Abstract
Rationale
The N-methyl-D-aspartate (NMDA) receptor antagonist ketamine provides a pragmatic approach to address the link between glutamate-mediated changes in brain function and psychosis-like experiences. Most studies using PET or BOLD fMRI have assessed these symptoms broadly, which may limit inference about specific mechanisms.
Objectives
The objective of this study is to identify the cerebral blood flow (CBF) correlates of ketamine-induced psychopathology, focusing on individual psychotomimetic symptom dimensions, which may have separable neurobiological substrates.
Methods
We measured validated psychotomimetic symptom factors following intravenous ketamine administration in 23 healthy male volunteers (10 given a lower dose and 13 a higher dose) and correlated ketamine-induced changes in symptoms with regional changes in CBF, measured non-invasively using arterial spin labelling (ASL).
Results
The main effect of ketamine paralleled previous studies, with increases in CBF in anterior and subgenual cingulate cortex and decreases in superior and medial temporal cortex. Subjective effects were greater in the high-dose group. For this group, ketamine-induced anhedonia inversely related to orbitofrontal cortex CBF changes and cognitive disorganisation was positively correlated with CBF changes in posterior thalamus and the left inferior and middle temporal gyrus. Perceptual distortion was correlated with different regional CBF changes in the low- and high-dose groups.
Conclusions
Here, we provide evidence for the sensitivity of ASL to the effects of ketamine and the strength of subjective experience, suggesting plausible neural mechanisms for ketamine-induced anhedonia and cognitive disorganisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ketamine is an uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist that can exacerbate symptoms in patients with schizophrenia (Lahti et al. 1995) and in healthy volunteers produces positive, negative and cognitive symptoms that are thought to resemble those observed in schizophrenia (Krystal et al. 1994). While not producing a phenocopy of the disorder, the ketamine model provides a pragmatic approach to address how glutamate-mediated changes in brain function can cause psychotomimetic symptoms (Corlett et al. 2011).
Brain imaging studies produced a mixture of findings when examining relationships between brain activity and the subjective effects of ketamine. Stone et al. used single photon emission tomography (SPET) to show that binding of ketamine to NMDA receptors in middle inferior frontal cortex (as measured by displacement of the radiolabelled marker [(123)I]CNS-1261) was correlated with negative symptoms as measured by the brief psychiatric rating scale—BPRS (Overall and Gorham 1962; Stone et al. 2008). Deakin et al. used the clinician-administered dissociative symptoms scale—CADSS (Bremner et al. 1998)—and BPRS to measure the effects of ketamine. Functional magnetic resonance imaging (fMRI) using the blood oxygen level-dependent phenomenon (BOLD) revealed signal reductions in medial OFC/subgenual cingulate correlated with scores of dissociative and psychotic symptomatology (using CADSS and BPRS) and BOLD signal reductions in temporal pole also correlated with dissociative symptomatology. Activations were correlated with CADSS and BPRS scores in the posterior cingulate and with BPRS scores in frontal pole and parahippocampal gyrus (Deakin et al. 2008). These findings differed from Holcomb and colleagues who used H2O15 PET and found a correlation between BPRS scores and increased anterior cingulate CBF following ketamine in a whole-brain analysis in healthy volunteers (Holcomb et al. 2001). A similar correlation between BPRS scores and anterior cingulate CBF following ketamine was found in a subsequent ROI analysis in patients with schizophrenia (Holcomb et al. 2005).
These studies have all examined correlations with scales measuring subjective effects in a relatively broad sense, each scale combining a number of separable psychotomimetic symptoms (e.g., perceptual abnormalities and delusional thinking). The aim of the present study was to measure validated psychotomimetic symptom factors (De Simoni et al. 2013; Mason et al. 2008) following low-dose ketamine administration in healthy volunteers and correlate changes in individual symptom factors with changes in regional cerebral blood flow (rCBF), measured non-invasively using arterial spin labelling (ASL). The use of symptom factors may provide more accurate delineation of regional correlates of ketamine effects. The use of ASL allowed the assessment of brain activity over a number of minutes of steady-state ketamine administration, uncontaminated by the rapid changes in brain activity observed using BOLD during a bolus administration (De Simoni et al. 2013) and independent of sources of low frequency noise (Aguirre et al. 2002).
ASL is a contrast-free MRI method that is directly sensitive to regional CBF, in a manner analogous to H2O15 positron emission tomography (PET), but without requiring exposure of the subjects to ionizing radiation. ASL allows the determination of absolute quantitative changes in a single physiological parameter, elicited by the drug. In contrast, BOLD is sensitive to both regional CBF and the regional rate of cerebral oxygen metabolism (CMRO2). Thus, ASL is suitable for directly comparing the state during which ketamine is continuously infused to achieve a steady plasma level, with the pre-infusion ketamine state. The utility of ASL for this study stems from the direct link between neuronal activity and regional microcirculation (also known as ‘neurovascular coupling’ (Attwell et al. 2010)); and has been confirmed by the results of recent investigations in which it was successfully employed to study the acute effects of various psychoactive compounds, including alcohol (Tolentino et al. 2011), psilocybin (Carhart-Harris et al. 2012), cocaine (Luo et al. 2009), cannabis (van Hell et al. 2011), propofol (Griffin et al. 2010), methylphenidate, atomoxetine (Marquand et al. 2012), fentanyl (Zelaya et al. 2012), aripiprazole and haloperidol (Handley et al. 2013).
All studies investigating the effect of sub-anaesthetic ketamine doses on CBF in humans to date have used H2O15 PET: these have demonstrated increases in CBF in thalamic regions and in prefrontal, orbitofrontal and cingulate cortices in both healthy volunteers and schizophrenic patients (Holcomb et al. 2005; Holcomb et al. 2001; Lahti et al. 1995; Langsjo et al. 2003; Rowland et al. 2010). The only study to measure ketamine-induced CBF changes using ASL was performed in rodents, and the authors assessed CBF changes in the striatum only (Bruns et al. 2009).
Given recent studies that have suggested a role for the anterior cingulate in mediating the psychotomimetic effects of ketamine (Holcomb et al. 2005; Stone et al. 2012), we chose also to take a ROI approach to see whether changes in any psychotomimetic symptoms correlated with changes in CBF within this region. Based on previous CBF studies (Holcomb et al. 2005; Holcomb et al. 2001), it was specifically predicted that CBF changes in the anterior cingulate cortex would correlate with ‘positive symptom’ dimensions. We also predicted that previously observed negative correlations with OFC activity (Deakin et al. 2008) would be replicated and that these would relate to negative symptom scores such as anhedonia, which are robustly induced by ketamine in healthy volunteers (Mason et al. 2008; Stone et al. 2008).
Methods
The data from this study were collected as part of two separate experiments with a low-dose and a high-dose ketamine administration protocol (De Simoni et al. 2013; Stone et al. 2012; Stone et al. 2013). Healthy male volunteers were recruited by advertisement. Ten volunteers (mean age 25.5 years, SD = 6.5) were recruited to the low-dose ketamine group and 13 volunteers to the high-dose ketamine group (mean age 27.0 years, SD = 6.9).
Exclusion criteria included positive urine drug screen for drugs of abuse, the consumption of more than five cups of coffee (or equivalent) per day, smoking more than five cigarettes per day, taking prescription drugs and any history of mental illness or serious medical condition that in the opinion of the study doctors prevented their participation in the study. Fulfillment of inclusion and exclusion criteria was assessed by a psychiatrist who completed a full psychiatric, neurological and medical examination of each participant (including electrocardiogram and urine drug screen). Written informed consent was provided by all participants prior to their inclusion in the study, which was approved by the Wandsworth and East London Research Ethics Committees.
Four patients in the high-dose ketamine group had already taken part as volunteers in the low-dose ketamine group. At least a year had elapsed since participation in the low-dose ketamine group.
The study had an open-label design. Subjects completed the psychotomimetic states inventory (PSI; 48 items consisting of six subscales—delusional thinking, perceptual distortion, cognitive disorganisation, anhedonia, mania and paranoia (Mason et al. 2008)) before entering the scanner. Following ketamine administration and the end of the scan, subjects completed the PSI again, answering the items with reference to the peak intensity of ketamine effects during the scan. We used the PSI as it has been developed for use in healthy volunteers, has excellent test–retest reliability and has sensitivity to low-dose effects of ketamine (De Simoni et al. 2013).
Imaging was performed using a 3.0T HDx MRI scanner (GE Medical Systems, Milwaukee, WI, USA). All subjects initially underwent a high-resolution T2-weighted structural scan.
The ASL acquisition protocol differed slightly between the low-dose and high-dose groups. This was due to modifications of the protocol to improve data quality between the earlier (low-dose) and later (high-dose) studies.
Low-dose ASL acquisition
A whole brain CBF map was obtained using a pseudo-continuous flow-driven adiabatic inversion labelling scheme during a 6-min ASL scan (labelling time 1.5 s, post-labelling delay 15 s, TE/TR = 32.256/5,500 ms, flip angle (FA) = 90°). Image data were acquired using a multi-shot, segmented 3D stack of spirals (eight arms) with a resultant spatial resolution of 2 × 2 × 3mm. Three control-label pairs were used to derive a perfusion weighted difference image. A proton density image was acquired in 48 s using the same acquisition parameters in order to compute the CBF map in standard physiological units (ml blood/100 g tissue/min). Total acquisition time for each ASL scan was 5:30 min.
High-dose ASL acquisition
ASL acquistion for the high-dose study used the same acquisition parameters as the low-dose study except that in the high-dose study the 3D volume of the image data was collected axially instead of coronally and used additional saturation bands.
All subjects then underwent an intravenous ketamine infusion, which was dynamically modelled using a laptop computer running Stanpump software, driving a Graseby 3400 syringe-driver. Infusion parameters used were based on the pharmacodynamics used by the Clements 250 model (Absalom et al. 2007). For the 10 subjects in the low-dose ketamine group, a target plasma level of 50–75 ng/mL was specified (in practice this approximated a rapid bolus of an average of 0.12 mg/kg over 20 s followed by a slow infusion of 0.31 mg/kg/h). For the 13 subjects in the high-dose ketamine group, a target plasma level of 150 ng/mL was specified (in practice this approximated a rapid bolus of 0.26 mg/kg over 20 s followed by a slow infusion of 0.42 mg/kg/h).
For both low- and high-dose ketamine groups, the ASL acquisitions were repeated at 10 min after the start of the ketamine infusion.
In order to warp the CBF maps into a standard (MNI) space, individual T2-weighted images were used. Initially, T2-weighted images were skull stripped using the FSL Brain Extraction Tool (BET). The remaining steps were performed using SPM8 (www.fil.ion.ucl.ac.uk/spm). Raw CBF maps for each subject (pre- and post-ketamine) were co-registered with their corresponding stripped T2-weighted image and the same skull stripping was applied to the CBF maps. The stripped co-registered CBF images were then normalised to MNI space using the subject’s original anatomical T2-weighted image as a source and a skull-stripped T2-weighted template image (slice thickness 2 mm). These images were then smoothed using a 10-mm FWHM Gaussian kernel.
Pre-ketamine CBF maps were subtracted from their corresponding post-ketamine CBF maps resulting in maps of ketamine-related change in CBF (Stewart et al. 2015).
For each subject, pre-ketamine scores were subtracted from post-ketamine scores for each of the PSI subscales, to obtain a value for the change in scores for each subscale. SPSS was used to identify outliers in subjective response to ketamine by applying the ‘outlier’ function to the total scaled PSI scores for each participant. This was repeated separately for the high-dose and the low-dose ketamine groups. Data points identified as outliers represented those with values greater than 1.5 times the interquartile range from the upper quartile value. Imaging data were analysed using SPM8. To assess the main effects of ketamine on CBF, paired t test analyses were performed using the pre- and post-ketamine CBF maps. An absolute threshold of 14 ml/100 mg/min was applied to remove non-physiologically plausible CBF values from the analysis and most of deep white matter tissue where ASL measurements are unreliable due to the long arterial blood transit times. This produced a binary mask that was used in the subsequent regression analysis. Global CBF was calculated as the average signal across all analysed voxels and the analysis was corrected for global CBF.
Separate whole-brain multiple regression analyses were carried out for the low-dose and high-dose ketamine groups using change in CBF as the dependent variable and the change scores for each PSI subscale as the independent variables, correcting for global CBF as above. For this analysis, in order to minimise the inclusion of physiologically implausible (either spatially or in terms of CBF value) voxels, we used an explicit grey matter mask that had been multiplied with the thresholded (at 14 ml/100 mg/min) binary mask from the t test analysis (to remove voxels with no data). For statistical inference, we used cluster corrected statistics, selecting only clusters which survived significance after family-wise error correction for multiple comparison based on cluster extent (p < 0.05); using a cluster forming threshold of p < 0.01 at the voxel level (as used in our previous studies: Mikita et al. 2015; Zelaya et al. 2012). We also confirmed that the results were unchanged using correction for non-stationarity of the data. For the ROI analysis, we used an anterior cingulate volume from the wfupickatlas toolbox (Maldjian et al. 2003). For statistical inference within the ROI, we used family-wise error corrected statistics at the voxel level.
Results
One subject in the high-dose ketamine group was identified as an outlier, having the highest rated subjective effects. This subject was excluded from the analyses.
In the low-dose group ketamine administration led to a significant (paired t test; [mean(SD); p value]) increase from baseline in subjective ratings on perceptual distortion [0.38(0.40); t(9) = 2.99; p = 0.015] and cognitive disorganisation [0.20(0.19); t(9) = 3.35; p < 0.01]. There was no significant increase for delusional thinking [0.03(0.05); t(9) = 1.50; p = 0.168], anhedonia [0.11(0.22); t(9) = 1.63; p = 0.137], mania [0.00(0.22); t(9) = 0.00; p = 0.999 ] or paranoia [0.00(0.10); t(9) = 0.00; p = 1.0].
In the high-dose group, ketamine administration led to a significant (paired t test; [mean difference(SD); p value]) increase from baseline in subjective ratings on delusional thinking [0.27(0.41); t(11) = 2.30; p = 0.042], perceptual distortion [0.68(0.44); t(11) = 5.32; p < 0.01], cognitive disorganisation [0.60(0.57); t(11) = 3.67; p < 0.01], anhedonia [0.39(0.33); t(11) = 4.00; p < 0.01] and mania [0.40(0.30); t(11) = 4.56; p < 0.01]. There was no significant increase for Paranoia [0.05(0.10); t(11) = 1.82; p = 0.096].
Ketamine-induced increases in subjective ratings were significantly greater in the high-dose group versus the low-dose group (independent samples t test; [scaled mean score in low-dose group (standard deviation) vs scaled mean score in high-dose group (standard deviation); p value] for cognitive disorganisation [0.20(0.19) vs 0.60(0.57); t(20) = 2.13; p = 0.046], anhedonia [0.11(0.22) vs 0.38(0.33); t(20) = 2.18; p = 0.042] and mania [0.00(0.22) vs 0.39(0.30) t(20) = 3.42; p < 0.01]. Changes in subjective ratings were not significantly different between dosages for delusional thinking [0.03(0.05) vs 0.27(0.41); t(11.4) = 2.06; p = 0.075], perceptual distortion [0.38(0.40) vs 0.68(0.44); t(20) = 1.65; p = 0.115] and paranoia [0.00(0.10) vs 0.05(0.09); t(20) = 1.21; p = 0.24]. See Fig. 1.
PSI subscale changes in low-dose and high-dose ketamine groups. Note that data pertaining to the low-dose ketamine group overlap with those previously reported by De Simoni et al. (2013)
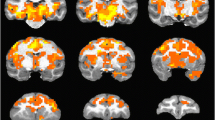
We used paired t tests to assess the main effect of ketamine on CBF. Ketamine-induced increases in CBF were observed in the low-dose group in the right anterior cingulate (p < 0.01; Table 1; Fig. 2a) and right ventromedial prefrontal cortex (p = 0.01; Table 1; Fig. 2a). In the high-dose group, ketamine-induced CBF increases were more restricted, reaching significance in the right subgenual cingulate (p < 0.01; Table 1; Fig. 2a). Ketamine-induced CBF decreases were observed in the low-dose group in the left retrosubicular hippocampal area (p < 0.01; Table 1; Fig. 2a). In the high-dose group CBF decreases were observed in the right superior temporal cortex (p < 0.01; Table 1; Fig. 2a).
a Changes in CBF with ketamine in the low- and high-dose groups overlaid on a high-resolution T1-weighted image from MRIcron. b Regions showing significant correlations with PSI subscales overlaid on a high-resolution T1-weighted image from MRIcron. The MNI Z axis is shown at the top. The colour bars show the t statistic
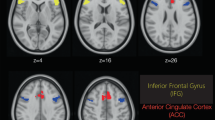
Changes in peak voxel activation with PSI subscale score (see Table 2a for peak voxel coordinates). a Anhedonia—high dose; b Cognitive disorganisation—low dose; c Cognitive disorganisation—high dose; d Perceptual distortion—high dose
We found a negative correlation between CBF changes and changes in anhedonia scores for the high-dose ketamine group in the right orbitofrontal cortex extending into the right middle prefrontal gyrus (p < 0.01; Table 2; Fig. 2b; Fig. 3a), such that greater ketamine-induced anhedonia was associated with increasingly negative CBF changes. There was a positive correlation between changes in CBF and changes in cognitive disorganisation scores for the high-dose ketamine group in the left posterior thalamus (p < 0.01; Table 2; Fig. 2b; Fig. 3c) extending into the left lingual gyrus as well as in the left inferior and middle temporal gyrus (p < 0.01; Table 2; Fig. 2b; Fig. 3b).
Correlations between CBF and changes in paranoia scores were not examined because of insufficient variance in paranoia scores in both the low- and high-dose ketamine groups (in general paranoia scores deviated only minimally from baseline under the influence of ketamine—see previous section and Fig. 1). Changes in perceptual distortion scores were correlated with different brain regions in the low- and high-dose ketamine groups. In the low-dose group, there was a significant negative correlation with CBF in right somatosensory association cortex (p < 0.01; Table 2). These correlations were not observed in the high-dose ketamine group; instead in this group a positive correlation between changes in CBF and changes in perceptual distortion scores was observed in the left medial thalamus (p = 0.02; Table 2; Fig. 3d).
The ROI analysis did not reveal any significant correlations between anterior cingulate CBF and changes in any of the PSI subscales.
Discussion
In this study, we used ASL to examine the relationships between different components of the subjective effects of ketamine infusion and rCBF. We found meaningful correlations that provide support for the future role of ASL in analysing the brain basis of subjective effects of ketamine and other psychoactive medications. We interpret these changes in CBF as reflecting proximate effects of ketamine-induced changes in neuronal activity on glucose and oxygen metabolism. This is evidenced by studies which indicate, using subanaesthetic doses in humans and anaesthetic doses in rats, that ketamine does not appear to disrupt neurovascular coupling (Cavazzuti et al. 1987; Langsjo et al. 2003; Langsjo et al. 2004).
The dosing protocol was designed such that ASL images were acquired when ketamine levels were at a steady state. Thus, this study differs significantly from previous BOLD studies where the effects of ketamine were captured in the period of infusion, including delivery of a bolus. In line with previous CBF studies of the effects of ketamine (Holcomb et al. 2001; Lahti et al. 1995; Rowland et al. 2010), we found a main effect of ketamine on CBF in anterior and subgenual areas of the cingulate cortex. Other effects were observed outside of this area, including decreases in CBF, although the areas of decrease were different in the high-dose and low-dose groups, i.e. significant CBF decreases in the left retrosubicular hippocampal area in the low-dose ketamine group and in the right superior temporal cortex in the high-dose ketamine group. This could reflect a dose-related difference in the ketamine response, although previous FDG-PET studies have not seen similar changes. The goal of this study was not to characterise the dose–response relationships of ketamine and CBF and it was not optimised for direct comparisons between doses; instead, we focussed on the relationship of those effects with the subjective measures acquired at steady state.
Most of the significant correlations occurred in the high-dose ketamine group. This is perhaps unsurprising given that subjects in the high-dose group experienced significantly greater subjective effects in most domains and that the post-ketamine scores were more clustered in the lower dose group. Because the low- and high-dose groups were not a single cohort, we were unable to combine them in the same correlational analysis to provide a broader view of the dose response relationships. Previous work has shown a dose–response effect for the BOLD (De Simoni et al. 2013) and CBF (H2O15 PET; (Langsjo et al. 2003)) response to ketamine and, with the relatively small numbers in the study, correlations were more likely to emerge when both subjective effects and CBF were more pronounced.
Unlike earlier studies, we did not include the CADSS as a measure of drug-induced dissociative experiences in this study because we previously demonstrated its poor reliability for the subjective effects of low-dose ketamine compared to the PSI (De Simoni et al. 2013). Using the PSI, we have extended the findings of Deakin et al. (2008), showing that orbitofrontal cortex activity is inversely related to the subjective effects of ketamine. Here we have extended that finding by showing that decreased CBF in the OFC, more specifically, is inversely related to ketamine-induced anhedonia.
Although we identified a more lateral part of OFC than Deakin and colleagues, the OFC is part of a network of regions involved in reward and value processing (Kringelbach 2005). This network also includes the insula, amygdala and ventral striatum, with the ventromedial PFC involved in secondary, domain independent processing of rewards and lateral, posterior parts of OFC associated more strongly with primary reward processing (Sescousse et al. 2013; Sescousse et al. 2010).
The ketamine-induced increase in anhedonia in this study is similar to that observed with previous use of the PSI with ketamine (Mason et al. 2008), but stands in apparent contrast to its emerging use in treatment-resistant depression, in which it has been demonstrated to exert a rapid and sometimes profound anti-anhedonic effect (Lally et al. 2015). Given the use of ketamine as an antidepressant, therefore, one might expect it to reduce anhedonia in our subjects. The PSI was not designed to assess depressive symptomatology in patient groups however and it is not clear that the acute, transient anhedonia experienced by subjects in this study has either phenomenological or neurobiological overlap with the chronic state experienced by patients with depression. Further, patients with depression have high baseline levels of anhedonia and therefore may show a differential response to acute ketamine administration compared to healthy subjects.
For the higher ketamine dose, the cognitive disorganisation factor was positively correlated with CBF changes in left inferior and middle temporal gyrus as well as the posterior thalamus. Inspection of the items comprising this subscale (e.g., ‘Your mind jumps a lot from one thing to another’; ‘Your speech is difficult to understand because your words are all mixed up’) suggest that what is being tapped is something close to psychotic ‘thought disorder’, a term that refers to abnormalities of internal thought and to their manifestation through abnormalities of speech production. The left inferior and middle temporal gyri contain regions essential for the processing and production of coherent speech and abnormalities of activation in this area, as measured by BOLD response, have been linked to formal thought disorder in patients with schizophrenia (Kircher et al. 2001). The pulvinar of the thalamus, located within the other significant cluster, projects to several cortical areas, including prefrontal and limbic regions, as well as having rich projections to and from sensory cortices; disruption to this region can produce deficits in verbal and non-verbal processing (Ojemann et al. 1968) and its function is abnormal in schizophrenia (Andrews et al. 2006).
Perceptual distortion was correlated with decreases in CBF in somatosensory association cortex under low-dose ketamine, but not at the higher dose. This subscale of the PSI includes items that relate to somatosensory distortions (e.g., ‘You feel as though your head, limbs or body have somehow changed’), suggesting that these decreases in CBF might underlie perceptual distortion in the somatosensory modality. Studies of hallucinations in psychotic patients and experimentally induced hallucinations in healthy controls have similarly implicated somatosensory areas (Blankenburg et al. 2006; Nemoto et al. 2010; Shergill et al. 2001). It is unclear why these correlations were not seen in the high-dose ketamine group, which instead saw a positive correlation with changes in CBF in the left medial thalamus. This ASL study was not suitable for a dose response connectivity analysis although thalamo-cortical connectivity is a candidate marker for dose response effects related to perceptual distortions based on our findings. The thalamus is an integral part of perceptual networks in both visual and somatosensory modalities and might be expected to undergo CBF changes under conditions of intense perceptual distortion with high-dose ketamine. Dawson et al. reported that ketamine administration in rats led to marked changes in the connectivity of multiple thalamic nuclei with the prefrontal cortex (Dawson et al. 2013). It is interesting to note that chronic ketamine users have lower levels of thalamic N-acetyl-aspartate suggesting a potential effect of long-term ketamine use on neural integrity in this brain region (Stone et al. 2013).
We were unable to replicate the association between CBF in anterior cingulate cortex and the overall subjective effects of ketamine that had been noted previously (Holcomb et al. 2005; Holcomb et al. 2001), although a main effect of ketamine at both doses was seen in this region. Notably these earlier studies, which used H2O15 PET, found a correlation with the psychosis subscale of the BPRS, the items of which relate to a number of phenomenologically separable psychotic symptoms. In fact the heterogeneity of items within this subscale means that it brings together elements which in the PSI are tapped by separable subscales. For example, items relating to symptoms of cognitive disorganisation and perceptual distortion, which in the PSI feature in separate subscales, would be subsumed within the psychosis subscale of the BPRS, which was used in the PET CBF studies. Although we did not find a correlation with subjective effects of ketamine in our ROI analysis, whole-brain analysis revealed correlations between regional changes in CBF and variables that may have more clinical and phenomenological meaning.
Limitations
The number of participants in the present study was relatively low, but to date there have been no investigations into the effects of ketamine on quantitative blood flow measurements. This study demonstrates the feasibility and utility of such techniques. This method differs from the related BOLD techniques (De Simoni et al. 2013; Deakin et al. 2008) because these assess the change in BOLD signal, incorporating the rapid changes at the time of infusion. Here, we use a quantitative technique to allow assessment of steady-state effects a number of minutes later.
Because quantitative blood flow measurements are a developing technique in MRI, optimisations have emerged over recent years and attempts to produce high-quality data in each study have occurred at the expense of standardisation. The fact that we used slightly different acquisition protocols for the low and high-dose groups limited formal comparison of the ketamine effect, although the focus here was the relationship with subjective ratings, which would be relatively unaffected as both methods have similar signal-to-noise characteristics.
Here, we have assumed a linear model for the effects of ketamine, whereas the effects may be non-linear. This is suggested by the perceptual distortion relationships differing in regions with dose. Investigations of multiple doses, preferably within subjects would be required to understand these relationships. To date, such a study has not been conducted.
Conclusion
We have demonstrated the utility of ASL as a non-invasive tool in the investigation of the physiological correlates of the subjective effects of ketamine, the first such study to use this modality to investigate psychoactive drug effects in this way. Meaningful correlations between changes in subjective experience and in CBF were identified. In particular, the correlations between anhedonia and orbitofrontal cortex CBF and that between perceptual abnormalities and areas subserving sensory processing point towards a plausible mechanism for striking aspects of the ketamine experience. This study is also the first to use ASL to replicate the central role, already established in other imaging modalities, of the anterior cingulate cortex in mediating the main effect of ketamine.
References
Absalom AR, Lee M, Menon DK, Sharar SR, De Smet T, Halliday J et al (2007) Predictive performance of the Domino, Hijazi, and Clements models during low-dose target-controlled ketamine infusions in healthy volunteers. Br J Anaesth 98(5):615–623. doi:10.1093/bja/aem063
Aguirre GK, Detre JA, Zarahn E, Alsop DC (2002) Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage 15(3):488--500. doi:10.1006/nimg.2001.0990
Andrews J, Wang L, Csernansky JG, Gado MH, Barch DM (2006) Abnormalities of thalamic activation and cognition in schizophrenia. Am J Psychiatry 163(3):463–469. doi:10.1176/appi.ajp.163.3.463
Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468(7321):232–243. doi:10.1038/nature09613
Blankenburg F, Ruff CC, Deichmann R, Rees G, Driver J (2006) The cutaneous rabbit illusion affects human primary sensory cortex somatotopically. PLoS Biol 4(3), e69. doi:10.1371/journal.pbio.0040069
Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS et al (1998) Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 11(1):125–136. doi:10.1023/A:1024465317902
Bruns A, Kunnecke B, Risterucci C, Moreau JL, von Kienlin M (2009) Validation of cerebral blood perfusion imaging as a modality for quantitative pharmacological MRI in rats. Magn Reson Med 61(6):1451–1458. doi:10.1002/mrm.21779
Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A et al (2012) Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A 109(6):2138–2143. doi:10.1073/pnas.1119598109
Cavazzuti M, Porro CA, Biral GP, Benassi C, Barbieri GC (1987) Ketamine effects on local cerebral blood flow and metabolism in the rat. J Cereb Blood Flow Metab 7(6):806–811. doi:10.1038/jcbfm.1987.138
Corlett PR, Honey GD, Krystal JH, Fletcher PC (2011) Glutamatergic model psychoses: prediction error, learning, and inference. Neuropsychopharmacology 36(1):294–315. doi:10.1038/npp.2010.163
Dawson N, Morris BJ, Pratt JA (2013) Subanaesthetic ketamine treatment alters prefrontal cortex connectivity with thalamus and ascending subcortical systems. Schizophr Bull 39(2):366–377. doi:10.1093/schbul/sbr144
De Simoni S, Schwarz AJ, O’Daly OG, Marquand AF, Brittain C, Gonzales C et al (2013) Test-retest reliability of the BOLD pharmacological MRI response to ketamine in healthy volunteers. Neuroimage 64:75–90. doi:10.1016/j.neuroimage.2012.09.037
Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM (2008) Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry 65(2):154–164. doi:10.1001/archgenpsychiatry.2007.37
Griffin KM, Blau CW, Kelly ME, O’Herlihy C, O’Connell PR, Jones JF et al (2010) Propofol allows precise quantitative arterial spin labelling functional magnetic resonance imaging in the rat. Neuroimage 51(4):1395–1404. doi:10.1016/j.neuroimage.2010.03.024
Handley R, Zelaya FO, Reinders AA, Marques TR, Mehta MA, O’Gorman R et al (2013) Acute effects of single-dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum Brain Mapp 34(2):272–282. doi:10.1002/hbm.21436
Holcomb HH, Lahti AC, Medoff DR, Weiler M, Tamminga CA (2001) Sequential regional cerebral blood flow brain scans using PET with H2(15)O demonstrate ketamine actions in CNS dynamically. Neuropsychopharmacology 25(2):165–172. doi:10.1016/S0893-133X(01)00229-9
Holcomb HH, Lahti AC, Medoff DR, Cullen T, Tamminga CA (2005) Effects of noncompetitive NMDA receptor blockade on anterior cingulate cerebral blood flow in volunteers with schizophrenia. Neuropsychopharmacology 30(12):2275–2282. doi:10.1038/sj.npp.1300824
Kircher TT, Liddle PF, Brammer MJ, Williams SC, Murray RM, McGuire PK (2001) Neural correlates of formal thought disorder in schizophrenia: preliminary findings from a functional magnetic resonance imaging study. Arch Gen Psychiatry 58(8):769–774
Kringelbach ML (2005) The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 6(9):691–702. doi:10.1038/nrn1747
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD et al (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51(3):199–214
Lahti AC, Holcomb HH, Medoff DR, Tamminga CA (1995) Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport 6(6):869–872
Lally N, Nugent AC, Luckenbaugh DA, Niciu MJ, Roiser JP, Zarate CA Jr (2015) Neural correlates of change in major depressive disorder anhedonia following open-label ketamine. J Psychopharmacol. doi:10.1177/0269881114568041
Langsjo JW, Kaisti KK, Aalto S, Hinkka S, Aantaa R, Oikonen V et al (2003) Effects of subanesthetic doses of ketamine on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology 99(3):614–623
Langsjo JW, Salmi E, Kaisti KK, Aalto S, Hinkka S, Aantaa R et al (2004) Effects of subanesthetic ketamine on regional cerebral glucose metabolism in humans. Anesthesiology 100(5):1065–1071
Luo F, Schmidt KF, Fox GB, Ferris CF (2009) Differential responses in CBF and CBV to cocaine as measured by fMRI: implications for pharmacological MRI signals derived oxygen metabolism assessment. J Psychiatr Res 43(12):1018–1024. doi:10.1016/j.jpsychires.2008.11.009
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19(3):1233–1239
Marquand AF, O’Daly OG, De Simoni S, Alsop DC, Maguire RP, Williams SC et al (2012) Dissociable effects of methylphenidate, atomoxetine and placebo on regional cerebral blood flow in healthy volunteers at rest: a multi-class pattern recognition approach. Neuroimage 60(2):1015–1024. doi:10.1016/j.neuroimage.2012.01.058
Mason OJ, Morgan CJ, Stefanovic A, Curran HV (2008) The psychotomimetic states inventory (PSI): measuring psychotic-type experiences from ketamine and cannabis. Schizophr Res 103(1–3):138–142. doi:10.1016/j.schres.2008.02.020
Mikita N, Mehta MA, Zelaya FO, Stringaris A (2015) Using arterial spin labeling to examine mood states in youth. Brain Behav 5(6), e00339. doi:10.1002/brb3.339
Nemoto K, Mizukami K, Hori T, Tachikawa H, Ota M, Takeda T et al (2010) Hyperperfusion in primary somatosensory region related to somatic hallucination in the elderly. Psychiatry Clin Neurosci 64(4):421–425. doi:10.1111/j.1440-1819.2010.02101.x
Ojemann GA, Fedio P, Van Buren JM (1968) Anomia from pulvinar and subcortical parietal stimulation. Brain 91(1):99–116
Overall JF, Gorham DR (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812
Rowland LM, Beason-Held L, Tamminga CA, Holcomb HH (2010) The interactive effects of ketamine and nicotine on human cerebral blood flow. Psychopharmacology (Berl) 208(4):575–584. doi:10.1007/s00213-009-1758-2
Sescousse G, Redoute J, Dreher JC (2010) The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci: Off J Soc Neurosci 30(39):13095–13104. doi:10.1523/JNEUROSCI.3501-10.2010
Sescousse G, Caldu X, Segura B, Dreher JC (2013) Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev 37(4):681–696. doi:10.1016/j.neubiorev.2013.02.002
Shergill SS, Cameron LA, Brammer MJ, Williams SC, Murray RM, McGuire PK (2001) Modality specific neural correlates of auditory and somatic hallucinations. J Neurol Neurosurg Psychiatry 71(5):688–690
Stewart SB, Koller JM, Campbell MC, Perlmutter JS, Black KJ (2015) Additive global cerebral blood flow normalization in arterial spin labeling perfusion imaging. Peer J 3, e834. doi:10.7717/peerj.834
Stone JM, Erlandsson K, Arstad E, Squassante L, Teneggi V, Bressan RA et al (2008) Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy: a [(123)I]CNS-1261 SPET study. Psychopharmacology 197(3):401–408. doi:10.1007/s00213-007-1047-x
Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ et al (2012) Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry 17(7):664–665. doi:10.1038/mp.2011.171
Stone JM, Pepper F, Fam J, Furby H, Hughes E, Morgan C et al (2013) Glutamate, N-acetyl aspartate and psychotic symptoms in chronic ketamine users. Psychopharmacology. doi:10.1007/s00213-013-3354-8
Tolentino NJ, Wierenga CE, Hall S, Tapert SF, Paulus MP, Liu TT et al (2011) Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol Clin Exp Res 35(6):1034–1040. doi:10.1111/j.1530-0277.2011.01435.x
van Hell HH, Bossong MG, Jager G, Kahn RS, Ramsey NF (2011) Methods of the pharmacological imaging of the cannabinoid system (PhICS) study: towards understanding the role of the brain endocannabinoid system in human cognition. Int J Methods Psychiatr Res 20(1):10–27. doi:10.1002/mpr.327
Zelaya FO, Zois E, Muller-Pollard C, Lythgoe DJ, Lee S, Andrews C et al (2012) The response to rapid infusion of fentanyl in the human brain measured using pulsed arterial spin labelling. Magma 25(2):163–175. doi:10.1007/s10334-011-0293-4
Acknowledgments
We thank Dr. David Alsop for making available to us the pCASL pulse sequences employed in this work, Professor Anthony Absalom for supplying the Stanpump software for the implementation of the Clements 250 infusion model and the radiographers at the Centre for Neuroimaging Sciences and Astrid Pauls for helping with the data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Funding
TP was supported by the National Institute for Health Research (NIHR). JMS was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King’s College London, and by the Yale Center for Clinical Investigation (UL1RR024139), U.S. Department of Veterans Affairs via its support for the National Center for Post Traumatic Stress Disorder and Consortium to Alleviate PTSD, and the U.S. National Institute on Alcohol Abuse and Alcoholism (P50AA012879). He has received honoraria from Janssen Pharmaceuticals, Behrenberg Bank, AstraZeneca, Pfizer, Sunovion and Hoffman-La Roche Ltd. MAM has consulted for Cambridge Cognition and Lundbeck and received payment for contribution towards educational materials for Shire in the past 5 years. This study was supported by a grant from Eli Lilly and Company. We also thank the Wellcome Trust and EPSRC for continued funding of the Centre for Neuroimaging Sciences.
Rights and permissions
About this article
Cite this article
Pollak, T.A., De Simoni, S., Barimani, B. et al. Phenomenologically distinct psychotomimetic effects of ketamine are associated with cerebral blood flow changes in functionally relevant cerebral foci: a continuous arterial spin labelling study. Psychopharmacology 232, 4515–4524 (2015). https://doi.org/10.1007/s00213-015-4078-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4078-8