Abstract
This study aimed to explore the role of miR-429 on the progression of oral squamous cell carcinoma (OSCC). OSCC cell lines were transfected with miR-429 mimic, pcDNA3.1-RUNX1, or pcDNA3.1-ITGB1, and their cell viability, apoptosis, migration, and invasion abilities were analyzed by cell counting, terminal deoxynucleotidyl transferase dUTP nick-end labeling staining, wound healing, and transwell assays, respectively. Furthermore, luciferase reporter assay, RNA pull-down, and ChIP were used to assess the regulation of miR-429, RUNX1, and ITGB1 expression in OSCC. Lastly, the biological role of the RUNX1/miR-429 feedback loop was explored in nude mice. The results revealed that miR-429 level was down-regulated, while RUNX1 and ITGB1 levels were up-regulated in OSCC tissues and that miR-429 was negatively correlated with RUNX1 and ITGB1 in OSCC tissues. Transfection of miR-429 mimic suppressed OSCC cell proliferation, migration, invasion, and epithelial–mesenchymal transition (EMT). Moreover, we found that miR-429 participated in OSCC progression by directly targeting ITGB1. Additionally, we found that RUNX1 negatively regulated miR-429 expression by binding to its promoter. Our results also revealed that the RUNX1/miR-429 feedback loop regulated ITGB1 expression and that RUNX1 overexpression rescued the inhibitory effects of miR-429 mimic on OSCC cells. In addition, miR-429 mimic significantly suppressed tumor growth, inflammatory cell infiltration, EMT, and ITGB1 expression in vivo, which were inhibited by RUNX1 overexpression. Altogether, these results indicate that the RUNX1/miR-429 feedback loop promoted growth, metastasis, and EMT in OSCC by targeting ITGB1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell carcinoma (HNSC) is one of the most common malignant tumors, accounting for 90% of all head and neck cancers (Johnson et al. 2020). Oral squamous cell cancer (OSCC), the primary subtype of HNSC, is a malignant tumor of the oral and maxillofacial region (Panarese et al. 2019). Approximately 350,000 new OSCC cases and 180,000 OSCC-related deaths are reported worldwide each year (Siegel et al. 2019). Surgical resection of the primary lesion combined with chemoradiotherapy are the main treatment modalities for OSCC (Bugshan and Farooq 2020). However, OSCC is prone to early metastasis and late diagnosis, resulting in poor prognosis. Over the years, the incidence of OSCC has increased gradually, while its five-year survival rate continues to remain low (Feng et al. 2019). However, the occurrence and progression of OSCC are poorly understood. Therefore, it is vital to explore the potential mechanisms and pathogenesis of OSCC to develop potential therapeutic strategies.
Epithelial–mesenchymal transition (EMT) is an important biological process in malignant tumor metastasis, including OSCC (Yao et al. 2020; Qin et al. 2022). EMT of OSCC transforms tumor cells into mesenchymal phenotypes, leading to invasion, lymphatic metastasis, and even distal metastasis of OSCC (Ling et al. 2021). EMT is characterized by the loss of adhesion molecules and an increase in mesenchymal markers (Chaw et al. 2012). Tumor occurrence is a complex multi-factor, multi-stage, and multi-step process that includes activation of oncogenes, inactivation of tumor suppressor genes, and mutation of mismatch repair genes (Sarode et al. 2018). Studies have used weighted correlation network analysis (WGCNA) to identify early diagnostic and prognostic biomarkers for OSCC (Zhang et al. 2018a, 2020a, 2021; Feng et al. 2020). For instance, Zhang X et al. (Zhang et al. 2018a) identified 10 hub genes that could serve as biomarkers and therapeutic targets for OSCC. Additionally, previous studies found that long noncoding RNAs SLC16A1-AS1 and LINC00472 may serve as novel diagnostic indicators for OSCC (Feng et al. 2020; Liu et al. 2022a).
MicroRNAs (miRNAs) are short (18–25 nt) endogenous non-coding RNAs that have gained increasing attention for over two decades (Chen et al. 2019). They have strong gene regulation abilities and play essential regulatory functions in cancer development (Yao et al. 2020; Jang et al. 2022). For instance, the expression of miR-128 is significantly down-regulated in OSCC, while its overexpression suppresses tumorigenesis and EMT in OSCC (Yao et al. 2020). Additionally, miR-485-5p level is significantly associated with the invasion and sphere-formation abilities of OSCC-derived cancer stem cells (Jang et al. 2022). Moreover, miR-429 level is associated with the progression of various cancers, including pancreatic cancer, bladder cancer, and colorectal cancer (Shen et al. 2019; Wu et al. 2018; Sun et al. 2014). However, recent studies have shown that miR-429 acts as a tumor suppressor in OSCC. Lei et al. (Lei et al. 2015) showed that miR-429 inhibits OSCC progression by targeting ZEB1. However, the regulatory effect and the underlying mechanism of miR-429 on OSCC EMT remain unclear.
ITGB1 is a laminin receptor protein and fibrinogen that negatively regulates cell adhesion and cell migration (Ju et al. 2017). Studies have confirmed that ITGB1 is one of the key genes that cause EMT in tumor cells (Wang and Chang 2020; Ren et al. 2020; Guo et al. 2019). Additionally, ITGB1 is crucial in OSCC progression (Takahara et al. 2017); however, its effect on the EMT of OSCC cells as well as its clinical significance needs to be further analyzed. RUNX1, a transcription factor involved in epithelial cell development, binds to the core locus of the promoter 5’-PYGPYGGT-3’ to regulate gene transcription (Lin 2022). Moreover, studies found that RUNX1 is a proto-oncogene that promotes tumor occurrence and EMT progression (Lin 2022; Li et al. 2019).
In this study, we found a RUNX1/miR-429 feedback loop in OSCC, resulting in a continuous increase in RUNX1 level and a decrease in miR-429 level, which ultimately promoted ITGB1 protein expression and EMT of OSCC cells.
Methods
Clinical samples
We collected 50 paired OSCC tissues and adjacent normal tissues from the Department of Stomatology, General Hospital of Ningxia Medical University (Ningxia, China). The inclusion criteria were as follows: (1) age: 40–65 years old; (2) diagnosis: OSCC with clear pathological data; and (3) data availability: complete general data. Patients who received treatment for OSCC before enrollment; had other malignant tumors and hematological diseases; or had diseases associated with infection and immunity were excluded from this study. Informed consent was obtained from all the participants included in the study. Our study was approved by the Ethics Committee of the General Hospital of Ningxia Medical University (Ethics approval number: KYLL-2021-772).
Immunohistochemistry (IHC) staining
The OSCC sections were treated with 3% hydrogen peroxide for 10 min. Thereafter, the sections were incubated with anti-RUNX1 (1:100, ab92336, Abcam) or anti-ITGB1 (1:1000, ab179471, Abcam, USA) antibodies at 4 °C overnight and secondary antibodies (1:2000, ab205718, Abcam, USA) at 37 °C for 1 h. The sections were then observed under an optical microscope (BX51, Olympus, Tokyo, Japan).
Cell culture
Human oral keratinocytes (HOKs) and oral squamous cell carcinoma (CSCC) cell lines (HN6, UM1, CAL27, and SCC9) were cultured in Dulbecco’s modified eagle medium (DMEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA) at 37 ℃ and 5% CO2.
Cell transfection
The cells were transfected with miR-429 mimic (5’-UAAUACUGUCUGGUAAAACCGU-3’) and miR-429 negative control (NC) mimic (5’-UUUGUACUACACAAAAGUACUG-3’). For overexpression of ITGB1 and RUNX1 expression, the cDNA sequences of human ITGB1 and RUNX1 were cloned into the pcDNA3.1 vector. All transfections were performed using Lipofectamine 3000 (Thermo, USA), according to the manufacturer’s instructions.
Cell counting assay
The CAL27 and SCC9 cells were incubated with 10 µL cell counting kit-8 (CCK-8) solution (Beyotime, China) at 37 ℃ for 2 h. Thereafter, their optical density was measured using a microplate reader at 450 nm.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining
After transfection, the CAL27 and SCC9 cells were fixed and incubated with 50 µl TUNEL test solution (Beyotime, China) at 37 ℃ for 1 h in the dark. Thereafter, the cells were treated with an anti-fluorescence quenching solution and observed under a fluorescence microscope (OLYMPUS, Tokyo, Japan).
Wound healing assay
The CAL27 and SCC9 cells were inoculated into 6-well plates for 24 h. Subsequently, a sterile micropipette tip was used to create a wound in the cell monolayer. Thereafter, the cells were cultured in fresh medium for 48 h and the wound width was observed and photographed to assess the cell migration ability.
Transwell assay
CAL27 and SCC9 cells were resuspended in DMEM and added to transwell chambers treated with or without Matrigel (BD Biosciences, USA) to assess in vitro cell invasion and migration, respectively. The culture medium containing 20% FBS was added into the lower chamber. After 24 h of incubation, the cells were stained and observed under an optical microscope (BX51, Olympus, Tokyo, Japan).
Luciferase reporter assay
The wild-type (WT) and mutant sequences of ITGB1 and RUNX1 were cloned into pGL3 luciferase reporter vectors, which were then transfected into CAL27 cells. The luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, USA).
Chromatin immunoprecipitation (ChIP) assay
ChIP was conducted using the ChIP IT Express kit (Active Motif, USA). Briefly, the chromatin was sheared by sonicating the cell lysate and then incubated with anti-RUNX1 antibodies (ab272456, Abcam, USA). Promoter binding was then analyzed by real-time quantitative reverse transcriptase-polymerase chain reaction (RT-qPCR) assay.
RNA pull-down assay
Cell lysates were incubated with miR-429 probe-coated beads at 4 ℃ overnight. Thereafter, the RNeasy Mini Kit (QIAGEN, Germany) was used to purify the eluted solution. The enrichment of ITGB1 and RUNX1 was analyzed by RT-qPCR.
In vivo experiments
Forty six-week-old female athymic nude mice were housed at 18–22 ℃. They were fed an ordinary diet and provided sufficient drinking water. The mice were subcutaneously injected with tumor cells (CAL27, 5 × 106 cells/mouse) and subjected to corresponding treatments. After 28 d, all mice were sacrificed and the tumor tissues were obtained for further study.
Hematoxylin and eosin (HE) staining
The tumor sections were paraffin-embedded and stained with 0.5% hematoxylin solution for 5 min and eosin solution for 3 min. Subsequently, the sections were observed under a light microscope.
Real-time quantitative reverse transcription PCR (RT-qPCR) assay
Total RNA was extracted from the tissue and cell samples using TRIzol reagent (Invitrogen, USA) and the RNA concentration was assessed by NanoDrop (Thermo, USA). The RT-qPCR assay was performed using the SYBR-Green kit (TaKaRa, Japan). The primers used for RT-qPCR are shown in Table 1. The relative gene expression levels were quantified using the 2−ΔΔCt method and normalized to that of U6 or GAPDH.
Western blot assay
Total protein was extracted from the tissue and cell samples using radioimmunoprecipitation assay buffer (Beyotime, China). The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. The membrane was then blocked with bovine serum albumin and incubated with anti-E-cadherin (1:1000, ab231303, Abcam, USA), anti-N-cadherin (1:5000, ab76011, Abcam, USA), anti-vimentin (1:1000, ab45939, Abcam, USA), anti-ITGB1 (1:2000, ab179471, Abcam, USA), anti-RUNX1 (1:1000, ab229482, Abcam, USA), and anti-GAPDH (1:5000, ab8245, Abcam, USA) antibodies overnight at 4 ℃. Subsequently, the membranes were incubated with goat anti-rabbit IgG (1:10000, ab6721, Abcam, USA) secondary antibodies for 2 h. Lastly, the membranes were treated with ECL chemiluminescence solution (Beyotime, Shanghai, China) and visualized with an enhanced chemiluminescence detection system. The development of specific protein blots was finished by treatment with enhanced chemiluminescence (ECL) reagent. Image J software (version 1.46r, ImageJ, NIH, Bethesda, MD, USA) was utilized to quantify the density of protein bands.
Statistical analysis
The data were presented as the mean ± standard deviation. One-way analysis of variance followed by Tukey’s post hoc test was used to analyze the data among multiple experimental groups. Significant differences between two groups were determined using a paired Student’s t-test. Statistical significance was set at P < 0.05.
Results
MiR-429 level was down-regulated and RUNX1 and ITGB1 levels were up-regulated in OSCC tissues
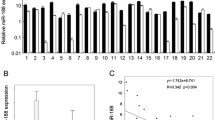
The RT-qPCR assay showed that miR-429 level was significantly decreased in OSCC tumor tissues (P < 0.001, Fig. 1A), while the IHC and RT-qPCR assays showed that RUNX1 and ITGB1 levels were significantly increased in OSCC tumor tissues (P < 0.001, Fig. 1B–E). Moreover, the results revealed that miR-429 level was significantly inversely associated with RUNX1 (P < 0.001) and ITGB1 (P < 0.05) levels in OSCC tissues (Fig. 1F, G). All these revealed that the down-expression of miR-429 and up-expression of RUNX1 and ITGB1 in OSCC tissues.
MiR-429 level was down-regulated and RUNX1 and ITGB1 levels were up-regulated in OSCC tissues. A MiR-429 mRNA expression in OSCC tumor tissues and adjacent tissues measured by RT-qPCR assay (n = 50). B RUNX1 protein level in OSCC tumor tissues and adjacent tissues assessed by IHC staining. C RUNX1 mRNA expression in OSCC tumor tissues and adjacent tissues measured by RT-qPCR assay (n = 50). D ITGB1 protein level in OSCC tumor tissues and adjacent tissues assessed by IHC staining. E ITGB1 mRNA expression in OSCC tumor tissues and adjacent tissues measured by RT-qPCR assay. (n = 50). F Pearson correlation analysis of miR-429 and RUNX1 expression in clinical samples of OSCC patients (n = 50). G Pearson correlation analysis of miR-429 and ITGB1 expression in clinical samples of OSCC patients (n = 50). ***P < 0.001 vs. Adjacent tissues group
MiR-429 overexpression suppressed OSCC cell proliferation, migration, invasion, and EMT
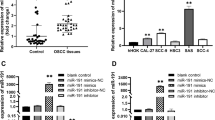
The RT-qPCR assay found that the miR-429 level was significantly decreased in various CSCC cell lines, including CAL27, HN6, UM1, and SCC9, compared with HOKs (P < 0.01, P < 0.001, Fig. 2A). Since miR-429 expression was relatively lower in CAL27 and SCC9 cell lines, these two were used in subsequent experiments. Successful transfection of miR-429 mimic was confirmed by the RT-qPCR assay (P < 0.001, Fig. 2B). MiR-429 mimic significantly suppressed cell viability (P < 0.001, Fig. 2C) and significantly increased apoptotic rate of CAL27 and SCC9 cells (P < 0.001, Fig. 2D). Wound healing assay revealed that miR-429 mimic significantly inhibited cell migration (P < 0.001, Fig. 2E), while transwell assay showed that miR-429 mimic significantly suppressed cell migration and invasion of CAL27 and SCC9 cells (P < 0.01, P < 0.001, Fig. 2F). Moreover, miR-429 mimic significantly promoted E-cadherin and inhibited N-cadherin and vimentin levels in CAL27 and SCC9 cells (P < 0.01, P < 0.001, Fig. 2G). Based on above, miR-429 overexpression inhibits the proliferation, migration, invasion, and EMT of OSCC cells.
MiR-429 overexpression suppressed OSCC cell proliferation, migration, invasion, and EMT. A MiR-429 mRNA expression in OSCC cell lines (including CAL27, HN6, UM1, and SCC9) and HOKs measured by RT-qPCR assay. **P < 0.01, ***P < 0.001 vs. HOKs group. MiR-429 mimic and miR-429 NC mimic were transfected into CAL27 and SCC9 cells. B MiR-429 mRNA expression in CAL27 and SCC9 cells measured by RT-qPCR assay. C Cell viability of CAL27 and SCC9 cells assessed by CCK-8 assay. D Cell apoptosis of CAL27 and SCC9 cells assessed by TUNEL staining. E Cell migration of CAL27 and SCC9 cells assessed by wound healing assay. F Cell migration and invasion of CAL27 and SCC9 cells assessed by transwell assay. G The protein levels of EMT-related markers (E-cadherin, N-cadherin, and vimentin) detected by western blot assay. **P < 0.01, ***P < 0.001 vs. NC mimic group
MiR-429 directly targeted ITGB1 in OSCC cells
StarBase, an online bioinformatics database, was used to retrieve miR-429-related genes, and the potential binding sites between miR-429 and ITGB1 are shown in Fig. 3A. Luciferase reporter assay revealed that miR-429 suppressed the luciferase activity of the ITGB1 WT (P < 0.01, P < 0.001, Fig. 3B). ITGB1 level was up-regulated in the biotin–miR-429 group (P < 0.001, Fig. 3C). Furthermore, miR-429 overexpression significantly down-regulated mRNA and protein expression of ITGB1 in CAL27 and SCC9 cells (P < 0.001, Fig. 3D, E). These findings confirm that in OSCC cells, miR-429 directly targets ITGB1.
MiR-429 directly targeted ITGB1 in OSCC cells. A StarBase was used to predict the binding sites of miR-429 on 3’-UTR of ITGB1. B The interaction between miR-429 and ITGB1 assessed by luciferase reporter assay. C The interaction between miR-429 and ITGB1 assessed by RNA pull-down assay. D The effect of miR-429 mimic on ITGB1 mRNA expression detected by RT-qPCR assay. E The effect of miR-429 mimic on ITGB1 protein expression detected by western blot assay. **P < 0.01, ***P < 0.001 vs. NC mimic group or Biotin-NC group
MiR-429 overexpression suppressed OSCC progression by targeting ITGB1
To confirm the functional relevance of ITGB1 in OSCC, we targeted miR-429. MiR-429 mimic significantly inhibited ITGB1 mRNA and protein expression (P < 0.01, P < 0.001), while ITGB1 overexpression significantly promoted ITGB1 expression (P < 0.01, P < 0.001, Fig. 4A, B). The miR-429 mimic + NC OE group prominently suppressed CAL27 cell viability compared with the NC mimic + NC OE group (P < 0.001), while the miR-429 NC mimic + ITGB1 OE group effectively blocked this effect induced by miR-429 mimic (P < 0.001, Fig. 4C). TUNEL assay showed that transfection with miR-429 mimic significantly increased cell apoptosis (P < 0.001), while transfection with pcDNA3.1-ITGB1 returned such phenomenon (P < 0.01, Fig. 4D). Furthermore, transfection with pcDNA3.1-ITGB1 reversed miR-429-induced migration, invasion, and EMT (P < 0.05, P < 0.01, P < 0.001, Fig. 4E–G). These results indicate that miR-429 mimic participates in OSCC progression by targeting ITGB1.
MiR-429 overexpression suppressed OSCC progression by targeting ITGB1. CAL27 cells were co-transfected with miR-429 mimic or/and pcDNA3.1-ITGB1. A ITGB1 mRNA expression in CAL27 cells measured by RT-qPCR assay. B ITGB1 protein expression in CAL27 cells measured by western blot assay. C Cell viability of CAL27 cells assessed by CCK-8 assay. D Cell apoptosis of CAL27 cells assessed by TUNEL staining. E Cell migration of CAL27 cells assessed by wound healing assay. F Cell migration and invasion of CAL27 cells assessed by transwell assay. G The protein levels of EMT-related markers (E-cadherin, N-cadherin, and vimentin) detected by western blot assay. **P < 0.01, ***P < 0.001 vs. NC mimic + NC OE group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. miR-429 mimic + NC OE group
RUNX1 regulated miR-429 expression via a feedback loop
StarBase also showed a complementary pairing region between miR-429 and RUNX1 that is located at the 3’-UTR of RUNX1 (Fig. 5A). Luciferase reporter assay revealed that miR-429 significantly decreased the luciferase activity of RUNX1 WT (P < 0.001, Fig. 5B), while RNA pull-down assay revealed that RUNX1 was precipitated by miR-429 probe (P < 0.001, Fig. 5C). In addition, miR-429 significantly decreased RUNX1 expression (P < 0.01, P < 0.001, Fig. 5D, E). Furthermore, ITGB1 overexpression increased miR-429 level in OSCC cell line, CAL27 cells transfected with pcDNA3.1-ITGB1. The transfection efficiency of RUNX1 overexpression was also verified (P < 0.001, Fig. 5F, G). RUNX1 overexpression significantly decreased miR-429 level (P < 0.01, Fig. 5H). JASPAR database was used to estimate RUNX1 binding sites on the miR-429 promoter region (Fig. 5I), and the results revealed that RUNX1 could target a region upstream of the pre-miR-429 promoter (Fig. 5J). RUNX1 overexpression significantly increased the luciferase activity in the WT miR-429 promoter (P < 0.001), while miR-429 knockdown significantly decreased this effect (P < 0.01, Fig. 5K, L). Altogether, these results indicate that RUNX1 binds to the miR-429 promoter and negatively regulates its expression.
RUNX1 regulated miR-429 expression via a feedback loop. A StarBase was used to predict the binding sites of miR-429 on 3’-UTR of RUNX1. B The interaction between miR-429 and RUNX1 assessed by luciferase reporter assay. C The interaction between miR-429 and RUNX1 assessed by RNA pull-down assay. D The effect of miR-429 mimic on RUNX1 mRNA expression detected by RT-qPCR assay. E The effect of miR-429 mimic on RUNX1 protein expression detected by western blot assay. F The transfection efficiency of RUNX1 overexpression verified by RT-qPCR assay. G The transfection efficiency of RUNX1 overexpression verified by western blot assay. H MiR-429 mimic expression in CAL27 cells transfected with pcDNA3.1-RUNX1 assessed by RT-qPCR assay. I The binding sites of the miR-429 promoter region on RUNX1 predicted using the JASPAR database. J The interaction between RUNX1 and miR-429 assessed by ChIP assay. K, L The miR-429 promoter-driven luciferase activity in CAL27 cells transfected with pcDNA3.1-RUNX1 and miR-429 inhibitor assessed by luciferase reporter assay. **P < 0.01, ***P < 0.001 vs. NC mimic group or Biotin-NC group; ##P < 0.01 vs. RUNX1 OE group
RUNX1/miR-429 feedback loop regulated ITGB1 expression and was associated with OSCC progression
Next, we determined whether RUNX1/miR-429 feedback loop was involved in OSCC progression. MiR-429 mimic remarkably suppressed RUNX1 and ITGB1 expression (P < 0.001), which was significantly inhibited by RUNX1 overexpression (P < 0.05, P < 0.001, Fig. 6A, B), suggesting that RUNX1/miR-429 feedback loop regulated ITGB1 expression. Therefore, RUNX1 overexpression rescued the miR-429 mimic-induced inhibition on OSCC cell viability, anti-apoptosis, cell migration, invasion, and EMT (P < 0.01, P < 0.001, Fig. 6C–G). Together, RUNX1/miR-429 feedback loop regulates ITGB1 expression and is involved in OSCC progression.
RUNX1/miR-429 feedback loop regulated ITGB1 expression and was associated with OSCC progression. CAL27 cells were co-transfected with miR-429 mimic and/or pcDNA3.1-RUNX1. A RUNX1 and ITGB1 mRNA expression in CAL27 cells detected by RT-qPCR assay. B RUNX1 and ITGB1 protein expression in CAL27 cells detected by western blot assay. C Cell viability of CAL27 cells assessed by CCK-8 assay. D Cell apoptosis of CAL27 cells assessed by TUNEL staining. E Cell migration of CAL27 cells assessed by wound healing assay. F Cell migration and invasion of CAL27 cells assessed by transwell assay. G The protein levels of EMT-related markers (E-cadherin, N-cadherin, and vimentin) detected by western blot assay. **P < 0.01, ***P < 0.001 vs. NC mimic + NC OE group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. miR-429 mimic + NC OE group
RUNX1/miR-429 feedback loop regulated OSCC progression in vivo
To explore the biological role of RUNX1/miR-429 feedback loop in OSCC progression, CAL27 cells with corresponding treatments were injected into nude mice. The results revealed that miR-429 mimic significantly suppressed tumor growth (P < 0.01), which was inhibited by RUNX1 overexpression (P < 0.001, Fig. 7A–C). Moreover, HE staining showed that RUNX1 overexpression partially rescued the miR-429 mimic-induced inhibitory effects on inflammatory cell infiltration (Fig. 7D). However, RUNX1 overexpression impaired miR-429-induced inhibition of RUNX1 and ITGB1 expression (Fig. 7E). The RT-qPCR assay suggested that miR-429 mimic increased miR-429 level and decreased RUNX1 and ITGB1 levels (P < 0.001), while RUNX1 overexpression decreased miR-429 level and increased RUNX1 and ITGB1 levels (P < 0.01, P < 0.001, Fig. 7F). Furthermore, RUNX1 overexpression impaired the miR-429 mimic-induced inhibition of EMT-related protein expression (P < 0.001, Fig. 7G). These above indicate RUNX1/miR-429 feedback loop regulates OSCC progression in vivo.
RUNX1/miR-429 feedback loop regulated OSCC progression in vivo. CAL27 cells with corresponding treatments were subcutaneously injected into nude mice. A The tumor volume = (length × width2)/2) was recorded once every week. B Images of the isolated tumors of the indicated group. C The weights of the isolated tumors of the indicated groups. D Histopathology of tumor sections stained with HE staining. E ITGB1 and RUNX1 protein expression in the tumor tissues detected by IHC staining. F MiR-429, ITGB1, and RUNX1 mRNA expression in the tumor tissues measured by RT-qPCR assay. G The protein levels of EMT-related markers (E-cadherin, N-cadherin, and vimentin) in the tumor tissues detected by western blot assay. **P < 0.01, ***P < 0.001 vs. NC mimic + NC OE group; ##P < 0.01, ###P < 0.001 vs. miR-429 mimic + NC OE group
Discussion
Due to the lack of effective diagnostic biomarkers, several OSCC patients are diagnosed with intermediate or advanced cancer at initial diagnosis (Bugshan and Farooq 2020). Many studies have highlighted the potential of tumor-related genes as non-invasive biomarkers for cancer diagnosis and therapeutics (Zhang et al. 2018a). For instance, a previous study used WGCNA to build a co-expression network and found two modules (turquoise and brown modules) and 10 hub genes (MMP1, TNFRSF12A, PLAU, FSCN1, PDPN, KRT78, EVPL, GGT6, SMIM5, and CYRST1) that were associated with OSCC tumorigenesis (Zhang et al. 2018a). A previous study found the presence of abnormally methylated differentially expressed genes and pathways in OSCC, thus shedding light on the underlying molecular processes in OSCC (Zhang et al. 2018b). A recent study reported that aspirin inhibits OSCC proliferation, increases apoptosis, and reduces migration and invasion via the PI3K-Akt and focal adhesion pathways (Zhang et al. 2018c). It is well established that OSCC invasion and metastasis are important factors leading to poor prognosis and that EMT of OSCC cells transforms tumor cells into interstitial phenotypes, leading to distal metastasis of OSCC (Ling et al. 2021). In this study, we found that the RUNX1/miR-429 feedback loop regulated ITGB1 expression and OSCC proliferation, migration, invasion, and EMT.
Aberrant expression of miR-429 in cancer cells contributes to cancer progression (Zhang et al. 2020b). For instance, down-regulation of miR-429 promotes proliferation, invasion, and EMT in breast cancer (Zhang et al. 2020b). However, miR-429 plays an inhibitory role in pancreatic cancer (Shen et al. 2019). Previous studies found that miR-429 can serve as a potential diagnostic, therapeutic, and prognostic biomarker in certain cancers (Guo et al. 2020; Garajei et al. 2023). In our study, we reported that miR-429 expression was decreased in OSCC tissues, indicating that the dysregulation of miR-429 expression may be associated with OSCC development and metastasis. Additionally, we found that miR-429 overexpression significantly inhibited the growth and metastasis of OSCC. These results are consistent with the findings of Lei W (Lei et al. 2015) and Sun et al. (2021a), who found that miR-429 played an inhibitory role in OSCC carcinogenesis. EMT is a typical feature of malignant tumors originating from epithelial cells and is characterized by the loss of cell-to-cell adhesion and the acquisition of spindle morphology to establish a motile and invasive phenotype (Joseph et al. 2018). EMT of OSCC cells can transform tumor cells into mesenchymal phenotypes, leading to invasion, lymphatic metastasis, and even distal metastasis of OSCC (Joseph et al. 2018). MiR-429 dysregulation has been implicated in the EMT of several types of cancers (Shen et al. 2019; Sun et al. 2021b). For instance, Sun Y et al. reported that miR-429 was significantly down-regulated in colorectal carcinoma tissues and that it reversed the TGF-β1-induced variations in the expression of EMT-related marker genes (Sun et al. 2021b). However, information about the effect of miR-429 on the EMT process in OSCC is limited. In our study, we found that miR-429 overexpression significantly promoted E-cadherin and inhibited N-cadherin and vimentin levels in OSCC cells. These results indicate that miR-429 suppressed cell proliferation, migration, invasion, and EMT in OSCC.
MiRNAs modulate cancer cell function by directly targeting mRNAs and negatively regulating mRNA expression (Liu et al. 2022b). A study found that miR-429 inhibits osteosarcoma development by targeting ZEB1 (Deng et al. 2017). Another study found that miR-429 suppresses gastric cancer development and invasion by targeting FSCN1 (Zhang et al. 2016). In this study, we used StarBase to predict miR-429-related target genes, and the results revealed that ITGB1 may interact with miR-429. Indeed, our results revealed that miR-429 directly targeted ITGB1 in OSCC. Furthermore, ITGB1 expression was increased, while miR-429 levels were decreased in the clinical samples of OSCC patients, indicating a negative correlation between ITGB1 and miR-429 in OSCC. Moreover, our results revealed that miR-429 suppressed the invasion and metastasis of OSCC by targeting ITGB1. Previous studies showed that ITGB1 expression is regulated by miRNAs and is involved in cancer growth and metastasis (Ding et al. 2017; Zhang et al. 2019). Ding et al. (2017) showed that miR-9-3p inhibited EMT of nasopharyngeal carcinoma cells by targeting ITGB1 protein expression, while Zhang et al. (2019) reported that miR-3653 inhibited EMT by targeting ITGB1 inhibition, thus reducing the risk of liver cancer metastasis.
StarBase also showed a complementary pairing region on RUNX1 (located at its 3’-UTR) that could bind to miR-429. Our results confirmed that miR-429 directly targeted and reduced RUNX1 expression in OSCC cells. A bioinformatics analysis based on clinical samples indicated that RUNX1 was overexpressed in OSCC and was associated with poor prognosis of OSCC patients (Feng et al. 2021). In this study, we found that RUNX1 expression was up-regulated in OSCC and that RUNX1 and miR-429 levels were inversely associated with OSCC.
Increasing evidence suggests that miRNA expression is regulated by transcription factors, which bind by recognizing specific sequences of gene promoters to promote or inhibit transcription levels (Jiang et al. 2016). Studies have also shown that RUNX1 can regulate the expression of both protein-coding genes and noncoding genes such as miRNAs in several biological processes (Cheng et al. 2017). For instance, up-regulation of RUNX1 reduces miR-93 level in pancreatic cancer cells, thus promoting cancer progression (Cheng et al. 2017). Another study showed that RUNX1 regulates miR-582-5p to suppress clear cell renal cell carcinoma progression (Xue et al. 2021). Recent studies have clarified the mechanism of RUNX1/miRNA feedback regulation in several cancers (Wang et al. 2015). Wang et al. (Wang et al. 2015) found that the expression of miR-101 and RUNX1 is negatively correlated in lung cancer and that miR-429 targets and regulates RUNX1 at the post-transcriptional level. Additionally, RUNX1 knockdown increases miR-101 level and increases miR-101 promoter-driven luciferase activity in lung cancer cells, suggesting that RUNX1 inhibits miR-101 transcription by directly binding to miR-101 promoter. This phenomenon was also observed in breast cancer, wherein RUXN1 negatively regulates miR-378 transcription, while miR-378 inhibits the expression of RUXN1 (Browne et al. 2016). These studies indicate the presence of a feedback loop between RUNX1 and miRNAs. Considering that RUNX1 is an important transcription factor that participates in cancer progression, it is necessary to verify whether RUNX1 feedback regulates miR-429 transcription in OSCC. Therefore, we transfected the OSCC cell line CAL27 with pcDNA3.1-ITGB1 and then detected miR-429 levels in cells overexpressing RUNX1. The results revealed that RUNX1 overexpression significantly decreased miR-429 expression. ChIP and luciferase activity analysis further confirmed the presence of RUNX1/miR-429 feedback loop in OSCC. Several studies confirmed the presence of the transcription factor–miRNA–mRNA axis in the regulation of many biological processes (Xue et al. 2021; Fischer et al. 2015). Xue et al. (2021) indicated that the RUNX1/miR-582-5p suppressed the growth and migration of clear cell renal cell carcinoma by targeting COL5A1. In this study, we found that RUNX1/miR-429 feedback loop regulated ITGB1 expression and that RUNX1 rescued the inhibitory effects of miR-429 mimic on OSCC growth and EMT.
To our knowledge, this is the first study to explore the regulatory mechanism of miR-429 on the EMT process in OSCC cells. The comprehensive analysis of the RUNX1/miR-429/ITGB1 axis can further improve our understanding of the underlying mechanism involved in OSCC metastasis and EMT and provide potential targets and theoretical basis for the diagnosis and treatment of OSCC.
Conclusion
In current study, we explored the effects of the RUNX1/miR-429 feedback loop on the expression of RUNX1, miR-429, and ITGB1 in OSCC, to analyze its effects on OSCC metastasis and EMT. Our results revealed that the RUNX1/miR-429 feedback loop promotes growth, metastasis, and EMT in OSCC by targeting ITGB1. This study may provide novel molecular targets and theoretical basis for the clinical diagnosis and treatment of OSCC.
Data availability
No datasets were generated or analysed during the current study.
References
Browne G, Dragon JA, Hong D, Messier TL, Gordon JA, Farina NH, Boyd JR, VanOudenhove JJ, Perez AW, Zaidi SK et al (2016) MicroRNA-378-mediated suppression of Runx1 alleviates the aggressive phenotype of triple-negative MDA-MB-231 human breast cancer cells. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine 37(7):8825–8839
Bugshan A, Farooq I (2020) Oral squamous cell carcinoma: metastasis, potentially associated malignant disorders, etiology and recent advancements in diagnosis. F1000Research 9:229
Chaw SY, Abdul Majeed A, Dalley AJ, Chan A, Stein S, Farah CS (2012) Epithelial to mesenchymal transition (EMT) biomarkers–E-cadherin, beta-catenin, APC and vimentin–in oral squamous cell carcinogenesis and transformation. Oral Oncol 48(10):997–1006
Chen L, Heikkinen L, Wang C, Yang Y, Sun H, Wong G (2019) Trends in the development of miRNA bioinformatics tools. Brief Bioinform 20(5):1836–1852
Cheng Y, Yang H, Sun Y, Zhang H, Yu S, Lu Z, Chen J (2017) RUNX1 promote invasiveness in pancreatic ductal adenocarcinoma through regulating miR-93. Oncotarget 8(59):99567–99579
Deng Y, Luan F, Zeng L, Zhang Y, Ma K (2017) MiR-429 suppresses the progression and metastasis of osteosarcoma by targeting ZEB1. EXCLI J 16:618–627
Ding Y, Pan Y, Liu S, Jiang F, Jiao J (2017) Elevation of MiR-9-3p suppresses the epithelial-mesenchymal transition of nasopharyngeal carcinoma cells via down-regulating FN1, ITGB1 and ITGAV. Cancer Biol Ther 18(6):414–424
Feng RM, Zong YN, Cao SM, Xu RH (2019) Current cancer situation in China: good or bad news from the 2018 Global Cancer statistics? Cancer Commun (London England) 39(1):22
Feng H, Zhang X, Lai W, Wang J (2020) Long non-coding RNA SLC16A1-AS1: its multiple tumorigenesis features and regulatory role in cell cycle in oral squamous cell carcinoma. Cell Cycle (Georgetown Tex) 19(13):1641–1653
Feng X, Zheng Z, Wang Y, Song G, Wang L, Zhang Z, Zhao J, Wang Q, Lun L (2021) Elevated RUNX1 is a prognostic biomarker for human head and neck squamous cell carcinoma. Experimental Biology and Medicine (Maywood NJ) 246(5):538–546
Fischer J, Rossetti S, Datta A, Eng K, Beghini A, Sacchi N (2015) miR-17 deregulates a core RUNX1-miRNA mechanism of CBF acute myeloid leukemia. Mol Cancer 14:7
Garajei A, Allameh A, Azadi M, Emami A, Atashbasteh M, Mostafavi M, Ghaderi B, Inchingolo F, Sadeghi M, Tadakamadla SK et al (2023) : Evaluation of the Expression Levels of miR-21-5p and miR-429 Genes in Biopsy Samples from Patients with Oral Squamous Cell Carcinoma. Diagnostics (Basel, Switzerland) 13(7)
Guo L, Sun C, Xu S, Xu Y, Dong Q, Zhang L, Li W, Wang X, Ying G, Guo F (2019) Knockdown of long non-coding RNA linc-ITGB1 inhibits cancer stemness and epithelial-mesenchymal transition by reducing the expression of snail in non-small cell lung cancer. Thorac cancer 10(2):128–136
Guo CM, Liu SQ, Sun MZ (2020) miR-429 as biomarker for diagnosis, treatment and prognosis of cancers and its potential action mechanisms: a systematic literature review. Neoplasma 67(2):215–228
Jang TH, Huang WC, Tung SL, Lin SC, Chen PM, Cho CY, Yang YY, Yen TC, Lo GH, Chuang SE et al (2022) MicroRNA-485-5p targets keratin 17 to regulate oral cancer stemness and chemoresistance via the integrin/FAK/Src/ERK/β-catenin pathway. J Biomed Sci 29(1):42
Jiang W, Mitra R, Lin CC, Wang Q, Cheng F, Zhao Z (2016) Systematic dissection of dysregulated transcription factor-miRNA feed-forward loops across tumor types. Brief Bioinform 17(6):996–1008
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR (2020) Head and neck squamous cell carcinoma. Nat Reviews Disease Primers 6(1):92
Joseph JP, Harishankar MK, Pillai AA, Devi A (2018) Hypoxia induced EMT: a review on the mechanism of tumor progression and metastasis in OSCC. Oral Oncol 80:23–32
Ju JA, Godet I, Ye IC, Byun J, Jayatilaka H, Lee SJ, Xiang L, Samanta D, Lee MH, Wu PH et al (2017) Hypoxia selectively enhances integrin α(5)β(1) receptor expression in breast Cancer to promote metastasis. Mol cancer Research: MCR 15(6):723–734
Lei W, Liu YE, Zheng Y, Qu L (2015) MiR-429 inhibits oral squamous cell carcinoma growth by targeting ZEB1. Med Sci Monitor: Int Med J Experimental Clin Res 21:383–389
Li Q, Lai Q, He C, Fang Y, Yan Q, Zhang Y, Wang X, Gu C, Wang Y, Ye L et al (2019) RUNX1 promotes tumour metastasis by activating the Wnt/β-catenin signalling pathway and EMT in colorectal cancer. J Experimental Clin cancer Research: CR 38(1):334
Lin TC (2022) RUNX1 and cancer. Biochim et Biophys acta Reviews cancer 1877(3):188715
Ling Z, Cheng B, Tao X (2021) Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: challenges and opportunities. Int J Cancer 148(7):1548–1561
Liu X, Ma X, Li H, Wang Y, Mao M, Liang C, Hu Y (2022a) LINC00472 suppresses oral squamous cell carcinoma growth by targeting miR-455-3p/ELF3 axis. Bioengineered 13(1):1162–1173
Liu J, Yang T, Huang Z, Chen H, Bai Y (2022b) : Transcriptional regulation of nuclear miRNAs in tumorigenesis (review). Int J Mol Med 50(1)
Panarese I, Aquino G, Ronchi A, Longo F, Montella M, Cozzolino I, Roccuzzo G, Colella G, Caraglia M, Franco R (2019) Oral and oropharyngeal squamous cell carcinoma: prognostic and predictive parameters in the etiopathogenetic route. Expert Rev Anticancer Ther 19(2):105–119
Qin SY, Li B, Chen M, Qin MQ, Liu JM, Lv QL (2022) MiR-32-5p promoted epithelial-to-mesenchymal transition of oral squamous cell carcinoma cells via regulating the KLF2/CXCR4 pathway. Kaohsiung J Med Sci 38(2):120–128
Ren L, Mo W, Wang L, Wang X (2020) Matrine suppresses breast cancer metastasis by targeting ITGB1 and inhibiting epithelial-to-mesenchymal transition. Experimental and Therapeutic Medicine 19(1):367–374
Sarode GS, Sarode SC, Maniyar N, Anand R, Patil S (2018) Oral cancer databases: a comprehensive review. J oral Pathol Medicine: Official Publication Int Association Oral Pathologists Am Acad Oral Pathol 47(6):547–556
Shen J, Hong L, Yu D, Cao T, Zhou Z, He S (2019) LncRNA XIST promotes pancreatic cancer migration, invasion and EMT by sponging miR-429 to modulate ZEB1 expression. Int J Biochem Cell Biol 113:17–26
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. Cancer J Clin 69(1):7–34
Sun Y, Shen S, Liu X, Tang H, Wang Z, Yu Z, Li X, Wu M (2014) MiR-429 inhibits cells growth and invasion and regulates EMT-related marker genes by targeting Onecut2 in colorectal carcinoma. Mol Cell Biochem 390(1–2):19–30
Sun B, Zheng X, Ye W, Zhao P, Ma G (2021a) : LncRNA LINC01303 Promotes the Progression of Oral Squamous Cell Carcinomas via the miR-429/ZEB1/EMT Axis. Journal of oncology 2021:7974012
Sun Y, Shen S, Liu X, Tang H, Wang Z, Yu Z, Li X, Wu M (2021b) Correction to: miR-429 inhibits cells growth and invasion and regulates EMT-related marker genes by targeting Onecut2 in colorectal carcinoma. Mol Cell Biochem 476(8):3215–3216
Takahara T, Kasamatsu A, Yamatoji M, Iyoda M, Kasama H, Saito T, Takeuchi S, Endo-Sakamoto Y, Shiiba M, Tanzawa H et al (2017) SIPA1 promotes invasion and migration in human oral squamous cell carcinoma by ITGB1 and MMP7. Exp Cell Res 352(2):357–363
Wang N, Chang LL (2020) Maspin suppresses cell invasion and migration in gastric cancer through inhibiting EMT and angiogenesis via ITGB1/FAK pathway. Hum Cell 33(3):663–675
Wang X, Zhao Y, Qian H, Huang J, Cui F, Mao Z (2015) The miR-101/RUNX1 feedback regulatory loop modulates chemo-sensitivity and invasion in human lung cancer. Int J Clin Exp Med 8(9):15030–15042
Wu CL, Ho JY, Hung SH, Yu DS (2018) miR-429 expression in bladder cancer and its correlation with tumor behavior and clinical outcome. Kaohsiung J Med Sci 34(6):335–340
Xue J, Zhu S, Qi F, Zhu K, Cao P, Yang J, Wang Z (2021) RUNX1/miR-582-5p pathway regulates the Tumor Progression in Clear Cell Renal Cell Carcinoma by Targeting COL5A1. Front Oncol 11:610992
Yao Y, Xu Q, Yan L, Jiao Y, Su Q, Li X, Liu C, Zhao F (2020) MiRNA-128 and MiRNA-142 regulate tumorigenesis and EMT in oral squamous cell carcinoma through HOXA10. Cancer Manage Res 12:9987–9997
Zhang M, Dong BB, Lu M, Zheng MJ, Chen H, Ding JZ, Xu AM, Xu YH (2016) miR-429 functions as a tumor suppressor by targeting FSCN1 in gastric cancer cells. OncoTargets and Therapy 9:1123–1133
Zhang X, Feng H, Li Z, Li D, Liu S, Huang H, Li M (2018a) Application of weighted gene co-expression network analysis to identify key modules and hub genes in oral squamous cell carcinoma tumorigenesis. OncoTargets and Therapy 11:6001–6021
Zhang X, Feng H, Li D, Liu S, Amizuka N, Li M (2018b) Identification of differentially expressed genes Induced by aberrant methylation in oral squamous cell carcinomas using Integrated Bioinformatic Analysis. Int J Mol Sci 19(6)
Zhang X, Feng H, Li Z, Guo J, Li M (2018c) Aspirin is involved in the cell cycle arrest, apoptosis, Cell Migration, and Invasion of oral squamous cell carcinoma. Int J Mol Sci 19(7)
Zhang L, Zhang T, Deng Z, Sun L (2019) MicroRNA–3653 inhibits the growth and metastasis of hepatocellular carcinoma by inhibiting ITGB1. Oncol Rep 41(3):1669–1677
Zhang X, Ren L, Yan X, Shan Y, Liu L, Zhou J, Kuang Q, Li M, Long H, Lai W (2020a) Identification of immune-related lncRNAs in periodontitis reveals regulation network of gene-lncRNA-pathway-immunocyte. Int Immunopharmacol 84:106600
Zhang L, Liu Q, Mu Q, Zhou D, Li H, Zhang B, Yin C (2020b) MiR-429 suppresses proliferation and invasion of breast cancer via inhibiting the Wnt/β-catenin signaling pathway. Thorac cancer 11(11):3126–3138
Zhang X, Zhang S, Yan X, Shan Y, Liu L, Zhou J, Kuang Q, Li M, Long H, Lai W (2021) m6A regulator-mediated RNA methylation modification patterns are involved in immune microenvironment regulation of periodontitis. J Cell Mol Med 25(7):3634–3645
Acknowledgements
None.
Funding
Ningxia Natural Science Foundation Project Plan (Project number: 2022AAC03565).
Author information
Authors and Affiliations
Contributions
Conceptualisation, XZ, and XL; formal analysis, YY, JC, and TZ; data curation, XZ, YY, JC, and TZ; writing, original draft preparation, XL; writing, review and editing, XZ, and XL; supervision, XZ; project administration, XZ, and XL; and funding acquisition, XZ. All authors have read and agreed to the published version of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
The consent agreement was signed by all patients and the project was approved by the Ethics Committee of General Hospital of Ningxia Medical University (Ethics approval number: KYLL-2021-772).
Consent to participate
All individuals have individual rights that are not to be infringed. Identifying details (names, dates of birth, identity numbers, biometrical characteristics (such as facial features, fingerprint, writing style, voice pattern, DNA or other distinguishing characteristic) and other information) of the participants in the study will not be published. When biological material is donated or data generated as part of a research project, as part of the informed consent process, we ensure that participants understand what kind of (personal) data will be processed, how it will be used and for what purpose. Informed written consent was obtained from all patients included in the study.
Consent for publication
All authors declare that they have seen and approved the submitted version of this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, X., Yang, Y., Chen, J. et al. RUNX1/miR-429 feedback loop promotes growth, metastasis, and epithelial-mesenchymal transition in oral squamous cell carcinoma by targeting ITGB1. Naunyn-Schmiedeberg's Arch Pharmacol 397, 5289–5302 (2024). https://doi.org/10.1007/s00210-024-02960-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-024-02960-9