Abstract
Purpose
The present study investigates child development following prenatal exposure to maternal use of selective serotonin reuptake inhibitors (SSRIs; N = 28), versus prenatal exposure to medically untreated depression (N = 42), and no exposure (N = 33).
Methods
When the children reached 5–6 years of age, child cognitive abilities were measured using selected tests from Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-r), Neuropsychological Assessment II (NEPSY-II), and the Attention Network Test. Maternal reports of child behavioral problems were collected using the Child Behavior Checklist (CBCL).
Conclusion
Analyses of variance revealed no effects of prenatal exposure to depression or SSRIs upon general cognition or inhibition. Regarding behavioral problems, there was a significant negative association between both SSRI and depression exposure upon externalizing, and between SSRI exposure and internalizing problems. The results are interpreted in light of theories on interactive specialization and reactivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increasing number of pregnant women use selective serotonin reuptake inhibitors (SSRIs) during pregnancy, as a treatment for major depression (Bakker et al. 2008; Kieler et al. 2012). SSRIs administered during pregnancy will initially enter the maternal blood stream before passing through the placenta and into the blood stream of the fetus (Iqbal et al. 2012). This transfer may cause an imbalance in fetal neurotransmission, which in turn may alter neural programming, plasticity, structural, and functional development (Gaspar et al. 2003; Homberg et al. 2010). There are mixed reports in terms of increased risk of birth defects following SSRI exposure (Tuccori et al. 2009). The risk of neonatal abstinence symptoms is fairly high (Klinger and Merlob 2008); however, recent population-based studies indicate no increased risk of major malformations (Furu et al. 2015; Nordeng et al. 2012). Further, a recent review revealed that studies on early cognitive development suggest no negative effects, while an increasing amount of studies report later child behavioral problems (Hermansen and Melinder 2014), indicating latent effects of exposure (cf., Lupien et al. 2009). This delayed effect may be explained by the nature of the serotonergic system and its projections to the prefrontal cortex (PFC), a region maturing throughout childhood and central for executive functions such as inhibition and in turn behavioral control (Anderson 2002).
Inhibition
The term executive function (EF) is a psychological construct referring to higher-order cognitive skills essential for goal-directed processes and self-regulation (Diamond 2013). Being a main component of EFs, inhibition is an important process in the regulation of behavior and social relationships (Miyake et al. 2000; Phillips et al. 2008).
The ability to inhibit thoughts and responses begins to emerge toward the end of the first postnatal year and undergoes rapid development across the toddler period and into the preschool years (Garon et al. 2008), a pattern coinciding with age-related changes in frontal lobe maturation and connectivity (Anderson 2002; Davidson et al. 2006). Reviewing studies on child inhibitory control, Casey et al. (2005) found that younger children generally recruit larger and less fine-tuned regions of the prefrontal cortex. As development proceeds, executive abilities are involved in fine-tuning the activity pattern relevant for the task at hand, involving structures such as the anterior cingulate and lateral prefrontal cortex (Bush et al. 2000; Fan et al. 2003). An effect of this improved efficiency is that as children get older, their ability to inhibit a response to task-irrelevant but salient stimuli is improved (McAuley et al. 2011; Rueda et al. 2004). This is an ability often labeled as interference suppression in contrast to the inhibition of motor responses (Nigg 2000). Furthermore, alterations in the serotonergic system, through tryptophan depletion, have been associated with increased interference effects (Munafo et al. 2006) and impulsivity (Fikke et al. 2013).
Although EFs such as inhibition rely on PFC functioning, these processes are also malleable and influenced by the social environment (Diamond 2011, 2012). Thus, research attempting to uncover possible negative effects of prenatal antidepressant exposure should be guided by theories of interactive specialization taking into account the interactional process between biological and social environment (Johnson 2001, 2005). As suggested by Kaiser and Sachser (2009), developmental differences are not necessarily deficits or problems, but rather adaptations following the given social environment. Along these lines, both the illness itself, i.e., depression, and the medical treatment mostly used to reduce symptoms of depression (e.g., SSRIs) may affect prenatal and postnatal development.
Maternal depression
Children exposed to maternal depression in utero may be at risk of atypical development due to prenatal and sometimes prolonged exposure throughout the toddler and preschool years (Korhonen et al. 2012). Depressed women are found to be less sensitive and more self-occupied in their parenting style (Cohn and Tronick 1989). Such a parenting style may be a source of insecure attachment and internalizing behaviors in the child (Madigan et al. 2013). From a transactional perspective, such child characteristics may in turn negatively affect the interplay between mother and child (Sameroff 1975), increasing the likelihood of later child psychopathology (Cicchetti and Toth 1998; W. Nilsen et al. 2013).
Child development and maternal depression: cognitive and behavioral effects
Previous studies have established a close link between maternal depression and child cognitive and behavioral problems (Choe et al. 2014; Evans et al. 2012; Korhonen et al. 2012; Van Batenburg-Eddes et al. 2013), with several lines of research suggesting both genetic influences, observational learning, and parenting behaviors as important predictors (for a meta analytic review on this topic see Goodman et al. 2011).
As mentioned, a direct association between prenatal SSRI exposure and atypical cognitive development has so far not been established. Unfortunately, previous studies have mainly focused on general cognitive abilities, rather than specific higher-order cognitive functions (for a review, see Hermansen and Melinder 2014). As suggested by recent literature on child cognitive development in an atypical environment such as maltreatment (Augusti and Melinder 2013) and prenatal methadone exposure (Konijnenberg and Melinder 2013), there are limited associations between early life stress and global deficits of cognition. Furthermore, among the previous studies, the age range is fairly broad (e.g., 2–7-year-olds), complicating the task of comparing across age groups and neurocognitive tests (Klinger and Merlob 2008; Nulman et al. 2012; Nulman et al. 1997; Nulman et al. 2002).
Regarding behavioral problems, the evidence is slightly more divergent. In some studies, no differences between SSRI-exposed children and unexposed controls are reported (Galbally et al. 2015; Misri et al. 2006; Nulman et al. 2002; Oberlander et al. 2007). These studies conclude that maternal levels of depressive symptoms are the main cause of behavioral differences. Other studies show that the increased risk of internalizing and externalizing behaviors is predominantly associated with prenatal exposure to SSRIs (Brandlistuen et al. 2015; Casper et al. 2011; Hanley et al. 2013; Pedersen et al. 2013). Thus, the question is still open as to whether it is the underlying mental illness or medical exposure that has the greatest impact on child development. In either case, understanding more about the core mechanism behind these behavioral problems is essential seeing as behavioral problems during childhood is an important mediator of later child psychopathology (Cicchetti et al. 1998; W. Nilsen et al. 2013).
The present study
The aim of the present paper is to investigate long-term developmental effects of prenatal exposure to SSRIs in contrast to medically untreated depression (DEP) and a comparison group (CON), in preschool-aged children. Abilities of inhibitory control are emerging at this age, which enables us to examine possible underlying mechanisms of behavioral problems previously observed among both groups of exposed children. We employ selected tests from the Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-r) and Neuropsychological Assessment II (NEPSY-II) test batteries, targeting general cognitive abilities and interference suppression, respectively. In addition, the children were tested on a modified version of the Attention Network task (ANT) developed to target interference control (Rueda et al. 2004).
Methods
Participants and attrition
All children were recruited from a prospective population-based pregnancy cohort study (The Norwegian Mother and Child Cohort Study, Norwegian Institute of Public Health 2010), for which participants were recruited in the time period 1999–2008. The women consented to participation in 40.6 % of the targeted pregnancies. The total cohort today includes 114.500 children, 95.200 mothers, and 75.200 fathers. Within this total cohort, approximately 0.9 % reported using antidepressants in the form of SSRIs (Nordeng et al. 2012). Both the cohort study in general and the current study were approved by the Regional Committee for Medical Research Ethics and utilizes version 8 of the quality-assured data files.
Based on responses given on questionnaires administered in the first (15th pregnancy week) and third trimester (30th pregnancy week), we invited three groups of women with children born in 2008 and 2009. Groups 1 and 2 consisted of depressed women with and without SSRI treatment, respectively. Drug exposure was classified and grouped according to the Anatomical Therapeutic Chemical (ATC) Classification System developed by the World Health Organization (WHO Collaborating Centre for Drugs Statistics Methodology). SSRI exposure was defined as exposure to drugs belonging to ATC code N06AB, or serotonergic agents within the N06AX code. A group of non-depressed women were included as a comparison group. Women were considered ineligible if they had any chronic illness. Regarding analgesics (ATC code N02A, N02BE01, M01A), participants recruited for the CON group were excluded if using any analgesics during pregnancy. As the population to recruit from was smaller for the DEP and SSRI groups, participants were included even though they had used analgesics during pregnancy. Unfortunately, we were not able to investigate whether the medication was used prior to, during, or after pregnancy; this information is therefore discarded in the following analyses. Multiple births were excluded in all groups.
In total, 1906 women were eligible for inclusion: 124 in the SSRI group, 400 in the DEP group, and 1382 in the comparison group. Two hundred women in each of the latter groups received invitations, as well as everyone eligible for the SSRI group. As response rate in the comparison group was low compared to the two others, we sent out an additional set of invitations to 143 women, totaling 667 invitations. A reminder was sent 2 months after the initial invitations to those who had not yet responded. Informed consent was obtained from each participant upon recruitment before we could make arrangements about the date and time of assessment.
The present study includes a total of 103 (15 % of the invited sample) children and their mothers, representing the three groups: SSRI-exposed (SSRI; N = 28 (22 %), 16 girls, M = 69.02 months, SD = 4.76); DEP-exposed (DEP; N = 42 (21 %), 17 girls, M = 68.41 months, SD = 5,16); and non-exposed controls (CON; N = 33 (10 %), 18 girls, M = 67.37 months, SD = 6.34). Most of the participants belonged to the ethnic majority (N = 91), but there were also children with a different background (N = 12). Given this sample size and depending on the contrasts, the probability of finding a medium effect (>.5) is approximately 98–99 %. Small effects (>.2) would be detectable with a 46–53 % probability (Cohen 1992).
One-way analysis of variance comparing the dyads included in the present study with those who did not wish to participate showed that there was no significant difference (i.e., p > .05) in terms of maternal age, relationship status, and socioeconomic status (data not shown). Further, there was no difference between the two exposure groups regarding symptom severity during pregnancy for any of the trimesters.
Measures
Maternal variables
Demographic information (Version 8, Magnus et al. and MoBa Study Group (2006))
Pregnancy-related and demographic variables of interest were all collected by researchers from the cohort study. The pregnant women fill in three questionnaires during pregnancy, two of which are included in the present study: Q1administered at 15th pregnancy week covers general health, exposures, and background data, and Q3 administered at week 30 and concerning health conditions during pregnancy. After birth, further questionnaires are sent to the participating families, but among these, only Q4 administered 6 months after birth is included in the present study. The present study includes only a subset of the data previously collected. In order to gain insight into the degree of previous depressive episodes, we used the Life Time History of Depression scale (LHD; Kendler et al. 1993), a six-item scale consisting of yes/no questions as to whether given symptoms were present. A high total score indicates higher levels of previous depressive episodes. We also included a short form of the symptom checklist for maternal anxiety and depressive symptoms during pregnancy (five items in Q1, and eight items in Q3 and Q4) (Tambs and Moun 1993; Tambs and Røysamb 2014). Each item on the SCL-5 is scored as 1 = “not bothered,” 2 = “a little bothered,” 3 = “quite bothered,” or 4 = “very bothered,” and an overall mean score is calculated to reflect the level of depressive symptoms (Strand et al. 2003). In addition, we collected information on other exposures during pregnancy such as alcohol and nicotine. Information on maternal and paternal educational levels as well as household income was collapsed across the selected variables to create a composite score of socio-economic status (SES).
Beck Depression Inventory II (BDI-II; Beck et al. 1996)
Maternal measures of concurrent depressive symptoms were assessed at the time of testing using BDI-II, a widely used self-report screening instrument of depressive symptoms, showing good reliability and validity (Sprinkle et al. 2002; Storch et al. 2004). The questions are composed of cognitive symptoms such as guilt, irritability, hopelessness, and physical symptoms such as lack of interest in sex, fatigue, and weight loss. A high score indicates increased symptom load, with a score above 17 considered borderline clinical depression, 21–30 as moderate depression, 31–40 severe depression, and above 40 as highly severe depression.
Child variables
Wechsler Preschool and Primary Scale of Intelligence-Revised
(WPPSI-r; Wechsler 1989). The subtests “Vocabulary,” “Similarities,” and “Reasoning” from WPPSI-r were used to test language development. In these verbal tasks, the children had to explain a selection of words, define the similarities between two concepts, and reason on a given subject, respectively. “Block design” was administered to test practical reasoning and problem solving. On this task, the child was presented with a block design and instructed to copy this with the given blocks. Level of performance was scored according to the WPPSI-r guidelines; the higher the score the child received on these tasks, the better their performance.
Neuropsychological Assessment II
(NEPSY-II; Korkman et al. 2000). Two subtests were selected from the NEPSY-II test battery: (1) “Visual attention” task, and (2) “Statue” task. The Visual attention task was administered to test the child’s visual attention and strategic planning, and the child had to search a picture to find a specific figure while resisting distracting similar information. Visual attention efficiency was scored by combining the amount of time spent on the task as a whole with the amount of correct items detected, subtracting incorrect items. Scores were scaled according to age-adjusted percentiles. A high score on this task reflects an inefficient search strategy with slow processing and more errors when presented with irrelevant but salient stimuli.
To target inhibition of motor responses, we administered the Statue test, where the child is instructed to stay completely still with eyes closed for 75 s. For every 5-s interval, a 2-point score is given when the child inhibits all responses, and a 1-point score is given if the child displays one inappropriate response. With more than one inappropriate response, that interval is scored 0. Scores were scaled according to age-adjusted percentiles.
Attentional Network Task
(ANT; Rueda et al. 2004). The ANT paradigm was originally developed to test processing efficiency of the attentional network (Rueda et al. 2004), comprising of the three components alerting, orienting, and interference control, using the principles of the Flanker task (Eriksen and Eriksen 1974). In our modified version, we have reduced the task to focus solely on interference control by removing cues for alerting and spatial orientation, which makes it more in line with the original Flanker test.
The task was presented to the children as a game where they had to catch an animal, fish, mouse, or bird, as fast as they could. More specifically, the stimulus consists of a line with five identical cartoon animals at the center of the screen (Fig. 1), with the central animal being the target, and the ones on the sides are flankers. The children were instructed to focus on the central animal while ignoring the flanker animals. To catch the animal, they were instructed to press the arrow key corresponding to the animals’ orientation. In the congruent trials, the five animals were oriented in the same direction, while in the incongruent trials, the flanker and target animals were oriented in opposite directions. Half of the trials were congruent and half incongruent.
Initially, the children were presented with a block of eight practice trials. There was no time limit on the practice trials and the children could repeat the practice block as many times as they needed until it was clear that they understood the instructions. During the practice trials, the experimenter gave verbal feedback and encouragement to the child. During the experimental session, the experimenter was in the room but no longer gave trial-by-trial encouragement. Each trial began with a fixation period of random variable duration of 500–1000 ms. The target display was presented until a response was made, or up to 2000 ms. Correct responses were followed by a “happy sound” and a 300-ms animation of the central animal moving into a net. Incorrect responses and trials of omission were followed by a “sad sound” as the animals disappeared from the screen. The inter-trial interval was 800 ms. A fixation cross was continuously displayed in the center of the screen except when targets and feedback were presented. In total, the task consisted of five experimental blocks of 16 trials per animal, and the task ended either when all blocks were completed or the child refused to continue. The experimental session lasted for a maximum of 19 min. During the experimental session, the mother was seated in the back of the room to provide comfort if necessary.
When analyzing these data, mean values for each condition are derived by including only trials with reaction times (RTs) equal to or larger than 200 ms, as responses prior to this are generally considered as prepotent responses (Jensen 2006). Similarly, the accuracy (ACC) score is based on data from trials of RT equal to or larger than 200 ms. Trials with no recorded response are considered as omissions and excluded. Next, an inverse inefficiency score (IES) was computed for both congruent and incongruent trials, due to the trade-off between reaction times and ACC (Townsend and Ashby 1978). A high IES indicates either more errors or slow processing. Finally, to assess the impact of interference, a conflict score (CS) was derived by subtracting performance on congruent from incongruent trials for RT, and IES. For ACC, conflict scores were derived by subtracting performance on incongruent from congruent trials. A larger CS reflects a greater interference effect, with a low CS indicating a speeded and more efficient processing of conflict with fewer errors (Stroop 1935).
Child Behavior Checklist
(CBCL; Achenbach and Rescorla 2001). CBCL is a widely used parent report standardized questionnaire in which the child is rated on various behavioral and emotional problems. It has demonstrated excellent reliability and validity (Achenbach and Rescorla 2001; Dutra et al. 2004). At time of testing, mothers filled out age-appropriate CBCL questionnaires; for children aged 5 years, the 99-item CBCL for Ages 1.5–5 was administered and, for children aged 6 years, the 112-item CBCL for Ages 6–18 was administered. Responses were scored according to provided guidelines, standardized for age and gender. In addition to a composite score of total behavioral problems, the CBCL assesses internalizing (i.e., anxious, depressive, and over controlled) and externalizing (i.e., aggressive, hyperactive, non-compliant, and under controlled) behaviors. Following conversion to t scores, the scales range from 1 to 100 for both versions of the questionnaires, with scores exceeding 60 considered borderline clinical, and exceeding 64 as clinical levels.
Procedure
Within the national cohort study, demographic information was collected throughout and after pregnancy. We extended this data collection by having each child visit the laboratory once, together with his or her parent. Upon arrival, the participants were given a short presentation of the study. The experimenter was naive as to which group the dyad was part of. The neuropsychological tests were administered in a quiet room, with the parent seated close by. The ANT-task was performed in an adjacent room with access to the necessary equipment. Depending on the needs of the child, short breaks were given in between the different tasks. The children received a small gift (approximately €10) for their participation after the tests were finished. For all but two children, they were accompanied by their biological mother; the biological father accompanied the last two. In these two cases, the BDI-II and CBCL questionnaires were sent by mail to the mothers after testing.
Apparatus
ANT stimuli were presented using E-Prime 2.0 software (Psychology Software Tools Inc., Sharpsburg, PA), and Windows XP professional, and shown on a 20-in color LCD monitor (Flex Scan L768) with 1280 by 1024 in screen resolution and 32-bit color quality. The child was seated approximately 45 cm from the monitor.
Statistical analyses
All data was analyzed using the statistical software package SPSS version 22 (SPSS Inc., Chicago, IL, USA).
The amount of missing data was minimal, with only one missing point on WPPSI-r Reasoning (SSRI; N = 1), two missing points on WPPSI-r Vocabulary (SSRI; N = 2), four missing data points on NEPSY-II Visual attention test (SSRI; N = 1, DEP; N = 3), and six data points on the ANT-task (SSRI; N = 1, DEP; N = 4). The missing data were due to different reasons; regarding NEPSY-II, two children did not understand the Visual attention task, while two others did not want to perform this exercise, the latter was the case for missing data on WPPSI-r Reasoning and Vocabulary as well as the ANT-task. All women returned the two first questionnaires administered during pregnancy, while five women failed to return the questionnaire at 6 months postpartum; all of which were part of the SSRI-group. Questionnaires received at testing were answered on the spot or returned by mail if they did not finish. Six women failed to return the CBCL questionnaires (SSRI; N = 1, DEP; N = 3, CON; N = 3). Missing data were excluded pairwise for the selected variables.
Normality and homogeneity of distribution were assessed in order to apply parametric or non-parametric tests. Extreme values exceeding ±3 SD were replaced with the value corresponding to ±2 SD. Shapiro–Wilk test indicated non-normal distributions for several of the continuous variables, although skewness only exceeded ±1 for the ACC variables, and two of the IES variables from the ANT (see Table 2).
Demographic information was compared between the three groups using chi-square for the categorical variables, and one-way analysis of variance (ANOVA) for the continuous variables. Variables with non-normal distributions were also analyzed using Kruskal–Wallis. As Kruskal–Wallis indicated no change in results, only data from the parametric statistics are reported. For significant associations, planned contrasts were performed between SSRI- and depression-exposed, as well as between either exposure group and controls. All tests were two-tailed, with p values <.05 considered statistically significant. Effect sizes are reported in terms of eta-square and considered small (>.02), medium (>.13), or large (>.26), according to Cohen (1992). When significant below the level of .05, results are reported and commented upon in the following sections.
Results
Demographic information
A detailed description of demographic information on maternal variables and test statistics is presented in Table 1. There were no significant differences between the three groups in terms of maternal age at delivery, and there was no difference in use of alcohol. There was no overall difference between the three groups on nicotine use; however, when collapsing across the two exposure groups, the comparison group showed significantly less nicotine use, t (1,40) = −2.22, p = .032, η 2 = .03. On measures of SES, there was a significant group difference, F (2, 100) = 9.29, p = .000, η 2 = .16, with the CON group being of higher SES. There were no significant differences between the two exposure groups on either measure.
Regarding measures of depressive symptoms, we found a significant group difference on symptom severity in the three different trimesters, F (2,97) = 16.89, p < .000, η 2 = .26, F (2,99) = 13.91, p < .000, η 2 = .22, and F (2,91) = 8.81, p < .000, η 2 = .16, respectively. The comparison group reported less symptoms in all trimesters as compared to both the SSRI, and depression group. Data from the BDI-II scale revealed that this group difference was still present at time of testing, F (2,100) = 9.10, p < .000, η 2 = 0.15, again with the comparison group reporting less symptoms than the two exposure groups. In terms of Lifetime History of Depression, there was a significant overall difference between the three groups, F (2,97) = 55.47, p < .000, η 2 = .92, with women in the SSRI group reporting a significantly higher score than the women in the DEP group, who in turn reported higher levels than the CON group (see Table 1 for details). There was no difference between the two exposure groups on any measure of depression either during or after pregnancy. These results confirm that the designated group membership, based on self-report, can be considered appropriate when comparing depression-exposed and controls in the following analyses. Further, it is plausible to infer that the use of SSRIs may have contributed to an improvement in maternal mental health among the women in the SSRI group, as their levels of depression during pregnancy were similar to those of the women in the DEP group.
Child characteristics are presented in the bottom section of Table 1 and reveal no significant difference between the groups on gender, gestational age, birth weight, birth length, or age at testing. With the exception of birth weight, preliminary analysis of correlation revealed no significant association between gender and the different dependent measures (data not shown). In the following analysis, we have thus not controlled for gender.
Effects of prenatal exposure
WPPSI-r
As indicated in Table 2, children’s performance on the WPPSI-r tasks (Reasoning, Block design, Vocabulary, Similarities) was very similar across the three groups and revealed no relationship with prenatal exposure.
NEPSY-II
Similarly, performance on the NEPSY-II tasks (Visual attention and Statue) was also similar across the three groups, indicating no effects of prenatal exposure.
ANT
To assess whether the different stimuli presented in the ANT paradigm were eliciting the intended interference effect, repeated measure ANOVAs were performed with flanker condition and stimuli type as the within-group variables, and group as between group variable for each of the dependent measures RT and ACC. There was a significant main effect of flanker on both RT, F (1,95) = 61.81, p < .000, η 2 = .39 and ACC, F (1,95) = 20.41, p < .000, η 2 = .17, with congruent trials yielding lower RT (M = 1209.56, SD = 23.21) and higher ACC (M = .94, SD = .01) than incongruent trials (RT, M = 1287.58, SD = 25.24; ACC, M = .91, SD = .01). There was no main effect of stimuli type on measures of ACC, or RT. There was no interaction effect between flanker and group or between stimuli type and group, on either RT or ACC measures. For the following analysis, we thus chose to include all three stimuli types.
On the ANT task, all groups showed the expected interference effect on measures of RT, with congruent trials yielding faster RT than incongruent (data not shown). However, in terms of ACC, only the CON and DEP groups revealed a significant interference effect. All groups showed a significant interference effect in terms of IES, indicating that the trade-off between speed and accuracy was larger for the incongruent than congruent trials. The between-group analysis revealed only marginal differences between the groups in terms of either measure; none of which reached statistical significance.
CBCL
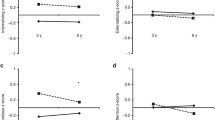
The overall between groups ANOVA of the behavioral data revealed a significant difference between the three groups of prenatal exposure on externalizing behaviors (F (2,93) = 3.43, p = .037, η 2 = .07), but only marginally so for internalizing behaviors (F (2,93) = 2.97, p = .056, η 2 = .06). Planned comparisons revealed that compared to controls, children in the SSRI-exposed group showed significantly more internalizing, t (1,55) = −2.41, p = .018, η 2 = .10; externalizing, t (1,55) = −1.96, p = .053, η 2 = .07; and total problems compared to controls, t (1, 55) = −2.21, p = .029, η 2 = .08. Children in the depression-exposed group on the other hand revealed a tendency of increased externalizing behavior compared to controls, t (1,67) = −2.50, p = .014, η 2 = .09. There was no difference between the comparison group and DEP exposed in terms of internalizing behaviors. There were no significant differences between the two exposure groups on either measure.
Similar to prenatal exposure (SSRI and DEP), maternal scores of LHD correlated significantly with child outcome on the CBCL scores, but not on any of the other measures (NEPSY and WPPSI). In order to explore the relationship between LHD and SSRI use during pregnancy and its impact on the different child outcome measures, we ran separate regression analyses for each of the tests. The interaction term (LHD*SSRI) never revealed a significant effect on child outcome, indicating no additively negative effect of the SSRIs
Discussion
Using antidepressants such as SSRIs during pregnancy remains a controversial topic, mostly due to the relatively scarce literature on long-term effects. The purpose of this study was to examine whether prenatal exposure to SSRIs in contrast to medically untreated depression had an adverse impact on measures of general cognitive abilities, inhibition, and behavioral problems in preschool children.
In line with previous studies (Klinger et al. 2011; Nulman et al. 2012; Nulman et al. 1997; Nulman et al. 2002), we found no significant difference in performance between the three groups of children in terms of general cognitive abilities as tested by the WPPSI-r. Further, regarding more specific aspects of inhibition, we found no association between prenatal exposure and performance on the two NEPSY-II tasks. Preliminary analysis of the ANT paradigm indicated that the desired conflict effect was elicited in all three groups, supporting the notion that this task is an appropriate measure of interference suppression in young age groups. Contrary to our initial prediction though, our results indicate no simple association between medical exposure and interference control on the ANT.
Regarding behavioral problems, we found increased externalizing problems among both the SSRI-exposed and the depression-exposed groups, compared to unexposed controls. Among the SSRI-exposed, we also found increased internalizing behaviors as compared to controls. This suggests that both SSRI and depression exposure impacts behavioral development, although the impact may be greater among the SSRI-exposed. There was, however, no significant difference between the SSRI and depression exposed; thus, we cannot reach any conclusions regarding the relative safety of SSRI use based on the current data.
The fact that we find increased behavioral problems among the prenatally exposed children suggests that there is a tendency toward poor self-regulation. As the literature indicates, these problems may stem from both biological and environmental influences (Goodman et al. 2011; Lovejoy et al. 2000), and the interaction between the two (Johnson 2001, 2005). By including a sample of children with depression exposure with and without the additional exposure to antidepressant medication, we sought to control for the underlying mental illness. There was no significant difference between the two exposure groups on depressive symptoms neither during nor after pregnancy or at time of testing.
There was no significant association between prenatal exposure and inhibition; thus, our hypothesis that inhibitory control could be the underlying mechanism of the observed behavioral problems is enfeebled. This could indicate that there are important aspects in the social environment of these children that mainly contributes to the developmental outcome observed. Alternatively, the lack of an anticipated association between inhibition and exposure group could be due to the valence of the stimuli used in the ANT paradigm. Alteration in the serotonergic system has been found to be especially associated with task performance during interference tasks with negative emotional valence, as compared to neutral or positive (Merens et al. 2008). As our stimuli were emotionally neutral, it may not have been ideal to target the specific inhibitory problems underlying self-regulation.
Overall, our findings from the cognitive tests indicate that the effect of prenatal SSRI exposure as contrasted with medically untreated depression and the non-exposed comparison group is fairly small (η 2 = .001–.04). Given that we had a 98–99 % chance of detecting a medium effect, our lack of findings in the current study indicates that any difference in effects between these groups is at least less than medium. Still, we did find increased reports of internalizing and externalizing behaviors among the exposed children and the control group, with medium effects (η 2 = .05–.10), underscoring that more effort needs to be directed toward uncovering the underlying cause of these problems.
Strengths and limitations of the present study
There are some important aspects of this particular study sample that must be considered before reaching conclusions about the relative safety of SSRI use during pregnancy. The women in this study are generally highly educated with the majority having studied at the university level, nearly all are in a relationship with the child’s father, and overall they have healthy pregnancies with little use of medications, nicotine, and alcohol, all of which are variables contributing to a good developmental outcome (Anderson and Arnstead 1995). This homogeneity is fairly rare in terms of studies of antidepressant use, and it is an important strength in terms of increased statistical control over the effects of SSRIs. At the same time, high socioeconomic status may be buffering against possibly unfortunate consequences of the prenatal exposure, as is suggested by several lines of research (Belsky and Pluess 2009; Branchi 2011; Hayden et al. 2013; Homberg and Lesch 2010; Pluess et al. 2011). Literature on the reciprocal interaction between serotonin and social behavior suggest that the environmental context affects the degree to which elevated serotonin levels are beneficial or not (Kiser et al. 2012). As is highlighted by Branchi (2011), this responsiveness toward the environment means that reactive individuals highly benefit from a nurturing environment, while an adverse environment affects them more negatively. In our homogenous sample, such buffering effects are difficult to investigate.
A second issue regarding this study sample is that there is no clinical evaluation of the mothers’ depression, only self-reports. Effects may therefore be less prominent than if all women in the exposed groups were prone to clinical levels of depression. However, studies report that even when maternal depressive symptoms are within subclinical levels, they represent a risk for child development (Conners-Burrow et al. 2015).
Finally, although our sample size is comparable to that of previous studies, it is still fairly small and thus limits us from exploring the impact of early, late, or prolonged exposure to SSRIs and/or depression. Also, we are unable to investigate the impact of different medications, dosages, and timings of exposure.
Concluding remarks
Adding to the existing literature on developmental effects following prenatal exposure to depression and SSRIs, the present study indicates no negative long-term effects upon executive function in general or inhibitory control in particular. Both groups of exposed children revealed higher levels of externalizing behaviors compared to non-exposed controls, the SSRI exposed also showed higher levels of internalizing behaviors. Due to the lack of group differences in terms of inhibitory control, we could not investigate any mediating effects between the behavioral scores and individual differences in inhibition. We suggest that this lack of association may be due to the ANT stimuli lacking emotional valence. Whether this is in fact the case should be further explored in future studies, in order to better address the underlying mechanisms of the behavioral problems observed. Future follow-up of these children will also be of interest, so as to understand more about the developmental trajectories contributing to resilience versus risk of later psychopathological outcomes.
References
Achenbach TM, Rescorla LA (2001) Manual for the ASEBA Preschool Forms & Profiles. University of Vermont, Research Center for Children, Youth & Families, Burlington
Anderson P (2002) Assessment and development of executive function (EF) during childhood. Child Neuropsychol 8(2):71–82. doi:10.1076/chin.8.2.71.8724
Anderson NB, Arnstead CA (1995) Toward understanding the association of socioeconomic status and health: a new challenge for the biopsychosocial approach. Psychosom Med 57(3):213–225. doi:10.1097/00006842-199505000-00003
Augusti E-M, Melinder A (2013) The effect of neutral and negative colour photographs on children’s item directed forgetting. Eur J Dev Psychol 10(3):378–391. doi:10.1080/17405629.2012.686741
Bakker MK, Kolling P, van den Berg PB, de Walle HEK, van den Berg L (2008) Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol 65(4):600–606. doi:10.1111/j.1365-2125.2007.03048.x
Beck AT, Steer RA, Brown GK (1996) Manual for the Beck Depression Inventory-II. Psychological Corporation, San Antonio
Belsky J, Pluess M (2009) Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull 135(6):885–908. doi:10.1037/a0017376
Branchi I (2011) The double edged sword of neural plasticity: Increasing serotonin levels leads to both greater vulnerability to depression and improved capacity to recover. Psychoneuroendocrinology 36(3):339–351. doi:10.1016/j.psyneuen.2010.08.011
Brandlistuen RE, Ystrom E, Eberhard-Gran M, Nulman I, Koren G, Nordeng H (2015) Behavioural effects of fetal antidepressant exposure in a Norwegian cohort of discordant siblings. Int J Epidemiol 0(0):1–11. doi:10.1093/ije/dyv030
Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4(6):215–222. doi:10.1016/S1364-6613(00)01483-2
Casey BJ, Galvan A, Hare TA (2005) Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol 15(2):239–244. doi:10.1016/j.conb.2005.03.012
Casper RC, Gilles AA, Fleisher BE, Baran J, Enns G, Lazzeroni LC (2011) Length of prenatal exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants: effects on neonatal adaptation and psychomotor development. Psychopharmacology (Berl) 217(2):211–219. doi:10.1007/s00213-011-2270-z
Choe DE, Shaw DS, Brennan LM, Dishion TJ, Wilson MN (2014) Inhibitory control as a mediator of bidirectional effects between early oppositional behavior and maternal depression. Dev Psychopathol 26(4):1129–1147. doi:10.1017/s0954579414000613
Cicchetti D, Toth SL (1998) The development of depression in children and adolescents. Am Psychol 53(2):221–241. doi:10.1037/0003-066x.53.2.221
Cicchetti D, Rogosch FA, Toth SL (1998) Maternal depressive disorder and contextual risk: contributions to the development of attachment insecurity and behavior problems in toddlerhood. Dev Psychopathol 10(2):283–300. doi:10.1017/s0954579498001618
Cohen J (1992) A power primer. Psychol Bull 112(1):155–159. doi:10.1037/0033-2909.112.1.155
Cohn JF, Tronick E (1989) Specificity of infants response to mothers of affective behavior. J Am Acad Child Adolesc Psychiatry 28(2):242–248. doi:10.1097/00004583-198903000-00016
Conners-Burrow NA, Swindle T, McKelvey L, Bokony P (2015) A little bit of the blues: low-level symptoms of maternal depression and classroom behavior problems in preschool children. Early Educ Dev 26(2):230–244. doi:10.1080/10409289.2015.979725
Davidson MC, Amso D, Anderson LC, Diamond A (2006) Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia 44(11):2037–2078. doi:10.1016/j.neuropsychologia.2006.02.006
Diamond A (2011) Biological and social influences on cognitive control processes dependent on prefrontal cortex. Gene Expr Neurobiol Behav Hum Brain Dev Dev Disord 189:319–339. doi:10.1016/b978-0-444-53884-0.00032-4
Diamond A (2012) Activities and programs that improve children’s executive functions. Curr Dir Psychol Sci 21(5):335–341. doi:10.1177/0963721412453722
Diamond A (2013) Executive functions. Annu Rev Psychol 64(64):135–168. doi:10.1146/annurev-psych-113011-143750
Dutra L, Campbell L, Western D (2004) Quantifying clinical judgement in the assessment of adolescent psychopathology: reliability, validity and factor structure of the Child Behavior Checklist for Clinician-Report. J Clin Psychol 60:65–85
Eriksen BA, Eriksen CW (1974) Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys 16(1):143–149
Evans J, Melotti R, Heron J, Ramchandani P, Wiles N, Murray L, Stein A (2012) The timing of maternal depressive symptoms and child cognitive development: a longitudinal study. J Child Psychol Psychiatry 53(6):632–640. doi:10.1111/j.1469-7610.2011.02513.x
Fan J, Fossella J, Sommer T, Wu YH, Posner MI (2003) Mapping the genetic variation of executive attention onto brain activity. Proc Natl Acad Sci U S A 100(12):7406–7411. doi:10.1073/pnas.0732088100
Fikke LT, Melinder A, Landro NI (2013) The effects of acute tryptophan depletion on impulsivity and mood in adolescents engaging in non-suicidal self-injury. Hum Psychopharmacol Clin Exp 28(1):61–71. doi:10.1002/hup.2283
Furu K, Kieler H, Haglund B, Engeland A, Selmer R, Stephansson O, Norgaard M (2015) Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. Bmj-Br Med J, 350. doi:10.1136/bmj.h1798
Galbally M, Lewis AJ, Buist A (2015) Child developmental outcomes in preschool children following antidepressant exposure in pregnancy. Aust N Z J Psychiatry 49(7):642–650. doi:10.1177/0004867415569800
Garon N, Bryson SE, Smith IM (2008) Executive function in preschoolers: a review using an integrative framework. Psychol Bull 134(1):31–60. doi:10.1037/0033-2909.134.1.31
Gaspar P, Cases O, Maroteaux L (2003) The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci 4(12):1002–1012. doi:10.1038/nrn1256
Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D (2011) Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev 14(1):1–27. doi:10.1007/s10567-010-0080-1
Hanley GE, Brain U, Oberlander TF (2013) Infant developmental outcomes following prenatal exposure to antidepressants, and maternal depressed mood and positive affect. Early Hum Dev 89(8):519–524. doi:10.1016/j.earlhumdev.2012.12.012
Hayden EP, Hanna B, Sheikh HI, Laptook RS, Kim J, Singh SM, Klein DN (2013) Child dopamine active transporter 1 genotype and parenting: evidence for evocative gene-environment correlations. Dev Psychopathol 25(1):163–173. doi:10.1017/S0954579412000971
Hermansen TK, Melinder AMD (2014) Prenatal SSRI exposure—effects on later child development. Child Neuropsychol. doi:10.1080/09297049.2014.942727
Homberg JR, Lesch KP (2010) Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry 69:513–519. doi:10.1016/j.biopsych.2010.09.024
Homberg JR, Schubert D, Gaspar P (2010) New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol Sci 31(2):60–65. doi:10.1016/j.tips.2009.11.003
Iqbal M, Audette MC, Petropoulos S, Gibb W, Matthews SG (2012) Placental drug transporters and their role in fetal protection. Placenta 33(3):137–142. doi:10.1016/j.placenta.2012.01.008
Jensen AR (2006) Clocking the mind: mental chronometry and individual differences. Elsevier, Oxford
Johnson MH (2001) Functional brain development in humans. Nat Rev Neurosci 2(7):475–483. doi:10.1038/35081509
Johnson MH (2005) Sensitive periods in functional brain development: problems and prospects. Dev Psychobiol 46(3):287–292. doi:10.1002/dev.20057
Kaiser S, Sachser N (2009) Effects of prenatal social stress on offspring development: pathology or adaptation? Curr Dir Psychol Sci 18(2):118–121. doi:10.1111/j.1467-8721.2009.01620.x
Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ (1993) The lifetime history of major depression in women: reliability of diagnosis and heritability. Arch Gen Psychiatr 50(11):863–870
Kieler H, Artama M, Engeland A, Ericsson O, Furu K, Gissler M, Haglund B (2012) Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. Br Med J, 344. doi:10.1136/bmj.d8012
Kiser D, SteemerS B, Branchi I, Homberg JR (2012) The reciprocal interaction between serotonin and social behaviour. Neurosci Biobehav Rev 36(2):786–798. doi:10.1016/j.neubiorev.2011.12.009
Klinger G, Merlob P (2008) Selective serotonin reuptake inhibitor induced neonatal abstinence syndrome. Isr J Psychiatry Relat Sci 45(2):107–113
Klinger G, Frankenthal D, Merlob P, Diamond G, Sirota L, Levinson-Castiel R, Inbar D (2011) Long-term outcome following selective serotonin reuptake inhibitor induced neonatal abstinence syndrome. J Perinatol 31(9):615–620. doi:10.1038/jp.2010.211
Konijnenberg C, Melinder A (2013) Neurodevelopmental investigation of the mirror neurone system in children of women receiving opioid maintenance therapy during pregnancy. Addiction 108(1):154–160. doi:10.1111/j.1360-0443.2012.04006.x
Korhonen M, Luoma I, Salmelin R, Tamminen T (2012) A longitudinal study of maternal prenatal, postnatal and concurrent depressive symptoms and adolescent well-being. J Affect Disord 136(3):680–692. doi:10.1016/j.jad.2011.10.007
Korkman M, Kirk U, Kemp S (2000) NEPSY: Neuropsykologisk Bedömning 3:10–12:11 år. Psykologiförlaget AB, Stockholm
Lovejoy MC, Graczyk PA, O’Hare E, Neuman G (2000) Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev 20(5):561–592. doi:10.1016/s0272-7358(98)00100-7
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10(6):434–445. doi:10.1038/nrn2639
Madigan S, Atkinson L, Laurin K, Benoit D (2013) Attachment and internalizing behavior in early childhood: a meta-analysis. Dev Psychol 49(4):672–689. doi:10.1037/a0028793
Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C, Moba Study Group (2006) Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 35(5):1146–1150. doi:10.1093/ije/dyl170
McAuley T, Christ SE, White DA (2011) Mapping the development of response inhibition in young children using a modified day-night task. 36. doi:10.1080/87565641.2010.549871
Merens W, Booij L, Haffmans PMJ, van der Does AJW (2008) The effects of experimentally lowered serotonin function on emotional information processing and memory in remitted depressed patients. J Psychopharmacol 22(6):653–662. doi:10.1177/0269881107081531
Misri S, Reebye P, Kendrick K, Carter D, Ryan D, Grunau RE, Oberlander TF (2006) Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatr 163(6):1026–1032. doi:10.1176/appi.ajp.163.6.1026
Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000) The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol 41(1):49–100. doi:10.1006/cogp.1999.0734
Munafo MR, Hayward G, Harmer C (2006) Selective processing of social threat cues following acute tryptophan depletion. J Psychopharmacol 20(1):33–39. doi:10.1177/0269881105056667
Nigg JT (2000) On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull 126(2):220–246. doi:10.1037//0033-2909.126.2.220
Nilsen W, Gustavson K, Roysamb E, Kjeldsen A, Karevold E (2013) Pathways from maternal distress and child problem behavior to adolescent depressive symptoms: a prospective examination from early childhood to adolescence. J Dev Behav Pediatr 34(5):303–313. doi:10.1097/DBP.0b013e318293ab05
Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M (2012) Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol 32(2):186–194
Norwegian Institute of Public Health (2010) The Norwegian Mother and Child Cohort Study. Oslo, Norway: Norwegian Institute of Public Health. Available at: http://www.fhi.no/eway/?pid=233; http://www.fhi.no/eway/default.aspx?pid=238&trg=MainArea5811&MainArea_5811=5903:0:15,4329:1:0:0:::0:0. Accessed May 5, 2010
Nulman I, Rovet J, Stewart DE, Wolpin J (1997) Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med 336:258–262. doi:10.1056/NEJM199701233360404
Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, Koren G (2002) Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatr 159(11):1889–1895. doi:10.1176/appi.ajp.159.11.1889
Nulman I, Koren G, Rovet J, Barrera M, Pulver A, Streiner D, Feldman B (2012) Neurodevelopment of children following prenatal exposure to venlafaxine, selective serotonin reuptake inhibitors, or untreated maternal depression. Am J Psychiatry 169(11):1165–1174. doi:10.1176/appi.ajp.2012.11111721
Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE (2007) Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med 161(1):22–29. doi:10.1001/archpedi.161.1.22
Pedersen LH, Henriksen TB, Bech BH, Licht RW, Kjaer D, Olsen J (2013) Prenatal antidepressant exposure and behavioral problems in early childhood—a cohort study. Acta Psychiatr Scand 127(2):126–135. doi:10.1111/acps.12032
Phillips M, Ladouceur C, Drevets W (2008) A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 13(9):833–857. doi:10.1038/mp.2008.65
Pluess M, Velders FP, Belsky J, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Jaddoe VWV, Tiemeier H (2011) Serotonin transporter polymorphism moderates effects of prenatal maternal anxiety on infant negative emotionality. Biol Psychiatry 69(6):520–525. doi:10.1016/j.biopsych.2010.10.006
Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, Posner MI (2004) Development of attentional networks in childhood. Neuropsychologia 42(8):1029–1040. doi:10.1016/j.neuropsychologia.2003.12.012
Sameroff AJ (1975) Early influences on development: fact or fancy? Merrill-Palmer Q J Dev Psychol 21(4):267–294
Sprinkle SD, Lurie D, Insko SL, Atkinson G, Jones GL, Logan AR, Bissada NN (2002) Criterion validity, severity cut scores, and test–retest reliability of the Beck Depression Inventory—II in a university counseling center sample. J Couns Psychol 49(3):381–385. doi:10.1037//0022-0167.49.3.381
Strand BH, Dalgard OS, Tambs K, Rognerud M (2003) Measuring the mental health status of the Norwegian population: a comparison of the instruments SCL-25, SCL-10, SCL-5 and MHI-5 (SF-36). Nord J Psychiatry 57(2):113–118. doi:10.1080/08039480310000932
Storch EA, Roberti JW, Roth DA (2004) Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory Second Edition in a sample of college students. Depression Anxiety 19:187–189. doi:10.1002/da.20002
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18(6):643–662. doi:10.1037/h0054651
Tambs K, Moun T (1993) How well can a few questionnaire items indicate anxiety and depression. Acta Psychiatr Scand 87:364–367. doi:10.1111/j.1600-0447.1993.tb03388.x
Tambs K, Røysamb E (2014) Selection of questions to short-form versions of original psychometric instruments in MoBa. Nor Epidemiol 24(1–2):195–201
Townsend J, Ashby FG (1978). Methods of modeling capacity in simple processing systems. In J. Castellan & F. Restle (Eds.), Cognitive Theory (Vol. 3, pp. 220–239). Erlbaum: Hillsdale, N. J
Tuccori M, Testi A, Antonioli L, Fornai M, Montagnani S, Ghisu N, Del Tacca M (2009) Safety concerns associated with the use of serotonin reuptake inhibitors and other serotonergic/noradrenergic antidepressants during pregnancy: a review. Clin Ther 31:1426–1453. doi:10.1016/j.clinthera.2009.07.009
Van Batenburg-Eddes T, Brion MJ, Henrichs J, Jaddoe VWV, Hofman A, Verhulst FC, Tiemeier H (2013) Parental depressive and anxiety symptoms during pregnancy and attention problems in children: a cross-cohort consistency study. J Child Psychol Psychiatry 54(5):591–600. doi:10.1111/jcpp.12023
Wechsler D (1989) WPPSI-R, Manual: Wechsler Preschool and Primary Scale of Intelligence, Revised. Psychological Corporation, San Antonio
WHO Collaborating Centre for Drugs Statistics Methodology. ATC/DDD index 2010. Available at: http://www.whocc.no/atc_ddd_index/. Accessed September 4, 2013
Acknowledgments
This study was supported by grants from the Norwegian Directorate for Children, Youth and Family Affairs (13/60525). The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no NO-ES-75558), NIH/NINDS (grant no. 1 UO1 NS 047537–01 and grant no. 2 UOI NS 047537-06A1). We are grateful to all the participating families in Norway who took part in MoBa in general, and in this study in particular.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Both the cohort study in general and the current study were approved by the Regional Committee for Medical Research Ethics and utilizes version 8 of the quality-assured data files
Rights and permissions
About this article
Cite this article
Hermansen, T.K., Røysamb, E., Augusti, EM. et al. Behavior and inhibitory control in children with prenatal exposure to antidepressants and medically untreated depression. Psychopharmacology 233, 1523–1535 (2016). https://doi.org/10.1007/s00213-016-4248-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4248-3