Abstract
Epidemiological studies have reported the relationship between vacuolating cytotoxin A (vacA) s-/m- region genotypes and duodenal ulcer (DU), but the results remained inconclusive. We performed the present meta-analysis to investigate a more authentic association between vacA s-/m- region genotypes and DU. Literature search was performed by searching Embase, PubMed and ISI Web of Science databases as well as checking references from identified articles, reviews and the abstracts presented at related scientific societies meetings. The association was assessed by combined odds ratio (OR) with 95 % confidence interval (CI). A total of 42 studies were included in our final meta-analysis. The combined ORs (95 % CIs) showed that vacA s1 (OR = 2.96, 95 % CI = 2.34–3.75), m1 (OR = 1.46, 95 % CI = 1.05–2.04) and s1m1 (OR = 1.89, 95 % CI = 1.47–2.42) were associated with increased DU risk significantly in the overall studied population. Subgroup analyses by ethnicity showed that vacA s1 increased the risk of DU in Asian countries (OR = 1.92, 95 % CI = 1.30–2.83), European countries (OR = 3.58, 95 % CI = 2.13–6.03) and Latin American countries (OR = 4.20, 95 % CI = 2.21–7.98); vacA m1 increased the risk of DU in Latin American countries (OR = 2.98, 95 % CI = 1.59–5.56); vacA s1m1 increased the risk of DU in Asian countries (OR = 2.04, 95 % CI = 1.12–3.73) and Latin American countries (OR = 2.05, 95 % CI = 1.20–3.48); vacA s2m1 increased the risk of DU in Latin American countries (OR = 2.30, 95 % CI = 1.17–4.50). The data suggest that genotype testing of vacA s- and m- region will be useful in screening susceptible individuals for DU development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Duodenal ulcer (DU) is one type of peptic ulcer in duodenum, which may result from infection of Helicobacter pylori (H. pylori) bacteria, overuse of alcohol and medications (aspirin and non-steroidal anti-inflammatory drugs) [1, 2]. Among these etiological factors, H. pylori infection was the most common one [3], which could lead to increased gastric acid, degradation of mucus barrier and eventual ulceration. However, it was reported that >20 % of patients with H. pylori infection would develop DU [4].
The reason why the H. pylori-induced pathologies are different is unclear. The vacuolating cytotoxin A (vacA), one of major virulence factors, has been reported to be involved. The vacA protein was encoded by the polymorphic H. pylori vacA gene. It is a secreted cytotoxin being capable to form vacuoles in gastric epithelial cells [5]. The vacA gene is polymorphic, distributed in three principal regions: the signal (s), intermediate (i) and middle (m) regions. Each type can be further divided into two main subtypes, numbered 1 and 2 [6, 7]. The two subtypes of the s-region (s1 and s2) and m-region (m1 and m2) were mostly studied. Different s or m genotypes of H. pylori varied in the vacuolating activities, which might contribute to different clinical outcomes [6]. It was reported that vacA s1 genotype produced a large amount of cytotoxin, whereas s2 secreted few or no cytotoxin at all. In addition, both s1m1 and s1m2 subtypes were able to produce high or moderate levels of vacA, whereas s2m2 subtype was not [6]. Until now, numerous studies have reported the association between DU and vacA gene s- and m- region genotypes [8–50], but the conclusions were inconsistent. In addition, the relatively small sample size in each single published study may limit the credibility of the conclusions. Therefore, we designed this meta-analysis to evaluate the association between vacA s-/m-region genotypes and DU risk.
Materials and methods
Literature search
The meta-analysis followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) criteria [51]. We collected all published studies on humans up to Aug. 15, 2013 by systematically searching Embase (1966 to Aug. 2013), PubMed (up to Aug. 2013) and ISI Web of Science (2003 to Aug. 2013) with search strategy: “duodenal ulcer” AND “vacA OR vacuolating cytotoxin A” AND “variation OR variant OR mutation OR polymorphism OR genotype”. The language was limited to English and Chinese. In addition, references from identified articles, reviews and the abstracts presented at related scientific societies meetings were also checking.
Inclusion criteria
The inclusion criteria were as follows: (1) studies of the association between vacA s- or m-region genotypes and DU; (2) studies being published; (3) studies with case–control design; (4) studies with sufficient data for estimating odds ratio (OR) and 95 % confidence interval (CI). Two investigators (Zhang BB and Yang B) screened the title, abstract and full text of each study to determine inclusion independently. If they could not reach a consensus, a third author (Li Y) was consulted.
Data extraction
The following data from the included studies were carefully extracted by the same two authors (Zhang BB and Yang B) independently: name of first author; publication year; country; total numbers, gender ratios and age of cases and controls; frequency of vacA s- or m-regions genotypes in cases and controls. Patients infected with strains of multiple vacA genotypes or undetected vacA genotypes were excluded from the meta-analysis.
Quality score assessment
The quality assessments of all the included studies were performed by the same two authors (Zhang BB and Yang B) independently using the Newcastle–Ottawa Scale (NOS) [52]. Disagreement was settled as described above. The NOS ranges from 0 (worst) to 9 points (best). Studies ≥7 points were assessed to be of high quality.
Statistical analysis
All the statistical analyses were performed using Stata 11.0. Pooled ORs with 95 % CIs were used to calculate the effects of vacA s- and m- region genotypes on the risk of DU respectively. Between-study heterogeneity was assessed by the I2 statistic and Q-test [53, 54]. When there was significant between-study heterogeneity (P < 0.10 and I2 > 50 %), the random effects model was used [55]. Otherwise, the fixed effects model was used [56]. Kappa statistic was used to evaluate the strength of agreement between reviewers regarding study selection. Subgroup analyses were performed according to ethnicity (Asia, Europe and Latin America). Galbraith plot was created to assess the source of heterogeneity graphically. Both Egger’s test and Begg’s funnel plot were applied to analyze publication bias [57].
Results
Study characteristics
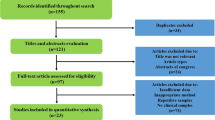
The study selection process is detailed in Fig. 1. There were 483 potentially relevant articles after the searching. Based on the titles and abstracts, we included 61 studies for full-text assessment with a Kappa value of 0.87. After full-text assessment, a total of 43 studies were included in qualitative synthesis. As a same population was studied in two studies [32, 33], we included the more comprehensive one [32]. Hence, 42 articles [8–32, 34–50] were finally included in the meta-analysis with a Kappa value of 0.85. The main characteristics of the studies included are shown in Table 1. The NOS results displayed that the average score was 5.69 (range 5–8). Studied countries included Cuba (one study), Bahrain (one study), Italy (one study), USA (one study), Poland (one study), France (one study), Netherlands (one study), Lithuania (one study), Thailand (one study), Iran (two studies), Korea (two studies), Japan (two studies), UK (two studies), Pakistan (two studies), India (three studies), Germany (four studies), Brazil (five studies), Turkey (five studies) and China (six studies). Six studies were from Latin America, 12 from Europe and 24 from Asia. One of the 42 articles was written in Chinese and the others in English.
Meta-analysis
There were 34 studies reporting the association between vacA s-region genotype and DU. The prevalence of vacA s1 was 90.9 % (1251/1376) in DU patients and 77.8 % (1190/1529) in controls. The combined OR and 95 %CI showed that vacA s1 increased the risk of DU significantly (OR = 2.96, 95 %CI = 2.34–3.75, Fig. 2). The prevalence of vacA s1 was 92.4 % (611/661) in DU patients and 87.1 % (676/776) in controls in Asian countries; 87.7 % (121/138) in DU patients and 63.3 % (88/139) in controls in European countries; 89.9 % (519/577) in DU patients and 69.4 % (426/614) in controls in Latin America countries. The combined ORs and 95 %CIs showed that vacA s1 increased the risk of DU in Asian countries (OR = 1.92, 95 %CI = 1.30–2.83), European countries (OR = 3.58, 95 %CI = 2.13–6.03) and Latin American countries (OR = 4.20, 95 %CI = 2.21–7.98).
There were 26 studies reporting the association between vacA m-region genotype and DU. The prevalence of vacA m1 is 48.8 % (503/1031) in DU patients and 41.7 % (458/1099) in controls. The combined OR and 95 %CI showed that vacA m1 increased the risk of DU significantly (OR = 1.46, 95 %CI = 1.05–2.04, Fig. 3). The prevalence of vacA m1 was 48.5 % (279/575) in DU patients and 41.9 % (247/590) in controls in Asian countries; 44.5 % (151/339) in DU patients and 40.3 % (162/402) in controls in European countries; 62.4 % (73/117) in DU patients and 47.6 % (49/103) in controls in Latin American countries. The combined ORs and 95 %CIs showed that vacA m1 increased the risk of DU in Latin American countries (OR = 2.98, 95 %CI = 1.59–5.56), whereas no significant associations were shown in Asian countries (OR = 1.27, 95 %CI = 0.75–2.16) and European countries (OR = 1.30, 95 %CI = 0.95–1.77).
There were 20 studies reporting the association between vacA s1m1 genotype and DU. The prevalence of vacA s1m1 is 63.3 % (451/712) in DU patients and 59.1 % (622/1053) in controls. The combined OR and 95 %CI showed that vacA s1m1 increased the risk of DU significantly (OR = 1.89, 95 %CI = 1.47–2.42, Fig. 4). The prevalence of vacA s1m1 was 72.2 % (322/446) in DU patients and 65.2 % (531/814) in controls in Asian countries; 34.4 % (42/122) in DU patients and 26.4 % (32/121) in controls in European countries; 60.4 % (87/144) in DU patients and 50.0 % (59/118) in controls in Latin America countries. The combined ORs and 95 %CIs showed that vacA s1m1 increased the risk of DU in Asian countries (OR = 2.04, 95 %CI = 1.12–3.73) and Latin American countries (OR = 2.05, 95 %CI = 1.20–3.48), whereas no significant association was shown in European countries (OR = 1.42, 95 %CI = 0.80–2.53).
There were 20 studies reporting the association between vacA s1m2 genotype and DU. The prevalence of vacA s1m2 was 28.9 % (206/712) in DU patients and 24.9 % (262/1053) in controls. The combined OR and 95 %CI showed no association between vacA s1m2 and DU (OR = 0.96, 95 %CI = 0.74–1.26, Fig. 5). The prevalence of vacA s1m2 was 25.3 % (113/446) in DU patients and 24.0 % (195/814) in controls in Asian countries; 49.2 % (60/122) in DU patients and 43.0 % (52/121) in controls in European countries; 22.9 % (33/144) in DU patients and 12.7 % (15/118) in controls in Latin American countries. The combined ORs and 95 %CIs showed no association between vacA s1m2 and DU in Asian countries (OR = 0.82, 95 %CI = 0.58–1.15), European countries (OR = 1.21, 95 %CI = 0.70–2.08) and Latin America countries (OR = 1.33, 95 %CI = 0.65–2.71).
There were 20 studies reporting the association between vacA s2m1 genotype and DU. The prevalence of vacA s2m1 was 25.0 % (178/712) in DU patients and 22.2 % (234/1053) in controls. The combined OR and 95 % CI showed no association between vacA s2m1 and DU (OR = 1.64, 95 % CI = 0.81–3.30, Fig. 6). The prevalence of vacA s2m1 was 24.7 % (110/446) in DU patients and 23.6 % (192/814) in controls in Asian countries; 9.0 % (11/122) in DU patients and 8.3 % (10/121) in controls in European countries; 39.6 % (57/144) in DU patients and 27.1 % (32/118) in controls in Latin America countries. The combined ORs and 95 % CIs showed that vacA s2m1 increased the risk of DU in Latin American countries (OR = 2.30, 95 % CI = 1.17–4.50), whereas no associations were shown in Asian countries (OR = 1.27, 95 % CI = 0.37–4.39) and European countries (OR = 1.54, 95 % CI = 0.59–4.03).
Heterogeneity analysis
Significant heterogeneity existed in m1 genotype (I2 = 60.0 %) and s2m1 genotype (I2 = 65.7 %). To explore the source of heterogeneity graphically, we created Galbraith plots. Two studies [23, 43] were identified as the main source of heterogeneity for m1 genotype. Four studies [23, 37, 44, 50] were identified as the main contributors to heterogeneity for s2m1 genotype (Fig. 7).
Publication bias
No significant evidence of asymmetry was revealed by the funnel plots visually (Fig. 8). In addition, no statistical evidence of publication bias was found using Egger’s regression: P = 0.96 for s1, P = 0.70 for m1, P = 0.59 for s1m1, P = 0.28 for s1m2 and P = 0.54 for s2m1, respectively.
Discussion
VacA is one of the most commonly studied virulence markers of H. pylori. To date, numerous studies have evaluated the association between vacA genotype and DU, but the conclusions remained inconsistent. In addition, the credibility of results from single case–control study is questionable due to their relatively small sample size. Meta-analysis is of benefit to increase the sample size generating more precise conclusions, which has been widely used in genetic association studies [58, 59]. To our knowledge, the present study is the first meta-analysis assessing the association between vacA s-/m- region genotype and DU. There were 1,876 patients and 2,704 controls in the present study. Results of our study showed that s1 genotype was associated with increased DU risk in overall studied population, and also Asian, European or Latin American population; m1 genotype increased the risk of developing DU in overall studied population and Latin American population; s1m1 genotype increased the risk of DU in overall studied population, Asian population and Latin American population; s2m1 genotype increased the risk of DU in Latin American population.
The s region encodes part of the cytotoxin’s signal peptide and N-terminus, while the m region encodes part of the 55 kDa C-terminal subunit [6]. There were two types of s region: s1 and s2. The s2 genotype was reported to block the vacuolating activity since it encodes a shorter extension of the N-terminal peptide on the mature protein. On the contrary, the s1 genotype was reported to increase cytotoxin activity and thereby lead to gastric inflammation and duodenal ulceration [60]. Similar to s region, m region also has two subtypes: m1 and m2. Type m1 strains demonstrated more toxin activity than m2 strains [6, 61]. In this meta-analysis, we identified 34 articles focusing on the vacA s region genotype and 26 articles focusing on the vacA m region genotype. Among them, ten studies reported that the s1 genotype could increase the risk of DU; five studies reported that the m1 genotype was associated with increased DU risk; one study provided evidence that m1 was the protective factor for DU; the others revealed no significant difference between DU and control. Our study showed that vacA s1 increased the risk of DU by 2.96-folds and vacA m1 increased the risk of DU by 1.46-folds. Regarding the combination of s- and m- region, previous reports showed that the s1m1 genotype was closely tied to a large amount of toxin with high vacuolating activity in gastric epithelial cells, whereas the s1m2 genotype was associated with moderate amounts of toxin and s2m2 was associated with very little or no toxin [6, 60]. In this meta-analysis, we identified 20 articles reporting data on the combination of s- and m- genotypes. The combined results showed that only the vacA s1m1 could increase the risk of DU by 1.89-fold in the overall population. Taken the above results into consideration, it suggests that s1 and m1 are indeed the risk factors during the development of DU. Patients with s1 and m1 genotype would increase DU risk by 1.46–2.96-folds compared those without the same genotype.
To explore a more precise relationship between vacA s-/m- region genotypes and DU, we performed subgroup analyses by ethnicity. Our results demonstrated that vacA s1 increased the risk of DU by 1.92–4.20-fold in all the three subgroups. VacA m1 increased the risk of DU only in Latin American population. In Latin American population, the s1m1 and s2m1 increased the risk of DU by 2.05 and 2.30-folds respectively. In Asian population, only s1m1 showed risk effect on DU. The above data suggest that there is region difference in the vacA m genotype distribution. In addition, gene-environment interaction may also influence the effect of vacA m1 on the development of DU.
Some limitations of this meta-analysis should be noted. Firstly, we could not obtain the original data, which may limit the further evaluation of potential interactions among gene–gene and gene–environment. Secondly, only studies published in English or Chinese were included, which may lead to some inevitable bias, as eligible studies unpublished or reported in other languages would be missed.
Nonetheless, our meta-analysis with robust data and unbiased results demonstrated convincingly that VacA genotypes of H. pylori was well correlated with the risk of developing DU, and the correlation extent was various in different genotypes and also affected by region factors. Genotype testing of vacA s- and m- regions will be useful in screening susceptible individuals for DU development.
References
Piper DW, Nasiry R, McIntosh J, Shy CM, Pierce J, Byth K (1984) Smoking, alcohol, analgesics, and chronic duodenal ulcer. A controlled study of habits before first symptoms and before diagnosis. Scand J Gastroenterol 19(8):1015–1021
Zapata-Colindres JC, Zepeda-Gomez S, Montano-Loza A, Vazquez-Ballesteros E, de Jesus Villalobos J, Valdovinos-Andraca F (2006) The association of Helicobacter pylori infection and nonsteroidal anti-inflammatory drugs in peptic ulcer disease. Canadian journal of gastroenterology = Journal canadien de gastroenterologie 20 (4):277-280
NIH Consensus Conference (1994) Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA, J Am Med Assoc 272(1):65–69
Go MF (2002) Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther 16(Suppl 1):3–15
Cover TL (1996) The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol 20(2):241–246
Atherton JC, Cao P, Peek RM, Jr, Tummuru MK, Blaser MJ, Cover TL (1995) Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem 270(30):17771–17777
Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, Atherton JC (2007) A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology 133(3):926–936. doi:10.1053/j.gastro.2007.06.056
Basso D, Navaglia F, Brigato L, Piva MG, Toma A, Greco E, Di Mario F, Galeotti F, Roveroni G, Corsini A, Plebani M (1998) Analysis of Helicobacter pylori vacA and cagA genotypes and serum antibody profile in benign and malignant gastroduodenal diseases. Gut 43(2):182–186
Go MF, Cissell L, Graham DY (1998) Failure to confirm association of vac A gene mosaicism with duodenal ulcer disease. Scand J Gastroenterol 33(2):132–136
Strobel S, Bereswill S, Balig P, Allgaier P, Sonntag HG, Kist M (1998) Identification and analysis of a new vacA genotype variant of Helicobacter pylori in different patient groups in Germany. J Clin Microbiol 36(5):1285–1289
Warburton VJ, Everett S, Mapstone NP, Axon AT, Hawkey P, Dixon MF (1998) Clinical and histological associations of cagA and vacA genotypes in Helicobacter pylori gastritis. J Clin Pathol 51(1):55–61
Yamaoka Y, Kodama T, Kita M, Imanishi J, Kashima K, Graham DY (1998) Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter 3(4):241–253
Hennig EE, Trzeciak L, Regula J, Butruk E, Ostrowski J (1999) VacA genotyping directly from gastric biopsy specimens and estimation of mixed Helicobacter pylori infections in patients with duodenal ulcer and gastritis. Scand J Gastroenterol 34(8):743–749
Sadakane Y, Kusaba K, Nagasawa Z, Tanabe I, Kuroki S, Tadano J (1999) Prevalence and genetic diversity of cagD, cagE, and vacA in Helicobacter pylori strains isolated from Japanese patients. Scand J Gastroenterol 34(10):981–986
Audibert C, Janvier B, Grignon B, Salaun L, Burucoa C, Lecron JC, Fauchere JL (2000) Correlation between IL-8 induction, cagA status and vacA genotypes in 153 French Helicobacter pylori isolates. Res Microbiol 151(3):191–200
De Gusmao VR, Nogueira Mendes E, De Magalhaes Queiroz DM, Aguiar Rocha G, Camargos Rocha AM, Ramadan Ashour AA, Teles Carvalho AS (2000) vacA genotypes in Helicobacter pylori strains isolated from children with and without duodenal ulcer in Brazil. J Clin Microbiol 38(8):2853–2857
Figueiredo C, Van Doorn LJ, Nogueira C, Soares JM, Pinho C, Figueira P, Quint WG, Carneiro F (2001) Helicobacter pylori genotypes are associated with clinical outcome in Portuguese patients and show a high prevalence of infections with multiple strains. Scand J Gastroenterol 36(2):128–135
Miehlke S, Yu J, Schuppler M, Frings C, Kirsch C, Negraszus N, Morgner A, Stolte M, Ehninger G, Bayerdorffer E (2001) Helicobacter pylori vacA, iceA, and cagA status and pattern of gastritis in patients with malignant and benign gastroduodenal disease. Am J Gastroenterol 96(4):1008–1013. doi:10.1111/j.1572-0241.2001.03685.x
Park SM, Park J, Kim JG, Yoo BC (2001) Relevance of vacA genotypes of Helicobacter pylori to cagA status and its clinical outcome. Korean J Intern Med 16(1):8–13
Wong BC, Yin Y, Berg DE, Xia HH, Zhang JZ, Wang WH, Wong WM, Huang XR, Tang VS, Lam SK (2001) Distribution of distinct vacA, cagA and iceA alleles in Helicobacter pylori in Hong Kong. Helicobacter 6(4):317–324
Chattopadhyay S, Datta S, Chowdhury A, Chowdhury S, Mukhopadhyay AK, Rajendran K, Bhattacharya SK, Berg DE, Nair GB (2002) Virulence genes in Helicobacter pylori strains from West Bengal residents with overt H. pylori-associated disease and healthy volunteers. J Clin Microbiol 40(7):2622–2625
Choe YH, Kim PS, Lee DH, Kim HK, Kim YS, Shin YW, Hwang TS, Kim HJ, Song SU, Choi MS (2002) Diverse vacA allelic types of Helicobacter pylori in Korea and clinical correlation. Yonsei Med J 43(3):351–356
Ashour AA, Magalhaes PP, Mendes EN, Collares GB, de Gusmao VR, Queiroz DM, Nogueira AM, Rocha GA, de Oliveira CA (2002) Distribution of vacA genotypes in Helicobacter pylori strains isolated from Brazilian adult patients with gastritis, duodenal ulcer or gastric carcinoma. FEMS Immunol Med Microbiol 33(3):173–178
Smith SI, Kirsch C, Oyedeji KS, Arigbabu AO, Coker AO, Bayerdoffer E, Miehlke S (2002) Prevalence of Helicobacter pylori vacA, cagA and iceA genotypes in Nigerian patients with duodenal ulcer disease. J Med Microbiol 51(10):851–854
Brito CA, Silva LM, Juca N, Leal NC, de Souza W, Queiroz D, Cordeiro F, Silva NL (2003) Prevalence of cagA and vacA genes in isolates from patients with Helicobacter pylori-associated gastroduodenal diseases in Recife, Pernambuco Brazil. Mem Inst Oswaldo Cruz 98(6):817–821
Leodolter A, Wolle K, Peitz U, Ebert M, Gunther T, Kahl S, Malfertheiner P (2003) Helicobacter pylori genotypes and expression of gastritis in erosive gastro-oesophageal reflux disease. Scand J Gastroenterol 38(5):498–502
Perng CL, Lin HJ, Sun IC, Tseng GY, Facg (2003) Helicobacter pylori cagA, iceA and vacA status in Taiwanese patients with peptic ulcer and gastritis. J Gastroenterol Hepatol 18(11):1244–1249
Qiao W, Hu JL, Xiao B, Wu KC, Peng DR, Atherton JC, Xue H (2003) cagA and vacA genotype of Helicobacter pylori associated with gastric diseases in Xi’an area. World J Gastroenterol 9(8):1762–1766
Ribeiro ML, Godoy AP, Benvengo YH, Mendonca S, Pedrazzoli J Jr (2003) Clinical relevance of the cagA, vacA and iceA genotypes of Helicobacter pylori in Brazilian clinical isolates. FEMS Immunol Med Microbiol 36(3):181–185
Yin Y, Zhang JZ, Wang ZY, Xia HX, Lin ZX (2003) Association between Helicobacter pylori virulence and duodenal ulcer disease in patients from Hong Kong in China. Zhonghua Liu Xing Bing Xue Za Zhi 24(2):123–126
Ando T, Tsuzuki T, Mizuno T, Minami M, Ina K, Kusugami K, Takamatsu J, Adachi K, El-Omar E, Ohta M, Goto H (2004) Characteristics of Helicobacter pylori-induced gastritis and the effect of H. pylori eradication in patients with chronic idiopathic thrombocytopenic purpura. Helicobacter 9(5):443–452. doi:10.1111/j.1083-4389.2004.00261.x
Lin HJ, Perng CL, Lo WC, Wu CW, Tseng GY, Li AF, Sun IC, Ou YH (2004) Helicobacter pylori cagA, iceA and vacA genotypes in patients with gastric cancer in Taiwan. World J Gastroenterol 10(17):2493–2497
Perng CL, Lin HJ, Lo WC, Tseng GY, Sun IC, Ou YH (2004) Genotypes of Helicobacter pylori in patients with peptic ulcer bleeding. World J Gastroenterol 10(4):602–605
Salih BA, Abasiyanik MF, Saribasak H, Huten O, Sander E (2005) A follow-up study on the effect of Helicobacter pylori eradication on the severity of gastric histology. Dig Dis Sci 50(8):1517–1522
Siavoshi F, Malekzadeh R, Daneshmand M, Ashktorab H (2005) Helicobacter pylori endemic and gastric disease. Dig Dis Sci 50(11):2075–2080. doi:10.1007/s10620-005-3010-1
Erzin Y, Koksal V, Altun S, Dobrucali A, Aslan M, Erdamar S, Dirican A, Kocazeybek B (2006) Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA, babA2 genotypes and correlation with clinical outcome in Turkish patients with dyspepsia. Helicobacter 11(6):574–580. doi:10.1111/j.1523-5378.2006.00461.x
Bolek BK, Salih BA, Sander E (2007) Genotyping of Helicobacter pylori strains from gastric biopsies by multiplex polymerase chain reaction. How advantageous is it? Diagn Microbiol Infect Dis 58(1):67–70. doi:10.1016/j.diagmicrobio.2006.12.001
Caner V, Yilmaz M, Yonetci N, Zencir S, Karagenc N, Kaleli I, Bagci H (2007) H. pylori iceA alleles are disease-specific virulence factors. World J Gastroenterol 13(18):2581–2585
Linpisarn S, Suwan W, Lertprasertsuk N, Koosirirat C, Steger HF, Prommuangyong K, Phornphutkul K (2007) Helicobacter pylori cagA, vacA and iceA genotypes in northern Thai patients with gastric disease. Southeast Asian J Trop Med Public Health 38(2):356–362
Proenca Modena JL, Lopes Sales AI, Olszanski Acrani G, Russo R, Vilela Ribeiro MA, Fukuhara Y, da Silveira WD, Modena JL, de Oliveira RB, Brocchi M (2007) Association between Helicobacter pylori genotypes and gastric disorders in relation to the cag pathogenicity island. Diagn Microbiol Infect Dis 59(1):7–16. doi:10.1016/j.diagmicrobio.2007.03.019
Tiwari SK, Khan AA, Manoj G, Ahmed S, Abid Z, Habeeb A, Habibullah CM (2007) A simple multiplex PCR assay for diagnosing virulent Helicobacter pylori infection in human gastric biopsy specimens from subjects with gastric carcinoma and other gastro-duodenal diseases. J Appl Microbiol 103(6):2353–2360. doi:10.1111/j.1365-2672.2007.03478.x
Miciuleviciene J, Calkauskas H, Jonaitis L, Kiudelis G, Tamosiunas V, Praskevicius A, Kupcinskas L, Berg D (2008) Helicobacter pylori genotypes in Lithuanian patients with chronic gastritis and duodenal ulcer. Medicina 44(6):449–454
Zhang Z, Zheng Q, Chen X, Xiao S, Liu W, Lu H (2008) The Helicobacter pylori duodenal ulcer promoting gene, dupA in China. BMC Gastroenterol 8:49. doi:10.1186/1471-230X-8-49
Bindayna KM, Al Mahmeed A (2009) vacA genotypes in Helicobacter pylori strains isolated from patients with and without duodenal ulcer in Bahrain. Indian J Gastroenterol 28(5):175–179. doi:10.1007/s12664-009-0069-1
Nagiyev T, Yula E, Abayli B, Koksal F (2009) Prevalence and genotypes of Helicobacter pylori in gastric biopsy specimens from patients with gastroduodenal pathologies in the Cukurova Region of Turkey. J Clin Microbiol 47(12):4150–4153. doi:10.1128/JCM.00605-09
Salehi Z, Abadi AS, Ismail PB, Kqueen CY, Jelodar MH, Kamalidehghan B (2009) Evaluation of Helicobacter pylori vacA genotypes in Iranian patients with peptic ulcer disease. Dig Dis Sci 54(11):2399–2403. doi:10.1007/s10620-008-0633-z
Torres LE, Melian K, Moreno A, Alonso J, Sabatier CA, Hernandez M, Bermudez L, Rodriguez BL (2009) Prevalence of vacA, cagA and babA2 genes in Cuban Helicobacter pylori isolates. World J Gastroenterol 15(2):204–210
Yakoob J, Abid S, Abbas Z, Jafri W, Ahmad Z, Ahmed R, Islam M (2009) Distribution of Helicobacter pylori virulence markers in patients with gastroduodenal diseases in Pakistan. BMC Gastroenterol 9:87. doi:10.1186/1471-230X-9-87
Dixit P, Sharma AK, Sinha SK, Prasad KK, Singh K (2011) Prevalence of cagA and vacA genotypes of H. pylori as a putative virulence marker in dyspeptic patients in northern India. J Gastroenterol Hepatol 26:276
Khan A, Farooqui A, Raza Y, Rasheed F, Manzoor H, Akhtar SS, Quraishy MS, Rubino S, Kazmi SU, Paglietti B (2013) Prevalence, diversity and disease association of Helicobacter pylori in dyspeptic patients from Pakistan. J Infect Dev Ctries 7(3):220–228. doi:10.3855/jidc.2942
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. doi:10.1371/journal.pmed.1000097
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwel lP (2011)The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Research Institute.www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed Dec. 1 2011
Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10:101–129
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. doi:10.1002/sim.1186
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188 0197-2456(86)90046-2 [pii]
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22(4):719–748
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Coppede F, Bosco P, Lorenzoni V, Migheli F, Barone C, Antonucci I, Stuppia L, Romano C, Migliore L (2013) The MTR 2756A>G polymorphism and maternal risk of birth of a child with Down syndrome: a case–control study and a meta-analysis. Mol Biol Rep. doi:10.1007/s11033-013-2810-1
Li YY, Wu XY, Xu J, Qian Y, Zhou CW, Wang B (2013) Apo A5–1131T/C, FgB -455G/A, -148C/T, and CETP TaqIB gene polymorphisms and coronary artery disease in the Chinese population: a meta-analysis of 15,055 subjects. Mol Biol Rep 40(2):1997–2014. doi:10.1007/s11033-012-2257-9
Letley DP, Atherton JC (2000) Natural diversity in the N terminus of the mature vacuolating cytotoxin of Helicobacter pylori determines cytotoxin activity. J Bacteriol 182(11):3278–3280
Atherton JC, Peek RM Jr, Tham KT, Cover TL, Blaser MJ (1997) Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 112(1):92–99
Acknowledgments
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, BB., Li, Y., Liu, XQ. et al. Association between vacA genotypes and the risk of duodenal ulcer: a meta-analysis. Mol Biol Rep 41, 7241–7254 (2014). https://doi.org/10.1007/s11033-014-3610-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3610-y