Abstract
Serotonin is a neurotransmitter, which is involved in memory via its receptors. The 5-HT1D and 5-HT1F receptors mainly exist in the hippocampus, which plays an important role in memory processing. However, few studies have assessed the effect of these serotonin receptors on memory. We evaluated the effect of a 5-HT1D receptor agonist, PNU142633, 5-HT1D receptor antagonist, BRL15572 hydrochloride, and 5-HT1F receptor agonist, LY344864, on the recognition and avoidance memory in the hippocampus area. Fifty adult male Wistar rats weighing 200–250 g were divided into the control, sham-operated, PNU, BRL, and LY groups (n=10 per group). Bilateral guide cannulas were implanted into the dentate gyrus area of the hippocampus. The drugs were administered at the dose of 1 μg/μl before the novel object recognition (NOR) and passive avoidance learning (PAL) tests. The results showed that in the NOR test, the administration of PNU and LY had no significant effect on recognition index; however, the recognition index was increased by BRL. In the PAL test, the administration of PNU had no significant effect on recognition index, but the administration of BRL and LY increased the time spent in the dark compartment of the apparatus and decreased the step-through latency into the dark compartment apparatus. It can be concluded that the inhibition of the hippocampal 5-HT1D receptor improved cognition memory but impaired avoidance memory. Activation of the hippocampal 5-HT1F receptor had no effect on cognitive memory but impaired avoidance memory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among many essential processes, learning and memory are the main functions of the brain (Amin et al., 2019). Learning and memory are cognitive functions that involve different stages, including acquisition, consolidation, and retrieval (More et al., 2016). The hippocampus, a limbic system structure, is located in the medial temporal lobe of the brain (Mu and Gage, 2011). Behavioral studies have suggested that the hippocampus plays a critical role in learning and memory (Kramer et al., 2004; Shahidi et al., 2017; Tahmasebi et al., 2015). Several neurotransmitters affect learning and memory through actions in different regions of the brain (Barzegar et al., 2015).
5-Hydroxytryptamine (5-HT, serotonin), a well-known monoamine, is an important neurotransmitter and neuromodulator in the central nervous system (CNS) (Osredkar and Kržan, 2009). In the CNS, serotonin is produced by serotonergic neurons located in the brain stem raphe nuclei (Butzlaff and Ponimaskin, 2016). Investigations on mammalian species have indicated high levels of 5-HT binding in the basal ganglia, substantia nigra, hippocampus, and related structures (Adham et al., 1993; Muchimapura et al. 2003; Waeber et al., 1989). Serotonin is also involved in regulating learning and memory and cognitive function (Butzlaff and Ponimaskin, 2016; Seyedabadi et al., 2014). The effects of 5-HT are due to its interaction with their receptors (Osredkar and Kržan, 2009). 5-HT receptors are currently classified into seven classes based on their structure, transduction mechanism, and pharmacological profile (Stasi et al., 2014). All serotonergic receptors, except the 5-HT3 receptor, are G-protein-coupled receptors (GPCRs) (Upadhyay, 2003). The class of 5-HT1 receptors includes several subtypes, such as 5-HT1A, 5-HT1B, 5-HT1C, 5-HT1D, 5-HT1E, and 5-HT1F (Filip and Bader, 2009; Stasi et al., 2014). The 5-HT1D and 5-HT1F receptors are attached to Gi/Go proteins to inhibit adenylyl cyclase and reduce cAMP levels (Berumen et al., 2012). According to the evidence from animal models, 5-HT1-7 receptors have numerous and complex effects on cognitive functions (Afshar et al., 2019; Hashemi-Firouzi et al., 2018; Liy-Salmeron and Meneses, 2007; Shahidi, S. et al., 2018).
The 5-HT1D receptors are expressed in various regions of the brain, especially in the raphe nuclei and cortex (Barnes and Sharp, 1999; Upadhyay, 2003). They negatively modulate the somatodendritic release of serotonin (Piñeyro et al., 1995). Also, the 5-HT1D receptor agonists exert their effects by reducing neurogenic inflammation and inhibiting neurotransmitter release (Neeb et al., 2010), leading to relieving migraine attacks (Wang et al., 2014) and attenuating hyperalgesia (Araldi et al., 2017). PNU142633, the 5-HT1D receptor agonist, and BRL15572 hydrochloride, the 5-HT1D receptor antagonist, bind with a high affinity and selectivity to the 5-HT1D receptor (Gomez-Mancilla et al., 2001; Price et al., 1997). PNU142633 and BRL15572 hydrochloride are effective in the acute treatment of migraine attacks (Barbanti et al., 2017).
On the other hand, the 5-HT1F receptor is prominently expressed in the neocortex, hippocampus, and dorsal raphe nucleus (Agosti, 2007; Muchimapura et al. 2003). Potent agonists for 5-HT1F receptors can be effective in the treatment of migraine (Neeb et al., 2010; Tfelt-Hansen and Olesen, 2012). However, the effect of 5-HT1D and 5-HT1F receptors on learning and memory has not been reported. LY 344864 is the selective 5-HT1F receptor agonist with a high affinity in humans and rabbits (Phebus et al., 1997). Activation of the 5-HT1F receptor by LY was effective in the treatment of migraine in animal models (Phebus et al., 1997), through the modulation of (anti) nociceptive impulses (Rubio-Beltrán et al., 2018) and attenuation of methamphetamine relapse (Shahidi, Siamak et al., 2018b).
The effect of 5-HT1F receptor activation or LY 344864 is not known in episodic-like memory. The aim of the present study was to investigate the role of 5-HT1D and 5-HT1F receptors in the retrieval stage of learning and memory processes by selected agonists or antagonists in the hippocampus area.
Materials and methods
Animals
Fifty adult male Wistar rats (200–250 g) were obtained from the laboratory animal center of Hamadan University of Medical Sciences, Hamadan, Iran. The rats were kept under standard laboratory conditions (temperature: 22 ± 2 °C and a 12:12-h light:dark cycle). All animals had free access to water and food. All experimental procedures were approved by the Ethics Committee of the Hamadan University of Medical Sciences (IR.UMSHA.REC.1394.358) and were performed according to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Drugs
PNU142633 (5-HT1D receptor agonist), BRL15572 hydrochloride (5-HT1D receptor antagonist), and LY344864 (5-HT1F receptor agonist) were purchased from Tocris Bioscience Company (Bristol, UK). All compounds were dissolved in saline dimethyl sulfoxide (DMSO). These chemicals were stored at −20 °C before use.
Experimental design
The animals were divided into five groups randomly, each consisting of ten rats: (1) Intact control with no surgical or injection procedure; (2) sham-operated group, which received bilateral intrahippocampal (IHP) injection of normal saline (1μl); (3) PNU group, which received a bilateral IHP injection of PNU-142633 (1 μg/μl); (4) BRL group, which received a bilateral IHP injection of BRL-15572 (1 μg/μl); and (5) LY group, which received a bilateral IHP injection of LY 344864 (1 μg/μl).
Surgical procedures
The animals were anesthetized intraperitoneally by a mixture of ketamine (100 mg/kg, BehbodDarou, Tehran, Iran) and xylazine (10 mg/kg, Alfasan, Woerden, and The Netherlands). After being fixed in the stereotaxic apparatus (Stoelting Co., Chicago, IL), cannulas (10 mm, 23- gauge) were implanted bilaterally into the hippocampus using the stereotaxic coordinates, AP: −3.8 mm from bregma; ML: ±2.3 mm from the midline, and DV: −2.8 mm from the skull surface (Beigi et al., 2018; Paxinos and Watson, 1998). Each rat was then returned to an individual cage (40×28×15 cm) and allowed to recover for 7 days. After recovery, the cages were replaced with other cages with diameters of 55 cm × 37 cm × 25 cm for a group of up to four rats.
Microinjection procedure
For the microinjection procedure, the cannula stylet was removed and replaced by an injection needle (30-gauge) connected with a short piece of polyethylene tubing to a Hamilton syringe. In order to prevent mechanical damage to the hippocampus, the needle was inserted 1 mm below the tip of the cannula so that it was just above or slightly within the target brain region. Then, the rat received infusions of either saline or drugs (1 μl per infusion) into the hippocampus over a 2-min period (Lashgari et al., 2008). The needle was left in place for another 60 s before it was slowly withdrawn. Then, the microinjection was performed on the other side. The doses of chemical substances were chosen according to previous studies (Centurión et al., 2001; Muñoz-Islas et al., 2006). After treatment, rats were subjected to the open field test, novel object recognition test, and the passive avoidance learning test. There was an interval of 5 days between behavioral tests (Fig. 1).
Open field test
The open field test was used to assess the rats’ locomotor activity (Afshar et al., 2018; Shahidi et al., 2019). The foursquare apparatus comprised a black wooden floor (48 cm×41.5 cm×36 cm) with low ambient light and a video recording system. The animal was placed in the middle of an open field and allowed to explore the whole field. The behavior of the animal was recorded using video recording for 10 min. The total distance traveled and mean velocity were analyzed using the Ethovision tracking program (Borj Sanat Azma, Iran).
Novel object recognition test
The novel object recognition test has been extensively used to measure memory in rodents. The apparatus used in this study consisted of a black square cage with a floor arena covered with wood tips. The test had the following three sessions: habituation, training, and retention. In the habituation session, the rats were individually placed in the box for 10 min without any objects to explore (day 1). The training session was performed 24 h after the habituation session, in which two identical objects were placed on one side of the box, and the rats were allowed to freely explore the two objects for 10 min. The retention session was performed 24 h after the training session, and 5 min prior to the retention session, animals received a single dose of saline or drugs. In the retention trial, one of the two familiar objects was replaced by a novel object, and the time taken to explore each of them was again recorded. Object exploration was defined as follows: sniffing, touching, and directing attention to the object. Animal behaviors were recorded with a video camera system and the results were manually analyzed. The recognition index (%) was calculated as the time spent exploring the novel object/total exploration time × 100 (Afshar et al., 2018).
Passive avoidance learning test
Apparatus
Passive avoidance learning was tested in an apparatus with two (light and dark) compartments of the same size (20×20×30 cm each) separated by a guillotine door. The light chamber was made of transparent plastic, and the dark chamber walls were made of dark opaque plastic. The floor of the dark chamber was equipped with stainless-steel rods, and electric shocks (50 Hz, 0.4 mA, 1.5 s) were delivered to the grid floor of the dark compartment by a stimulator (Hasanein and Shahidi, 2012).
Training
All rats were habituated to the experiment room for 30 min before the training and testing experiments. First, all groups were given two trials to habituate and acclimatize them to the apparatus. We habituated the rats to the apparatus as follows: the rats were placed in the lighted compartment of the apparatus for 30 s, and then, the guillotine door was opened to allow access to the dark compartment. The rat has a natural preference for the dark environment. Once the rat entered the dark compartment, the door was closed, and after 30 s, the rat was removed from the dark compartment and placed in its home cage. This habituation trial was repeated 30 min later and followed by the first acquisition trial after the same interval.
For the training of the animals, when they had spontaneously entered the dark compartment, the door was closed and after 30 s, a mild electrical shock (0.4 mA, 1.5 s) was applied. The entrance latency to the dark compartment (step-through latency in acquisition, STLa) was recorded when the animal had all four paws in the dark compartment. After 20 s, the rat was removed from the apparatus and placed in the home cage. Two minutes later, the procedure was repeated. If the rat did not enter the dark compartment in 120 s, successful acquisition of inhibitory avoidance response was recorded. Otherwise, when the rat entered the dark compartment (before 120 s), the door was closed, and the animal received the same shock. The number of trials (entries into the dark chamber) was recorded.
Retention
The retention test was performed 24 h after the passive avoidance training trial to evaluate long-term memory. Five minutes prior to the retention session, animals received a single dose of saline or drugs. The rat was placed in the light compartment as in the passive avoidance training session, the door was opened after 10 s, and the step-through latency during the retention trial and the time spent in the dark compartment were recorded up to 300 s. The test session was terminated when the rat either entered the dark compartment or remained in the light compartment for 300 s. During the retention test, the electric shocks were not applied to the grid floor (Shahidi et al., 2019).
Statistical analysis
Statistical analyses were performed using SPSS 16.0 statistical software. The Kolmogorov–Smirnov test was used to check the data normality. The data were analyzed using a one-way analysis of variance (ANOVA) followed by a Tukey post-hoc test. A P < 0.05 was considered statistically significant. The results were expressed as mean ± SD.
Results
Open field test
One-way ANOVA results showed no significant difference in the total distance moved (F (4,49) = 0.47, 0.75, P > 0.05) and the velocity [F (4, 49) =0.37, P=0.82 > 0.05] between the groups (data are not shown).
Novel object recognition test
One-way ANOVA results indicated significant differences between the recognition indices of objects between groups [F (4, 49) = 9.735, P< 0.001] (Fig. 2). Tukey’s test results showed that the recognition index of the BRL group was significantly higher than that of the control (P<0.05) and sham (P<0.05) groups. The recognition index in rats that received LY was not significantly different compared to the control and sham groups. The rats receiving PNU had a significantly lower recognition index than the BRL and LY groups (P < 0.001).
The effect of treatment with PNU (5-HT1D receptor agonist), BRL (5-HT1D receptor antagonist), and LY (5-HT1F receptor agonist) on the recognition index. Data are shown as mean ± SD. Data were analyzed using one-way ANOVA followed by Tukey’s post-hoc test (n=10 per group). *P < 0.05 compared to the control group; #P < 0.05 compared to the sham group, and !!!P < 0.001 compared to the PNU group
Passive avoidance learning test
One-way ANOVA results showed no significant difference in step-through latency in acquisition between the experimental groups in the first acquisition trial (before the electrical shock) [F (4,49) = 1.021, P=0.407> 0.05; Fig. 3]. There was also no significant difference in the number of trials during the first acquisition between the experimental groups [F (4,49) = 1.164, P=0.339> 0.05; Fig. 4].
The effect of treatment with PNU (5-HT1D receptor agonist), BRL (5-HT1D receptor antagonist), and LY (5-HT1F receptor agonist) on the step-through latency in acquisition in the passive avoidance learning test. Data are expressed as mean ± SD. Data were analyzed using one-way ANOVA followed by Tukey’s post-hoc test (n=10 per group)
The effect of treatment with PNU (5-HT1D receptor agonist), BRL (5-HT1D receptor antagonist), and LY (5-HT1F receptor agonist) on the number of trials in the passive avoidance learning test. Data are expressed as mean ± SD. Data were analyzed using one-way ANOVA followed by Tukey’s post-hoc test (n=10 per group)
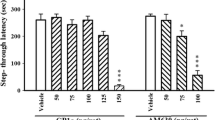
According to one-way ANOVA results, there was a significant difference in the step-through latency during the retention trial between the groups [F (4,49) = 12.369, P<0.001] (Fig. 5). Also, Tukey’s test results showed that STLr was significantly lower in the BRL group than in the control (P < 0.001), sham (P < 0.01), and PNU (P < 0.001) groups. In addition, STLr in rats that received LY was significantly lower than step-through latency during the retention trial in the control (P < 0.01), sham (P < 0.01), and PNU (P < 0.001) groups. There was no significant difference between the PNU, control, and sham groups in the memory test.
The effect of treatment with PNU (5-HT1D receptor agonist), BRL (5-HT1D receptor antagonist), and LY (5-HT1F receptor agonist) on step-through latency in the retention trial in the passive avoidance learning test. Data are expressed as mean ± SD. Data were analyzed using one-way ANOVA followed by Tukey’s post-hoc test (n=10 per group). **P < 0.01 and ***P < 0.001 compared to the control group; ##P < 0.01 compared to the sham group; and !!!P < 0.001 compared to the PNU group
A statistically significant difference was detected in the time spent in the dark compartment between the experimental groups evidenced by one-way ANOVA [F (4,48) = 2.657, P=0.045<0.05]. As shown in Fig. 6, the time spent in the dark compartment of the BRL group was significantly greater than that of the control (P < 0.01) and PNU (P < 0.001) groups. The time spent in the dark compartment in the LY group was significantly higher than time spent in the dark compartment in the control (P<0.05) and PNU (P < 0.01) groups.
The effect of treatment with PNU (5-HT1D receptor agonist), BRL (5-HT1D receptor antagonist), and LY (5-HT1F receptor agonist) on the time spent in the dark compartment (TDC) in the passive avoidance learning test. Data are expressed as mean ± SD. Data were analyzed using one-way ANOVA followed by Tukey’s post-hoc test (n=10 per group). *P < 0.05 and **P < 0.01 compared to the control group and !!P < 0.01 and !!!P < 0.001 compared to the PNU group
Discussion
The present study investigated the role of serotonin receptors in the retrieval stage of learning and memory by PNU (5-HT1D receptor agonist), BRL (5-HT1D receptor antagonist), and LY (5-HT1F receptor agonist). Briefly, the results indicated that IHP injection of BRL increased the recognition index in the novel object recognition test. BRL decreased the step through latency during the retention trial and increased the time spent in the dark compartment in the passive avoidance learning test. It can be concluded that acute inhibition of the 5-HT1D receptor potentiated the recognition memory but attenuated the avoidance memory. The acute activation of the 5-HT1D receptor by an IHP injection of PNU had no effects on memory. Also, the administration of LY decreased the step-through latency during the retention trial and increased the time spent in the dark compartment in the passive avoidance learning test.
These conflicting results could be due to the different brain structures involved in the memory processes. The recognition memory is a form of non-spatial memory in the object and passive avoidance. The novel object recognition test is a valuable measurement of cognition in rodents (Antunes and Biala, 2012). The prefrontal cortex and hippocampus are involved in object memory (Chao et al., 2020). The hippocampus plays a main role in the encoding, consolidation, and retrieval stages in object memory (Cohen and Stackman Jr, 2015). The hippocampus is responsible for long-term object recognition by receiving inputs from the perirhinal cortex (Reger et al., 2009). In addition, hippocampal formation has the main role in memory for contextual information (Antunes and Biala, 2012). On the other hand, the entorhinal cortex processes object information (Clemow et al., 2020). The hippocampus and prefrontal cortex contribute to forming memory (Rolls, 2022).
According to Shahidi et al., the intracerebroventricular administration of PNU decreased long-term potentiation components and synaptic transmission in methamphetamine-treated rats (Shahidi, Siamak et al., 2018a). Also, the administration of GR46611 (a 5-HT1B and 5-HT1D receptor agonist) impaired the consolidation of learning in the autoshaping learning task (Meneses, 2001; Meneses et al., 1997). Najar et al. reported that intra-septum post-training administration of CP94253 (5-HT1B and 5-HT1D receptor agonist) reduced the step-through latency during the retention trial in the passive avoidance learning test, while GR127935 (5-HT1B and 5-HT1D receptor antagonist) did not alter memory consolidation (Najar et al., 2015).
PNU142633 is a selective and high-affinity 5-HT1D receptor agonist, which can activate the receptor by the inhibition of adenylyl cyclase activity (Hensler, 2012). Central 5-HT1D receptors are found at low densities and low levels in the cortex and hippocampus (Blackburn, 2009; Hoyer, 2019). Our results showed that PNU142633 had no effects on memory tests. The activation of the brain’s 5-HT1D receptors is linked to a reduction in serotonin, glutamate, and acetylcholine release in many brain regions and can impair some aspects of learning and memory (Filip and Bader, 2009). The low concentration of PNU142633 (1 μg/μl) can be the reason for no effect of 5-HT1D receptor on memory in the present study.
Our finding show that 5-HT1D receptor antagonist enhanced recognition memory. The hippocampus contains a high density of 5-HT1D sites (Bruinvels et al., 1994). 5-HT1D receptors are negatively coupled to adenylate cyclase (Hoyer et al., 1990) and act as inhibitory autoreceptors in axon terminals of serotonergic neurons (Sari, 2004). 5-HT1D receptors are found at pre- and postsynaptic sites but presynaptic receptors are predominantly located on 5-HT hippocampal nerve terminals (Fink and Göthert, 2007; Muchimapura et al. 2003). Presynaptic 5-HT1D receptors participate in the release of 5-HT and other neurotransmitters such as, acetylcholine and glutamate (Bruinvels et al., 1994; De Deurwaerdere and Di Giovanni, 2021; Pauwels, 1997; Rutz et al., 2006). Selective blockade of 5-HT1D autoreceptors facilitates serotonin neurotransmission (Pauwels, 1997) and elevated extracellular 5-HT concentrations (Pullar et al., 2004). Inhibition of 5-HT1D receptor enhances release of neurotransmitter and improves memory impairments (Meneses et al., 1997).
On the other hand, the present finding shows that 5-HT1D receptor antagonist attenuates avoidance memory. The 5-HT1D receptors are located in postsynaptic neurons (Pauwels, 1997). 5-HT1D receptors negatively modulate the somatodendritic release of 5-HT (Piñeyro et al., 1995). BRL15572 blockade the postsynaptic sites of 5-HT1D receptor, and inhibition of 5-HT1D receptors induces a serotonergic activation on adrenergic neurotransmission (García-Pedraza et al., 2013). These events may modulate the avoidance memory.
The different effects of 5-HT1D receptor antagonist in object recognition and passive avoidance tests is related to the hippocampus and other brain area, which link to cognition or avoidance memory. The hippocampus plays an important role in memory, and in this process, performs via synapses, neuronal circuits, and pathways. Serotonin has positive role in memory. The cognitive deficits are associated with reduced serotonin levels (Cowen and Sherwood, 2013; Mendelsohn et al., 2009). Hippocampus, entorhinal system, and neocortex involved in cognition memory (Lisman et al., 2017).
Memory of inhibitory avoidance is processed by the hippocampus, amygdala, entorhinal cortex, striatum, and parietal cortex (González-Salinas et al., 2018; Izquierdo and Medina, 1997). Memory of habituation to a novel environment is performed only by the hippocampus (Izquierdo and Medina, 1993). Retrieved inhibitory avoidance memory is performed through memory reconsolidation by interactions between the amygdala prefrontal cortex and hippocampus (Fukushima et al., 2021). The entorhinal cortex is the gate to control the flow of information into and out of the hippocampus (Lei, 2012).
Hippocampal injection of 5-HT1D receptors antagonist decreases the avoidance memory. Although, the hippocampus contains a high density of 5-HT1 sites (Waeber et al., 1989) and serotonin receptors express at high density in CA3, low density in dentate gyrus, and lowest density in CA1 (Hensler, 2006), but 5-HT1D binding sites in hippocampus have very low presence (Bruinvels et al., 1993). Distribution of 5-HT1D receptors is high concentrations in the nigro-striatal pathway, entorhinal cortex, and cortex (Hoyer et al., 1990). It seems that the low binding sites of 5-HT1D in hippocampus are responsible for the decrease on avoidance memory in shuttle box.
In the current study, the hippocampal injection of LY decreased avoidance memory in the passive avoidance learning task but had no effects on object recognition memory. These results may be due to the differences in cell signaling mechanisms and the neuronal circuits involved in each task. However, no studies have investigated the role of LY in memory function. LY attenuated the reinstatement of methamphetamine-seeking behavior (Shahidi, Siamak et al., 2018b). Moreover, 5-HT1 receptor subtypes improved cognitive function. Also, 5-HT1A receptor blockade by NAD-299 (Afshar et al., 2018; Lüttgen et al., 2005) and WAY-100635 (Misane and Ögren, 2003) improved memory in rats in the passive avoidance test.
LY344864 is a selective 5-HT1F receptor agonist. LY344864 administration leads to the inhibition of cyclic AMP accumulation in cells following in vitro electrical stimulation (Phebus et al., 1997). Also, the 5-HT1F agonist exerts its effect on memory by inhibiting neuropeptide and neurotransmitter release (Clemow et al., 2020). Although the protein and neurotransmitter were not measured in our study, BRL15572 and LY344864 had similar effects on the recognition and avoidance memory.
BRL and LY had similar effects on the performed tests. BRL is the 5-HT1D receptor antagonist and LY is the 5-HT1F receptor agonist. It means that the inhibition of the 5-HT1D receptor and stimulation of the 5-HT1F receptor can lead to similar effects on memory.
There were some limitations in this study that should be discussed. This study investigated the effect of drugs in the learning phase (before the learning test) and preliminary information was obtained. More studies should be done on different stages of learning and memory in impaired memory models. It is necessary to investigate the effect of the receptor on neuronal plasticity and changes in the expression of proteins, such as brain-derived neurotrophic factor. This study was further limited by the choice of behavioral test. Although the passive avoidance task and novel object recognition test evaluated memory and cognition, they did not test spatial memory, which can provide more information about memory. As mentioned in the methods section, acute treatment with the agonist and antagonist was done using microinjections into the hippocampus so that the stimulation or inhibition of the serotonin receptor was observed over a relatively short period of time. The effectiveness of the substance through systemic consumption was not studied. Examining the effect of long-term treatment with 5-HT1D receptor agonist and 5-HT1F receptor antagonist is essential in understanding the function of the serotonin receptors.
Conclusion
In conclusion, the present study showed that the inhibition of the hippocampal 5-HT1D receptor improved the recognition memory but impaired avoidance memory. Activation of the hippocampal 5-HT1F receptor had no effect on recognition memory but impaired aversive memory. However, more studies should be performed to confirm these results. Also, further investigations, such as molecular and behavioral experiments, are required to clarify the more detailed mechanism of our findings.
References
Adham N, Kao H-T, Schecter L, Bard J, Olsen M, Urquhart D, Durkin M, Hartig PR, Weinshank RL, Branchek TA (1993) Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc Nat Acad Sci 90(2):408–412
Afshar S, Shahidi S, Rohani AH, Asl SS, Komaki A (2019) Protective effects of 5-HT1A receptor antagonist and 5-HT2A receptor agonist on the biochemical and histological features in a rat model of Alzheimer’s disease. J Chem Neuroanat 96:140–147
Afshar S, Shahidi S, Rohani AH, Komaki A, Asl SS (2018) The effect of NAD-299 and TCB-2 on learning and memory, hippocampal BDNF levels and amyloid plaques in streptozotocin-induced memory deficits in male rats. Psychopharmacol 235(10):2809–2822
Agosti RM (2007) 5HT1F-and 5HT7-receptor agonists for the treatment of migraines. CNS Neurolog Disord-drug Targets (formerly current drug targets-CNS neurological disorders) 6(4):235–237
Amin SA, Adhikari N, Kotagiri S, Jha T, Ghosh B (2019) Histone deacetylase 3 inhibitors in learning and memory processes with special emphasis on benzamides. Eur J Med Chem
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13(2):93–110
Araldi D, Ferrari LF, Green P, Levine JD (2017) Marked sexual dimorphism in 5-HT1 receptors mediating pronociceptive effects of sumatriptan. Neuroscience 344:394–405
Barbanti P, Fofi L, Aurilia C, Egeo G, Caprio M (2017) Ketogenic diet in migraine: rationale, findings and perspectives. Neurol Sci 38(1):111–115
Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacol 38(8):1083–1152
Barzegar S, Komaki A, Shahidi S, Sarihi A, Mirazi N, Salehi I (2015) Effects of cannabinoid and glutamate receptor antagonists and their interactions on learning and memory in male rats. Pharmacol Biochem Behav 131:87–90. https://doi.org/10.1016/j.pbb.2015.02.005
Beigi B, Shahidi S, Komaki A, Sarihi A, Hashemi-Firouzi N (2018) Pretraining hippocampal stimulation of melatonin type 2 receptors can improve memory acquisition in rats. Int J Neurosci:(just-accepted), 1-14
Berumen LC, Rodríguez A, Miledi R, García-Alcocer G (2012) Serotonin receptors in hippocampus. Sci World J 2012
Blackburn, T. (2009). Serotonin (5-hydroxytryptamine; 5-HT): receptors.
Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM (1994) Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology 33(3-4):367–386. https://doi.org/10.1016/0028-3908(94)90067-1
Bruinvels AT, Palacios JM, Hoyer D (1993) Autoradiographic characterisation and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn Schmiedeberg's Arch Pharmacol 347(6):569–582. https://doi.org/10.1007/bf00166939
Butzlaff M, Ponimaskin E (2016) The role of serotonin receptors in Alzheimer’s disease. Opera Medica et Physiologica 2(1):77–86
Centurión D, Sánchez-López A, De Vries P, Saxena PR, Villalón CM (2001) The GR127935-sensitive 5-HT1 receptors mediating canine internal carotid vasoconstriction: resemblance to the 5-HT1B, but not to the 5-HT1D or 5-ht1F, receptor subtype. Br J Pharmacol 132(5):991–998
Chao OY, de Souza Silva MA, Yang Y-M, Huston JP (2020) The medial prefrontal cortex-hippocampus circuit that integrates information of object, place and time to construct episodic memory in rodents: behavioral, anatomical and neurochemical properties. Neurosci Biobehav Rev 113:373–407
Clemow DB, Johnson KW, Hochstetler HM, Ossipov MH, Hake AM, Blumenfeld AM (2020) Lasmiditan mechanism of action–review of a selective 5-HT1F agonist. J Headache Pain 21(1):1–13
Cohen SJ, Stackman RW Jr (2015) Assessing rodent hippocampal involvement in the novel object recognition task. A review Behav Brain Res 285:105–117
Cowen P, Sherwood AC (2013) The role of serotonin in cognitive function: evidence from recent studies and implications for understanding depression. J Psychopharmacol (Oxford, England) 27(7):575–583. https://doi.org/10.1177/0269881113482531
De Deurwaerdere P, Di Giovanni G (2021) 5-HT interaction with other neurotransmitters: an overview. Prog Brain Res 259:1–5
Filip M, Bader M (2009) Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacolog Rep : PR 61(5):761–777
Fink KB, Göthert M (2007) 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev 59(4):360–417. https://doi.org/10.1124/pr.107.07103
Fukushima H, Zhang Y, Kida S (2021) Interactions between the amygdala and medial prefrontal cortex as upstream regulators of the hippocampus to reconsolidate and enhance retrieved inhibitory avoidance memory. 14(1), 44. https://doi.org/10.1186/s13041-021-00753-2
García-Pedraza J, García M, Martín ML, Gómez-Escudero J, Rodríguez-Barbero A, Román LS, Morán A (2013) Peripheral 5-HT1D and 5-HT7 serotonergic receptors modulate sympathetic neurotransmission in chronic sarpogrelate treated rats. Eur J Pharmacol 714(1-3):65–73. https://doi.org/10.1016/j.ejphar.2013.05.045
Gomez-Mancilla B, Cutler N, Leibowitz MA, Spierings E, Klapper J, Diamond S, Goldstein J, Smith T, Couch J, Fleishaker J (2001) Safety and efficacy of PNU-142633, a selective 5-HT1D agonist, in patients with acute migraine. Cephalalgia 21(7):727–732
González-Salinas S, Medina AC, Alvarado-Ortiz E, Antaramian A, Quirarte GL, Prado-Alcalá RA (2018) Retrieval of inhibitory avoidance memory induces differential transcription of arc in striatum, hippocampus, and amygdala. Neurosci 382:48–58. https://doi.org/10.1016/j.neuroscience.2018.04.031
Hasanein P, Shahidi S (2012) Preventive effect of Teucrium polium on learning and memory deficits in diabetic rats. Med Sci Monitor : Int Med J Exp Clin Res 18(1):Br41–Br46. https://doi.org/10.12659/msm.882201
Hashemi-Firouzi N, Shahidi S, Soleimani-Asl S, Komaki A (2018) 5-Hydroxytryptamine receptor 6 antagonist, SB258585 exerts neuroprotection in a rat model of streptozotocin-induced Alzheimer’s disease. Metab Brain Dis. https://doi.org/10.1007/s11011-018-0228-0
Hensler JG (2006) Serotonergic modulation of the limbic system. Neurosci Biobehav Rev 30(2):203–214. https://doi.org/10.1016/j.neubiorev.2005.06.007
Hensler JG (2012) Serotonin. In Basic Neurochemistry (Elsevier):300–322
Hoyer D (2019) Serotonin receptors nomenclature. In The Serotonin System. (Elsevier), pp. 63-93
Hoyer D, Schoeffter P, Waeber C, Palacios JM (1990) Serotonin 5-HT1D receptors. Ann N Y Acad Sci 600, 168-181 discussion 181-162. https://doi.org/10.1111/j.1749-6632.1990.tb16880.x.
Izquierdo I, Medina JH (1993) Role of the amygdala, hippocampus and entorhinal cortex in memory consolidation and expression. Brazilian J Med Biolog Research = Revista brasileira de pesquisas med Biologicas 26(6):573–589
Izquierdo I, Medina JH (1997) Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem 68(3):285–316. https://doi.org/10.1006/nlme.1997.3799
Kramer JH, Schuff N, Reed BR, Mungas D, Du A-T, Rosen HJ, Jagust WJ, Miller BL, Weiner MW, Chui HC (2004) Hippocampal volume and retention in Alzheimer’s disease. J Int Neuropsychol Soc 10(4):639–643
Lashgari R, Khakpour-Taleghani B, Motamedi F, Shahidi S (2008) Effects of reversible inactivation of locus coeruleus on long-term potentiation in perforant path-DG synapses in rats. Neurobiol Learn Mem 90(2):309–316. https://doi.org/10.1016/j.nlm.2008.05.012
Lei S (2012) Serotonergic modulation of neural activities in the entorhinal cortex. Int J Physiol, Pathophysiol Pharmacol 4(4):201–210
Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Ranganath C, Redish AD (2017) Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci 20(11):1434–1447. https://doi.org/10.1038/nn.4661
Liy-Salmeron G, Meneses A (2007) Role of 5-HT1–7 receptors in short-and long-term memory for an autoshaping task: intrahippocampal manipulations. Brain Res 1147:140–147
Lüttgen M, Elvander E, Madjid N, Ögren SO (2005) Analysis of the role of 5-HT1A receptors in spatial and aversive learning in the rat. Neuropharmacol 48(6):830–852
Mendelsohn D, Riedel WJ, Sambeth A (2009) Effects of acute tryptophan depletion on memory, attention and executive functions: a systematic review. Neurosci Biobehav Rev 33(6):926–952. https://doi.org/10.1016/j.neubiorev.2009.03.006
Meneses A (2001) Could the 5-HT1B receptor inverse agonism affect learning consolidation? Neurosci Biobehav Rev 25(2):193–201
Meneses A, Terrón JA, Hong E (1997) Effects of the 5-HT receptor antagonists GR127935 (5-HT1B/1D) and MDL100907 (5-HT2A) in the consolidation of learning. Behav Brain Res 89(1-2):217–223
Misane I, Ögren SO (2003) Selective 5-HT 1A antagonists WAY 100635 and NAD-299 attenuate the impairment of passive avoidance caused by scopolamine in the rat. Neuropsychopharmacol : Off Pub Ame Col Neuropsychopharmacol 28(2):253–264
More SV, Kumar H, Cho D-Y, Yun Y-S, Choi D-K (2016) Toxin-induced experimental models of learning and memory impairment. Int J Mol Sci 17(9):1447
Mu Y, Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener 6(1):85
Muchimapura S, Mason R, Marsden CA (2003) Effect of isolation rearing on pre- and post-synaptic serotonergic function in the rat dorsal hippocampus. Synapse (New York, N.Y.) 47(3):209–217. https://doi.org/10.1002/syn.10167
Muñoz-Islas E, Gupta S, Jiménez-Mena L, Lozano-Cuenca J, Sánchez-López A, Centurión D, Mehrotra S, MaassenVanDenBrink A, Villalón C (2006) Donitriptan, but not sumatriptan, inhibits capsaicin-induced canine external carotid vasodilatation via 5-HT1B rather than 5-HT1D receptors. British J Pharmacol 149(1):82–91
Najar F, Nasehi M, Haeri-Rohani S-A, Zarrindast M-R (2015) The involvement of medial septum 5-HT1 and 5-HT2 receptors on ACPA-induced memory consolidation deficit: possible role of TRPC3, TRPC6 and TRPV2. J Psychopharmacol 29(11):1200–1208
Neeb L, Meents J, Reuter U (2010) 5-HT 1F receptor agonists: a new treatment option for migraine attacks? Neurotherapeutics 7(2):176–182
Osredkar D, Kržan M (2009) Expression of serotonin receptor subtypes in rat brain and astrocyte cell cultures: an ageand tissue-dependent process. Period Biol 111(1), 129-135
Pauwels PJ (1997) 5-HT 1B/D receptor antagonists. Gen Pharmacol 29(3):293–303. https://doi.org/10.1016/s0306-3623(96)00460-0
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic, San Diego
Phebus LA, Johnson KW, Zgombick JM, Gilbert PJ, Van Belle K, Mancuso V, Nelson DL, Calligaro DO, Kiefer AD Jr, Branchek TA (1997) Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life Sci 61(21):2117–2126
Piñeyro G, de Montigny C, Blier P (1995) 5-HT1D receptors regulate 5-HT release in the rat raphe nuclei: in vivo voltammetry and in vitro superfusion studies. Neuropsychopharmacol : Off Pub Ame Col Neuropsychopharmacol 13(3):249–260
Price G, Burton M, Collin L, Duckworth M, Gaster L, Göthert M, Jones B, Roberts C, Watson J, Middlemiss D (1997) SB-216641 and BRL-15572–compounds to pharmacologically discriminate h5-HT1B and h5-HT1D receptors. Naunyn-Schmiedeberg's Archiv Pharmacol 356(3):312–320
Pullar IA, Boot JR, Broadmore RJ, Eyre TA, Cooper J, Sanger GJ, Wedley S, Mitchell SN (2004) The role of the 5-HT1D receptor as a presynaptic autoreceptor in the guinea pig. Eur J Pharmacol 493(1-3):85–93. https://doi.org/10.1016/j.ejphar.2004.04.029
Reger ML, Hovda DA, Giza CC (2009) Ontogeny of rat recognition memory measured by the novel object recognition task. Dev Psychobiol: J Int Soc Develop Psychobiol 51(8):672–678
Rolls ET (2022) The hippocampus, ventromedial prefrontal cortex, and episodic and semantic memory. Prog Neurobiol, 102334
Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, MaassenVanDenBrink A (2018) Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Therapeut 186:88–97
Rutz S, Riegert C, Rothmaier AK, Buhot MC, Cassel JC, Jackisch R (2006) Presynaptic serotonergic modulation of 5-HT and acetylcholine release in the hippocampus and the cortex of 5-HT1B-receptor knockout mice. Brain Res Bull 70(1):81–93. https://doi.org/10.1016/j.brainresbull.2006.04.004
Sari Y (2004) Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev 28(6):565–582. https://doi.org/10.1016/j.neubiorev.2004.08.008
Seyedabadi M, Fakhfouri G, Ramezani V, Mehr SE, Rahimian R (2014) The role of serotonin in memory: interactions with neurotransmitters and downstream signaling. Exp Brain Res 232(3):723–738
Shahidi S, Hashemi-Firouzi N, Asl SS, Komaki A (2019) Serotonin type 6 receptor antagonist attenuates the impairment of long-term potentiation and memory induced by Abeta. Behav Brain Res 364:205–212
Shahidi S, Komaki A, Sadeghian R, Asl SS (2018a) Effect of a 5-HT1D receptor agonist on the reinstatement phase of the conditioned place preference test and hippocampal long-term potentiation in methamphetamine-treated rats. Brain Res 1698:151–160
Shahidi S, Sadeghian R, Komaki A, Asl SS (2018b) Intracerebroventricular microinjection of the 5-HT1F receptor agonist LY 344864 inhibits methamphetamine conditioned place preference reinstatement in rats. Pharmacol Biochem Behav 173:27–35
Shahidi S, Soleimani Asl S, Komaki A, Hashemi-Firouzi N (2018) The effect of chronic stimulation of serotonin receptor type 7 on recognition, passive avoidance memory, hippocampal long-term potentiation, and neuronal apoptosis in the amyloid β protein treated rat. Psychopharmacol 235(5):1513–1525. https://doi.org/10.1007/s00213-018-4862-3
Shahidi S, Zargooshnia S, Asl SS, Komaki A, Sarihi A (2017) Influence of N-acetyl cysteine on beta-amyloid-induced Alzheimer’s disease in a rat model: a behavioral and electrophysiological study. Brain Res Bull 131:142–149. https://doi.org/10.1016/j.brainresbull.2017.04.001
Stasi C, Bellini M, Bassotti G, Blandizzi C, Milani S (2014) Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Techniq Coloproctol 18(7):613–621
Tahmasebi L, Komaki A, Karamian R, Shahidi S, Sarihi A, Salehi I, Nikkhah A (2015) The interactive role of cannabinoid and vanilloid systems in hippocampal synaptic plasticity in rats. Eur J Pharmacol 757:68–73. https://doi.org/10.1016/j.ejphar.2015.03.063
Tfelt-Hansen PC, Olesen J (2012) The 5-HT 1F receptor agonist lasmiditan as a potential treatment of migraine attacks: a review of two placebo-controlled phase II trials. J Headache Pain 13(4):271–275
Upadhyay S (2003) Serotonin receptors, agonists and antagonists. Indian J Nucl Med 18(481):1–11
Waeber C, Died M, Hoyer D, Palacios J (1989) 5. HT 1 receptors in the vertebrate brain. Naunyn-Schmiedeberg’s Archives Pharmacol 340(5):486–494
Wang X, Fang Y, Liang J, Yan M, Hu R, Pan X (2014) 5-HT 7 receptors are involved in neurogenic dural vasodilatation in an experimental model of migraine. J Mol Neurosci 54(2):164–170
Data availability
All data and material will be made available on request.
Funding
This study was funded by a grant (Grant Number: 9410015225) of the Hamadan University of Medical Sciences, Hamadan, Iran.
Author information
Authors and Affiliations
Contributions
Simin Afshar, Nasrin Hashemi-Firouzi, and Siamak Shahidi contributed to study design and conducted the experiments. Hemen Baooshi, Mahdieh Hoseini, and Mahsa Esmaeili performed the experiments. Simin Afshar and Nasrin Hashemi-Firouzi contributed to analyze and interpret the data. Nasrin Hashemi-Firouzi and Siamak Shahidi wrote the main manuscript and editing. Siamak Shahidi was responsible for the funding. Alireza Komaki provided the technical assistance.
Corresponding authors
Ethics declarations
Ethical approval
The present study was approved by the Ethics Committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1394.358).
Consent to participate
Not applicable.
Consent for publication
All the authors have approved the manuscript. They agree with submission to your esteemed journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afshar, S., Shahidi, S., Baooshi, H. et al. The role of hippocampal 5-HT1D and 5-HT1F receptors on learning and memory in rats. Naunyn-Schmiedeberg's Arch Pharmacol 396, 1451–1460 (2023). https://doi.org/10.1007/s00210-023-02411-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02411-x