Abstract
Gastric cancer, as the fifth most frequent disease and the fourth foremost cause of cancer-related death worldwide, remains a main clinical challenge due to its poor prognosis, limited treatment choices, and ability to metastasize. Combining siRNAs to suppress lncRNA with chemotherapeutic medications is a novel treatment approach that eventually increases the therapeutic efficacy of the drug while lessening its adverse effects. This study was performed with the purpose of examining the impact of inhibiting DLGAP1-AS2 expression on gastric cancer cells’ drug chemosensitivity. AGS cells were cultured as the study cell line and were transfected with an optimum dose of DLGAP1-AS2 siRNA and then treated with oxaliplatin. Cell viability was examined using the MTT technique. Apoptosis and cell cycle were evaluated using Annexin V/PI staining and flow cytometry. Later, the scratch test was conducted to investigate the ability of cells to migrate, and the inhibition of the stemness of AGS cells was further investigated through the colony formation method. Finally, the qRT-PCR technique was used to assess the expression of Bax, Bcl-2, Caspase-3, p53, MMP-2, and CD44 genes. The MTT test indicated the effect of gene therapy with siRNA and oxaliplatin in combination reduced the chemotherapy drug dose to 29.92 µM and increased AGS cells’ sensitivity to oxaliplatin. Also, the combination therapy caused a significant increase in apoptosis. However, it reduced the stemness feature, the rate of cell viability, proliferation, and metastasis compared to the effect of each treatment alone; the results also showed the arrest of the cell cycle in the Sub G1 phase after the combined treatment and a further reduction in the number and size of the formed colonies. Suppressing the expression of lncRNA DLGAP1-AS2 by siRNA followed by treatment with oxaliplatin can be utilized as an effective and new therapeutic technique for gastric cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC), one of the deadliest cancers, is the fifth most common cancer and the fourth reason of death worldwide in that genetic and environmental factors influence its growth (Sung et al. 2021). Gastritis of the stomach membrane is prone to inflammation and can lead to stomach ulcer and subsequently cause stomach cancer (Gao et al. 2018). Approximately 90% of gastric cancers are adenocarcinomas with the origination of the gastric mucosa, which are differentiated into two subgroups, intestinal and diffuse, based on Lauren’s system (Karimi et al. 2014; Machlowska et al. 2020). Most GCs occur in the distal part of the stomach (non-cardia GC), while about 18% happen in the cardia (cardia GC) (Morgan et al. 2022). Treatment of GC is highly dependent on tumor location and size, which gets more complicated as cancer progresses and spreads to other organs such as the liver, lungs, and bones. Chemotherapy, radiotherapy, surgery, immunotherapy, and targeted therapy are all effective treatments for gastric adenocarcinoma. These methods can be utilized independently or in combination with other methods (Joshi and Badgwell 2021; Society, A.C. Stomach Cancer n.d.).
Oxaliplatin is used as antineoplastic medication in chemotherapy treatments for cancer. In all tissues, oxaliplatin is dispersed after becoming bonded to plasma proteins. This medication has non-specific cell cycle toxicity and is an alkylating agent. Following the medication’s platinum complex binding to DNA, cross-links between adjacent guanines or guanine and adenine are formed. Cell death is eventually brought on by these linkages, which stop DNA replication, transcription, and cell cycle arrest (André et al. 2004; Devanabanda and Kasi 2022). Resistance to chemotherapy is the most frequent occurrence that reduces the treatment efficacy. Novel targeted cancer therapies are made possible by genome editing technology that is more precise and quicker. By decreasing the regulation of mutant genes and turning off undesirable gene expression, siRNA-based gene therapy inhibits tumor cell proliferation and invasion (Singh et al. 2018; Biagioni et al. 2019).
It has been demonstrated that silencing many lncRNAs can be effectively accomplished by siRNAs with a length of 19 to 30 nucleotides (Cantile et al. 2020). LncRNAs are long noncoding RNAs that often have poly-A tails and 5′ caps and are over 200 nucleotides long. LncRNAs can act as tumor suppressors or oncogenes, participate in a variety of tumor signaling pathways, and have an aberrant or dysregulated expression that interferes with a variety of cellular processes, making them prospective targets for cancer therapy (Gutschner and Diederichs 2012; Gu et al. 2015; Bhan et al. 2017). The location of the lncRNA DLGAP1-AS2 is on the human chromosome 18p11.31, transcribed in the antisense of the DLGAP1 gene. Studies have revealed a considerable increase in the expression of DLGAP1-AS2 in gastric cancer tumor plasma and tissues. Consequently, inhibiting this lncRNA decreases the proliferation, invasion, and migration of gastric cancer cells (Lu 2020; Soltani et al. 2022; National Library of Medicine 2024). Recent research has shown a substantial correlation between DLGAP1-AS2 expression and vascular and lymphatic invasion, which promotes the progression and metastasis of GC (Soltani et al. 2022).
Finding a way to achieve the most impact in the shortest amount of time is one of the most critical concerns in cancer treatment. Considering these pieces of information, this study aimed to investigate the effect of lncRNA DLGAP1-AS2 inhibition on GC cells’ chemosensitivity to oxaliplatin, the effective dose of the medication, determine the cytotoxic effects of chemotherapy, and also examine the synergistic effects of combining chemotherapy with gene therapy.
Materials and methods
TCGA gastric cancer data analysis
DLGAP1-AS2 gene expression data were gathered and evaluated using the UALCAN tool, used for the analysis of TCGA (the Cancer Genome Atlas) data related to cancer. The TCGA database was utilized to investigate and compare the expression of this gene in both normal and gastric cancer tissues.
Cell culture
AGS cells, selected as the studied cell line, were obtained from the cell bank of Pasteur Institute of Iran in the form of vials and stored as frozen vials in the cell bank of the Immunology Research Center of Medical Sciences in Tabriz. To ensure the viability of the cells, they were counted through trypan blue staining; in the following, they cultured through transferring to a 25-cm2 flask filled with culture medium that was RPMI-1640 (Gibco, USA) including 10% fetal bovine serum (FBS; Gibco, USA), penicillin, and streptomycin. The flask of cells was kept at 37 °C, 5% CO2, and 95% humidity.
Transfection of siRNA DLGAP1-AS2
The designed siRNA sequence specificity was checked using nucleotide blast on the human RNAseq dataset and the powder form of siRNA was purchased.
The Gene Pulser electroporation device (Bio-Rad, USA) was used to transfect siRNA at several doses to find the most optimal dose of it. In the following step, transfected cells were seeded on different plates. One hundred picomoles of siRNA was utilized as the optimum concentration during transfection.
MTT assay
The MTT test is a technique for assessing metabolic activity and viability of cells. The basis of this technique is the deduction and conversion of yellow tetrazolium salt (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) or MTT into purple formazan crystals in active cells. In this method, due to the tiny size of AGS cells, 15 × 103 cells were sown in each well of a 96-well plate. The cells were split into three groups: the control group, which did not receive any siRNA transfection; the transfected group, which received a dose of 100 pmol of siRNA; and the negative control group, which received scramble siRNA transfection. After 24 h, the supernatant from each well was taken out, and the cells were then given various amounts of the chemotherapy medication oxaliplatin. Twenty-four hours following the treatment, the supernatant liquid was removed; 50 µl of MTT solution (2 mg/ml) and 100 µl of medium were added to each well. For 4 h, the plate was incubated at 37 °C. Each well’s supernatant was discarded, 200 μL of DMSO was added, and re-incubation was conducted. The amount of light absorbed by each well was measured using the ELISA microplate reader device (Tecan, Switzerland) at wavelengths 570 and 620 nm.
Apoptosis assay
The procedure begins with the seeding of 5 × 105 AGS cells into a 6-well plate in groups of transfected and non-transfected cells. The 6-well plate was divided into four groups consisting of negative control, transfected with siRNA, treated with oxaliplatin, and the combination of siRNA and oxaliplatin. After 24 h, the IC50 dose of oxaliplatin was administered to treat the wells containing the drug and combined treatment. After incubation, AGS cells were washed with PBS and then stained using the Annexin V/PI staining kit (Exbio-Czech) according to the kit protocol. Finally, a MACS Quant Authorized 10 flow cytometry device was used to determine cell apoptosis and necrosis.
Cell cycle assay
A 6-well plate was grouped into four subgroups containing negative control, siRNA-transfected, oxaliplatin-treated, and combined treatment. Afterward, 5 × 105 transfected and non-transfected cells were seeded in respective wells. Twenty-four hours later, the medication and combination treatment groups received the IC50 dose of oxaliplatin. In the next step of the protocol, the cells were washed with PBS, fixed with 70% alcohol, and left overnight at − 20 °C. After the requisite time, 500 µl of PBS and RNase A enzyme was slowly added to the cells, incubated for 30 min at 37 °C, and stained with DAPI. Following the incubation, the samples were analyzed using a MACS Quant Authorized 10 flow cytometry device.
Wound healing assay
Cancer cell migration and invasion was checked through the wound healing assay or scratch test. In order to achieve this, 5 × 105 transfected and non-transfected AGS cells per well, consisting of negative control, gene therapy, oxaliplatin drug, and combined treatment group, were seeded in a 6-well plate. After 24 h of incubation, the drug and combination treatment groups were treated with IC50 dose of oxaliplatin. After that, a linear scratch was made along all the wells with the tip of a sterile yellow pipette tip of a sampler. In order to evaluate the ability of cells to migrate from the edge of the scratch to the middle space, photographs were taken of the wells at 0, 24, and 48 h using an inverted microscope (OPTIKA, Italy).
Colony formation assay
In this technique, for the purpose of examining colony formation in cancer cells, first, transfected and non-transfected AGS cells were seeded in their respective groups in a 6-well plate. Next, the plate was categorized into negative control, siRNA-transfected, drug-treated, and combination treatment groups. The cells were given the optimum dose of oxaliplatin following incubation at 37 °C and 5% CO2. The medium’s quality and colony formation process were compared daily with the control group while the plate was incubated for 10 days. In addition, the colonies were PBS-washed and fixed with paraformaldehyde, crystal violet was used to stain them, and they were incubated for 30 min. In the last step, the AGS cells’ potential to form colonies was analyzed through taking pictures of the wells.
Quantitative real‑time PCR
The 5 × 105 siRNA-transfected and non-transfected cells were seeded into four groups: negative control, siRNA transfected, oxaliplatin treated, and combination treatment in a 6-well plate and treated with the drug. The purity and concentration of the extracted RNA were determined utilizing a nanodrop device (DeNovix, USA) at a wavelength of 260 nm. The following phase was complementary DNA synthesis (cDNA), which was done by Addbio kit to assess the relative expression of linked genes. The expression of target genes, such as MMP-2, Bax, Bcl-2, Caspase-3, CD44, and P53, was investigated with the StepOnePlus real time PCR machine (Thermo Fisher Scientific, USA) and using 2 × Real-Time Master Mix (BioFact, South Korea). Analysis of the target genes’ expression was performed using the obtained CTs and the equation 2−ΔΔCt. The internal control gene for this experiment was GAPDH. The sequences of utilized primers are listed on Table 1.
Statistical analysis
This study’s tests were conducted in triplicate. Data analysis was carried out using FlowJo, GraphPad Prism 6, one-way ANOVA, two-way ANOVA, and T-tests. A statistically significant difference was defined as a P-value of 0.05.
Results
DLGAP1-AS2 is upregulated in gastric cancer
The findings of analyzing TCGA data with Ualcan indicated that stomach cancer (STAD) had a significant overexpression of DLGAP1-AS2 (Fig. 1A). In addition, compared to normal tissues, the expression of DLGAP1-AS2 was considerably higher in gastric cancer samples.
Cell line selection
Investigation of DLGAP1-AS2 gene expression in three cell lines—AGS, MKN-45, and KATO3—resulted in AGS cells expressing this gene at a higher level than the other two cell lines. As a result, the AGS cell line was chosen to begin the study (Fig. 2).
Quantitative evaluation of siRNA transfection into AGS cells
In order to evaluate the efficacy of siRNA transfection, scramble siRNA containing FAM was transfected into AGS cells. The findings revealed a transfection effectiveness of approximately 99% (Fig. 3A, B).
Investigating the reduction of lncRNA DLGAP1-AS2 expression by siRNA at different doses and times
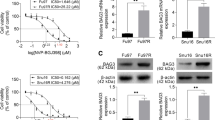
AGS cells were transfected with 40-, 60-, and 100-pmol doses of siRNA to determine the optimum dose to use at the proper time. Following transfection with 100 pmol of siRNA, DLGAP1-AS2 expression levels significantly decreased, according to the results of qRT-PCR; furthermore, the expression of lncRNA DLGAP1-AS2 was evaluated at 24, 48, and 72 h. Based on qRT-PCR, siRNA significantly downregulated the expression of DLGAP1-AS2 within 48 h compared to other groups (Fig. 4A, B).
A The effect of siRNA on the expression of lncRNA DLGAP1-AS2 at different doses of 40, 60, and 100 pmol. One hundred picomoles was the optimum siRNA concentration. B The effect of siRNA on the expression of lncRNA DLGAP1-AS2 at 24, 48, and 72 h. It has been demonstrated that siRNA reduces lncRNA DLGAP1-AS2 expression in 48 h. (****P < 0.0001, **P < 0.01, *P < 0.05; ns, not significant; NC, scramble siRNA transfected negative control)
Effect of siRNA transfection alone and combined treatment with siRNA and oxaliplatin on the viability of AGS cells
The MTT test demonstrates the impact of siRNA transfection on AGS cells’ survival rate. It is also used to examine the toxicity of oxaliplatin and the sensitivity of AGS cells to the medication following siRNA-mediated knockdown and downregulation of the lncRNA DLGAP 1-AS2 expression. The analysis of the data revealed that the percentage of cell viability and survival was considerably reduced (**P < 0.01) in the group transfected with 100 pmol of siRNA compared to the control group (Fig. 5A). The findings of this study indicated the effect of siRNA transfection on reducing AGS cells’ survival. It also showed how siRNA-mediated reducing DLGAP1-AS2 expression further increased these cells’ sensitivity to oxaliplatin by decreasing the expression of the lncRNA DLGAP1-AS2. The IC50 for the oxaliplatin-treated group and combination treatment group was reported as 54.91 µM and 29.92 µM respectively, indicating the critical contribution of siRNA to increasing sensitivity of gastric cancer cells to chemotherapy drug (Fig. 5B).
The impact of siRNA transfection combined with oxaliplatin therapy on the survival rate of AGS cells. A The cell viability decreased after transfection of siRNA. B Reduction of IC50 of oxaliplatin drug from 54.91 µM in sole treatment to 29.92 µM in combined treatment of siRNA and chemotherapy. (***P < 0.001, **P < 0.01; ns, not significant; NC, scramble siRNA transfected negative control)
Combining DLGAP1-AS2 knockdown with oxaliplatin could make AGS cells more vulnerable to apoptosis
Flow cytometry and annexin V/PI staining revealed that apoptosis rates were 16.54% in the siRNA-transfected group and 29.1% in the group receiving oxaliplatin treatment. In comparison to the control group, which had an apoptosis rate of 3.56%, the combined therapy group’s rate raised to 43.8%, which was significantly higher (Fig. 6A). Additionally, using qRT-PCR, genes implicated in apoptosis molecular mechanisms were studied and examined. While Bcl-2 expression was reduced in the combination treatment group compared to other groups, the expression of Bax was significantly enhanced in the combined treatment group when compared to each therapy alone and the control group (Fig. 6B, C). On the other hand, in comparison to the individual treatment and control groups, the expression of Caspase-3 increased following siRNA transfection and when used in combination with oxaliplatin (Fig. 6D). The conclusion drawn from these findings points to an increase in cell death following simultaneous siRNA transfection and treatment with oxaliplatin. Furthermore, siRNA DLGAP1-AS2 has a crucial role in inducing apoptosis by increasing AGS cells’ sensitivity to oxaliplatin.
The impact of siRNA transfection and treatment with oxaliplatin on the apoptotic rate of AGS cells. A Apoptosis test by Annexin-V/PI staining confirmed a significantly higher rate of cell apoptosis after siRNA transfection and in combination with oxaliplatin treatment. B The expression level of Bax markedly increased following combination therapy compared to the control group. C Compared to the control group, Bcl-2 expression became lower after transfection with siRNA and oxaliplatin treatment. D Comparing the combined therapy group to the control group and solo treatment, the combined therapy increased expression of Caspase-3. (****P < 0.0001, ***P < 0.001, **P < 0.01; ns, not significant; NC, scramble siRNA transfected negative control)
AGS cells arrested in the sub-G1 phase through the combination of siRNA transfection and oxaliplatin treatment
Flow cytometry data indicated that compared to the control group, the siRNA transfected group had a higher percentage of cells in the Sub-G1 phase (5.99%). The population of cells arrested in Sub-G1 phase grew and reached 6.05% in the oxaliplatin-treated group. The number of cells arrested in this phase increased significantly (12.6%) in the group that received both siRNA transfection and oxaliplatin treatment (Fig. 7A). qRT-PCR was used to investigate the expression of the p53 gene, a gene associated with the cell cycle. According to the findings, oxaliplatin therapy, siRNA transfection, and combination treatment enhanced the p53 expression level compared to the control group (Fig. 7B).
Simultaneous siRNA transfection and oxaliplatin treatment reduces the migration of AGS cancer cells
Pictures of migration assay showed that compared to the control group, both the siRNA and oxaliplatin independently reduced the migration and invasion of AGS cells toward the scratch space. In addition, a significant reduction in the migration of cells was also observed in the combined treatment group (Fig. 8A). MMP-2 gene expression was assessed using qRT-PCR; the results revealed that in two groups that had received siRNA transfection and oxaliplatin treatment, the expression of this gene had decreased. The combination therapy group experienced a considerable reduction than the other two individual groups (Fig. 8B).
The stemness feature of AGS cells is reduced after siRNA transfection and oxaliplatin therapy, and alterations in the colony formation result
Comparing the resulting images revealed that oxaliplatin treatment in combination with siRNA transfection remarkably reduced the number and size of colonies as compared to each group acting independently (Fig. 9A). CD44 gene expression as a gene involved in the stemness property was assessed; a significant decrease in the number and size of the formed colonies accompanied the reduction in the expression of this gene (Fig. 9B).
Investigating the effect of combination therapy on the stemness features and formed colonies. A Compared to the control group, a significant decrease occurred in the size and number of the former colonies in the combined treatment group. B The combined therapy group showed a reduction in the expression of the CD44 gene, a gene associated with cell stemness, compared to other groups. (****P < 0.0001; NC, scramble siRNA transfected negative control)
Discussion
Cancer early detection, diagnosis, and treatment have been the subject of numerous studies and medical advances. However, the disease still remains a serious threat to global health (Pantel et al. 2009). There are few effective ways to treat GC; it is typically detected at an advanced stage, so surgery is not advised. Additionally, the efficiency of chemotherapy and radiotherapy is similarly less (Ye et al. 2020, 2021; Soltani et al. 2022). Therefore, it is essential to develop more specialized, innovative, and effective cancer treatment methods that are also achievable economically. Non-specific targeting of actively proliferating cells using monotherapy approaches results in the death of both malignant and healthy cells. Basically, chemotherapy does not kill cancer stem cells or other resistant cancer cells because of this non-specific activity. Consequently, after therapy, the disease frequently recurs and metastasizes. Combination therapy significantly decreases toxicity by targeting various pathways. Because of its synergistic effect, a lower dose of the drug is required. Additionally, a more effective response can be achieved with fewer cycles of this type of treatment by reducing drug resistance. Furthermore, combined treatment can decrease cancer stem cell numbers, induce apoptosis, and arrest the cell cycle, thereby reducing metastasis (Mokhtari et al. 2017). Recent research revealed a substantial relationship between the expression of DLGAP1-AS2 and lymphatic and vascular invasion, which induces the progression and metastasis of GC (Soltani et al. 2022).
Finding a procedure with the highest possible effectiveness in the shortest amount of time is one of the most critical and main challenges in cancer treatment. We, therefore, provided a treatment strategy based on combination therapy in this study. We investigated the interaction between the medicine oxaliplatin and the siRNA DLGAP1-AS2 for the first time in this approach. At the beginning of this study, expression levels of DLGAP1-AS2 in different cell lines including MKN-45, AGS, and KATO III were evaluated and AGS cell line with highest expression of DLGAP1-AS2 was chosen as the main cell lines to conduct subsequent experiments. According to the results of this study, DLGAP1-AS2-siRNA can suppress the division and proliferation of cancer cells by time and dose. Based on the evaluation of the expression levels of the DLGAP1-AS2 following siRNA transfection, the optimal dose of siRNA DLGAP1-AS2 with a concentration of 100 pmol in 48 h which significantly reduced DLGAP1-AS2 expression, can significantly reduce the viability of AGS cells. Moreover, the use of oxaliplatin in combination with gene therapy decreased its IC50 from 54.91 to 29.92 µM, demonstrating the synergistic effect of combined treatment on increasing sensitivity of AGS cells to oxaliplatin and lowering the viability of AGS cells. It can be inferred that the viability and cytotoxicity assay demonstrated that siRNA transfection decreases cell survival and viability and increases the sensitivity of malignant cells to oxaliplatin drug, due to the suppression of lncRNA DLGAP1-AS2. According to a study done in 2022 by Kumar and his colleagues, the simultaneous use of siRNA with other anticancer medications improved the efficiency of the therapy by overcoming multidrug resistance and creating a synergistic effect. It also improved the therapeutic response by affecting various proteins and pathways (Kumar et al. 2022).
In the current study, the wound healing assay also indicated a decrease in the migration and invasion of tumor cells. In addition, real-time PCR showed that expression of the MMP-2 decreased by transfection of siRNA individually and further combined with oxaliplatin therapy. In a 2021 investigation, Liu and his coworkers researched the role of the lncRNA DLGAP1-AS2 in cholangiocarcinoma cells. This research shows that DLGAP1-AS2 increases cancer cell proliferation, migration, and invasion by inhibiting miR505, which decreases the expression of N-acetylgalactosaminyltransferase 10 (GALNT10). In order to treat cholangiocarcinoma, knocking out GALNT10 and DLGAP1-AS2 together was introduced as a novel target. This work also showed the function of the lncRNA DLGAP1-AS2 in the development and growth of cholangiocarcinoma tumors (Liu et al. 2021). Another study was conducted in 2021 by Jie Luo and colleagues on ovarian cancer cells. Overexpression of DLGAP1-AS2 was found to decrease mature miR-16 levels in OC cells; thus, this overexpression of DLGAP1-AS2 may reduces miR-16 inhibitory effect on the invasion and migration of OC cells (Luo et al. 2021). Previous research by Wei Miao et al. in 2020 was published with focusing on evaluating the expression of DLGAP1-AS2 in glioma patients. According to the qRT-PCR results, DLGAP1-AS2 expression was extremely increased in glioma cancer cells. Therefore, depletion of DLGAP1-AS2 in glioma cells could substantially prevent cell growth as well as cell migration and induce apoptosis, thereby lowering the development of glioma. These results suggest that DLGAP1-AS2 may function as an oncogene in glioma progression (Miao et al. 2020).
A 2020 study by Jiawei LU et al. on two gastric cancer cell lines, HGC‐27 and AGS, indicated that high expression of DLGAP1-AS2 in GC patients was associated with poor disease prognosis and progression. Data analysis indicated a notable decrease in cell proliferation when lncRNA DLGAP1-AS2 was knocked down. Apoptosis tests also revealed that the suppression of DLGAP1-AS2 promotes cancer cell apoptosis compared to the control group. Colony formation assay and Transwell assay further showed that the knockdown of DLGAP1-AS2 remarkably reduced the number of colonies. This transfection also resulted in inhibiting GC cell migration and invasion (Lu et al. 2021).
The study we conducted was a continuation of these results and showed that the combination of siRNA transfection and oxaliplatin drug, compared to a single treatment, increased the sensitivity of cancer cells to chemotherapy drugs and even reduced the effective dose of the medication. Apoptosis test through flow cytometry also proved that combined treatment induces cell death in this group more than other groups; in addition, the expression of apoptosis-related genes including Bax, Bcl-2, and Caspase-3 after combined treatment had more significant changes compared to the control and each monotherapy groups. Further tests showed a marked lowering in migration, size, and number of colonies following combination therapy. Also, qRT-PCR results showed a significant reduction in expression of CD44, a gene which is involved in stemness. Furthermore, the combination of DLGAP-1-AS2 siRNA and oxaliplatin, significantly increased Sub-G1 arrested cells compared to sole treatments. Evaluating expression of P53 also confirmed cell cycle results.
Conclusion
Our study demonstrated that targeting lncRNA DLGAP1-AS2 using siRNA could have a significant effect on malignant cells. Combination of siRNA-mediated inhibition of DLGAP1-AS2 with oxaliplatin can increase the efficacy of the treatment, consequently reducing drug resistance and increasing the chemosensitivity of tumor cells to chemotherapy. Through the combination method, proliferation, growth, and migration of the cells were reduced and apoptosis was induced. Taken together, our results implied the potential of DLGAP1-AS2 knockdown and oxaliplatin combination as a new therapeutic method to improve gastric cancer treatment.
Data availability
The results will be available upon the reasonable request from the corresponding author.
References
André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343–2351
Bhan A, Soleimani M, Mandal SS (2017) Long noncoding RNA and cancer: a new paradigm. Can Res 77:3965–3981
Biagioni A, Skalamera I, Peri S, Schiavone N, Cianchi F, Giommoni E, Magnelli L, Papucci L (2019) Update on gastric cancer treatments and gene therapies. Cancer Metastasis Rev 38:537–548
Cantile M, Di Bonito M, Cerrone M, Collina F, De Laurentiis M, Botti G (2020) Long non-coding RNA HOTAIR in breast cancer therapy. Cancers 12:1197
Devanabanda B, Kasi A (2022) Oxaliplatin. StatPearls [Internet]. StatPearls Publishing
Gao J-P, Xu W, Liu W-T, Yan M, Zhu Z-G (2018) Tumor heterogeneity of gastric cancer: from the perspective of tumor-initiating cell. World J Gastroenterol 24:2567
Gu Y, Chen T, Li G, Yu X, Lu Y, Wang H, Teng L (2015) LncRNAs: emerging biomarkers in gastric cancer. Future Oncol 11:2427–2441
Gutschner T, Diederichs S (2012) The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol 9:703–719
Joshi SS, Badgwell BD (2021) Current treatment and recent progress in gastric cancer. CA Cancer J Clin 71:264–279
Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F (2014) Gastric cancer: descriptive epidemiology, risk factors, screening, and preventiongastric cancer. Cancer Epidemiol Biomark Prev 23:700–713
Kumar K, Rani V, Mishra M, Chawla R (2022) New paradigm in combination therapy of siRNA with chemotherapeutic drugs for effective cancer therapy. Curr Res Pharmacol Drug Discov 3:100103. https://doi.org/10.1016/j.crphar.2022.100103
Liu Z, Pan L, Yan X, Duan X (2021) The long noncoding RNA DLGAP1-AS2 facilitates cholangiocarcinoma progression via miR-505 and GALNT10. FEBS Open Bio 11:413–422
Lu J, Xu Y, Xie W, Tang Y, Zhang H, Wang B, Mao J, Rui T, Jiang P, Zhang W (2021) Long noncoding RNA DLGAP1-AS2 facilitates Wnt1 transcription through physically interacting with Six3 and drives the malignancy of gastric cancer. Cell Death Discov 7:255
Lu J (2020) Expression and mechanism of long non-coding RNA DLGAP1-AS2 in gastric cancer. Tumor 153–162
Luo J, Zhang Y, Zheng T, Jing Y, Cao R, Wu M, Fan D, Tao Y, Zhao M (2021) LncRNA DLGAP1-AS2 suppresses the maturation of miR-16 to suppress cell invasion and migration of ovarian cancer cells. https://doi.org/10.21203/rs.3.rs-142142/v1
Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R (2020) Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci 21:4012
Miao W, Li N, Gu B, Yi G, Su Z, Cheng H (2020) LncRNA DLGAP1-AS2 modulates glioma development by up-regulating YAP1 expression. J Biochem 167:411–418
Mokhtari RB, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yeger H (2017) Combination therapy in combating cancer. Oncotarget 8:38022
Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, Meheus F, Verhoeven RH, Jo V, Laversanne M (2022) The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: a population-based modelling study. EClinicalMedicine 47:101404
National Library of Medicine (2024) DLGAP1-AS2: DLGAP1 antisense RNA 2 [Homo sapiens (human)]. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=84777. Accessed 2022
Pantel K, Alix-Panabières C, Riethdorf S (2009) Cancer micrometastases. nature reviews. Clin Oncol 6:339–351
Singh A, Trivedi P, Jain NK (2018) Advances in siRNA delivery in cancer therapy. Artif Cells Nanomed Biotechnol 46:274–283
Society, A.C. Stomach Cancer (n.d.). https://www.cancer.org/cancer/types/stomach-cancer.html
Soltani R, Amini M, Mazaheri Moghaddam M, Jebelli A, Ahmadiyan S, Bidar N, Baradaran B, MotieGhader H, Asadi M, Mokhtarzadeh A (2022) LncRNA DLGAP1-AS2 overexpression associates with gastric tumorigenesis: a promising diagnostic and therapeutic target. Mol Biol Rep 49(7):6817–6826. https://doi.org/10.1007/s11033-021-07038-w
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 71:209–249
Ye DM, Xu G, Ma W, Li Y, Luo W, Xiao Y, Liu Y, Zhang Z (2020) Significant function and research progress of biomarkers in gastric cancer. Oncol Lett 19:17–29
Ye J, Li J, Zhao P (2021) Roles of ncRNAs as ceRNAs in gastric cancer. Genes 12:1036
Acknowledgements
The authors wish to thank the financial support from the National Institute for Medical Research Development (NIMAD) of Iran (project no. 4021422). Also, the authors are thankful for the support of the Immunology Research Center, Tabriz University of Medical Science
Funding
The authors wish to thank the financial support from the National Institute for Medical Research Development (NIMAD) of Iran (project no. 4021422). Also, the authors are thankful for the support of the Immunology Research Center, Tabriz University of Medical Science.
Author information
Authors and Affiliations
Contributions
S.S.A, the first author of the manuscript, performed the experiment, contributed to the cellular and molecular assays, and wrote the manuscript. R.S revised the main text of the manuscript and revised the work critically for important intellectual content. M.A assisted in the cellular and molecular assays and analyzed the data. M.A.H.F revised the main text of the manuscript and revised the work critically for important intellectual content. S.A assisted in the molecular assays. S.N helped with the data categorization and interpreted the results. S.Z.B.M assisted in the cellular assays and revised the main text of manuscript. A.Y and L.N helped with the data categorization and interpreted the results. B.B revised the main text of the manuscript and revised the work critically for important intellectual content. A.A.M, the corresponding author of the manuscript, designed and supervised the project, and revised the main text of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Human ethics declaration
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azadi, S.S., Safaralizadeh, R., Amini, M. et al. Investigating the effect of LncRNA DLGAP1-AS2 suppression on chemosensitivity of gastric cancer to chemotherapy. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03130-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03130-7