Abstract
Asperuloside (ASP) and geniposide (GP) are iridoids that have shown various biological properties, such as reduction of inflammation, oxidative stress, and neuroprotection. The aim of this study was to investigate the mechanism of action of ASP and GP through the experimental model of pilocarpine-induced seizures. Mice were treated daily with saline, valproic acid (VPA), GP (5, 25, or 50 mg/kg), or ASP (20 or 40 mg/kg) for 8 days. Pilocarpine (PILO) treatment was administered after the last day of treatment, and the epileptic behavior was recorded for 1 h and analyzed by an adapted scale. Afterward, the hippocampus and blood samples were collected for western blot analyses, ELISA and comet assay, and bone marrow to the micronucleus test. We evaluated the expression of the inflammatory marker cyclooxygenase-2 (COX-2), GluN2B, a subunit of the NMDA receptor, pGluR1, an AMPA receptor, and the enzyme GAD-1 by western blot and the cytokine TNF-α by ELISA. The treatments with GP and ASP were capable to decrease the latency to the first seizure, although they did not change the latency to status epilepticus (SE). ASP demonstrated a genotoxic potential analyzed by comet assay; however, the micronuclei frequency was not increased in the bone marrow. The GP and ASP treatments were capable to reduce COX-2 and GluN2B receptor expression after PILO exposure. This study suggests that GP and ASP have a protective effect on PILO-induced seizures, decreasing GluN2B receptor and COX-2 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept of epilepsy is applied to a variety of conditions, very heterogeneous in terms of etiology, clinical expression, severity, and prognosis, united only by the recurrence of epileptic seizures (Fisher et al. 2014). It can be defined as a brain disorder characterized by a long-lasting predisposition to generate epileptic seizures and by the neurobiological, cognitive, psychological, and social consequences of this condition. Epilepsy has emerged as a global health concern, affecting around 70 million of the population worldwide, mostly in underserved regions. Its incidence and prevalence vary throughout life, with higher rates in children under 5 years old, a lower incidence of new cases in early adulthood, and an increase in new cases in adults over 55 years of age (Johnson 2019; Akyuz et al. 2021).
Despite the rapid progression in clinical and preclinical epilepsy research, its pathogenesis remains elusive. Epilepsy is characterized by unexpected recurrent seizures associated with abnormal electrical activity in the brain due to an imbalance between excitatory and inhibitory neurotransmitters, e.g., glutamate and gamma-aminobutyric acid (γ-aminobutyric acid, GABA), which can result in an overexcitation of the neurons (Santulli et al. 2016; Johnson 2019; Alachkar et al. 2020). The classic antiepileptic medication (AEM) exerts only symptomatic relief rather than promoting changes in the pathological process. Current drugs aim to restore the balance between neurotransmitters by different mechanisms, thereby providing symptomatic relief. Although most patients achieve permanent remission, 30% still are resistant to pharmacological treatment. Likewise, adverse events related to AEM are common, with as many as 60% of patients reporting issues such as cognitive dysfunction, incoordination, sedation, and mood disturbance (Akyuz et al. 2021; Dang et al. 2021). These facts clearly reflect the need for a better understanding of the mechanisms involved in epilepsy, as well as the development of more effective therapies against epileptic seizures.
In this context, the search for new drugs from natural products becomes a suitable process, where plants are a natural reservoir of secondary metabolites, chemical entities that demonstrate many biological activities (Manzione et al. 2020). Several classes of natural compounds have been explored, one of them being iridoids, which are metabolites commonly found in a wide variety of plants. These compounds are known to represent an extensive group of natural secondary metabolites that have a bicyclic structure consisting of a cyclopentane ring fused to a six-membered heterocyclic ring containing an acetal linkage (Chan et al. 2020; Manzione et al. 2020).
GP and ASP, iridoid glycoside compounds, can be found in several plant species, such as Gardenia jasminoides, Eucommia ulmoides, Rehmanniaglutinosa, and Achyranthes bidentata (Dinda et al. 2019). Studies have shown that GP produces a diversity of in vitro and in vivo pharmacological effects, including neuroprotective, antidiabetic, hepatoprotective, anti-inflammatory, analgesic, antidepressant-like, cardioprotective, antioxidant, immune-regulatory, antithrombotic, and antitumoral effects (Zhou et al. 2019b). It has been proposed that GP seems to be a promising compound for the treatment of neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease (Zhang et al. 2015; Su et al. 2016; Zhou et al. 2019a). Furthermore, the GP attenuated electroshock-induced epileptic seizures, significantly reducing the incidence and increasing the latency for clonic seizures in a preclinical model (Wei et al. 2018).
Investigations have reported on various medicinal ASP properties, such as in the treatment of obesity, inflammatory diseases, cancer, and bacterial infections (Chan et al. 2020). Likewise, studies have shown that ASP showed to possess an effect on anti-inflammation, including through nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways. Alike, the protective effects of ASP were observed in LPS-induced acute lung injury in mice (Chen et al. 2021). Furthermore, it was observed that ASP significantly decreased levels of nitric oxide and prostaglandins in a model of inflammation, exerting an anti-inflammatory effect (He et al. 2018). However, few studies have been performed analyzing the ASP neuroprotective activity. Thus, the aim of this study was to evaluate the ASP and GP mechanisms of action of ASP in the experimental model of PILO in mice.
Materials and methods
Animals and experimental design
To carry out the experiments, 112 adult male CF1 mice were used (2- to 3-month old, weighing 35–50 g). The animals were obtained and kept at the Center for Reproduction and Experimentation of Laboratory Animals at the Federal University of Rio Grande do Sul (UFRGS). Mice were housed in plastic cages (four per cage), with water and food ad libitum, under a 12-h light/dark cycle (lights on at 7:00 a.m.) and at a constant temperature of 23 ± 2 °C. All experiments were designed aiming to reduce the number of animals used to a minimum, as well as to minimize their suffering.
The sample size was calculated using a previous study and G* power version 3.1 software, the effect size (f) = 0.38; α = 0.05 (type 1 error); 95% confidence interval; power = 0.95; and total sample size = 112. It employs a simple randomization method, and mice were divided into eight groups (fourteen animals per group): SAL (0.9% saline), PILO (saline 0.9% + PILO, 280 mg/kg), VPA (valproic acid 300 mg/kg + PILO), GP5 (geniposide 5 mg/kg + PILO), GP25 (geniposide 25 mg/kg + PILO), GP50 (geniposide 50 mg/kg + PILO), ASP20 (asperuloside 20 mg/kg + PILO), and ASP40 (asperuloside 40 mg/kg + PILO).
The present work was approved by the Federal University of Rio Grande do Sul Ethics Committee (CEUA/UFRGS; 37,769).
Drugs and pharmacological procedures
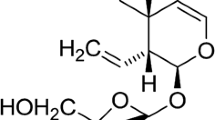
GP and ASP were extracted from Genipa americana and Escallonia bifida, respectively, and isolated by chromatographic methods. Their structures were confirmed by spectroscopic methods and can be seen in Fig. 1 (Vendruscolo et al. 2018). Both compounds were extracted in the Pharmacognosy laboratory in the Faculty of Pharmacy of the Federal University of Rio Grande do Sul. Diazepam (DZP) (Compaz®) was purchased from Cristália, VPA (Depakene®), our positive control, was purchased from Abbot, and PILO was acquired from Sigma-Aldrich. All drugs were dissolved in saline (NaCl 0.9%) solution. Mice treatments were administered once daily for 8 days by gavage (Fig. 2). The drugs were given in a volume not exceeding 10 ml/kg. PILO (280 mg/kg, intraperitoneally, i.p.) was used to induce seizures 24 h after the last treatment with ASP, GP, or ASP. The decision to treat the animals before the SE generation was based on previous studies (Wang et al. 2017; Asgharzade et al. 2020). The PILO dose was chosen according to pilot test results conducted in our laboratory, and GP and ASP doses were chosen based on previous results (Qiu et al. 2016; Wei et al. 2018; Zhao et al. 2018).
Asperuloside toxicity test
ASP was investigated for in vivo toxicological properties in a 14-day acute oral toxicity study. As per OECD 423 guidelines, if there is no information on a substance to be tested, for animal welfare reasons, it is recommended to use the starting dose of 300 mg/kg body weight.
The parameters evaluated were skin and mucosal changes, salivation, tremors, lethargy, convulsions, and diarrhea. These parameters were assessed by visual analysis performed by two researchers independently. All animals were observed individually every 30 min for 24 h after administration of the single dose. The total time of observation was 14 days. The tested doses were 300 and 2000 mg/kg. Each dose was tested on six animals.
Induction of status epilepticus (SE)
Mice were treated with scopolamine methyl bromide (SMB) (1 mg/kg, i.p.) 20 min previously to PILO injection to limit peripheral effects (Curia et al. 2008). After the PILO injection, the animals were isolated in plastic cages, and their behavior was recorded by video for 60 min. Behavioral changes were graded according to the following scale: 0, no abnormality; 1, mouth and facial movements; 2, head nodding; 3, forelimb clonus; 4, rearing and bilateral forelimb clonus; 5, rearing, falling, and jumping; 6, death (Racine 1972; Chen et al. 2019; Wang et al. 2019; Lima et al. 2020; Yang et al. 2020). Seizure latency was defined as the time between the administration of PILO and the occurrence of the first score 3 seizure. The beginning of SE was defined as the onset of continuous stage 4 or 5 seizures or multiple stage 4/5 seizures without regaining normal behavior between seizures. DZP was injected to end the seizure in all the animals.
To carry out the comet assay and micronucleus test, forty-eight mice (six animals per group) were sacrificed by decapitation, 60 min after PILO injection, to prevent cell repair mechanisms from decreasing detected damage (Azqueta et al. 2014; Jiang et al. 2019). After the behavioral analysis, sixty-four animals (eight animals per group) that were used in western blot and ELISA assay received DZP (2 mg/kg) to end their seizures.
Tissue sample
Hippocampus samples were collected at the end of the experiment to evaluate the expression of protein scyclooxygenase-2 (COX-2), GluN2B, a subunit of the NMDA receptor, pGluR1 an AMPA receptor, and the enzyme GAD-1 by western blot. Likewise, hippocampus samples were used to evaluate the cytokine TNF-α by ELISA. Frozen hippocampus (− 80 °C) from each treated mouse was homogenized in lysis buffer (pH 7.4) containing protease inhibitors and detergents (150 mM NaCl, 20 Mm Tris–HCl, 1 mM EDTA, 10% glycerol, 1% Nonidet P40, and 0.2 mM phenylmethylsulphonyl fluoride). The homogenates were centrifuged at 7000 g for 10 min at 4 °C to discard cell debris, and the supernatant fraction obtained was used for TNF-α measure (ELISA) and western blotting assay. The protein levels were measured by the method described by Bradford (Bradford 1976). Tissue samples were frozen in liquid nitrogen and stored at − 80 °C previously for analysis.
Peripheral blood samples were collected for the comet assay. For the micronucleus test, bone marrow was used. Hippocampus, blood, and bone marrow samples were collected at different times after treatment to allow a better analysis of different biochemical parameters. The comet assay assesses acute damage, so it was necessary to collect blood samples soon after the end of treatment. As for the evaluation of protein expression by western blot and TNF-α analysis by ELISA, the time was longer, according to previous studies.
TNF-α measure
The levels of TNF-α were obtained by using a commercially available Enzyme-linked immunosorbent assay kit (R&D system catalog number: DY510-05) and read using a microplate reader set to 450 nm, according to the supplier’s recommendations. The test presents 2.4% cross-reactivity and can detect 100–10,000 pg/mL. Data were calculated as ng/mL and were expressed as percentages of the control.
Western blotting
For the western blot analysis, a 30-μg sample of total proteins was separated on an SDS–polyacrylamide gel 10% and transferred to a nitrocellulose membrane. The membranes were blocked by incubation in 5% (w/v) BSA in TBS-T (Tris-buffered saline with Tween 20 0.1%) for 30 min at room temperature. Then, membranes were blotted overnight at 4 °C with the primary antibodies using rabbit polyclonal antibodies. For these experiments, we used antibodies, anti-phospho-GluR1 (Ser845) clone EPR2148 (Merck Millipore 106 kDa; 1:1000; catalog no. 04–1073), anti-GluN2B (190 kDa; 1:1000, Cell Signaling Technology, catalog no. #D15B3), anti-COX-2 (69 kDa; 1:1000. Abcam catalog number ab15191), anti-GAD1 (67 kDa 1:1000; Cell Signaling Technology, catalog no. #5305), and anti-β-actin (45 kDa; 1:500; Cell Signaling Technology, catalog no. #8457). The blots were washed three times with TBS-T for 10 min and developed with peroxidase-linked secondary antibodies goat antirabbit secondary (Millipore, Billerica, Massachusetts, USA) (1:10,000 dilution) and washed with TBS (20 mM Tris–HCl, 140 mM NaCl, pH = 7.4) for 10 min. Protein bands were then visualized using an enhanced chemiluminescent reagent (ECL) in the iBright™ Imaging System. Relative protein levels were determined by normalizing to the β-actin band signal, and the gray value of the pictures was quantified by ImageJ 1.52a software.
Comet assay
The alkaline comet assay was performed as previously described by Singh et al. (Singh et al. 1988). Briefly, 10 μL of the peripheral blood was mixed with 90 μL LMP agarose, spread on a normal agarose precoated microscope slide, and kept at 4 °C for 5 min to allow for solidification. Cells were lysed in a high-concentration salt and detergent solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris with 1% Triton X-100, and 10% DMSO freshly added) for 2 h. Subsequently, cells were exposed to alkali conditions (300 mM NaOH/1 mM Na2EDTA, pH > 13, 30 min, (4 °C) to allow DNA unwinding and expression of alkali labile sites. Electrophoresis was conducted for 20 min at 25 V and 300 mA (94 V/cm). Then, the slides were neutralized and silver stained. One hundred cells were scored visually according to tail length and the amount of DNA present in the tail. Each comet was given an arbitrary value of 0–4 (0, undamaged; 4, maximally damaged), as described by (Collins 2004). A damage score was thus assigned to each sample, ranging from 0 (completely undamaged: 100 cells × 0) to 400 (with maximum damage: 100 cells × 4). International guidelines and recommendations for the comet assay consider that visual scoring of comets is a good-validated evaluation method, as it is highly correlated with computer-based image analysis (Collins 2004).
Micronucleus test
The micronucleus test was performed according to the US Environmental Protection Agency Gene-Tox Program guidelines (Mavournin et al. 1990). Bone marrow from both mouse femurs was suspended in fetal calf serum, and smears were prepared on clean glass slides. Slides were air dried, fixed in methanol, stained in 10% Giemsa, and coded for blind analysis. Cyclophosphamide 40 mg/kg (i.p.) was used as a positive control in three mice. To avoid false-negative results and to obtain a value of bone marrow toxicity, the polychromatic erythrocyte/normochromatic erythrocyte (PCE/NCE) ratio was determined in 1000 cells. The incidence of micronuclei was observed in 2000 PCE for each animal using bright-field optical microscopy at × 1000 magnification.
Statistical analysis
All data were analyzed using GraphPad Prism software. Evaluation of data from a percentage of seizures was carried out using Fisher’s exact test because they were categorical data. Latency to the first seizure, the comet assay results, micronucleus test, TNF-α, and western blot were tested for data normality by the Kolmogorov–Smirnov test and for homogeneity by the Levene test. The data were analyzed by one-way ANOVA followed by Dunnett’s test. In all comparisons, a significance level of 5% (p < 0.05) was adopted. All the collected data can be seen in Supplementary material 1.
Results
Evaluation of ASP toxicity in mice
The visual observations revealed that there were no salivation, tremors, convulsions, lethargy, diarrhea, sleep, and coma, and it was not found visible changes in the skin, fur, and eyes in the mice treated with ASP (300 or 2000 mg/kg). During the 14 days of the experiment, there was a gradual increase in the mice’s mean body weight (Fig. 3). In the RM, one-way ANOVA observed a difference between groups (F4,40 = 15.31; p = 0.0001). At the dose of 300 mg/kg, the last two weight measurements on days 12 and 14 differed from the first measurement (p = 0.0043 and 0.0001, respectively). As well as at the dose of 2000 mg/kg, the weighing of days 12 and 14 was different from the initial weighing (p = 0.0011 and p = 0.0005). No mortality was observed. As per the OECD standard, ASP demonstrates low toxicity at the doses tested.
Weight measurement of mice for 14 days after single dose ASP treatment (OECD 423 guidelines), the values represent means ± SEM. Data were analyzed by RM one-way * p < 0.05 in comparison with the first measure of the group. N = 12. ASP 300: mice receive one dose of 300 mg/kg of asperuloside. ASP 2000: mice receive one dose of 2000 mg/kg of asperuloside
Effect of GP and ASP on PILO-induced seizure in mice
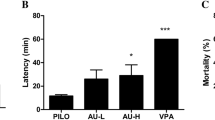
As shown in Fig. 4a, GP and ASP treatments were able to increase the latency for a score 3 seizure. Data were analyzed by one-way ANOVA (F6,90 = 2.585; p = 0.0234) followed by Dunnett’s test. In the VPA group, the mean latency was 42.19 min, different from the PILO group, whose mean latency was 20.76 min (p = 0.0129). The GP50 and ASP40 groups were different from the PILO group (p = 0.0150 and p = 0.0068), increasing the latency to 41.99 and 43.59 min, respectively.
Effect of GP and ASP treatment on PILO-induced seizures in mice; a on latency to first score 3 seizures, values represent means ± SEM. b Frequency of score 3 seizures, data expressed as a percentage of animals that reached seizure 3 in each group. c Frequency of SE and data expressed as a percentage of animals that reached SE in each group; d Average of scores, values represent means ± SEM. Analyzed by one-way ANOVA followed by Dunnett’s test *p < 0.05 in comparison with the PILO group. **p < 0.01 in comparison with the PILO group. Each circle represents one mouse. N = 14. VPA: mice received valproic acid (300 mg/kg) for 8 days and pilocarpine; PILO: saline (0.9%) for 8 days and pilocarpine; GP5: 5 mg/kg of geniposide for 8 days and pilocarpine; GP25: 25 mg/kg of geniposide for 8 days and pilocarpine; GP50: 50 mg/kg of geniposide for 8 days and pilocarpine; ASP20: 20 mg/kg of asperuloside for 8 days and pilocarpine; ASP40: 40 mg/kg of asperuloside for 8 days and pilocarpine
As seen in Fig. 4b, 100% of the animals in the PILO group had scored 3 seizures. Data were analyzed in a 2 × 2 contingency table by Fisher’s exact test (χ2 = 9.539; df = 6). Although the VPA group showed a reduction in the percentage of seizures, the result was not statistically different from the PILO group (p = 0.22). The GP25, GP50, and ASP40 groups differed statistically from the PILO group (p = 0.0407 for both groups), but not GP5 and ASP20 (p = 0.99 and 0.97, respectively).
From the group treated only with PILO, 69% of the animals reached SE (Fig. 4c). In the VPA and ASP40 groups, 36% of the treated animals reached SE, but this difference was not significant in the Fisher’s exact test (p = 0.216).
The mean scores of seizures in each group were calculated and compared to the PILO control group by Fisher’s exact test (Fig. 4d). The groups that achieved the highest scores in our scale were PILO and GP5 with means of 4.02 and 3.8, respectively. The group with the lowest score was the VPA, with a mean of 3.05. Nevertheless, no statistical difference between groups was observed in Fisher’s exact test (p = 0.1077).
The mortality after intraperitoneal application of PILO (280 mg/kg) was 23% in the PILO group and 14% in the ASP20 group. The other groups that received GP or ASP did not die at the time of observation.
Effect of GP and ASP on TNF-α expression
To analyze whether the GP and ASP properties are mediated by pro-inflammatory mechanisms, we measured the hippocampus levels of TNF-α. The results can be seen in Fig. 5. Data were analyzed by one-way ANOVA, but there was no statistical difference between groups (p = 0.5357).
Effect of GP and ASP on the relative expression of TNF-α in mice hippocampus after PILO injection. Each circle represents one measure in duplicate. N = 3–4. SAL: mice received only saline (0.9%); VPA: mice received valproic acid (300 mg/kg) for 8 days and pilocarpine; PILO: saline for 8 days and pilocarpine; GP5: 5 mg/kg of geniposide for 8 days and pilocarpine; GP25: 25 mg/kg of geniposide for 8 days and pilocarpine; GP50: 50 mg/kg of geniposide for 8 days and pilocarpine; ASP20: 20 mg/kg of asperuloside for 8 days and pilocarpine; ASP40: 40 mg/kg of asperuloside for 8 days and pilocarpine
Effects of GP and ASP on GluN2B, COX-2, pGluR1, and GAD1 expression
To analyze the mechanisms of action of GP and ASP, we evaluated COX-2, GluN2B, pGluR1, and GAD expression by western blot. The results of western blot analysis can be seen in Fig. 6 and Supplementary material 2.COX-2 expression was analyzed by one-way ANOVA (F7,28 = 3.002; p = 0.0176) followed by Dunnet’s test. The expression was increased in the PILO mice compared to the SAL group (p = 0.0040). A reduction in COX-2 expression was observed in all treated groups (Fig. 6a). GluN2B relative expression was analyzed by one-way ANOVA (F7,30 = 3.002; p = 0.0058) followed by Dunnet’s test. NMDA receptor was upregulated in the PILO group, compared to the SAL group (p = 0.0010). In GP- and ASP-treated groups, we observed a decrease in GluN2B expression after PILO injection (Fig. 6c). The expression of GAD1 and pGluR1 did not differ between groups (p = 0.7842 and p = 0.9669, respectively) (Fig. 6b and d).
Effect of GP and ASP treatment in PILO-induced seizures in mice on a Cox-2 relative expression b GluN2B relative expression; c GAD1 relative expression and d pGluR1 relative expression e Representative bands of western blot analysis. Values represent means ± SEM. Analyzed by one-way ANOVA followed by Dunnett’s test. Each circle represents one measure in duplicate. N = 3–6. *p < 0.05 in comparison to the SAL group. #p < 0.05 in comparison to the PILO group. SAL: mice received only saline (0.9%); VPA: mice received valproic acid (300 mg/kg) for 8 days and pilocarpine; PILO: saline for 8 days and pilocarpine; GP5: 5 mg/kg of geniposide for 8 days and pilocarpine; GP25: 25 mg/kg of geniposide for 8 days and pilocarpine; GP50: 50 mg/kg of geniposide for 8 days and pilocarpine; ASP20: 20 mg/kg of asperuloside for 8 days and pilocarpine; ASP40: 40 mg/kg of asperuloside for 8 days and pilocarpine
The genotoxic potential of the treatments
The comet assay was used to assess the possible genotoxic effect of GP and ASP after PILO administration. The data was analyzed by one-way ANOVA (F7,29 = 2.771; p = 0.0246) followed by Dunnet’s test. PILO did not significantly increase the DNA damage compared to SAL (p = 0.4356) (Fig. 7). The groups previously treated with VPA or GP (all doses) showed DF- and DI-like SAL and PILO groups. DNA damage was observed in the ASP20 and ASP40 when compared to the SAL group (p = 0.0110 and p = 0.0139, respectively), suggesting a genotoxic effect of this compound.
Peripheral blood comet assay in mice submitted to a PILO-induced seizure model. Data expressed as mean ± SEM. Damage index (a) was ranging from 0 (completely undamaged, 100 cells × 0) to 400 (with maximum damage 100 × 4). Damage frequency (b) was calculated based on the number of cells with a tail versus those with no tail. *p < 0.05 in comparison to the SAL group. Each circle represents one measure in duplicate. N = 3–6. SAL: mice received only saline (0.9%); VPA: mice received valproic acid (300 mg/kg) for 8 days and pilocarpine; PILO: saline for 8 days and pilocarpine; GP5: 5 mg/kg of geniposide for 8 days and pilocarpine; GP25: 25 mg/kg of geniposide for 8 days and pilocarpine; GP50: 50 mg/kg of geniposide for 8 days and pilocarpine; ASP20: 20 mg/kg of asperuloside for 8 days and pilocarpine; ASP40: 40 mg/kg of asperuloside for 8 days and pilocarpine
The mutagenic potential of the treatments
The micronucleus test showed that no group studied was able to increase the micronucleus frequency in polychromatic erythrocytes (MNPCE) of bone marrow (Table 1). The polychromatic erythrocytes/normochromatic erythrocytes (PCE/NCE) ratio was similar in all groups. Data were analyzed by one-way ANOVA (F8,42 = 34.34; p = 0.0001), followed by Dunnet’s test. The only group that differed from the SAL group was a positive control, in which the cells were treated with cyclophosphamide (p = 0.0001).
Discussion
This study aimed to evaluate the GP and ASP mechanism of action after PILO-induced seizures in male mice. The results demonstrated that GP and ASP did not block PILO-induced SE. However, they were able to reduce the COX-2 and GluN2B receptor expression after seizure induction.
GP and ASP are iridoid compounds found in a variety of plant species, mainly in the Rubiaceae family (Zhou et al. 2019b; Chan et al. 2020). Studies have shown that these compounds exhibit a broad spectrum of pharmacological effects, including neuroprotective, antidiabetic, hepatoprotective, anti-inflammatory, analgesic, and antidepressant (Zhao et al. 2018; He et al. 2018; Chan et al. 2020; Shen et al. 2020; Zhang et al. 2021; Wu et al. 2022). Thus, considering the protective profile previously demonstrated by GP and ASP, the evaluation of possible mechanisms of action becomes important, and it was the objective of this study.
Considering the lack of studies on ASP, we evaluated the toxicity of this compound to determine which doses would be used in the pharmacological investigation. So, the potential acute oral toxicity of ASP was evaluated. Doses of 300 and 2000 mg/kg of ASP did not induce mortality in mice during the 14 days of the experimental period, meaning that this compound has no identified oral toxicity up to 2000 mg/kg.
GP and ASP were able to increase the latency for PILO-induced seizures in mice (Fig. 4a), and GP (25 and 50 mg/kg) and ASP (40 mg/kg) decreased the occurrence of score 3 seizures (Fig. 4b), suggesting some ability to alter the threshold for clonic seizures. Several anticonvulsant drugs delay the latency of the first seizure in rodent models, like phenobarbital (Fleck et al. 2016), pregabalin (Lotarski et al. 2014), and levetiracetam (Oliveira et al. 2005). Likewise, in a previous study, GP (10 and 20 mg/kg) was able to increase the latency for electroshock-induced seizures in mice, probably through negative regulation of inflammatory pathways (Wei et al. 2018).
The SE is characterized by continuous epileptic seizures or rapid recurrence without a recovery period between them. This state may have long-term consequences, including neuronal death, neuronal injury, and altered neural networks (Trinka et al. 2015). In the present study, the SE was determined by the observation of score 4–5 seizures that continued for more than 5 min. Despite the reduction in the percentage of animals that reached SE, none of the treatments were effective in significantly decreasing the percentage of the score 4–5 seizures (Fig. 4c). These results suggest that GP and ASP do not demonstrate a protective effect in relation to the main behavioral parameter evaluated in the PILO model, which is the SE.
GP anti-inflammatory activity was evaluated in previous studies, which demonstrated its effectiveness in regulating inflammatory pathways. In a model of hypoxia/reperfusion-induced blood–brain barrier impairment, GP was able to regulate inflammatory mediators by reducing pro-inflammatory cytokines (such as IL-6) in rat astrocyte culture (Li et al. 2019). Similarly, ASP (20, 40, and 80 mg/kg) exhibited anti-inflammatory activity in an LPS-induced inflammation model in mice, reducing the expression of IL-1β and IL-6 and TNF-α in lung tissue (Qiu et al. 2016).
Due to the importance of neuroinflammatory mechanisms in the onset and prolongation of epileptic seizures, it is suggested that the anti-inflammatory properties of GP and ASP can attenuate PILO-induced epileptic seizures in mice. To contribute to the elucidation of the anti-inflammatory mechanisms of GP and ASP, we evaluated TNF-α levels in the hippocampus of mice after induction of epileptic seizures (Fig. 5), however, no differences were found between the groups. Besides, COX-2 expression was evaluated in hippocampus samples (Fig. 6a). PILO increased COX-2 expression in mice hippocampus, a result already observed in other studies (Lee et al. 2007; Youssef et al. 2020). COX-2 is rapidly induced in principal brain neurons in response to injury or excessive neuronal activity, and its overexpression is often associated with neurotoxicity and tissue injury in acute conditions, including seizures (Takemiya et al. 2006). VPA, GP, and ASP were capable to decrease COX-2 expression after seizure induction, suggesting that compounds can affect inflammatory signaling.
To evaluate the involvement of the GABAergic system in the GP and ASP mechanism of action, we evaluated the expression of the enzyme GAD-1 by western blot (Fig. 6b). GAD-1 is responsible for the decarboxylation of glutamate in GABA (Buddhala et al. 2009). The inhibition of this enzyme leads to a decrease in GABA concentration resulting in an imbalanced glutamate/GABA ratio in the hippocampus, which may lead to seizures and in the long-term, excitotoxicity and neurodegeneration (Salazar and Tapia 2015). However, here no difference was detected in the expression of GAD-1 in the hippocampus of mice treated with PILO. This result is interesting, as some studies found a reduction in GAD1 levels in the piriform cortex (Freichel et al. 2006) and dentate gyrus (Esclapez and Houser 1993) after PILO exposure. However, another study showed that pretreatment with scopolamine (10 mg/kg) prevented decreases in GAD activity induced by PILO in the hippocampus (Turski et al. 1986). Therefore, we suggest that the result observed could be due to the pretreatment with scopolamine used in our study, which could be investigated in future investigations.
Here, we evaluated the effect of GP and ASP on phosphorylating GLUR1 and GluN2B receptor expression in hippocampus samples (Fig. 6c and d). The results demonstrated no difference between groups in the pGluR1 expression. However, the GluN2B receptor was overexpressed in the PILO group, in line with the results reported by Dubey et al. (2022). On the other hand, VPA, GP, and ASP treatments were capable to decrease GluN2B expression, suggesting that the increase in the latency for score 3 seizures promoted by these compounds is related, at least in part, to the increase in the expression of GluN2B receptors.
It is possible that increased COX 2 and GluN2B expression may be related since there are several studies that relate these two mediators (Stachowicz 2021). The NMDAR upregulation increases neuronal COX-2 expression, while prolonged stimulation leads to the inhibition of enzymatic activity by suppression of arachidonic acid (Stark and Bazan 2011). In this way, the GP and ASP could modulate the GluN2Brexpression and consequently decreasing the expression of COX2 and reducing the inflammatory process. In fact, one study evaluated GP activity in rats subjected to an ischemia model and observed that it could attenuate postischemic LTP induction through NR2A-containing NMDARs (Yao et al. 2021).
The genotoxic and mutagenic potentials are important endpoints frequently assessed to evaluate the safety of new drug candidates. As observed in previous studies (da Costa e Silva et al. 2018), in this study, PILO (a single dose, at 280 mg/kg) did not induce genotoxicity in the blood (Fig. 7). However, DNA damage was observed in the ASP groups, suggesting a genotoxic effect of this compound at the doses tested. It has already been reported that some antiepileptic drugs, such as topiramate and levetiracetam, can impair the antioxidant defense system, which can trigger DNA damage, while the same drugs produce a disease-modifying effect with antioxidant potential in some epilepsy models (Shishmanova-Doseva et al. 2021). The micronucleus test is recommended by the US Food and Drug Administration and is frequently used for evaluating the mutagenicity of various types of substances (He and Xu 2022). Our results showed that the incidence of MNPCE was similar in all groups studied. It is important to emphasize that the mice were killed 1 h after the PILO injection. Hence, PILO probably had no influence in the mice that received ASP and GP since the formation of a new micronucleus needs a mitotic division, which occurs in approximately 24 h (OECD 474, 2016). Thus, the results observed in those mice were due to treatment with GP and ASP and both did not induce chromosomal breaks or mitotic spindle dysfunctions that would lead to an increase of MNPCE in bone marrow, suggesting GP and ASP are not mutagenic substances (Table 1). In addition, ASP did not cause mortality in the oral toxicity assay. This is the first study showing ASP safety profile aspects.
ASP and GP were effective to alter score 3 after PILO injection, but they were not able to prevent the establishment of the SE. ASP and GP seem to have an anti-inflammatory effect by decreasing COX-2 expression after seizure induction. Both GP and ASP were capable to decrease GluN2B expression after seizure induction by PILO. However, there were some limitations in our research, such as the small number of animals used to carry out the Elisa and western blot assays and the lack of control groups treated only with GP and ASP. This is due to the attempt to reduce the number of animals used in the study, considering the OECD principles for reducing the number of animals used in research. It used the smallest number of animals that statistically would give us a reliable answer, but with the death of some animals during the experimental process, our N became lower than we would like, this should be taken into consideration in future research. Further studies with the GP and ASP are required to supplement the data obtained in this survey, particularly as it relates to the safety of ASP administration.
Conclusion
The GP and ASP did not demonstrate a neuroprotective profile in the PILO model since they did not block the SE. These iridoid compounds have been shown to decrease the COX-2 and GluN2B receptor expression, suggesting that they modulate an important component of the inflammatory pathway, as well as a glutamate receptor subunit. In addition, the GP did not induce toxicity in the comet assay, whereas ASP at higher doses was shown to induce genotoxicity.
Data availability
The datasets generated during the current study are available in the supplementary material.
References
Akyuz E, Polat AK, Eroglu E et al (2021) Revisiting the role of neurotransmitters in epilepsy: an updated review. Life Sci 265:1–21. https://doi.org/10.1016/J.LFS.2020.118826
Alachkar A, Azimullah S, Lotfy M et al (2020) Antagonism of histamine H3 receptors alleviates pentylenetetrazole-induced kindling and associated memory deficits by mitigating oxidative stress, central neurotransmitters, and c-Fos protein expression in rats. Molecules 25:1–16. https://doi.org/10.3390/MOLECULES25071575
Asgharzade S, Rabiei Z, Rabiei S et al (2020) Therapeutic effects of oleuropein in improving seizure, oxidative stress and cognitive disorder in pentylenetetrazole kindling model of epilepsy in mice. Iran J Pharm Res 19:98–110. https://doi.org/10.22037/ijpr.2019.14212.12209
Azqueta A, Slyskova J, Langie SAS et al (2014) Comet assay to measure DNA repair: approach and applications. Front Genet 5:1–8. https://doi.org/10.3389/fgene.2014.00288
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Buddhala C, Hsu CC, Wu JY (2009) A novel mechanism for GABA synthesis and packaging into synaptic vesicles. Neurochem Int 55:9–12. https://doi.org/10.1016/J.NEUINT.2009.01.020
Chan Y, Ng SW, Xin Tan JZ et al (2020) Emerging therapeutic potential of the iridoid molecule, asperuloside: a snapshot of its underlying molecular mechanisms. Chem Biol Interact 315:108911. https://doi.org/10.1016/j.cbi.2019.108911
Chen S, Zeng X, Zong W et al (2019) Aucubin alleviates seizures activity in Li-pilocarpine-induced epileptic mice: involvement of inhibition of neuroinflammation and regulation of neurotransmission. Neurochem Res 44:472–484. https://doi.org/10.1007/s11064-018-2700-y
Chen Y er, Xu S jie, Lu Y yu et al (2021) Asperuloside suppressing oxidative stress and inflammation in DSS-induced chronic colitis and RAW 264.7 macrophages via Nrf2/HO-1 and NF-κB pathways. Chem Biol Interact 344:1–13. https://doi.org/10.1016/J.CBI.2021.109512
Collins AR (2004) The comet assay for DNA damage and repair: principles, applications, and limitations. Appl Biochem Biotechnol - Part B Mol Biotechnol 26:249–261. https://doi.org/10.1385/MB:26:3:249
Curia G, Longo D, Biagini G et al (2008) The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods 172:143–157
da Costa e Silva LD, Pereira P, Regner GG et al (2018) DNA damage and oxidative stress induced by seizures are decreased by anticonvulsant and neuroprotective effects of lobeline, a candidate to treat alcoholism. Metab Brain Dis 33:53–61. https://doi.org/10.1007/s11011-017-0130-1
Dang YL, Foster E, Lloyd M et al (2021) Adverse events related to antiepileptic drugs. Epilepsy Behav 115:1–10. https://doi.org/10.1016/J.YEBEH.2020.107657
Dinda B, Dinda M, Kulsi G et al (2019) Therapeutic potentials of plant iridoids in Alzheimer’s and Parkinson’s diseases: a review. Eur J Med Chem 169:185–199. https://doi.org/10.1016/j.ejmech.2019.03.009
Dubey V, Dey S, Dixit AB et al (2022) Differential glutamate receptor expression and function in the hippocampus, anterior temporal lobe and neocortex in a pilocarpine model of temporal lobe epilepsy. Exp Neurol 347:113916. https://doi.org/10.1016/j.expneurol.2021.113916
Esclapez M, Houser CR (1993) Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci 3:4470–4485
Fisher RS, Acevedo C, Arzimanoglou A et al (2014) ILAE official report: a practical clinical definition of epilepsy. Epilepsia 55:475–482. https://doi.org/10.1111/epi.12550
Fleck J, Temp FR, Marafiga JR et al (2016) Montelukast reduces seizures in pentylenetetrazol-kindledmice. Brazilian J Med Biol Res 49:1–7. https://doi.org/10.1590/1414-431X20155031
Freichel C, Potschka H, Ebert U et al (2006) Acute changes in the neuronal expression of GABA and glutamate decarboxylase isoforms in the rat piriform cortex following status epilepticus. Neuroscience 141:2177–2194. https://doi.org/10.1016/J.NEUROSCIENCE.2006.05.040
He D, Xu Y (2022) Toxicological evaluation of Camellia euphlebia leaves aqueous extract using acute and subacute toxicity studies in mice and genotoxicity studies. Evid Based Complement Alternat Med 2022:1–10. https://doi.org/10.1155/2022/7889199
Jiang H, Panda S, Gekara NO (2019) Comet and micronucleus assays for analyzing DNA damage and genome integrity. Methods Enzymol 625:299–307. https://doi.org/10.1016/BS.MIE.2019.05.015
Johnson EL (2019) Seizures and epilepsy. Med Clin North Am 103:309–324. https://doi.org/10.1016/J.MCNA.2018.10.002
Lee B, Dziema H, Lee KH et al (2007) CRE-mediated transcription and COX-2 expression in the pilocarpine model of status epilepticus. Neurobiol Dis 25:80. https://doi.org/10.1016/J.NBD.2006.08.015
Li C, Wang X, Cheng F et al (2019) Geniposide protects against hypoxia/reperfusion-induced blood-brain barrier impairment by increasing tight junction protein expression and decreasing inflammation, oxidative stress, and apoptosis in an in vitro system. Eur J Pharmacol 854:224–231. https://doi.org/10.1016/J.EJPHAR.2019.04.021
Lima IV de A, Bellozi PMQ, Batista EM et al (2020) Cannabidiol anticonvulsant effect is mediated by the PI3Kγ pathway. Neuropharmacol 176:1–12. https://doi.org/10.1016/j.neuropharm.2020.108156
Lotarski S, Hain H, Peterson J et al (2014) Anticonvulsant activity of pregabalin in the maximal electroshock-induced seizure assay in α2δ1 (R217A) and α2δ2 (R279A) mouse mutants. Epilepsy Res 108:833–842. https://doi.org/10.1016/J.EPLEPSYRES.2014.03.002
Manzione MG, Martorell M, Sharopov F et al (2020) Phytochemical and pharmacological properties of asperuloside, a systematic review. Eur J Pharmacol 883:1–9. https://doi.org/10.1016/J.EJPHAR.2020.173344
Mavournin KH, Blakey DH, Cimino MC et al (1990) The in vivo micronucleus assay in mammalian bone marrow and peripheral blood. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res Genet Toxicol 239:29–80
OECD (2016) Test No. 474: mammalian erythrocyte micronucleus test, OECD guidelines for the testing of chemicals, Section 4. Paris: OECD. https://doi.org/10.1787/9789264264762-en
Oliveira AA, Nogueira CRA, Nascimento VS et al (2005) Evaluation of levetiracetam effects on pilocarpine-induced seizures: cholinergic muscarinic system involvement. Neurosci Lett 385:184–188. https://doi.org/10.1016/J.NEULET.2005.05.048
Qiu J, Chi G, Wu Q et al (2016) Pretreatment with the compound asperuloside decreases acute lung injury via inhibiting MAPK and NF-κB signaling in a murine model. Int Immunopharmacol 31:109–115. https://doi.org/10.1016/j.intimp.2015.12.013
Racine RJ (1972) Modification of seizure activity by electrical stimulation: II Motor Seizure. Electroencephalogr Clin Neurophysiol 32:281–294. https://doi.org/10.1016/0013-4694(72)90177-0
Salazar P, Tapia R (2015) Epilepsy and hippocampal neurodegeneration induced by glutamate decarboxylase inhibitors in awake rats. Epilepsy Res 116:27–33. https://doi.org/10.1016/J.EPLEPSYRES.2015.06.014
Santulli L, Coppola A, Balestrini S, Striano S (2016) The challenges of treating epilepsy with 25 antiepileptic drugs. Pharmacol Res 107:211–219. https://doi.org/10.1016/J.PHRS.2016.03.016
Shen B, Feng H, Cheng J et al (2020) Geniposide alleviates non-alcohol fatty liver disease via regulating Nrf2/AMPK/mTOR signalling pathways. J Cell Mol Med 24:5097–5108. https://doi.org/10.1111/JCMM.15139
Shishmanova-Doseva M, Peychev L, Yoanidu L et al (2021) Anticonvulsant effects of topiramate and lacosamide on pilocarpine-induced status epilepticus in rats: a role of reactive oxygen species and inflammation. Int J Mol Sci 22:1–13. https://doi.org/10.3390/IJMS22052264
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191. https://doi.org/10.1016/0014-4827(88)90265-0
Stachowicz K (2021) Deciphering the mechanisms of regulation of an excitatory synapse via cyclooxygenase-2. A Review Biochem Pharmacol 192:114729. https://doi.org/10.1016/J.BCP.2021.114729
Stark DT, Bazan NG (2011) Synaptic and extrasynaptic NMDA receptors differentially modulate neuronal cyclooxygenase-2 function, lipid peroxidation, and neuroprotection. J Neurosci 31:13710–13721. https://doi.org/10.1523/JNEUROSCI.3544-11.2011
Su C, Yang X, Lou J (2016) Geniposide reduces α-synuclein by blocking microRNA-21/lysosome-associated membrane protein 2A interaction in Parkinson disease models. Brain Res 1644:98–106. https://doi.org/10.1016/j.brainres.2016.05.011
Takemiya T, Maehara M, Matsumura K et al (2006) Prostaglandin E2 produced by late induced COX-2 stimulates hippocampal neuron loss after seizure in the CA3 region. Neurosci Res 56:103–110. https://doi.org/10.1016/J.NEURES.2006.06.003
Trinka E, Cock H, Hesdorffer D et al (2015) A definition and classification of status epilepticus - report of the ILAE task force on classification of status epilepticus. Epilepsia 56:1515–1523. https://doi.org/10.1111/epi.13121
Turski L, Cavalheiro EA, Sieklucka-Dziuba M et al (1986) Seizures produced by pilocarpine: neuropathological sequelae and activity of glutamate decarboxylase in the rat forebrain. Brain Res 398:37–48. https://doi.org/10.1016/0006-8993(86)91247-3
Vendruscolo MH, das Neves GM, Kagami LP et al (2018) In vitro and in silico activity of iridoids against Leishmania amazonensis. Curr Drug Discov Technol 16:173–183. https://doi.org/10.2174/1570163814666171002102058
Wang J, Li Y, Huang WH et al (2017) The protective effect of aucubin from Eucommia ulmoides against status epilepticus by inducing autophagy and inhibiting necroptosis. Am J Chin Med 45:557–573. https://doi.org/10.1142/S0192415X17500331
Wang Z, Zhou L, An D et al (2019) TRPV4-induced inflammatory response is involved in neuronal death in pilocarpine model of temporal lobe epilepsy in mice. Cell Death Dis 10:1–10. https://doi.org/10.1038/s41419-019-1612-3
Wei H, Duan G, He J et al (2018) Geniposide attenuates epilepsy symptoms in a mouse model through the PI3K/Akt/GSK-3β signaling pathway. Exp Ther Med 15:1136–1142. https://doi.org/10.3892/etm.2017.5512
Wu Q, Gai S, Zhang H (2022) Asperulosidic acid, a bioactive iridoid, alleviates placental oxidative stress and inflammatory responses in gestational diabetes mellitus by suppressing NF-κB and MAPK signaling pathways. Pharmacology 107:1–9. https://doi.org/10.1159/000521080
Yang N, Zhang Y, Wang JT et al (2020) Effects of dexamethasone on remodeling of the hippocampal synaptic filamentous actin cytoskeleton in a model of pilocarpine-induced status epilepticus. Int J Med Sci 17:1683–1691. https://doi.org/10.7150/ijms.44927
Yao H, Liu W et al (2021) Geniposide attenuates postischemic long-term potentiation via GluN2A. Pak J Pharm Sc 34:909–914. https://doi.org/10.36721/PJPS.2021.34.3.REG.909-914.1
Youssef FS, Menze ET, Ashour ML (2020) A potent lignan from prunes alleviates inflammation and oxidative stress in lithium/pilocarpine-induced epileptic seizures in rats. Antioxidants 9:1–15. https://doi.org/10.3390/ANTIOX9070575
Zhang W, Zhang F, Hu Q et al (2021) The emerging possibility of the use of geniposide in the treatment of cerebral diseases: a review. Chin Med 16:1–16. https://doi.org/10.1186/S13020-021-00486-3
Zhang Y, Yin F, Liu J et al (2015) Geniposide attenuates insulin-deficiency-induced acceleration of β-amyloidosis in an APP/PS1 transgenic model of Alzheimer’s disease. Neurochem Int 89:7–16. https://doi.org/10.1016/J.NEUINT.2015.04.002
Zhao Y, Li H, Fang F et al (2018) Geniposide improves repeated restraint stress-induced depression-like behavior in mice by ameliorating neuronal apoptosis via regulating GLP-1R/AKT signaling pathway. Neurosci Lett 676:19–26. https://doi.org/10.1016/j.neulet.2018.04.010
Zhou R, Lu G, Yan Z et al (2019a) Bioaccumulation and biochemical effects of ethylhexyl methoxy cinnamate and its main transformation products in zebrafish. Aquat Toxicol 214:1–9. https://doi.org/10.1016/j.aquatox.2019.105241
Zhou YX, Zhang RQ, Rahman K et al (2019b) Diverse pharmacological activities and potential medicinal benefits of geniposide. Evidence-based Complement Altern Med 2019:1–15. https://doi.org/10.1155/2019
Acknowledgements
This work was supported by the Higher Education Personnel Improvement Coordination (CAPES) and the Federal University of Rio Grande do Sul (UFRGS).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Mariana Uczay and Pricila Pflüger carried out the assays in the study and participated in the drafting of the manuscript. Gilsane von Poser and Maria Helena Vendruscolo extracted the compounds and participated in the drafting of the manuscript. Iraci Torres provided reagents and participated in the drafting of the manuscript. Helouise Richardt Medeiros helped with the measurement of TNF-α and João Denis Medeiros de Oliveira the comet assay. Jaqueline Nascimento Picada and Patrícia Pereira conceived, designed, supervised the study, and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experimental procedures were carried out in accordance with the Guidelines of the Brazilian Council of Animal Experimentation—CONCEA, with the approval of the Committee on the Ethical Use of Animals of UFRGS (authorization number: 37769).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uczay, M., Pflüger, P., Picada, J.N. et al. Geniposide and asperuloside alter the COX-2 and GluN2B receptor expression after pilocarpine-induced seizures in mice. Naunyn-Schmiedeberg's Arch Pharmacol 396, 951–962 (2023). https://doi.org/10.1007/s00210-022-02367-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02367-4