Abstract
Cisplatin (CP) is a highly effective broad-spectrum chemotherapeutic agent for several solid tumors. However, its clinical use is associated with ovarian toxicity. Icariin (ICA) is a bioactive flavonoid of Epimedium brevicornum with reported protective activities against inflammation, oxidative stress and ovarian failure. This study aimed to explore the protective effects of ICA against CP-associated ovarian toxicity in rats. Rats were randomized into five groups and treated for 17 days: control, ICA (10 mg/kg/day, for 17 days. p.o.), CP (6 mg/kg, i.p. on days 7 and 14), CP + ICA (CP 6 mg/kg i.p. on days 7 and 14 and ICA 5 mg/kg p.o. daily), and CP + ICA (CP 6 mg/kg i.p. on days 7 and 14 and ICA 10 mg/kg p.o. daily). Our results indicated that ICA effectively improved ovarian reserve as indicated by attenuating CP-induced histolopathological changes and enhancing serum anti-müllerian hormone (AMH). Furthermore, co-administration of ICA with CP showed restoration of the oxidant-anti-oxidant balance in ovarian tissues, evidenced by decreased malondialdehyde (MDA) concentrations and elevated superoxide dismutase (SOD) and catalase (CAT) activities. Also, ICA suppressed ovarian inflammation as evidenced by down-regulation of the expression of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and nuclear factor kappa B (NF-κB). ICA inhibited ovarian apoptosis in CP-treated rats by down-regulation of CASP3 and Bax and up-regulation of Bcl-2 mRNA expression. Further, ICA enhanced PTEN, p-AKT, p-mTOR, and p-AMPKα expression. In conclusion, ICA possesses a protective activity against CP-induced ovarian toxicity in rats by exhibiting antioxidant, antiinflammatory, anti-apoptotic activities and modulating NF-κB expression and PTEN/AKT/mTOR/AMPK axis in ovarian tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adverse effects of cancer chemotherapy on women of reproductive ages (15–44 years) are well-documented (Vo and Kawamura 2021; Hoefgen et al. 2023). The ovaries possess a predetermined number of follicular reserves, which diminish progressively from birth until menopause (Broekmans et al. 2009). Therefore, exposure to chemotherapeutic regimens can negatively influence the depletion rate of these follicular reserves (Spears et al. 2019). In particular, treatments involving platinum complexes, such as cisplatin (CP), alkylating agents, and taxanes, have been identified to cause significant ovarian toxicity (van Dorp et al. 2018; van denBoogaard et al. 2022). Consequently, the likelihood of pregnancy among women post-cancer diagnosis and treatment is 38% lower compared to the general female population (Anderson et al. 2018).
CP, a potent broad-spectrum chemotherapeutic agent, is efficaciously employed against numerous solid tumors and gynecological cancers (Ozols et al. 2003; Jang et al. 2016; Kumar et al. 2017). Once inside the cell, CP binds to DNA bases, disrupting DNA replication and inducing apoptosis in cancer cells (Forgie et al. 2022). Despite its clinical efficacy, CP is associated with a significant risk of ovarian failure (Blumenfeld 2012). Several studies have consistently shown that CP leads to depletion of the ovarian primordial follicle reserve, leading to premature ovarian failure (POF) and infertility (Chang et al. 2015; Jang et al. 2016). The condition of POF is marked by reduced levels of gonadal hormones, notably the anti-müllerian hormone (AMH) (Pouresmaeili and Fazeli 2014; Bedenk et al. 2020). Studies have demonstrated that CP ovarian toxicity involves the induction of oxidative stress (Altuner et al. 2013; Yan et al. 2022), inflammation (Ibrahim et al. 2021; Algandaby 2021), and apoptosis (kaygusuzoglu et al. 2018; Algandaby 2021; Yan et al. 2022; Xing et al. 2022). Further, adverse ovarian effects of CIS were reported to involve modulated phosphorylation and expression of key proteins including PTEN and Akt, eventually lead to loss of primordial follicles, accelerated activation of primordial follicles, follicular atresia, and damage to the vasculature (Spears et al. 2019).
Epimedium brevicornum is is a Chinese medicine that is commonly used for treatment of sexual dysfunction and osteoporosis (Agrawal et al. 2024). Also, it is also a component of modern proprietary traditional Chinese medicine products for the treatment of several ailments including arthritis, amnesia, infertility, and impotence (Ma et al. 2011). The plant belongs to the genus Epimedium that has been reported to contain flavonoids, lignans, ionones, phenol glycosides, phenethyl alcohol glycosides and sesquiterpenes (Ma et al. 2011; Zhuang et al. 2023). Icariin (ICA) was found to be the major constituent of Epimedium brevicornum and responsible for almost every pharmacological activity (Agrawal et al. 2024).. Also, ICA possesses a rich a wide range of applications in traditional medicine (He et al. 2020). It is commonly used as an aphrodisiac agent and for the treatment of erectile dysfunction through its ability to increase blood flow and enhance sexual function (Chen et al. 2014). Beyond its traditional uses, ICA has been extensively reported to offer various pharmacological properties. These include protective effects on the cardiovascular and immune systems and anti-depressant and anti-tumor properties (Li et al. 2015a). Moreover, ICA protects gainst experimentally-induced ovarian failure (Wang et al. 2019; Li et al. 2024) and ovarian insufficiency (Chen et al. 2014). It exhibits potent antioxidant activities, contributing further to its therapeutic profile (El-Shitany and Eid 2019; Xia et al. 2022). Additionally, ICA has been shown to exert significant anti-inflammatory effects by inhibiting nuclear factor kappa B (NF-κB) signaling pathways (Deng et al. 2017; El-Shitany and Eid 2019; Zhang et al. 2021). It also demonstrates anti-apoptotic activities, highlighting its potential for cellular protection (Deng et al. 2017). Thus, the current study aimed to investigates the protective potential of ICA against CP-induced ovarian toxicity in rats as well as its impact on expression of key proteins ovarian injury.
Materials and methods
Chemicals
ICA (> 97% purity) was acquired from Royal Pharm (Hangzhou, China). CP was sourced from EBEWE Pharma G.m.b.H. Nfg. KG (Unterach Am Attersee, Austria). All other chemicals used were of the highest commercial analytical quality.
Animals
Female Wistar rats (12–14-week-old, 200–220 g) were kept in our animal facility for two weeks to acclimate to laboratory conditions. The humidity and temperature in the animal house were maintained at 50% and 22 ± 2 °C, respectively. A 12-h cycle of darkness and light was provided. There were plenty of standard food pellets and drinking water. The study was ethically approved by the Research Ethics Committee, the Faculty of Pharmacy, King Abdulaziz University (PH-1442–53).
Experimental design
A total of 30 female Wistar rats were allocated randomly into five groups (6 rats per group) as follows: Group I (Control) was the control group and received normal saline daily p.o. for 17 days and i.p. on the 7th and 14th days. Group II (ICA 10 mg) received ICA (10 mg/kg/day p.o.) by oral gavage once daily for 17 days. Group III (CP group) was injected by CP only (6 mg/kg/day; i.p.) as a single dose on the 7th and 14th days. Group IV (ICA 5 mg + CP) received ICA (5 mg/kg/day, p.o.) by gavage once daily for 17 days and was injected with CP 6 mg/kg/day i.p. one hour after ICA oral administration on the 7th and 14th days. Group V (ICA 10 mg + CP) received ICA (10 mg/kg/day, p.o.) by gavage once daily for 17 days and was injected with CP 6 mg/kg/day i.p. one hour after ICA oral administration on the 7th and 14th days (Algandaby 2021). The chosen doses were based on a pilot experiment.
At the end of the study, animals were anesthetized using ketamine (80 mg/kg, i.p.), and blood was collected from the retro-orbital plexus and centrifuged for 15 min at 3000 rpm to separate the sera, which were subsequently stored at -80°C for future analyses. Next, animals were sacrificed by decapitation, and the ovaries were carefully removed, cleansed with normal saline, and weighed. The right ovaries were homogenized in phosphate buffered saline (0.1 M, pH 7.4) to yield a 10% w/v homogenate for the biochemical tests. The homogenate was kept at 4 °C for 15 min before being centrifuged at 10,000 rpm, and the supernatant was kept at -80 °C until the assays were performed. Part of the left ovaries were kept in 10% neutral formalin for histological and immunohistochemical examinations. The other part of the left ovary was flash-frozen in liquid nitrogen and kept in RNA later for molecular biomarker investigations.
Determination of the serum levels of AMH and estradiol
According to the vendor's instructions, the serum levels of AMH and Estradiol were measured using an ELISA kit (MBS726534, MyBioSource, USA) and (K4266-100, Biovision, USA), respectively, following the manufacturer's instructions.
Histological assessments
Portions of the left ovaries that were fixed in formalin were dehydrated using ascending concentrations of alcohol. This was followed by paraffinization of the ovarian tissues and sectioning at a thickness of 5 \(\mu\)m. Xylene was used to deparaffinize the sections, after which they were rehydrated and stained with hematoxylin and eosin (H&E). The slides were studied and photographed by light microscopy (Carl Zeiss Axiostar plus, Oberkochen, Germany). A pathologist blindly examined the slides and classified the follicles as primordial, pre-antral, antral, or atretic. Then, the percentage of healthy follicles was calculated as follows (Algandaby 2021):
Assessment of ovarian oxidative stress biomarkers
Oxidative injury markers such as content of malondialdehyde (MDA) (Cat. No. MD 25 29, Biodiagnostic, Giza, Egypt) and activities of the enzymes superoxide dismutase (SOD) (Cat. No. SD 25 21, Biodiagnostic, Giza, Egypt) and catalase (CAT) (Cat. No. CA 25 17, Biodiagnostic, Giza, Egypt) in the homogenates of the ovary were assessed using commercial kits.
Immunohistochemical analysis
Paraffin-embedded blocks were deparaffinized by immersion of the tissue sections twice in 100% xylene for 15 min each time. Graded ethanol was used to complete a rehydration stage. Tissues were rehydrated and then rinsed with phosphate buffered saline (PBS) for 5 min. For antigen retrieval, sections were then incubated in boiling citrate buffer for 5 min. Endogenous peroxidases were quenched by incubation in 3% H2O2 for 10 min. The sections were then exposed to one of the following primary antibodies: TNF-α (Catalog # ab307164, ABCAM), IL-6 (Catalog # ab9324, ABCAM), NF-κB (Catalog # 8242, CST), PTEN (Catalog # ab170941, ABCAM), p-AKT (Catalog # 4060, CST), p-mTOR (Catalog # 2976, CST), and p-AMPKα (Catalog # 2535, CST) for overnight incubation at 4 °C, followed by a 20-min incubation with the secondary antibody at room temperature. Hematoxylin was used to counteract staining caused by peroxidase activity using DAB. Light microscopy was employed to assess the immunostaining. Image J software (1.46a, NIH, Bethesda, MD, USA) was used to quantify the immunostaining.
qRT-PCR analysis
To determine the effect of ICA on CP-induced ovarian apoptosis, qRT-PCR was used to evaluate the mRNA expression levels of CASP3, Bax, and Bcl-2. Direct-zol RNA Miniprep Plus kit (R2072, ZYMO RESEARCH CORP., USA) was used to extract the total RNA from the ovary homogenate. A spectrophotometer was used to determine the total RNA, which was then reverse-transcribed to cDNA using an appropriate kit (12594100, Thermo Fisher Scientific, Waltham, MA, USA). This was followed by amplification using the SYBR Green Master Mix Kit (204143, Qiagen, MD, USA). In the analysis of qRT-PCR data using the 2−ΔΔCt method, β-actin was used as a housekeeping gene. The primers are listed in Table 1 (Wang et al. 2015; Hareeri et al. 2023).
Statistical analysis
GraphPad Prism version 8 (GraphPad, La Jolla, CA, USA) was used for the statistical analysis. Mean ± standard deviation (SD) was used to present the results. One-way ANOVA and the Tukey-post hoc test were used to determine the statistical significance between groups for each biomarker. Significance was taken at P < 0.05.
Results
Assessment of serum estradiol and AMH levels
Serum AMH and estradiol levels were evaluated as indicators of ovarian function to determine the protective effects of ICA against CP-induced ovarian damage. As shown in Fig. 1, administration of ICA (10 mg/kg) alone had no impact on serum AMH levels. Cisplatin (7 mg/kg) injection significantly reduced serum AMH by 49% compared to the control group. Conversely, co-administration of ICA with CP mitigated this effect and enhanced AMH levels by 44 and 52% as compared to CP-alone group. There were no significant differences in serum estradiol levels between all treatment groups.
Effect of ICA administration on serum hormones level in CP-induced ovarian impairment in rats. (A) AMH (ng/ml) level and (B) estradiol (pg/mg) level in different experimental groups. Data are expressed as mean ± SD (n = 6). a, b, c indicate significant differences from control, ICA (10 mg/kg), or CP (7 mg/kg), respectively, at p < 0.05 using one-way ANOVA followed by Tukey post-analysis
Histopathological examination
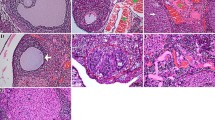
Microscopic examination of ovaries from the control group (Fig. 2A) revealed normal histology of rat ovaries that exhibited numerous corpora lutea with growing follicles in different stages of maturation and a few interstitial tissue cells. Likewise, the ovaries of rats from the ICA alone (10 mg/kg) group (Fig. 2B) were also histologically normal. Regarding the CP (7 mg/kg) treated group (Fig. 2C), the examined sections showed marked histopathological alterations; interstitial cell hyperplasia was noticed, manifested by large polygonal pale vacuolated cells in between growing follicles. Ovarian fibroplasia and fibrous strands in the ovarian tissue were also seen. Moderate improvement was noticed in the CP + ICA (5 mg/kg) co-treated group (Fig. 2D), as the examined sections exhibited mild and limited interstitial cell hyperplasia. The best protective action was detected in the CP + ICA (10 mg/kg) co-treated group (Fig. 2E), as all examined sections showed normal architecture.
Representative photomicrographs of ovarian sections showing the effect of ICA administration on CP-induced ovarian impairment in rats. The ovarian sections stained with hematoxylin and eosin (H&E) are shown from the following groups: (A) The control group has a normal architecture of the ovaries. (B) ICA 10 mg/kg group with no observable histological changes. (C) CP 7 mg/kg group showing inflammation in the ovarian follicles. Arrows point to marked interstitial cells hyperplasia as cords and clusters of vacuolated cells occupying the ovarian tissue with increasing numbers of atretic follicles. (D) CP group co-treated with ICA 5 mg/kg showing almost normal follicles and corpora lutea with mild interstitial cells hyperplasia. (E) CP group co-treated with ICA 10 mg/kg showing healthier development. (F) Effect of ICA on the percentage of healthy follicles in CP-induced ovarian impairment in rats. Data are expressed as mean ± SD (n = 6). a, b, c, d indicate significant differences from control, ICA (10 mg/kg), CP (7 mg/kg), or CP + ICA (5 mg/kg), respectively, at p < 0.05 using one-way ANOVA followed by Tukey post-analysis
Figure 2F demonstrates the effects of the four treatments on the ovarian tissues of rats as a % of healthy follicles. The combined administration of CP and ICA helped in retaining the health of the follicles. The group that was administered ICA (10 mg/kg) did not show a change in the percentage of healthy follicles compared with the control group. Cisplatin (7 mg/kg) reduced the follicle count by 52% compared to the control group. In contrast, the groups treated with CP + ICA (5 mg/kg) and CP + ICA (10 mg/kg) had significantly more healthy follicles (52% and 80%, respectively) than the CP group.
Assessment of oxidative stress biomarkers
To assess the effect of ICA on CP-induced ovarian oxidative damage, the lipid peroxidation biomarker MDA, along with antioxidant enzymes SOD and CAT, were measured in ovarian tissues using ELISA kits. Table 2 demonstrates that ICA treatment (10 mg/kg) did not significantly alter oxidative stress markers. However, the ovarian MDA content was significantly increased by 55%, while the levels of ovarian SOD and CAT were markedly reduced by 48% and 47% respectively as compared to control values following CP administration (7 mg/kg). Notably, the oxidant/antioxidant balance in the ovaries of CP-intoxicated rats was effectively restored by ICA co-treatment (5 and 10 mg/kg), as shown by a significant decrease in MDA levels (approximately 27% and 38% respectively) as compared to CP-alone group. This was paralled by enhancements of of SOD (37% & 63%) and CAT (43% & 65%) activities in the ovarian tissues by ICA (5 or 10 mg/kg, respectively) as compared to CP-alone values.
Immunohistochemical determination of IL-6, TNF-α and NFκB (p65)
The anti-inflammatory effects of ICA against CP-induced ovarian inflammation were investigated. Figure 3 demonstrates that the control group displayed only slight immunopositivity for IL-6, TNF-α, and NF-κB. The expression levels of these inflammatory markers were unchanged when rats were treated with ICA alone (10 mg/kg). However, CP injection (7 mg/kg) significantly increased the expression of inflammatory markers IL-6, TNF-α, and NF-κB (in the evaluated nuclei), highlighted by strong brown staining. Quantification of optical densities showed significant increases in IL-6, TNF-α, and NF-κB (p65) expression by 132%, 135%, and 118%, respectively, compared to respective control values. Co-administration of ICA (5 mg/kg) to CP treatment significantly reduced the immunostaining intensity for IL-6, TNF-α, and NF-κB (p65), which dropped by 35%, 31% and 25%, respectively, compared to the CP group. Increasing the ICA dose to 10 mg/kg further decreased the immunostaining for IL-6, TNF-α, and NF-κB (p65), which declined by 50%, 47%, and 36%, respectively, compared to the CP group.
Effect of ICA administration on the expression of inflammatory markers in ovarian sections. This figure presents photomicrographs of IL-6 (upper panel), TNF-α (middle panel), and NF-κB (lower panel) immune reactions in different experimental groups. Bar charts represent the semi-quantification of the expression of corresponding proteins. Data are expressed as mean ± SD (n = 6). a, b, c, d indicate significant differences from control, ICA (10 mg/kg), CP (7 mg/kg), or CP + ICA (5 mg/kg), respectively, at p < 0.05 using one-way ANOVA followed by Tukey post-analysis
Assessment of mRNA expression of Bax, Bcl-2 and CASP3
To assess the effect of ICA on apoptosis, the mRNA expression levels of the apoptotic markers Bax, Bcl-2, and CASP3 were evaluated by RT-PCR in ovarian tissues (Fig. 4). Treating rats with ICA alone (10 mg/kg) did not affect the expression level of these apoptosis markers relative to the control. Conversely, the administration of CP (7 mg/kg) resulted in an increased mRNA expression of Bax (a pro-apoptotic protein) by 280% in comparison to the control (Fig. 4A). Interestingly, co-treatment of the CP group with ICA at doses of 5 and 10 mg/kg significantly reduced this increase in Bax mRNA expression by 42% and 66%, respectively, compared to the CP group. Moreover, the CP (7 mg/kg) group exhibited a significant decrease in the mRNA expression level of Bcl-2 (an anti-apoptotic protein) by 57% compared to the control (Fig. 4B). In contrast, ICA co-administration at 5 and 10 mg/kg doses markedly enhanced the Bcl-2 mRNA expression by 69% and 81%, respectively, compared to the CP group.
Effect of ICA administration on mRNA expression of apoptotic markers in CP-induced ovarian impairment in rats. (A) Bax, (B) Bcl-2, and (C) CASP3 in different experimental groups. Data are expressed as mean ± SD (n = 6). a, b, c, d indicate significant differences from control, ICA (10 mg/kg), CP (7 mg/kg), or CP + ICA (5 mg/kg), respectively, at p < 0.05 using one-way ANOVA followed by Tukey post-analysis
Additionally, CP (7 mg/kg) injection led to a significant elevation in CASP3 mRNA expression by 176% relative to the control (Fig. 4C). As expected, co-administration with ICA at 5 and 10 mg/kg provided considerable protection against this increase by 34% and 39%, respectively, compared to the CP group.
Immunohistochemical determination of PTEN, AKT, mTOR and AMPK
Figure 5 demonstrates no notable difference between the ICA and the control groups. However, rats that received CP (7 mg/kg) exhibited a significant decrease in immunostaining for PTEN, p-AKT, p-mTOR, and p-AMPKα with reductions of 60%, 60%, 57%, and 58%, respectively, when compared to the control group. When ICA (5 mg/kg) was co-administered, there was a marked increase in the levels of PTEN, p-AKT, p-mTOR, and p-AMPKα increasing by 68%, 34%, 26%, and 37%, respectively, in comparison to the CP treated group. Importantly, using a higher dose of ICA (10 mg/kg) with CP led to even more significant enhancements in PTEN, p-AKT, p-mTOR, and p-AMPKα expression by 113%, 95%, 84%, and 68%, respectively, compared to the CP treated group.
Effect of ICA administration on ovarian PTEN/Akt/mTOR/AMPK signaling pathway in CP-induced ovarian impairment in rats. Bar charts represent the semi-quantification of the expression of corresponding proteins. Data are expressed as mean ± SD (n = 6). a, b, c, d indicate significant differences from control, ICA (10 mg/kg), CP (7 mg/kg), or CP + ICA (5 mg/kg), respectively, at p < 0.05 using one-way ANOVA followed by Tukey post-analysis
Discussion
The advent of chemotherapeutic agents like CP has markedly improved cancer survival rates. However, the resultant off-target effects, particularly ovarian toxicity, pose significant challenges, especially for female patients of reproductive age (Chow et al. 2016). This has driven a search for mitigating strategies and increased interest in phytochemicals known for their extensive therapeutic potential and minimal side effects. Our study focused on ICA, a flavonoid known for its multifaceted pharmacological activities, including anti-oxidative and anti-inflammatory effects (Li et al. 2010; He et al. 2020; Seyedi et al. 2023). Here, we demonstrated ICA's effectiveness in alleviating CP-induced ovarian damage by reducing histological damage and improving ovarian function markers such as AMH while modulating key signaling pathways involved in cellular survival and inflammation.
In evaluating the protective effects of ICA against CP-induced ovarian toxicity, this study assessed biochemical markers and histological changes in ovarian tissues. It was observed that serum AMH levels, indicative of the quantity of primordial ovarian follicles, were significantly declined following CP administration (Kevenaar et al. 2006). ICA treatment restored serum AMH levels to almost normal values, highlighting its capacity to shield against CP-induced ovarian damage—a finding supported by earlier reports (Li et al. 2015b; Jiang et al. 2019). Despite the observed changes in AMH levels, serum estradiol levels remained stable across all groups. This suggests that the protective mechanism of ICA may specifically target the preservation of ovarian reserve without altering the overall hormonal balance. Histological analysis revealed substantial ovarian damage in response to CP exposure, notably a reduced count of healthy primordial ovarian follicles. This observation aligns with previous findings indicating CP's impact on ovarian primordial follicle numbers (Chang et al. 2015). However, our data revealed that ICA treatment effectively mitigated these histopathological changes. The administration of ICA, particularly at higher doses, significantly preserved the count of healthy follicles in a dose-dependent manner. These findings are in line with previous studies marking the ability of ICA to protect against experimentally-induced ovarian injury induced by D-galactose (Li et al. 2019) and CP (Li et al. 2024).
Oxidative stress is a critical factor in CP-induced toxicities, substantially impairing the antioxidant defense system and raising the production of ROS, which in turn triggers apoptosis through increased release of pro-apoptotic factors (Santos et al. 2007; Hassanein et al. 2022; Ali et al. 2023). Specifically, CP has been shown to elevate MDA levels while decreasing the activities of critical free radical scavenging enzymes such as SOD and CAT in ovarian tissues, confirming its role in ovarian toxicities in rat models (Altuner et al. 2013). This insight builds on our prior findings, where ICA was shown to mitigate carrageenan-induced acute inflammation by enhancing both enzymatic and non-enzymatic antioxidants (El-Shitany and Eid 2019). Additionally, previous reports emphasize ICA's effectiveness in countering CP-induced oxidative stress and cell damage (Zhou et al. 2019). ICA has been shown to explicitly attenuate oxidative stress by modulating antioxidants such as glutathione peroxidase (GSH-Px), CAT, and SOD and lowering MDA levels (Yoon et al. 2021), thereby diminishing the detrimental effects of CP (Zhou et al. 2019; Xia et al. 2022). Our current study extends these observations by confirming ICA's capacity to alleviate oxidative damage, as evidenced by its impact on enhancing antioxidant enzyme activities (SOD and CAT) and reducing MDA levels in the ovaries of CP-treated rats.
CP-induced release of oxidative stress has been linked to inflammatory pathways, notably activating NF-κB, which is implicated in organ toxicities (Abdel-Wahab et al. 2021; Ali et al. 2021; Sami et al. 2022; Ramadan et al. 2023). In line with these findings, a previous study showed that CP injection elevated expression of pro-inflammatory cytokines like IL-6, IL-1β, and TNF-α in ovarian tissues (Algandaby 2021). Interestingly, our findings showed that ICA effectively reduces NF-κB expression along with diminishing the expression of IL-6 and TNF-α in ovarian tissues in a dose-related fashion. This suggests ICA's effectiveness in reducing the pro-inflammatory impacts triggered by CP. Such findings align with prior research recognizing ICA's anti-inflammatory effects, further establishing its role as an effective countermeasure to CP-induced inflammatory harm (Deng et al. 2017; El-Shitany and Eid 2019; Zhang et al. 2021).
This study explored ICA’s critical function in protecting ovarian tissues from CP-induced apoptosis, specifically through its interactions with the Bcl-2 family of proteins essential for regulating apoptosis. This family includes pro-apoptotic proteins such as Bax, which initiate apoptosis via caspase activation, and anti-apoptotic proteins like Bcl-2, which block apoptosis by preventing Bax translocation to mitochondria (Deng et al. 2017). Our findings revealed that CP significantly altered the critical balance within ovarian tissues, enhancing Bax and decreasing Bcl-2 mRNA expression, amplifying apoptotic activities. This highlights the complex regulation of apoptosis essential for cellular health, reflecting similar outcomes observed in previous research (Hengartner 2000; Cory and Adams 2002; Deng et al. 2017). Further, a rise in CASP3 mRNA expression was observed following CP insult, confirming apoptosis progression. However, these observations were significantly mitigated by ICA treatment. This aligns with previous studies, reinforcing ICA's anti-apoptotic efficacy (Shao et al. 2022; Wu et al. 2023). The ability of ICA to reverse CP-induced changes in gene expression related to apoptosis suggests a complex mechanism of action.
PTEN/AKT/mTOR/AMPK signaling pathway plays a vital role in cellular defenses against chemotherapy's oxidative and inflammatory impacts, influencing crucial cellular processes such as proliferation, survival, and metabolism (Zhou et al. 2017). Previous studies highlighted CP’s detrimental effects on this pathway, mainly showing that a significant reduction in the mRNA expression levels of AKT, mTOR, and PTEN in ovarian tissues, contributes to ovarian toxicity (Al-Shahat et al. 2022). Our immunohistochemical analysis corroborates these findings, revealing a substantial decrease in the immunostaining of PTEN, p-AKT, p-mTOR, and p-AMPKα in CP-treated rats. This disruption in signaling pathways is likely pivotal in reducing ovarian primordial follicles and the subsequent onset of premature ovarian failure (Chang et al. 2015). In contrast, the co-administration of ICA with CP effectively counteracted the negative impacts of CP, showing a dose-dependent upregulation of PTEN, p-AKT, p-mTOR, and p-AMPKα. Data on ICA's impact on PTEN expression are controversial (Zou et al. 2020; Al-Shahat et al. 2022). Notably, ICA has been shown to enhance the expression of the Akt/mTOR signaling pathway and reduce autophagy and apoptosis in cisplatin-resistant ovarian cancer cells (Jiang et al.2019). Additionally, ICA influences the proliferation and apoptosis of human ovarian cancer cells by increasing the expression of targeting proteins such as PTEN and Bcl-2 (Li et al. 2015b). These findings suggest that ICA's modulation of this signaling pathway could be a promising strategy for protecting against CP-induced ovarian toxicity, highlighting its potential therapeutic benefits in enhancing the efficacy and safety of chemotherapy treatments.
Conclusion
In conclusion, our study highlights the potential of ICA to mitigate the adverse effects of CP on ovarian tissues, demonstrating its potential as a viable adjunct therapy in chemotherapy. Administration of ICA was associated with attenuation of the histopathological changes, enhancing serum AMH levels, and restoring the oxidant-anti-oxidant balance in ovarian tissues. Furthermore, ICA effectively exhibited anti-inflammatory and anti-apoptotic activities. Additionally, ICA enhanced PTEN, p-AKT, p-mTOR, and p-AMPKα expression. These multifaceted protective effects justify further substantiation of ICA's efficacy in preserving ovarian reserve in CP-treated patients.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abdel-Wahab BA, Walbi IA, Albarqi HA et al (2021) Roflumilast protects from cisplatin-induced testicular toxicity in male rats and enhances its cytotoxicity in prostate cancer cell line. Role of NF-κB-p65, cAMP/PKA and Nrf2/HO-1, NQO1 signaling. Food Chem Toxicol 151:112133. https://doi.org/10.1016/j.fct.2021.112133
Agrawal N, Goyal D, Bansal D (2024) A Comprehensive review on chemistry and contribution of Chinese herb epimedium brevicornum maxim in medicine. Curr Tradit Med 10:102–114. https://doi.org/10.2174/2215083810666230607151656
Algandaby MM (2021) Quercetin attenuates cisplatin-induced ovarian toxicity in rats: emphasis on anti-oxidant, anti-inflammatory and anti-apoptotic activities. Arab J Chem 14:103191. https://doi.org/10.1016/j.arabjc.2021.103191
Ali FEM, Hassanein EHM, Abd El-Ghafar OAM et al (2023) Exploring the cardioprotective effects of canagliflozin against cisplatin-induced cardiotoxicity: role of iNOS/NF-κB, Nrf2, and Bax/cytochrome C/Bcl-2 signals. J Biochem & Molecular Tox 37:e23309. https://doi.org/10.1002/jbt.23309
Ali FEM, Hassanein EHM, El-Bahrawy AH et al (2021) Nephroprotective effect of umbelliferone against cisplatin-induced kidney damage is mediated by regulation of NRF2, cytoglobin, SIRT1/FOXO-3, and NF- k B-p65 signaling pathways. J Biochem & Molecular Tox 35:e22738. https://doi.org/10.1002/jbt.22738
Al-Shahat A, Hulail MAE, Soliman NMM et al (2022) Melatonin mitigates cisplatin-induced ovarian dysfunction via altering steroidogenesis, inflammation, apoptosis, oxidative stress, and PTEN/PI3K/Akt/mTOR/AMPK signaling pathway in female rats. Pharmaceutics 14:2769. https://doi.org/10.3390/pharmaceutics14122769
Altuner D, Gulaboglu M, Yapca OE, Cetin N (2013) The Effect of mirtazapine on cisplatin-induced oxidative damage and infertility in rat ovaries. Sci World J 2013:1–6. https://doi.org/10.1155/2013/327240
Anderson RA, Brewster DH, Wood R et al (2018) The impact of cancer on subsequent chance of pregnancy: a population-based analysis. Hum Reprod 33:1281–1290. https://doi.org/10.1093/humrep/dey216
Bedenk J, Vrtačnik-Bokal E, Virant-Klun I (2020) The role of anti-Müllerian hormone (AMH) in ovarian disease and infertility. J Assist Reprod Genet 37:89–100. https://doi.org/10.1007/s10815-019-01622-7
Blumenfeld Z (2012) Chemotherapy and fertility. Best Pract Res Clin Obstet Gynaecol 26:379–390. https://doi.org/10.1016/j.bpobgyn.2011.11.008
Broekmans FJ, Soules MR, Fauser BC (2009) Ovarian aging: mechanisms and clinical consequences. Endocr Rev 30:465–493. https://doi.org/10.1210/er.2009-0006
Chang EM, Lim E, Yoon S et al (2015) Cisplatin induces overactivation of the dormant primordial follicle through PTEN/AKT/FOXO3a pathway which leads to loss of ovarian reserve in mice. PLoS ONE 10:e0144245. https://doi.org/10.1371/journal.pone.0144245
Chen M, Hao J, Yang Q, Li G (2014) Effects of icariin on reproductive functions in male rats. Molecules 19:9502–9514. https://doi.org/10.3390/molecules19079502
Chow EJ, Stratton KL, Leisenring WM et al (2016) Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: a report from the childhood cancer survivor study cohort. Lancet Oncol 17:567–576. https://doi.org/10.1016/S1470-2045(16)00086-3
Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–656. https://doi.org/10.1038/nrc883
Deng X, Wu W, Liang H et al (2017) Icariin prevents IL-1β-induced apoptosis in human nucleus pulposus via the PI3K/AKT pathway. Evid Based Complement Alternat Med 2017:2198323. https://doi.org/10.1155/2017/2198323
El-Shitany NA, Eid BG (2019) Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed Pharmacother 120:109567. https://doi.org/10.1016/j.biopha.2019.109567
Forgie BN, Prakash R, Telleria CM (2022) Revisiting the anti-cancer toxicity of clinically approved platinating derivatives. Int J Mol Sci 23:15410. https://doi.org/10.3390/ijms232315410
Hareeri RH, Alam AM, Bagher AM et al (2023) Protective effects of 2-methoxyestradiol on acute isoproterenol-induced cardiac injury in rats. Saudi Pharm J 31:101787. https://doi.org/10.1016/j.jsps.2023.101787
Hassanein EHM, Sayed GA, Alzoghaibi AM et al (2022) Azithromycin mitigates cisplatin-induced lung oxidative stress, inflammation and necroptosis by upregulating SIRT1, PPARγ, and Nrf2/HO-1 signaling. Pharmaceuticals 16:52. https://doi.org/10.3390/ph16010052
He C, Wang Z, Shi J (2020) Pharmacological effects of icariin. In: : Du G (ed) Advances in pharmacology. Academic Press, pp 179–203
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407:770–776. https://doi.org/10.1038/35037710
Hoefgen HR, Benoit J, Chan S et al (2023) Female reproductive health in pediatric, adolescent, and young adult cancer survivors. Pediatr Blood Cancer 70:e29170. https://doi.org/10.1002/pbc.29170
Ibrahim MA, Albahlol IA, Wani FA et al (2021) Resveratrol protects against cisplatin-induced ovarian and uterine toxicity in female rats by attenuating oxidative stress, inflammation and apoptosis. Chem Biol Interact 338:109402. https://doi.org/10.1016/j.cbi.2021.109402
Jang H, Lee O-H, Lee Y et al (2016) Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J Pineal Res 60:336–347. https://doi.org/10.1111/jpi.12316
Jiang S, Chang H, Deng S, Fan D (2019) Icariin enhances the chemosensitivity of cisplatin-resistant ovarian cancer cells by suppressing autophagy via activation of the AKT/mTOR/ATG5 pathway. Int J Oncol 54:1933–1942. https://doi.org/10.3892/ijo.2019.4785
Kaygusuzoglu E, Caglayan C, Kandemir FM et al (2018) Zingerone ameliorates cisplatin-induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female wistar rats. Biomed Pharmacother 102:517–530. https://doi.org/10.1016/j.biopha.2018.03.119
Kevenaar ME, Meerasahib MF, Kramer P et al (2006) Serum anti-müllerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 147:3228–3234. https://doi.org/10.1210/en.2005-1588
Kumar P, Barua CC, Sulakhiya K, Sharma RK (2017) Curcumin ameliorates cisplatin-induced nephrotoxicity and potentiates its anticancer activity in SD rats: potential role of curcumin in breast cancer chemotherapy. Front Pharmacol 8:132
Li C, Li Q, Mei Q, Lu T (2015a) Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci 126:57–68. https://doi.org/10.1016/j.lfs.2015.01.006
Li F, Zhu F, Wang S et al (2024) Icariin alleviates cisplatin-induced premature ovarian failure by inhibiting ferroptosis through activation of the Nrf2/ARE pathway. Sci Rep 14:17318. https://doi.org/10.1038/s41598-024-67557-x
Li J, Jiang K, Zhao F (2015b) Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol Rep 33:2829–2836. https://doi.org/10.3892/or.2015.3891
Li N, Wang J, Wang X et al (2019) Icariin exerts a protective effect against d-galactose induced premature ovarian failure via promoting DNA damage repair. Biomed Pharmacother 118:109218. https://doi.org/10.1016/j.biopha.2019.109218
Li S, Dong P, Wang J et al (2010) Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Lett 298:222–230. https://doi.org/10.1016/j.canlet.2010.07.009
Ma H, He X, Yang Y et al (2011) The genus Epimedium: an ethnopharmacological and phytochemical review. J Ethnopharmacol 134:519–541. https://doi.org/10.1016/j.jep.2011.01.001
Ozols RF, Bundy BN, Greer BE et al (2003) Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a gynecologic oncology group study. J Clin Oncol 21:3194–3200. https://doi.org/10.1200/JCO.2003.02.153
Pouresmaeili F, Fazeli Z (2014) Premature ovarian failure: a critical condition in the reproductive potential with various genetic causes. Int J Fertil Steril 8:1–12
Ramadan SA, Kamel EM, Ewais MA et al (2023) Flavonoids of Haloxylon salicornicum (Rimth) prevent cisplatin-induced acute kidney injury by modulating oxidative stress, inflammation, Nrf2, and SIRT1. Environ Sci Pollut Res 30:49197–49214. https://doi.org/10.1007/s11356-023-25694-2
Sami DH, Soliman AS, Khowailed AA et al (2022) 7-hydroxycoumarin modulates Nrf2/HO-1 and microRNA-34a/SIRT1 signaling and prevents cisplatin-induced oxidative stress, inflammation, and kidney injury in rats. Life Sci 310:121104. https://doi.org/10.1016/j.lfs.2022.121104
Santos NAG, Catão CS, Martins NM et al (2007) Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol 81:495–504. https://doi.org/10.1007/s00204-006-0173-2
Seyedi Z, Amiri MS, Mohammadzadeh V et al (2023) Icariin: a promising natural product in biomedicine and tissue engineering. JFB 14:44. https://doi.org/10.3390/jfb14010044
Shao Y, Sun L, Yang G et al (2022) Icariin protects vertebral endplate chondrocytes against apoptosis and degeneration via activating Nrf-2/HO-1 pathway. Front Pharmacol 13:937502. https://doi.org/10.3389/fphar.2022.937502
Spears N, Lopes F, Stefansdottir A et al (2019) Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 25:673–693. https://doi.org/10.1093/humupd/dmz027
van den Boogaard WMC, Komninos DSJ, Vermeij WP (2022) Chemotherapy side-effects: not all DNA damage is equal. Cancers 14:627. https://doi.org/10.3390/cancers14030627
van Dorp W, Haupt R, Anderson RA et al (2018) Reproductive function and outcomes in female survivors of childhood, adolescent, and young adult cancer: a review. J Clin Oncol 36:2169–2180. https://doi.org/10.1200/JCO.2017.76.3441
Vo KCT, Kawamura K (2021) Female oncofertility: current understandings, therapeutic approaches, controversies, and future perspectives. J Clin Med 10:5690. https://doi.org/10.3390/jcm10235690
Wang J-L, Liu B, Zhang C et al (2019) Effects of icariin on ovarian function in d-galactose-induced aging mice. Theriogenology 125:157–167. https://doi.org/10.1016/j.theriogenology.2018.10.028
Wang LI, Liu F, Luo Y et al (2015) Effect of acute heat stress on adrenocorticotropic hormone, cortisol, interleukin-2, interleukin-12 and apoptosis gene expression in rats. Biomed Rep 3:425–429. https://doi.org/10.3892/br.2015.445
Wu X, Zhang X, Sun L et al (2023) Icariin prevents depression-like behaviors in chronic unpredictable mild stress-induced rats through Bax/cytoplasm C/caspase-3 axis to alleviate neuronal apoptosis. Cell Mol Biol (Noisy-le-grand) 69:196–204. https://doi.org/10.14715/cmb/2023.69.7.32
Xia J, Hu J, Zhang R et al (2022) Icariin exhibits protective effects on cisplatin-induced cardiotoxicity via ROS-mediated oxidative stress injury in vivo and in vitro. Phytomedicine 104:154331. https://doi.org/10.1016/j.phymed.2022.154331
Xing F, Wang M, Ding Z et al (2022) Protective effect and mechanism of melatonin on cisplatin-induced ovarian damage in mice. J Clin Med 11:7383. https://doi.org/10.3390/jcm11247383
Yan F, Zhao Q, Li Y et al (2022) The role of oxidative stress in ovarian aging: a review. J Ovarian Res 15:100. https://doi.org/10.1186/s13048-022-01032-x
Yoon J-W, Lee S-E, Park Y-G et al (2021) The antioxidant icariin protects porcine oocytes from age-related damage in vitro. Anim Biosci 34:546–557. https://doi.org/10.5713/ajas.20.0046
Zhang H, Zhuo S, School of Medicine & Holistic Integrative Medicine, Nanjing University of Chinese Medicine, Nanjing, China et al (2021) Icariin inhibits intestinal inflammation of DSS-induced colitis mice through modulating intestinal flora abundance and modulating p-p65/p65 molecule. Turk J Gastroenterol 32:382–392. https://doi.org/10.5152/tjg.2021.20282
Zhou L, Xie Y, Li S et al (2017) Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J Ovarian Res 10:56. https://doi.org/10.1186/s13048-017-0350-3
Zhou Y, Hou J, Yang G et al (2019) Icariin ameliorates cisplatin-induced cytotoxicity in human embryonic kidney 293 cells by suppressing ROS-mediated PI3K/Akt pathway. Biomed Pharmacother 109:2309–2317. https://doi.org/10.1016/j.biopha.2018.11.108
Zhuang W, Sun N, Gu C et al (2023) A literature review on Epimedium, a medicinal plant with promising slow aging properties. Heliyon 9:e21226. https://doi.org/10.1016/j.heliyon.2023.e21226
Zou X, Feng X, Fu Y et al (2020) Icariin attenuates amyloid-β (Aβ)-induced neuronal insulin resistance through PTEN downregulation. Front Pharmacol 11:880. https://doi.org/10.3389/fphar.2020.00880
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, under grant no. (IFPHI-278–166-2020). The authors, therefore, acknowledge with thanks DSR's technical and financial support.
Author information
Authors and Affiliations
Contributions
B.E.: Project administration, Data curation, Methodology, Formal analysis, Funding acquisition. L.B.: Formal analysis, Data curation, Methodology. R.S.: Methodology, Investigation, Validation. A.B.: Data analysis, Investigation, Methodology, Software. A.S.: Data curation, Investigation, Methodology. A.A.: Funding acquisition, Methodology, Investigation. All authors reviewed and revised the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study received ethical approval from the Research Ethics Committee, Faculty of Pharmacy, King Abdulaziz University (PH-1442–53).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eid, B.G., Binmahfouz, L.S., Shaik, R.A. et al. Icariin inhibits cisplatin-induced ovarian toxicity via modulating NF-κB and PTEN/AKT/mTOR/AMPK axis. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03395-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03395-y