Abstract
Rationale
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by memory impairment, neuronal death, and synaptic loss in the hippocampus. Long-term potentiation (LTP), a type of synaptic plasticity, occurs during learning and memory. Serotonin receptor type 7 (5-HTR7) activation is suggested as a possible therapeutic target for AD.
Objective
The aim of the present study was to examine the effects of chronic treatment with the 5-HTR7 agonist, AS19, on cognitive function, memory, hippocampal plasticity, amyloid beta (Aβ) plaque accumulation, and apoptosis in an adult rat model of AD.
Methods
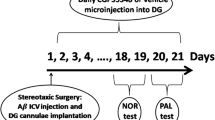
AD was induced in rats using Aβ (single 1 μg/μL intracerebroventricular (icv) injection during surgery). The following experimental groups were included: control, sham-operated, Aβ + saline (1 μL icv for 30 days), and Aβ + AS19 (1 μg/μL icv for 30 days) groups. The animals were tested for cognition and memory performance using the novel object recognition and passive avoidance tests, respectively. Next, anesthetized rats were placed in a stereotaxic apparatus for electrode implantation, and field potentials were recorded in the hippocampal dentate gyrus. Lastly, brains were removed and Aβ plaques and neuronal apoptosis were evaluated using Congo red staining and TUNEL assay, respectively.
Results
Administration of AS19 in the Aβ rats increased the discrimination index of the novel object recognition test. Furthermore, AS19 treatment decreased time spent in the dark compartment during the passive avoidance test. AS19 also enhanced both the population spike (PS) amplitude and the field excitatory postsynaptic potential (fEPSP) slope evoked potentials of the LTP components. Aβ plaques and neuronal apoptosis were decreased in the AS19-treated Aβ rats.
Conclusions
These results indicate that chronic treatment with a 5-HTR7 agonist can prevent Aβ-related impairments in cognition and memory performance by alleviating Aβ plaque accumulation and neuronal apoptosis, hence improving neuronal plasticity. AS19 may be useful as a therapeutic agent for AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serotonin (5-hydroxytryptamine; 5-HT) acts as a neurotransmitter and neuromodulator in the central nervous system (CNS) (Fidalgo et al. 2013). It is involved in physiological functions and pathophysiological conditions including schizophrenia, depression, anxiety, and Alzheimer’s disease (AD) (Butzlaff and Ponimaskin 2016; Filip and Bader 2009).
The behavioral effects of 5-HT are mediated by a large family of specific 5-HT receptors (Butzlaff and Ponimaskin 2016; Filip and Bader 2009). 5-HT receptor type 7 (5-HTR7) is located in different brain regions, such as the hypothalamus, thalamus, cerebral cortex, and hippocampus (Leopoldo et al. 2011). It was shown that a 5-HTR7 agonist improved motor coordination, cognition, spatial memory, and synaptic plasticity in rodents (De Filippis et al. 2015; Stiedl et al. 2015). Also, 5-HT7R is involved in depression, schizophrenia (Nikiforuk et al. 2013; Wesolowska et al. 2006; Wesołowska et al. 2006), and drug abuse (Hauser et al. 2014; Shahidi and Hashemi-Firouzi 2014), and improves cognitive dysfunction (Waters et al. 2012), indicating that 5-HTR7 agonists may be used as pharmacological tools for the treatment of neurological disorders. Moreover, 5-HTR7 activation is suggested as a possible therapeutic targets strategy for AD (Meneses 2014; Xiang et al. 2016). Nevertheless, 5-HTR7’s role in AD is unclear.
AD is a neurodegenerative disease of the CNS characterized by progressive cognitive decline and dysfunction, aggregation of amyloid β-peptide (Aβ) plaques, neuronal loss, and neuronal apoptosis (Alberghina and Colangelo 2006; Obulesu and Lakshmi 2014; Querfurth and LaFerla 2010; Radi et al. 2014). The cholinergic system is severely impaired in AD (Xu et al. 2012). Moreover, it has been reported that the serotonergic system also contributes to this impairment. AD is associated with decreases in serotonergic synthesis and receptors (Butzlaff and Ponimaskin 2016; Dengler-Crish et al. 2017; Garcia-Alloza et al. 2004; Palmer et al. 1987). In addition, AD disturbs neural plasticity and synaptic function (Park et al. 2012; Vasefi et al. 2013; Xiang et al. 2016; Zhang et al. 2002).
Long-term potentiation (LTP) is a type of synaptic plasticity (Bliss and Collingridge 1993). LTP is the physiological basis for memory formation and has been used as a neuronal model of neuroplasticity (Bliss and Collingridge 1993; Bliss and Gardner-Medwin 1973). 5-HT is involved in most forms of synaptic plasticity (Costa et al. 2012; Costa et al. 2012; Tokarski et al. 2005; Tokarski et al. 2003; Vasefi et al. 2013), and in vivo data show that 5-HTR7 activation reduced Aβ levels in the interstitial fluid of the brain (Fisher et al. 2016). Additionally, 5-HTR7 activation has been shown to promote the formation of dendritic spines and neurite elongation (Li et al. 2017; Speranza et al. 2015; Speranza et al. 2017). Stimulation of 5-HTR7 reverses long-term synaptic depression in animal models of fragile X syndrome (Costa et al. 2015; Costa et al. 2012). However, in vivo data regarding 5-HTR7 activation in LTP formation in AD is not available.
Therefore, the aim of this study was to show the effect of 1 month of selective 5-HTR7 agonist, AS19, on cognition, memory, neuronal apoptosis, Aβ accumulation, and LTP induction in the hippocampal dentate gyrus in a rat model of AD.
Materials and methods
Animals
Adult male Wistar rats weighing 250–300 g were used. Rats were housed at a temperature of 20 °C ± 2 °C on a 12 h light/dark cycle with free access to food and water. Rats were maintained in cages with two rats per cage. Animal care, treatment, and surgical procedures were approved by the ethics committees of the Hamadan University of Medical Sciences (Ref. No.: 6383), and performed according to the Guide for Care and Use of laboratory animals published by the National Institute of Health, USA (NIH Publication No. 85-23, revised 1985).
The rats were randomly distributed into four groups as follows (n = 10 per group): the control group, which did not undergo any operation or treatment; the sham group, which received 6 μL of 0.9% saline (Shahid Ghazi Co, Tabriz, Iran) via intracerebroventricular (icv) injection during stereotaxic surgery, and subsequently received 1 μL saline via icv injection daily for 30 consecutive days; the Aβ group, which received Aβ peptides via icv injection during stereotaxic surgery, and subsequently received 1 μL saline via icv injection daily for 30 consecutive days; and the Aβ + AS19 group, which received Aβ peptides via icv injection during stereotaxic surgery, and subsequently received AS19 (a selective 5-HTR7 agonist, 1 μg/μL) via icv injection daily for 30 consecutive days.
Chemicals
Aβ (1–42) and AS19 were purchased from Tocris (UK). Ketamine and xylazine were purchased from Behbod Darou (IR) and Alfasan (Holland), respectively. Urethane was acquired from Sigma.
AD induction
Surgery and AD induction were performed as previously described (Shahidi et al. 2017). In brief, rats were anesthetized using xylazine (10 mg/kg) and ketamine (100 mg/kg), and placed into a stereotaxic device. A guide cannula was lowered into the right lateral ventricle, and relative to the bregma, the coordinates for the icv injection were: anterior-posterior (AP), 1.6 mm from bregma; mediolateral (ML), + 0.9 mm from the midline; and dorsoventral (DV), 2 mm from the skull surface (Paxinos, 2007). The guide cannula was secured in place using two stainless steel screws anchored to the skull with dental acrylic cement. Animals individually recovered in their home cages.
The Aβ was solubilized in sterile water at a 1 μg/μL concentration and 6 μL of Aβ fragment solution were used for each single injection (Babri et al. 2014; Shahidi et al. 2017). Aβ solution was unilaterally injected into the region using a 10-μL microsyringe (Hamilton-Reno, NV, USA). Chemical or saline microinjections were performed using a 30-gauge injector cannula (1 mm below the tip of the guide cannula) with a Hamilton syringe (Hamilton Laboratory Products, Switzerland) attached to the injector cannula with polyethylene micro-tubing (PE-20). To treat the Aβ group, AS19 powder (1 μg/μl) was dissolved in saline and microinjected via icv at a volume of 1 μL for 30 consecutive days (Han et al. 2016; Perez-Garcia and Meneses 2009; Zhang et al. 2015). The sham rats received saline at the same volume.
The novel object-recognition test
The procedure was performed as described in our previous studies (Hashemi-Firouzi et al. 2015). The apparatus consisted of a wooden open brown box (48 cm × 41.5 cm × 36 cm) with a black floor. The training phase was done with two similar objects symmetrically fixed to the floor of the box, 10 cm apart for 10 min. One day later, the retention test was performed in which one of the objects was replaced by a novel object. The animals’ behavior was recorded with a video-camera system for 10 min. The exploration time for an object was defined as sniffing or placing the nose within 1 cm of the object while being oriented towards the object. The discrimination index was defined as the exploration time spent on the novel object divided by the total exploration time (Fig. 1). The box and the objects were cleaned with wet tissue paper (water) before each session or test (Takuma et al. 2014; Wang et al. 2014).
Passive avoidance learning
The passive avoidance apparatus and procedure were similar to our previous studies (Ghahremanitamadon et al. 2014; Khodamoradi et al. 2015). The transparent plastic apparatus consisted of a two-chamber dark/light shuttle box (20 × 30 × 20 cm) with a rectangular opening (6 cm × 8 cm) located between the two chambers. The floor of the dark chamber was lined with stainless steel rods 1 cm apart from each other. A shock generator was used to deliver electric shocks (50 Hz, 0.2 mA, 0.5 s) on the floor of the dark chamber.
This test has three stages: habituation, acquisition, and retention. In order to habituate the groups to the apparatus, rats were given two habituation trials. The rats entered into a lighted section of the apparatus and 5 s later, the guillotine door was raised. Because of the natural tendency of rats to seek dark environments, they tried to enter the dark compartment (Barzegar et al. 2015). This trial was repeated after 30 min. The latency to enter the dark compartment (the step-through latency) was recorded after the rats entered freely into the dark compartment. Then, the door was closed, an electrical shock was administered, and the experimental rat was returned to its cage. This procedure was repeated again after 2 min. If the rats did not enter the dark chamber within 120 s, the acquisition trial was completed. The number of trials was recorded (Hasanein and Shahidi 2012; Jabbarpour et al. 2014).
The following day the retention test was performed. During the retention test, rats were placed in the light chamber and allowed to explore for 20 s before the guillotine door was opened. Step through latency, total time spent in the dark compartment, and the number of total entrances into the dark compartment were recorded for 600 s during the test day (Fig. 1).
Electrophysiological recording
Rats were anesthetized with urethane (1.5 g/kg, intraperitoneally (ip)) and fixed in a stereotaxic apparatus. During surgery, a heating pad was used to maintain the body temperature of the anesthetized rat. Cement was slowly removed and the skull was exposed. Two bipolar electrodes (0.008 in. in diameter, Teflon-coated stainless steel, A-M Systems, USA) were used as follows. The stimulating electrode was positioned at coordinates 8.1 mm posterior to the bregma, 4.3 mm lateral to the midline, and 2.7–3.2 mm from the skull surface into the perforant pathway (PP). The recording electrode was placed at coordinates 3.8 mm posterior to the bregma, 2.3 mm from the midline, and 2.7–3.2 mm from the surface of the skull into the dentate gyrus of hippocampus (Paxinos) (Fig. 1).
Baseline responses were elicited by single 0.1 ms biphasic square wave pulses (0.1 Hz frequency, current isolation unit A365WPI) which were delivered to the perforant pathway via the stimulating electrode, and the recording electrode obtained the evoked field potentials in the granular cells of the dentate gyrus (Karamian et al. 2015; Karimi et al. 2015; Lashgari et al. 2008). An input/output (I/O) response curve was constructed using the intensity of a single-pulse stimulation of the perforant pathway and averaging 10 responses in the dentate gyrus per intensity. The evoked field potential response was equal to 50% of the maximum response for all subsequent stimuli. The baseline response recorded was considered as the time point “0 min” just before high-frequency stimulation (HFS). The HFS (400 Hz, 10 bursts of 20 stimuli, 0.2 ms stimulus duration, and 10 s inter-burst interval) induced LTP (Komaki et al. 2014; Tahmasebi et al. 2015). After high-frequency stimulation, evoked responses were recorded at 5, 30, and 60 min (Karamian et al. 2015; Komaki et al. 2015; Salehi et al. 2015; Tahmasebi et al. 2015). The peak field excitatory postsynaptic potential (fEPSP) amplitude and population spike (PS) were measured. PS amplitude was analyzed as the distance from the negative peak to halfway between two positive peaks. The slope on the rising part of the first positive peak was used as the fEPSP slope.
Apoptosis (TUNEL assay)
After all rats had completed behavioral testing and electrophysiological recording (postoperative day 30), they were anesthetized (Fig. 1). Brains were fixed through perfusion with a paraformaldehyde solution. Subsequently, the brains were dehydrated, embedded in paraffin, and cut into consecutive 5-μm transverse sections. Alternate sections were used for TdT-mediated dUTP end labeling (TUNEL) staining.
One method for detecting DNA damage is TUNEL staining. TUNEL labels cells that have undergone DNA fragmentation and programmed cell death (Anderson et al. 2000). In order to detect apoptotic neurons in brain sections, the TUNEL staining was performed using a TUNEL Detection Kit (Roche) as previously described (Pourheydar et al. 2016) and according to the manufacturer’s instructions. After staining, the sections were analyzed under a light microscope (× 400, Olympus). A cell clearly displaying dark brown colored particles in the nucleus was defined as an apoptotic neuron in the hippocampal cornu ammonis 1 (CA1) region. For each animal, the mean number of apoptotic cells (dark brown cells) was obtained by counting five coronal sections with 120 μm intervals.
Congo red staining
Congo red staining was used to show Aβ plaque production in the brain tissue (Gorevic et al. 2011). The fixed brains were serially sectioned into 5-μm coronal sections. The paraformaldehyde-fixed brain tissues were embedded in paraffin, hydrated, and prepared for the staining of amyloid plaque using the Congo red kit according to the manufacturer’s protocol (Asianpajuhesh, Iran). Briefly, the brain sections were incubated in Congo red for 20 min, and then differentiated in an alkaline alcohol solution, followed by counterstaining with hematoxylin for 30 s, and were then mounted onto slides. The number of Congo red stained plaques was calculated for statistical analysis.
Data analysis
All data are presented as mean ± standard error of the mean (SEM). Behavioral and histological data were statistically analyzed using a one-way analysis of variance (ANOVA) followed by a least significant difference (LSD) test. Electrophysiology data were analyzed using two-way ANOVA with repeated-measures analysis on the time points of the same animals. Values of p < 0.05 were considered to be significant.
Results
Effects of treatments on the novel object recognition test
One-way ANOVA also showed that there was no significant difference in the total time spent exploring objects in proximity on the training day between all of the experimental groups (F (3, 39) = 0.96, p > 0.05, Fig. 2a).
Effect of chronic treatment of AS19 on total time exploration (a) and discrimination index (b) in object recognition test. ***p < 0.001 compared with the control group. ###p < 0.001 as compared with the sham group. &&&p < 0.001 as compared with the Aβ + AS19 group (n = 10 in each group). Each column represents mean ± SEM
There was a stronger tendency to explore the new object among all of the experimental groups on test day. One-way ANOVA showed significant differences in the discrimination index between the groups 1 day after training (F (3, 39) = 31.981, p < 0.001; Fig. 2b). A significant difference was also found between the Aβ rats and the other groups. The LSD posttest determined that the discrimination index of the Aβ rats was significantly lower than in the control and sham groups (p < 0.001). The LSD test also determined that the discrimination index of Aβ rats receiving AS19 was significantly higher than in the Aβ group (p < 0.001). One-way ANOVA clarified that there was no significant difference in the discrimination index between the control, sham, and the Aβ group receiving AS19.
Effects of treatments on the passive avoidance learning test
One-way ANOVA revealed that in the first acquisition trial there was no significant difference in the step-through latency in acquisition among the experimental groups of rats (F (3, 39) = 1.47, p > 0.05), indicating that there is no difference in the exploratory behavior of the groups into the dark compartment (Fig. 3a). Figure 3b shows significant differences among the experimental groups with respect to the number of trials to acquisition (F (3, 39) = 9.818, p < 0.001). LSD posttest showed that this number for the Aβ group was significantly higher than that of the control and sham groups (p < 0.001). However, the Aβ rats receiving AS19 also required a higher number of trials compared to the control and sham groups (p < 0.01).
Effect of chronic treatment of AS19 on the step through latency in acquisition (a), number of trials to acquisition in acquisition (b), step-through latency in retention (c), and time spent in the dark compartment (d) on passive avoidance learning task. ***p < 0.001, **p < 0.01, and *p < 0.05 as compared with the control group. ###p < 0.001, ##p < 0.01, and #p < 0.05 as compared with the sham group. &&&p < 0.001) and &p < 0.05 as compared with the Aβ + AS19 group (n = 10 in each group). Each column represents mean ± SEM There are not symboles in Fig 3.D. Therefore the Fig 3.D was attached.
There was a significant difference in the step-through latency between all groups in the retention test (F (3, 39) = 9.4, p < 0.001; Fig. 3c). LSD posttest showed that the Aβ group had lower step-through latency in retention than the control and sham groups (p < 0.001, p < 0.001, and p < 0.05 respectively; Fig. 3b). The AS19-treated Aβ group had higher step-through latencies in retention compared to the Aβ group (p < 0.05); however, step-through latency in retention of AS19 rats was lower than those of control and sham groups (p < 0.05).
There was also a significant difference in time spent in the dark compartment among the experimental groups (F (3, 39) = 46.495, p < 0.001). LSD posttest revealed that the Aβ group spent more time in the dark compartment compared to the control and sham groups (p < 0.001). AS19-treated Aβ rats spent less time in the dark compartment compared to the Aβ group (p < 0.001). Conversely, AS19-treated Aβ rats had greater time in dark compartment values than the control and sham groups (p < 0.01) (Fig. 3d).
Measurement of evoked potentials
Figure 4a clarifies sample traces from the control, sham, Aβ, and Aβ + AS19 groups before and after the high-frequency stimulation. The traces of population spike (PS) amplitude and fEPSP slope in the control, sham, and Aβ + AS19 groups were increased following high-frequency stimulation, but not in the Aβ group.
a Indicates sample traces of the evoked field potentials recorded in the hippocampal dentate gyrus in the experimental groups before high-frequency stimulation (BHSF) (solid lines) and 5, 30, 30 min (dashed lines) after high-frequency stimulation applied in the perforant pathway. b Time-dependent changes in the population spikes (PS) amplitude of dentate gyrus responses to perforant pathway stimulation following chronic icv treatment of AS19 or vehicle for 30 days. Line shows the control (black dashed line) (n = 10 in each group), sham (blue dashed line) (n = 10 in each group), Aβ (Pink dashed line) (n = 10 in each group), and Aβ + AS19 (violet solid line) groups (n = 8 in each group). ***p < 0.001 significant difference in comparison between different time, ###p < 0.001 significant difference in comparison between the different group, &&&p < 0.001 significant difference in comparison between (time × group). The significant difference in the Aβ group comparison with control ♪♪♪p < 0.001, sham ♣♣♣p < 0.001, and Aβ + AS19 groups ♥♥p < 0.01. Data are expressed as mean ± SEM, % of baseline. c Time-dependent changes in the excitatory post-synaptic potentials (fEPSP) slope of dentate gyrus responses to perforant pathway stimulation following chronic icv treatment of AS19 or vehicle for 30 days. Line shows the control (black dashed line) (n = 10 in each group), sham (blue dashed line) (n = 10 in each group), Aβ (pink dashed line) (n = 10 in each group), and Aβ + AS19 (violet solid line) groups (n = 8 in each group). ***p < 0.001 significant difference in comparison between different time, ###p < 0.001 significant difference in comparison between different group, &&&p < 0.001 significant difference in comparison between (time × group). The significant difference in the Aβ group in comparison with the control ♪♪♪p < 0.001, sham ♣♣p < 0.001, and Aβ + AS19 groups ♥p < 0.01. Data are expressed as mean ± SEM, % of baseline
Effects of treatments on population spike amplitude
Figure 4b shows the effects of Aβ and AS19 on the amplitude of the population spike induced by high-frequency stimulation in the perforant pathway-dentate gyrus area of the hippocampus. The time point “0 min” represents the baseline measurements of population spike amplitude just before high-frequency stimulation induction. Statistical analysis with two-way ANOVA (groups × time) determined that there were significant differences in the population spike amplitude between experimental groups ((F (3, 34) = 8.12, p < 0.001), and time points ((F (3, 114) = 83.3, p < 0.001), and their interaction ((F (9, 102) = 5.21, p < 0.001). Pairwise comparisons revealed that the population spike amplitude of the Aβ group was significantly less than that of the control and sham groups (p < 0.001 and p < 0.001, respectively). The population spike amplitude of the AS19-treated Aβ group was significantly higher than that of the Aβ group (p < 0.01). There was no significant difference in population spike amplitude among control and sham groups (p > 0.05). In addition, there were no significant differences in population spike amplitude between AS19-treated Aβ and control and sham groups (p > 0.05 and p > 0.05, respectively).
Pairwise comparisons revealed that high-frequency stimulation elicited a main enhancement of population spike amplitude from base time compared to 5 min (p < 0.001). Population spike amplitude at 5 min was significantly higher than at 30 min and 60 min (p < 0.001, p < 0.001 respectively), and population spike amplitude at 30 min was significantly higher than at 60 min (p < 0.001).
Effects of treatments on the fEPSP slope
Figure 4c illustrates the effects of Aβ and AS19 on the fEPSP slope induced by high-frequency stimulation in the perforant pathway-dentate gyrus area of the hippocampus. The baseline responses recorded were considered as the time point “0 min” in the measurement of fEPSP slope just before high-frequency stimulation.
Statistical analysis with two-way ANOVA (groups × time) determined that there were significant differences in fEPSP slope between experimental groups ((F (3, 34) = 6.68, p < 0.001) and time points ((F (3, 114) = 58.26, p < 0.001), and their interaction ((F (9, 102) = 4.11, p < 0.001). Pairwise comparisons revealed that the fEPSP slope of the Aβ group was significantly less than that of the control and sham groups (p < 0.001 and p < 0.01, respectively). The fEPSP slope of the AS19-treated Aβ group was significantly more than that of the Aβ group (p < 0.05). There were no significant differences among control and sham groups in PS amplitude between groups (p > 0.05). In addition, there were no significant differences in PS amplitude between the AS19-treated Aβ groups and the control and sham groups (p > 0.05 and p > 0.05, respectively). Pairwise comparisons revealed that high-frequency stimulation elicited a main enhancement of fEPSP slope from base time compared to 5 min (p < 0.001), 30 min (p < 0.001), and 60 min (p < 0.001). Additionally, EPSP slope at 5 min was significantly higher than at 30 min and 60 min (p < 0.001, p < 0.001, respectively) and EPSP slope at 30 min was significantly higher than at 60 min (p < 0.001).
Effects on hippocampal apoptotic cells
As mentioned earlier, TUNEL staining was performed to detect apoptotic neurons in the brain sections. Figure 5a shows a representative neuronal section stained using the TUNEL kit. One-way ANOVA indicated a significant difference in the number of apoptotic cells between groups (F (3, 39) = 174.2, p < 0.001). Tukey’s test showed that the brain sections of the rats in the Aβ group contained more apoptotic neurons than those in the control and sham groups (p < 0.01). The mean number of apoptotic neurons in the brain sections in the AS19-treated Aβ group was significantly decreased compared to the Aβ rats (p < 0.01). However, the AS19-treated Aβ rats had more apoptotic neurons compared to control and sham groups (p < 0.01 and p < 0.01, respectively). There was no significant difference in apoptotic neurons counted among control and sham groups (Fig. 5b).
Light micrographs of cell apoptosis in the hippocampal CA1 region. a Sections derived from the “a: control; b: sham; c: Aβ; and d: Aβ + AS19” groups stained by TUNEL. The arrow shows an apoptotic neuron. Scale bar = 100 μm, magnification: ×400. The neuron apoptotic mean was calculated (b). **p < 0.01 as compared with the control group. ##p < 0. 01) as compared with the sham group. &&p < 0.01 as compared with the AD group. Each column represents mean ± SEM
Effects of AS19 on brain Aβ plaque
Figure 6a–d shows the Aβ plaques in the coronal sections of the cortex region. The plaques were detected in the brain sections of the Aβ group. Congo red staining showed that the amyloid plaque deposits in the AS19-treated Aβ rats were significantly decreased compared with the Aβ rats. There was no recognizable plaque in the control and sham groups.
Discussion
The major results of this study are as follows: (1) icv Aβ injections caused a decrease in cognition, learning, and memory in the novel object recognition and passive avoidance tests; (2) 30 consecutive days of treatment with the 5-HTR7 agonist, AS19, ameliorated the recognition and passive avoidance memory impairment caused by the Aβ injections; (3) icv Aβ injections resulted in an impairment of fEPSP slope and population spike amplitude in the hippocampal dentate gyrus granule cells; (4) 30 consecutive days of AS19 administration caused an increase in the population spike amplitude and fEPSP slope in Aβ-treated rats; (5) icv Aβ injections enhanced the number of apoptotic neurons in the hippocampus; (6) 30 consecutive days of AS19 administration decreased the number of apoptotic neurons in the hippocampus of Aβ-treated rats; (7) icv Aβ injections caused Aβ plaque accumulation in the brain; and (8) 30 consecutive days of AS19 administration diminished Aβ plaque accumulation in the brains of Aβ-treated rats.
AD rats exhibited a decline in cognitive and memory functions, as well as synaptic dysfunction, loss of neurons, and Aβ plaque formation. Our results are in agreement with other data indicating that Aβ induces memory deficits and causes inhibition of neuronal plasticity in the hippocampus (Knobloch et al. 2007; Shahidi et al. 2017). Memory loss and cognitive impairment may result from synaptic dysfunction and dendrite loss in AD (Chapman et al. 1999; Chen et al. 2000; Mufson et al. 2016). In transgenic animal models, synapse abnormalities and memory impairments are correlated with plaque formation (Jacobsen et al. 2006; Mucke et al. 2000). Aβ damages neuronal cell, dendritic spines, and ultimate highlighted AD pathology (Krafft and Klein 2010; Lacor et al. 2004). Therefore, cognitive and memory impairment in the AD rats may be due to the accumulation of beta amyloids in the brain which promote neuronal apoptosis and neuronal plasticity deficits.
The chronic administration of AS19 improved cognitive ability and memory in Aβ-treated rats. These results agree with the beneficial effects of AS19, which include improved memory consolidation in naive animals and inhibited scopolamine or dizocilpine amnesic-induced effects (Perez-Garcia and Meneses 2005). In addition, impaired memory has been reported in mice lacking 5-HTR7 (Hedlund 2009; Sarkisyan and Hedlund 2009). Furthermore, in rats, treatment with a 5-HTR7 antagonist resulted in reduced hippocampal-dependent learning (Gasbarri et al. 2008). The 5-HTR7 is expressed in the hippocampal formation and cortex (Hedlund 2009) and participates in memory consolidation (Meneses 2014; Perez-Garcia et al. 2006; Perez-Garcia and Meneses 2009; Perez-Garcia and Meneses 2005). AS19 activates 5-HTR7, increases cyclic adenosine monophosphate (cAMP), and then activates protein kinase A (Kawasaki et al. 1998) and facilitates memory consolidation (Perez-Garcia et al. 2006; Perez-Garcia and Meneses 2009; Perez-Garcia and Meneses 2005). In addition, our results indicated that the improved memory and better cognitive function may be due to improved synaptic function and neuronal plasticity. Aβ rats that received daily microinjections of AS19 exhibited potentiation of hippocampal neuronal plasticity. Long-term treatment with AS19 positively shifted the fEPSP slope and population spike amplitude. Our present electrophysiological data are in agreement with previous studies indicating that 5-HTR7 contributes to the inhibition of hyperpolarization, and hence facilitates depolarization and neuronal excitability (Bacon and Beck 2000; Bickmeyer et al. 2002; Tokarski et al. 2005). Activation of 5-HTR7 decreases slow after-hyperpolarization amplitude in hippocampal neurons (Bickmeyer et al. 2002; Tokarski et al. 2005; Tokarski et al. 2003) and induces a positive shift in the activation curve (Bickmeyer et al. 2002) and neuronal excitability (Tokarski et al. 2003). The population spike amplitude of LTP reveals the number of granule cell discharges (Andersen et al. 1971; Anderson et al. 2000; Sloviter and Lomo 2012), and the fEPSP slope is started through neurotransmitter release from presynaptic receptors in the synaptic process (Bliss and Collingridge 1993; Bliss and Gardner-Medwin 1973; Sloviter and Lomo 2012). 5-HTR7 agonists potentiate hippocampal burst firing activity and synaptic transmission (Bacon and Beck 2000; Bickmeyer et al. 2002; Chen et al. 2011; Costa et al. 2012; Costa et al. 2012; Matsumoto et al. 2002; Speranza et al. 2017; Tokarski et al. 2003). In addition, the 5-HTR7 expression is located in the postsynaptic hippocampal neurons (Berumen et al. 2012). LTP occurs on the postsynaptic side of the synapse (Taube and Schwartzkroin 1988), and is a result of an increase in the number of glutamate receptors in the postsynaptic neuron (Luscher and Malenka 2012). Chronic 5-HTR7 stimulation elevates basal neuronal excitability (Kobe et al. 2012) via enhancing NMDA receptor activity (Xiang et al. 2016) and increasing AMPA glutamate receptor transmission (Berumen et al. 2012). Accordingly, chronic 5-HTR7 stimulation elevates basal neuronal excitability (Kobe et al. 2012) and enhances memory and cognitive function.
In the present study, AS19 diminished Aβ-induced programmed cell death and Aβ plaque accumulation. AS19 activated 5-HTR7 and enhanced the level of secondary messenger cAMP via stimulated adenylyl cyclase (Hoyer and Lannert 2007). This cAMP reduced Aβ levels in the brain’s interstitial fluid (Fisher et al. 2016; Speranza et al. 2015) and attenuated Aβ pathologies that ultimately lead to the reduction of AD pathology (Kazim et al. 2014). The activation of the 5-HTR7 induced new synaptic growth via coupling with the G12 protein signaling pathways (Citri and Malenka 2008), and also increased neural dendritic growth (Nativio et al. 2015; Speranza et al. 2015) and prevented apoptosis (Soga et al. 2007).
Conclusions
In summary, the results of the present study show that 1 month of icv administration of AS19 (5-HTR7 agonist) improved cognition and memory in an AD rat model and is associated with decreasing Aβ plaque accumulation, reduced neuronal apoptosis, and improving synaptic plasticity.
References
Alberghina L, Colangelo AM (2006) The modular systems biology approach to investigate the control of apoptosis in Alzheimer’s disease neurodegeneration. BMC Neurosci 7(Suppl 1):S2. https://doi.org/10.1186/1471-2202-7-s1-s2
Andersen P, Bliss TV, Skrede KK (1971) Lamellar organization of hippocampal pathways. Exp Brain Res 13:222–238
Anderson AJ, Stoltzner S, Lai F, Su J, Nixon RA (2000) Morphological and biochemical assessment of DNA damage and apoptosis in Down syndrome and Alzheimer disease, and effect of postmortem tissue archival on TUNEL. Neurobiol Aging 21:511–524
Babri S, Mohaddes G, Feizi I, Mohammadnia A, Niapour A, Alihemmati A, Amani M (2014) Effect of troxerutin on synaptic plasticity of hippocampal dentate gyrus neurons in a beta-amyloid model of Alzheimers disease: an electrophysiological study. Eur J Pharmacol 732:19–25. https://doi.org/10.1016/j.ejphar.2014.03.018
Bacon WL, Beck SG (2000) 5-Hydroxytryptamine7 receptor activation decreases slow after hyperpolarization amplitude in CA3 hippocampal pyramidal cells. J Pharmacol Exp Ther 294:672–679
Barzegar S, Komaki A, Shahidi S, Sarihi A, Mirazi N, Salehi I (2015) Effects of cannabinoid and glutamate receptor antagonists and their interactions on learning and memory in male rats. Pharmacol Biochem Behav 131:87–90. https://doi.org/10.1016/j.pbb.2015.02.005
Berumen LC, Rodriguez A, Miledi R, Garcia-Alcocer G (2012) Serotonin receptors in hippocampus TheScientificWorldJOURNAL 2012:823493 doi:https://doi.org/10.1100/2012/823493, 1, 15
Bickmeyer U, Heine M, Manzke T, Richter DW (2002) Differential modulation of I(h) by 5-HT receptors in mouse CA1 hippocampal neurons. Eur J Neurosci 16:209–218
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39. https://doi.org/10.1038/361031a0
Bliss TV, Gardner-Medwin AR (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. The J Physiol 232:357–374
Butzlaff M, Ponimaskin E (2016) The role of serotonin receptors in Alzheimer’s disease. Opera Medica et Physiologica 2:77–86
Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TVP, Hyman BT, Younkin SG, Hsiao KK (1999) Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci 2:271–276. https://doi.org/10.1038/6374
Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RGM (2000) A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature 408:975–979. https://doi.org/10.1038/35050103
Chen KH, Reese EA, Kim HW, Rapoport SI, Rao JS (2011) Disturbed neurotransmitter transporter expression in Alzheimer’s disease brain. Journal of Alzheimer’s disease : JAD 26:755–766. https://doi.org/10.3233/jad-2011-110002
Citri A, Malenka RC (2008) Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 33:18–41. https://doi.org/10.1038/sj.npp.1301559
Costa L, Sardone LM, Lacivita E, Leopoldo M, Ciranna L (2015) Novel agonists for serotonin 5-HT7 receptors reverse metabotropic glutamate receptor-mediated long-term depression in the hippocampus of wild-type and Fmr1 KO mice, a model of Fragile X Syndrome. Front Behav Neurosci 9:65. https://doi.org/10.3389/fnbeh.2015.00065
Costa L, Spatuzza M, D’Antoni S, Bonaccorso CM, Trovato C, Musumeci SA, Leopoldo M, Lacivita E, Catania MV, Ciranna L (2012) Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of Fragile X syndrome. Biol Psychiatry 72:924–933. https://doi.org/10.1016/j.biopsych.2012.06.008
Costa L, Trovato C, Musumeci SA, Catania MV, Ciranna L (2012) 5-HT(1A) and 5-HT(7) receptors differently modulate AMPA receptor-mediated hippocampal synaptic transmission. Hippocampus 22:790–801. https://doi.org/10.1002/hipo.20940
De Filippis B et al (2015) Long-lasting beneficial effects of central serotonin receptor 7 stimulation in female mice modeling Rett syndrome. Front Behav Neurosci 9:86. https://doi.org/10.3389/fnbeh.2015.00086
Dengler-Crish CM, Smith MA, Wilson GN (2017) Early evidence of low bone density and decreased serotonergic synthesis in the dorsal raphe of a tauopathy model of Alzheimer’s disease. Journal of Alzheimer’s disease : JAD 55:1605–1619. https://doi.org/10.3233/jad-160658
Fidalgo S, Ivanov DK, Wood SH (2013) Serotonin: from top to bottom. Biogerontology 14:21–45. https://doi.org/10.1007/s10522-012-9406-3
Filip M, Bader M (2009) Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacological reports : PR 61:761–777
Fisher JR, Wallace CE, Tripoli DL, Sheline YI, Cirrito JR (2016) Redundant Gs-coupled serotonin receptors regulate amyloid-beta metabolism in vivo. Mol Neurodegener 11:45. https://doi.org/10.1186/s13024-016-0112-5
Garcia-Alloza M, Hirst WD, Chen CP, Lasheras B, Francis PT, Ramirez MJ (2004) Differential involvement of 5-HT(1B/1D) and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer’s disease. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 29:410–416. https://doi.org/10.1038/sj.npp.1300330
Gasbarri A, Cifariello A, Pompili A, Meneses A (2008) Effect of 5-HT 7 antagonist SB-269970 in the modulation of working and reference memory in the rat. Behav Brain Res 195:164–170
Ghahremanitamadon F, Shahidi S, Zargooshnia S, Nikkhah A, Ranjbar A, Soleimani Asl S (2014) Protective effects of Borago officinalis extract on amyloid beta-peptide(25-35)-induced memory impairment in male rats: a behavioral study Biomed Res Int 2014:798535 doi:https://doi.org/10.1155/2014/798535, 1, 8
Gorevic PD, Schur PH, Romain PL (2011) Overview of amyloidosis. PATHOGENESIS 5:6
Han LN, Zhang L, Sun YN, du CX, Zhang YM, Wang T, Zhang J, Liu J (2016) Serotonin 7 receptors in the lateral habenular nucleus regulate depressive-like behaviors in the hemiparkinsonian rats. Brain Res 1644:79–87
Hasanein P, Shahidi S (2012) Preventive effect of Teucrium polium on learning and memory deficits in diabetic rats Medical science monitor : international medical journal of experimental and clinical research 18:Br41-46
Hashemi-Firouzi N, Akhavan M, Komaki A, Shahidi S (2015) Effects of acute administration of Urtica dioica on the novel object-recognition task in mice. Avicenna Journal of Neuro Psych Physiology 2
Hauser SR, Hedlund PB, Roberts AJ, Sari Y, Bell RL, Engleman EA (2014) The 5-HT7 receptor as a potential target for treating drug and alcohol abuse. Front Neurosci 8:448. https://doi.org/10.3389/fnins.2014.00448
Hedlund PB (2009) The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology 206:345–354. https://doi.org/10.1007/s00213-009-1626-0
Hoyer S, Lannert H (2007) Long-term abnormalities in brain glucose/energy metabolism after inhibition of the neuronal insulin receptor: implication of tau-protein. J Neural Transm Suppl:195–202
Jabbarpour Z, Shahidi S, Saidijam M, Sarihi A, Hassanzadeh T, Esmaeili R (2014) Effect of tempol on the passive avoidance and novel object recognition task in diabetic rats. Brain Res Bull 101:51–56. https://doi.org/10.1016/j.brainresbull.2013.12.013
Jacobsen JS et al. (2006) Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease Proceedings of the National Academy of Sciences of the United States of America 103:5161-5166 doi:https://doi.org/10.1073/pnas.0600948103
Karamian R, Komaki A, Salehi I, Tahmasebi L, Komaki H, Shahidi S, Sarihi A (2015) Vitamin C reverses lead-induced deficits in hippocampal synaptic plasticity in rats. Brain Res Bull 116:7–15. https://doi.org/10.1016/j.brainresbull.2015.05.004
Karimi SA, Komaki A, Salehi I, Sarihi A, Shahidi S (2015) Role of group II metabotropic glutamate receptors (mGluR2/3) blockade on long-term potentiation in the dentate gyrus region of hippocampus in rats fed with high-fat diet. Neurochem Res 40:811–817. https://doi.org/10.1007/s11064-015-1531-3
Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM (1998) A family of cAMP-binding proteins that directly activate Rap1. Science 282:2275–2279
Kazim SF, Blanchard J, Dai CL, Tung YC, LaFerla FM, Iqbal IG, Iqbal K (2014) Disease modifying effect of chronic oral treatment with a neurotrophic peptidergic compound in a triple transgenic mouse model of Alzheimer’s disease. Neurobiol Dis 71:110–130. https://doi.org/10.1016/j.nbd.2014.07.001
Khodamoradi N, Komaki A, Salehi I, Shahidi S, Sarihi A (2015) Effect of vitamin E on lead exposure-induced learning and memory impairment in rats. Physiol Behav 144:90–94. https://doi.org/10.1016/j.physbeh.2015.03.015
Knobloch M, Farinelli M, Konietzko U, Nitsch RM, Mansuy IM (2007) Abeta oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. The Journal of neuroscience : the official journal of the Society for Neuroscience 27:7648–7653. https://doi.org/10.1523/jneurosci.0395-07.2007
Kobe F, Guseva D, Jensen TP, Wirth A, Renner U, Hess D, Muller M, Medrihan L, Zhang W, Zhang M, Braun K, Westerholz S, Herzog A, Radyushkin K, el-Kordi A, Ehrenreich H, Richter DW, Rusakov DA, Ponimaskin E (2012) 5-HT7R/G12 signaling regulates neuronal morphology and function in an age-dependent manner. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:2915–2930. https://doi.org/10.1523/jneurosci.2765-11.2012
Komaki A, Karimi SA, Salehi I, Sarihi A, Shahidi S, Zarei M (2015) The treatment combination of vitamins E and C and astaxanthin prevents high-fat diet induced memory deficits in rats. Pharmacol Biochem Behav 131:98–103. https://doi.org/10.1016/j.pbb.2015.02.008
Komaki A, Khalili A, Salehi I, Shahidi S, Sarihi A (2014) Effects of exposure to an extremely low frequency electromagnetic field on hippocampal long-term potentiation in rat. Brain Res 1564:1–8. https://doi.org/10.1016/j.brainres.2014.03.041
Krafft GA, Klein WL (2010) ADDLs and the signaling web that leads to Alzheimer’s disease. Neuropharmacology 59:230–242. https://doi.org/10.1016/j.neuropharm.2010.07.012
Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL (2004) Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. The Journal of neuroscience : the official journal of the Society for Neuroscience 24:10191–10200. https://doi.org/10.1523/jneurosci.3432-04.2004
Lashgari R, Khakpour-Taleghani B, Motamedi F, Shahidi S (2008) Effects of reversible inactivation of locus coeruleus on long-term potentiation in perforant path-DG synapses in rats. Neurobiol Learn Mem 90:309–316. https://doi.org/10.1016/j.nlm.2008.05.012
Leopoldo M, Lacivita E, Berardi F, Perrone R, Hedlund PB (2011) Serotonin 5-HT 7 receptor agents: structure-activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol Ther 129:120–148
Li YH, Han L, Wu KLK, Chan YS (2017) Activation of 5-HT7 receptors reverses NMDA-R-dependent LTD by activating PKA in medial vestibular neurons. Neuropharmacology 123:242–248. https://doi.org/10.1016/j.neuropharm.2017.05.005
Luscher C, Malenka RC (2012) NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol 4. https://doi.org/10.1101/cshperspect.a005710
Matsumoto M, Kojima T, Togashi H, Mori K, Ohashi S, Ueno K, Yoshioka M (2002) Differential characteristics of endogenous serotonin-mediated synaptic transmission in the hippocampal CA1 and CA3 fields of anaesthetized rats. Naunyn Schmiedeberg's Arch Pharmacol 366:570–577. https://doi.org/10.1007/s00210-002-0634-y
Meneses A (2014) Memory formation and memory alterations: 5-HT6 and 5-HT7 receptors, novel alternative. Rev Neurosci 25:325–356. https://doi.org/10.1515/revneuro-2014-0001
Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L (2000) High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 20:4050–4058
Mufson EJ, Ikonomovic MD, Counts SE, Perez SE, Malek-Ahmadi M, Scheff SW, Ginsberg SD (2016) Molecular and cellular pathophysiology of preclinical Alzheimer’s disease. Behav Brain Res 311:54–69. https://doi.org/10.1016/j.bbr.2016.05.030
Nativio P et al. (2015) Stimulation of 5-HT7 receptor during adolescence determines its persistent upregulation in adult rat forebrain areas Synapse (New York, NY) 69:533-542 doi:https://doi.org/10.1002/syn.21846
Nikiforuk A, Kos T, Fijal K, Holuj M, Rafa D, Popik P (2013) Effects of the selective 5-HT7 receptor antagonist SB-269970 and amisulpride on ketamine-induced schizophrenia-like deficits in rats. PLoS One 8:e66695. https://doi.org/10.1371/journal.pone.0066695
Obulesu M, Lakshmi MJ (2014) Apoptosis in Alzheimer’s disease: an understanding of the physiology, pathology and therapeutic avenues. Neurochem Res 39:2301–2312. https://doi.org/10.1007/s11064-014-1454-4
Palmer AM, Francis PT, Benton JS, Sims NR, Mann DMA, Neary D, Snowden JS, Bowen DM (1987) Presynaptic serotonergic dysfunction in patients with Alzheimer’s disease. J Neurochem 48:8–15
Park SW, Jang HJ, Cho KH, Kim MJ, Yoon SH (2012) Rhie DJ, Developmental switch of the serotonergic role in the induction of synaptic long-term potentiation in the rat visual cortex. The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of Pharmacology 16:65–70. https://doi.org/10.4196/kjpp.2012.16.1.65
Paxinos GW C. (2007) The rat brain in stereotaxic coordinates Burlington MA Elsevier Inc
Perez-Garcia G, Gonzalez-Espinosa C, Meneses A (2006) An mRNA expression analysis of stimulation and blockade of 5-HT7 receptors during memory consolidation. Behav Brain Res 169:83–92. https://doi.org/10.1016/j.bbr.2005.12.013
Perez-Garcia G, Meneses A (2009) Memory time-course: mRNA 5-HT1A and 5-HT7 receptors. Behav Brain Res 202:102–113. https://doi.org/10.1016/j.bbr.2009.03.027
Perez-Garcia GS, Meneses A (2005) Effects of the potential 5-HT7 receptor agonist AS 19 in an autoshaping learning task. Behav Brain Res 163:136–140. https://doi.org/10.1016/j.bbr.2005.04.014
Pourheydar B, Soleimani Asl S, Azimzadeh M, Rezaei Moghadam A, Marzban A, Mehdizadeh M (2016) Neuroprotective Effects of Bone Marrow Mesenchymal Stem Cells on Bilateral Common Carotid Arteries Occlusion Model of Cerebral Ischemia in Rat. Behavioural Neurology 2016:2964712–2964710. https://doi.org/10.1155/2016/2964712
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362:329–344. https://doi.org/10.1056/NEJMra0909142
Radi E, Formichi P, Battisti C, Federico A (2014) Apoptosis and oxidative stress in neurodegenerative diseases. Journal of Alzheimer’s disease : JAD 42(Suppl 3):S125–S152. https://doi.org/10.3233/jad-132738
Salehi I, Karamian R, Komaki A, Tahmasebi L, Taheri M, Nazari M, Shahidi S, Sarihi A (2015) Effects of vitamin E on lead-induced impairments in hippocampal synaptic plasticity. Brain Res 1629:270–281. https://doi.org/10.1016/j.brainres.2015.10.007
Sarkisyan G, Hedlund PB (2009) The 5-HT7 receptor is involved in allocentric spatial memory information processing. Behav Brain Res 202:26–31. https://doi.org/10.1016/j.bbr.2009.03.011
Shahidi S, Hashemi-Firouzi N (2014) The effects of a 5-HT7 receptor agonist and antagonist on morphine withdrawal syndrome in mice. Neurosci Lett 578:27–32. https://doi.org/10.1016/j.neulet.2014.06.027
Shahidi S, Zargooshnia S, Asl SS, Komaki A, Sarihi A (2017) Influence of N-acetyl cysteine on beta-amyloid-induced Alzheimer’s disease in a rat model: a behavioral and electrophysiological study. Brain Res Bull 131:142–149. https://doi.org/10.1016/j.brainresbull.2017.04.001
Sloviter RS, Lomo T (2012) Updating the lamellar hypothesis of hippocampal organization. Frontiers in neural circuits 6:102. https://doi.org/10.3389/fncir.2012.00102
Soga F, Katoh N, Inoue T, Kishimoto S (2007) Serotonin activates human monocytes and prevents apoptosis. The Journal of investigative dermatology 127:1947–1955. https://doi.org/10.1038/sj.jid.5700824
Speranza L, Giuliano T, Volpicelli F, de Stefano ME, Lombardi L, Chambery A, Lacivita E, Leopoldo M, Bellenchi GC, di Porzio U, Crispino M, Perrone-Capano C (2015) Activation of 5-HT7 receptor stimulates neurite elongation through mTOR, Cdc42 and actin filaments dynamics. Front Behav Neurosci 9:62. https://doi.org/10.3389/fnbeh.2015.00062
Speranza L, Labus J, Volpicelli F, Guseva D, Lacivita E, Leopoldo M, Bellenchi GC, di Porzio U, Bijata M, Perrone-Capano C, Ponimaskin E (2017) Serotonin 5-HT7 receptor increases the density of dendritic spines and facilitates synaptogenesis in forebrain neurons. J Neurochem 141:647–661. https://doi.org/10.1111/jnc.13962
Stiedl O, Pappa E, Konradsson-Geuken A, Ogren SO (2015) The role of the serotonin receptor subtypes 5-HT1A and 5-HT7 and its interaction in emotional learning and memory. Front Pharmacol 6:162. https://doi.org/10.3389/fphar.2015.00162
Tahmasebi L, Komaki A, Karamian R, Shahidi S, Sarihi A, Salehi I, Nikkhah A (2015) The interactive role of cannabinoid and vanilloid systems in hippocampal synaptic plasticity in rats. Eur J Pharmacol 757:68–73. https://doi.org/10.1016/j.ejphar.2015.03.063
Takuma K, Hara Y, Kataoka S, Kawanai T, Maeda Y, Watanabe R, Takano E, Hayata-Takano A, Hashimoto H, Ago Y, Matsuda T (2014) Chronic treatment with valproic acid or sodium butyrate attenuates novel object recognition deficits and hippocampal dendritic spine loss in a mouse model of autism. Pharmacol Biochem Behav 126:43–49. https://doi.org/10.1016/j.pbb.2014.08.013
Taube JS, Schwartzkroin PA (1988) Mechanisms of long-term potentiation: EPSP/spike dissociation, intradendritic recordings, and glutamate sensitivity. The Journal of neuroscience : the official journal of the Society for Neuroscience 8:1632–1644
Tokarski K, Zahorodna A, Bobula B, Grzegorzewska M, Pitra P, Hess G (2005) Repeated administration of citalopram and imipramine alters the responsiveness of rat hippocampal circuitry to the activation of 5-HT7 receptors. Eur J Pharmacol 524:60–66. https://doi.org/10.1016/j.ejphar.2005.09.014
Tokarski K, Zahorodna A, Bobula B, Hess G (2003) 5-HT7 receptors increase the excitability of rat hippocampal CA1 pyramidal neurons. Brain Res 993:230–234
Vasefi MS, Yang K, Li J, Kruk JS, Heikkila JJ, Jackson MF, MacDonald JF, Beazely MA (2013) Acute 5-HT7 receptor activation increases NMDA-evoked currents and differentially alters NMDA receptor subunit phosphorylation and trafficking in hippocampal neurons. Molecular brain 6:24. https://doi.org/10.1186/1756-6606-6-24
Wang W, Pan YW, Zou J, Li T, Abel GM, Palmiter RD, Storm DR, Xia Z (2014) Genetic activation of ERK5 MAP kinase enhances adult neurogenesis and extends hippocampus-dependent long-term memory. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:2130–2147. https://doi.org/10.1523/jneurosci.3324-13.2014
Waters KA, Stean TO, Hammond B, Virley DJ, Upton N, Kew JN, Hussain I (2012) Effects of the selective 5-HT(7) receptor antagonist SB-269970 in animal models of psychosis and cognition. Behav Brain Res 228:211–218. https://doi.org/10.1016/j.bbr.2011.12.009
Wesolowska A, Nikiforuk A, Stachowicz K (2006) Potential anxiolytic and antidepressant effects of the selective 5-HT7 receptor antagonist SB 269970 after intrahippocampal administration to rats. Eur J Pharmacol 553:185–190. https://doi.org/10.1016/j.ejphar.2006.09.064
Wesołowska A, Nikiforuk A, Stachowicz K, Tatarczyńska E (2006) Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology 51:578–586. https://doi.org/10.1016/j.neuropharm.2006.04.017
Xiang K, Zhao X, Li Y, Zheng L, Wang J, Li YH (2016) Selective 5-HT7 receptor activation may enhance synaptic plasticity through N-methyl-D-aspartate (NMDA) receptor activity in the visual cortex. Curr Neurovasc Res 13:321–328
Xu Y, Yan J, Zhou P, Li J, Gao H, Xia Y (2012) Wang Q, Neurotransmitter receptors and cognitive dysfunction in Alzheimer’s disease and Parkinson’s disease. Progress in neurobiology 97:1–13. https://doi.org/10.1016/j.pneurobio.2012.02.002
Zhang QJ, du CX, Tan HH, Zhang L, Li LB, Zhang J, Niu XL, Liu J (2015) Activation and blockade of serotonin7 receptors in the prelimbic cortex regulate depressive-like behaviors in a 6-hydroxydopamine-induced Parkinson’s disease rat model. Neuroscience 311:45–55. https://doi.org/10.1016/j.neuroscience.2015.10.016
Zhang Y, McLaughlin R, Goodyer C, LeBlanc A (2002) Selective cytotoxicity of intracellular amyloid beta peptide1-42 through p53 and Bax in cultured primary human neurons. J Cell Biol 156:519–529. https://doi.org/10.1083/jcb.200110119
Acknowledgments
This work was supported by a grant (Grant number: 9312186737) from the Neurophysiology Research Centre, Hamadan University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal care, treatment, and surgical procedures were approved by the ethics committees of the Hamadan University of Medical Sciences (Ref. No.: 6383), and performed according to the Guide for Care and Use of laboratory animals published by the National Institute of Health, USA (NIH Publication No. 85-23, revised 1985).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shahidi, S., Asl, S.S., Komaki, A. et al. The effect of chronic stimulation of serotonin receptor type 7 on recognition, passive avoidance memory, hippocampal long-term potentiation, and neuronal apoptosis in the amyloid β protein treated rat. Psychopharmacology 235, 1513–1525 (2018). https://doi.org/10.1007/s00213-018-4862-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-4862-3