v. 3.17) with muscles as a reference region. BPND values were corrected for differences between brain tissue VND and muscle VT using equation (6) from (Le Foll et al. 2007a).
Previously, using an averaged 2-FA volumes of distribution (VD), VDT value in muscle (3.02 ± 0.24; n = 15) and an averaged VDND value for thalamus, cortex, and midbrain obtained from blocking studies with nicotine pumps in squirrel monkeys (4.06 ± 0.21, n = 4), we obtained the α value (the ratio of VDND over muscle VDT) of 1.34 (Le Foll et al. 2007a).
First, BPND values for the whole brain were compared before and after self-administration using paired t test. Subsequently, cerebellum, thalamus, pons, amygdala, putamen, and temporal cortex regions were compared before and after self-administration using paired t test. Results were corrected for multiple comparisons using Holm-Bonferroni method.
Intravenous nicotine self-administration
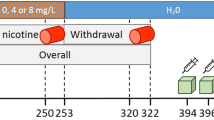
Several days after the first PET scan, acquisition sessions were initiated during which the monkeys were allowed to self-administer nicotine intravenously under a fixed-ratio schedule of reinforcement. The ratio requirement was gradually increased up to the final ratio requirement (FR-10). Session duration was 1 h, and SA sessions were conducted during weekdays. Once self-administration was stable, the monkeys got access to 4 weeks of nicotine self-administration with a unit dose of 10 μg/kg per injection. The unit dose of 10 μg/kg nicotine per injection was selected, as this dose had previously been reported to maintain self-administration at high rates under both fixed and progressive ratio schedule of reinforcement in squirrel monkeys under those conditions (Le Foll et al. 2007b). Ten micrograms/kilograms per injection was also the unit dose that maintained the highest level of responding under FR10 schedule of reinforcement (Le Foll et al. 2007b). The following week after 4 days, the post-exposure PET sessions were performed. The 4-day interval between nicotine access and PET was chosen as previous studies indicates that binding of imaging tracers could be affected in early withdrawal (Staley et al. 2006). Previous studies with human subjects and 2-FA indicated that 4 days is sufficient to decrease plasma nicotine to levels at which it will not compete with the PET radioligand for binding sites (Mukhin et al. 2008), and some pilot data (Le Foll et al., unpublished personal observations) supported the use of the 4-day withdrawal phase in squirrel monkeys.
Results
Behaviorally, the nicotine self-administration behavior remained stable over the 4-week exposure period. There was a significantly higher number of FR completed on the active vs the inactive lever (see Fig. 1, P < 0.00001), and there was no fluctuation of the number of active lever presses over the 4 weeks of testing (NS).
The typical distribution of 2FA BPND in the squirrel monkey brain before and after nicotine self-administration of nicotine is shown in Fig. 2. ROI data analysis indicated a 31 % increase of 2-FA BPND in the whole brain compared to baseline, P = 0.005 (Fig. 3a). After correcting for multiple testing, there was a significant increase of BNND in the amygdala (+36 %, P = 0.006) and putamen (+62 %, P = 0.002). Increased binding in the cerebellum, thalamus, pons, and temporal cortex did not survive multiple comparison corrections.
BPND images of squirrel monkeys brain acquired with 2FA before (Pre SA) and after (Post SA) nicotine self-administration. The images illustrate the representative results from a single animal. Similar results were obtained in five additional animals. The first column represents the structural T1 brain MRI images
We performed correlations between the number of nicotine infusions (average infusions during the 4 weeks and average infusions during the last 2 days of the 4 weeks) and the percentage of α4β2* nAChRs upregulation. Unexpectedly, no significant correlations were found (data not shown). However, due to the small sample size, we may have been underpowered to detect such relationship between upregulation and nicotine exposure.
Discussion
Here, we report that stable 4 weeks of nicotine self-administration (10 ug/kg/injection) in squirrel monkeys is sufficient to produce upregulation of α4β2* nAChRs. That nicotine is an effective reinforcer in squirrel monkeys has been previously shown (Goodwin et al. 2015; Le Foll et al. 2007b). Indeed, the first report of nicotine’s reinforcing properties was generated in squirrel monkeys trained to respond under a second-order schedule of reinforcement (Goldberg et al. 1981). This seminal report had a tremendous influence in the field and led subsequently to the Surgeon General report indicating that nicotine was addictive (Department of Health and Human Services 1988). The conditions under which nicotine functions as an effective reinforcer of drug use have been clearly identified and are used to study nicotine addiction processes (Justinova et al. 2015a; Le Foll et al. 2007b, 2009; Mascia et al. 2011). The present findings are in agreement with those previous reports. Although the monkeys in our study had a slightly different exposure to nicotine (due to their differential number of voluntarily self-administer infusions of nicotine), we felt that it was more valid to use a contingent method of administration as compared to a non-contingent method of nicotine administration (Jacobs et al. 2003).
The innovative aspect of this research consists of combining the PET imaging with the extended nicotine SA paradigm. The distribution and density of α4β2* nicotinic receptors assessed by 2-FA BPND before and after self-administration is consistent with previously published data in an acute nicotine access model (Le Foll et al. 2007a, 2009). Notably, we found that the highest density of a α4β2* receptors is observed in the thalamus, whereas other brain areas displayed lower BPND. The lowest binding was observed in the cerebellum. This result is in agreement with previous PET studies performed in humans (Kimes et al. 2003; Mukhin et al. 2008). Although the overall pattern of expression of nAChRs was not dramatically changed following the self-administration sessions, we observed a significant elevation of BPND in the whole brain as well as in the amygdala and putamen. The brain area with the lowest upregulation was the thalamus, and this result is consistent with the absence of higher densities determined with 2-FA reported in the thalamus in smokers vs non-smokers (Mukhin et al. 2008). In other brain areas, the upregulation was measured from 22 to 62 %, which is about two times smaller than that observed using PET and 2FA in human smokers (Mukhin et al. 2008). It is possible that the smaller upregulation observed in some areas may be related to underestimation of radioactivity concentration in squirrel monkey brain structures as a result of greater partial volume effect compared with humans.
Concerning the analysis of the PET data, in this study, we were not able to use CB as a reference region for receptor quantifications. Though previously we were able to demonstrate that untreated squirrel monkeys have very low levels of nAChRs in CB and that this region can be used for calculation of brain BPND at this condition (Le Foll et al. 2007a), it seems that such an approach is not suitable for brain nAChR quantification after treatment with nicotine. As shown in Fig. 3, nicotine self-administration results in the upregulation of nAChRs in CB, which would result in the underestimation of brain region BPND values after treatment and therefore leads to the underestimation of nicotine-induced receptor upregulation. To overcome this obstacle in the present study we have employed our previously developed and evaluated method utilizing neck muscle as a reference region (Le Foll et al. 2007a).
This study has several limitations. The first one is the small sample size. Second, we have only included male squirrel monkeys and sex may be a factor influencing 2-FA binding, so we cannot determine that similar results would have been obtained in female subjects. Another limitation is that changes in BPND can be produced not only by the changes in receptor density but also could be produced by changes in receptor affinity and/or receptor occupancy by acetylcholine, which we did not explore here. Finally, we did not measure plasma nicotine or the concentration of nicotine in the brain at the time of post-exposure scan. Therefore, we cannot exclude the presence of residual nicotine in the brain which may have interfered with the binding of 2-FA (Staley et al. 2006). It is also possible that during the 4-day interval between the cessation of nicotine exposure and the PET scan, the receptor upregulation may have decreased, but we could not perform the scan earlier due to the possible presence of nicotine within the brain. Another clear limitation is the fact that those findings do not allow any conclusions to be drown about the functional role of this upregulation. However, a recent study evaluating the response to nicotine patches in human smokers indicates that the smokers with less upregulation of available α4β2* nAChRs are more likely to quit after treatment, as compared to smokers with more upregulation (Brody et al. 2014). This suggests that this receptor’s upregulation may be of importance in the smoking cessation process. Nonetheless, the additional studies are required to determine if this upregulation could represent a therapeutic target.
Conclusion
Although these results should be duplicated with a larger group of animals, this report is the first to explore upregulation of nAChRs in non-human primates trained to self-administer nicotine. It appears that the model of short access to nicotine as used to screen for medication discovery (see Justinova et al. 2015a, b; Mascia et al. 2011) is capable of inducing the same upregulation as described in the brains of human smokers. This research suggests that this upregulation may be associated with the early development of nicotine addiction and its functional role should be explored further.
References
Benwell ME, Balfour DJ, Anderson JM (1988) Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem 50:1243–1247
Besson M, Granon S, Mameli-Engvall M, Cloez-Tayarani I, Maubourguet N, Cormier A, Cazala P, David V, Changeux JP, Faure P (2007) Long-term effects of chronic nicotine exposure on brain nicotinic receptors. Proc Natl Acad Sci U S A 104:8155–8160
Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S (1997) Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther 282:7–13
Brody AL, Mukhin AG, La Charite J, Ta K, Farahi J, Sugar CA, Mamoun MS, Vellios E, Archie M, Kozman M, Phuong J, Arlorio F, Mandelkern MA (2013) Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int J Neuropsychopharmacol 16:957–966
Brody AL, Mukhin AG, Mamoun MS, Luu T, Neary M, Liang L, Shieh J, Sugar CA, Rose JE, Mandelkern MA (2014) Brain nicotinic acetylcholine receptor availability and response to smoking cessation treatment: a randomized trial. JAMA Psychiatry 71:797–805
Chefer SI, London ED, Koren AO, Pavlova OA, Kurian V, Kimes AS, Horti AG, Mukhin AG (2003) Graphical analysis of 2-[18F]FA binding to nicotinic acetylcholine receptors in rhesus monkey brain. Synapse 48:25–34
Department of Health and Human Services (1988) The Health Consequences of Smoking. Nicotine Addiction. Department of Health and Human Services (DHHS) Publication No. (CDC) 88–8406, Washington DC
Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ (1992) A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol 41:31–37
Flores CM, Davila-Garcia MI, Ulrich YM, Kellar KJ (1997) Differential regulation of neuronal nicotinic receptor binding sites following chronic nicotine administration. J Neurochem 69:2216–2219
Gergen GA, MacLean PD (1962) A Stereotaxic atlas of the Brain of the squirrel monkey (Saimiri Sciureus), U.S. Department of Health, Education, and Welfare. Public Health Service. National Institutes of Health. Bethesda, Maryland. U.S. Government Printing Office. Washington, D.C.
Goodwin AK, Hiranita T, Paule MG (2015) The reinforcing effects of nicotine in humans and nonhuman primates: a review of intravenous self-administration evidence and future directions for research. Nicotine Tob Res 17(11):1297–310
Goldberg SR, Spealman RD, Goldberg DM (1981) Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science 214:573–575
Gould RW, Duke AN, Nader MA (2014) PET studies in nonhuman primate models of cocaine abuse: translational research related to vulnerability and neuroadaptations. Neuropharmacology 84:138–151
Govind AP, Vezina P, Green WN (2009) Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol 78:756–765
Horti AG, Scheffel U, Koren AO, Ravert HT, Mathews WB, Musachio JL, Finley PA, London ED, Dannals RF (1998) 2-[18F]Fluoro-A-85380, an in vivo tracer for the nicotinic acetylcholine receptors. Nucl Med Biol 25:599–603
Horti AG, Kuwabara H, Holt DP, Dannals RF, Wong DF (2013) Recent PET radioligands with optimal brain kinetics for imaging nicotinic acetylcholine receptors. J Label Compd Radiopharm 56:159–166
Howell LL, Wilcox KM (2002) Functional imaging and neurochemical correlates of stimulant self-administration in primates. Psychopharmacology (Berlin) 163:352–361
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539
Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN (2003) Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci 24:566–573
Justinova Z, Panlilio LV, Moreno-Sanz G, Redhi GH, Auber A, Secci ME, Mascia P, Bandiera T, Armirotti A, Bertorelli R, Chefer SI, Barnes C, Yasar S, Piomelli D, Goldberg SR (2015a) Effects of fatty acid amide hydrolase (FAAH) inhibitors in non-human primate models of nicotine reward and relapse. Neuropsychopharmacology 40:2185–2197
Justinova Z, Le Foll B, Redhi GH, Markou A, Goldberg SR (2015a) Differential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on nicotine versus cocaine self-administration and relapse in squirrel monkeys. Psychopharmacology (Berlin)
Kimes AS, Horti AG, London ED, Chefer SI, Contoreggi C, Ernst M, Friello P, Koren AO, Kurian V, Matochik JA, Pavlova O, Vaupel DB, Mukhin AG (2003) 2-[18F]F-A-85380: PET imaging of brain nicotinic acetylcholine receptors and whole body distribution in humans. FASEB J Off Publ Fed Am Soc Exp Biol 17:1331–1333
Kuryatov A, Luo J, Cooper J, Lindstrom J (2005) Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol Pharmacol 68:1839–1851
Le Foll B, Chefer SI, Kimes AS, Shumway D, Goldberg SR, Stein EA, Mukhin AG (2007a) Validation of an extracerebral reference region approach for the quantification of brain nicotinic acetylcholine receptors in squirrel monkeys with PET and 2-18F-fluoro-A-85380. J Nucl Med 48:1492–1500
Le Foll B, Wertheim C, Goldberg SR (2007b) High reinforcing efficacy of nicotine in non-human primates. PLoS ONE 2:e230
Le Foll B, Chefer SI, Kimes AS, Shumway D, Stein EA, Mukhin AG, Goldberg SR (2009) Baseline expression of alpha4beta2* nicotinic acetylcholine receptors predicts motivation to self-administer nicotine. Biol Psychiatry 65:714–716
Lester HA, Xiao C, Srinivasan R, Son CD, Miwa J, Pantoja R, Banghart MR, Dougherty DA, Goate AM, Wang JC (2009) Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery. AAPS J 11:167–177
Lomazzo E, Hussmann GP, Wolfe BB, Yasuda RP, Perry DC, Kellar KJ (2011) Effects of chronic nicotine on heteromeric neuronal nicotinic receptors in rat primary cultured neurons. J Neurochem 119:153–164
Marks MJ, Burch JB, Collins AC (1983) Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther 226:817–825
Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le Foll B, Tanda G, Piomelli D, Goldberg SR (2011) Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry 69:633–641
Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP (2005) Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436:103–107
Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, Vaupel DB, Pavlova O, Stein EA (2008) Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2-18F-FA-85380. J Nucl Med 49:1628–1635
Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ (1999) Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther 289:1545–1552
Picciotto MR, Mineur YS (2014) Molecules and circuits involved in nicotine addiction: The many faces of smoking. Neuropharmacology 76(Pt B):545–553
Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177
Picciotto MR, Addy NA, Mineur YS, Brunzell DH (2008) It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84:329–342
Schwartz RD, Kellar KJ (1983) Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science 220:214–216
Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O’Malley S, van Dyck CH (2006) Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci 26:8707–8714
Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA (2004) Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science 306:1029–1032
Valette H, Bottlaender M, Dolle F, Coulon C, Ottaviani M, Syrota A (2003) Long-lasting occupancy of central nicotinic acetylcholine receptors after smoking: a PET study in monkeys. J Neurochem 84:105–111
Valette H, Bottlaender M, Dolle F, Coulon C, Ottaviani M, Syrota A (2005) Acute effects of physostigmine and galantamine on the binding of [18F]fluoro-A-85380: a PET study in monkeys. Synapse 56:217–221
Vaupel DB, Stein EA, Mukhin AG (2007) Quantification of alpha4beta2* nicotinic receptors in the rat brain with microPET and 2-[18F]F-A-85380. Neuroimage 34:1352–1362
Wonnacott S (1990) The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci 11:216–219
Wullner U, Gundisch D, Herzog H, Minnerop M, Joe A, Warnecke M, Jessen F, Schutz C, Reinhardt M, Eschner W, Klockgether T, Schmaljohann J (2008) Smoking upregulates alpha4beta2* nicotinic acetylcholine receptors in the human brain. Neurosci Lett 430:34–37
Zambrano CA, Salamander RM, Collins AC, Grady SR, Marks MJ (2012) Regulation of the distribution and function of [(125)I]epibatidine binding sites by chronic nicotine in mouse embryonic neuronal cultures. J Pharmacol Exp Ther 342:245–254
Zambrano CA, Short CA, Salamander RM, Grady SR, Marks MJ (2015) Density of alpha4beta2* nAChR on the surface of neurons is modulated by chronic antagonist exposure. Pharmacol Res Perspect 3:e00111
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Financial support
This study was supported by the Intramural Research Program of the National Institute on Drug Abuse, NIH, DHHS.
Additional information
Steven R. Goldberg In memoriam
Rights and permissions
About this article
Cite this article
Le Foll, B., Chefer, S.I., Kimes, A.S. et al. Impact of short access nicotine self-administration on expression of α4β2* nicotinic acetylcholine receptors in non-human primates. Psychopharmacology 233, 1829–1835 (2016). https://doi.org/10.1007/s00213-016-4250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4250-9