Abstract
Peptic ulcers are lesions in the gastric and duodenal mucosa generated by an imbalance between protective factors (gastroduodenal mucus secretion, bicarbonate production, adequate blood flow) and harmful factors (excess pepsin or hydrochloric acid). Some drugs used in peptic ulcer therapy are associated with adverse effects. The aim of this study was to evaluate the antiulcerogenic and healing activity of hecogenin acetate (HA) in acute and chronic models of gastric lesions in rodents. The antiulcerogenic activity of HA was evaluated in models of gastric lesions induced by absolute ethanol and in acidified ethanol with HA (5, 10, and 20 mg/kg). For the model of gastric lesions induced by ischemia and reperfusion, rats were pre-treated with HA (5, 10, 20 mg/kg). After that, they were submitted to 30 min of ischemia, followed by 1 h of reperfusion. To evaluate the healing activity was induced gastric ulcer using acetic acid (80%) in rats. After 24 h, they were treated for 7 consecutive days with HA (10 and 20 mg/kg). They were evaluated the possible signs of toxicity, measurement of the lesions, collagen deposition, and histological analysis. HA significantly reduced the area of the lesion in models of gastric lesions induced by absolute and acidified ethanol, ischemia-induced gastric lesions and reperfusion, and regarding healing. In the collagen deposition, the presence and increase of collagen demonstrate the healing effect. The AH has antiulcerogenic and healing potential demonstrated by the decrease in gastric injury and presence of collagen fibers, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peptic ulcers are lesions that occur in the gastric and duodenal mucosa caused by an imbalance between protective (gastroduodenal mucus secretion, bicarbonate production, adequate blood flow) and harmful factors (excess pepsin or hydrochloric acid, continuous and even inappropriate use of some medications such as non-steroidal anti-inflammatory drugs (NSAIDs), unbalanced diet rich in processed foods and caffeine, addictive behavior with alcohol and/or cigarette use, and also Helicobacter pylori infection) in the gastric and duodenal mucosa (Malfertheiner and Schulz 2020; Périco et al. 2020).
Complications of peptic ulcer disease include perforation and bleeding and remain a significant healthcare problem, which can consume considerable financial resources. Management may involve various subspecialties including surgeons, gastroenterologists, and radiologists. Successful management of patients with complicated peptic ulcer (CPU) involves prompt recognition, resuscitation when required, appropriate antibiotic therapy, and timely surgical/radiological treatment (Tarasconi et al. 2021).
Centuries ago, peptic ulcer was surgically controlled and caused high morbidity and mortality rates. The introduction of histamine H2 receptor antagonists (cimetidine and famotidine as the most used in this class of drugs) led to a decrease of up to 85% in the surgical processes for the treatment of peptic ulcer. Proton pump inhibitors (IBPs) (omeprazole, esomeprazole, lansoprazole, and pantoprazole) are also currently widely used in the treatment of gastric ulcer, as well as H2 antagonists and antiacids (Fazalda et al. 2018; Kuna et al. 2019).
However, some adverse effects are associated with such therapies, such as vitamin B12 deficiency; increased risk of dementia; the accumulation of beta-amyloid peptides involved in Alzheimer’s disease, as studies have shown that Lansoprazol led to higher levels of beta-amyloid in the brain of mice; and increased risk of fracture (Nehra et al. 2018). Because the therapies used to treat peptic ulcers have such adverse effects, research has been stimulated to study alternative therapies that generate fewer unwanted effects and that may be effective, and in this context, the plant kingdom has been gaining prominence, especially when considered secondary metabolites of plant species (Rosa 2013).
Among the secondary metabolites produced by plants, saponins constitute one of the most prominent classes due to their wide distribution in the plant kingdom and their important biological activities (Kaiser et al. 2010). Hecogenin, which is isolated from Agave sisalana, belongs to the class of steroidal saponins or sapogenins, in dried sisal leaves (Cripps and Bluden 1978; Zullo et al. 1984; Zullo et al. 1989), anti-inflammatory (Ingawale and Patel, 2016), and gastroprotective (Cerqueira 2012).
Hecogenin, like other sapogenins obtained, is relatively unstable in the environment and can easily hydrolyze, and therefore, chemical modifications are needed in this molecule to improve these unstable spots. Starting from this point, hecogenin acetate is an acetylated hecogenin, and the inclusion of the acetate group has made the hecogenin chemically more stable, contributing, for example, presenting same or elevated activity previously observed in the original structure of the molecule and present new properties (Quintans et al. 2014). Therefore, the present study evaluated the antiulcerogenic and healing activity of hecogenin acetate in models of induced gastric lesions in rodents.
Materials and methods
Obtaining the test substance
The hecogenin acetate (HA) used in the research was obtained by Sigma-Aldrich Merck® and used in the protocols as test substance.
Animals
Mice (Mus musculus) from the Swiss lineage, where males were approximately 2 months old and weighing between 25 and 30 g, and rats (Rattus norvegicus) from the Wistar lineage, where males were weighing between 250 and 300 g, from the Sectorial Bioterium of Federal University of Piauí, were used for the experiments. All of animal experiments were conducted similarly like Cerqueira (2012), except collagen measurement (Xing et al. 2020).
Ethical approval
The use of animals followed the principles and standards for the use of animals in research projects and all experimental procedures of the project were submitted to the Ethics Committee on Animal Experimentation of UFPI (CEEA/UFPI), in which it was approved generating the protocol number CEUA/UFPI: 2018/516.
Gastric lesions induced by absolute ethanol in mice
Animals were divided and pre-treated with vehicle (distilled water + 2% tween 80 p.o.), hecogenin acetate (HA) in three different doses (2,5; 5 and 10 mg/kg p.o.) or carbenoxolone (100 mg/kg p.o.) 60 min before administration oral ulcerogenic agent: absolute ethanol in a volume of 0.2 ml per animal. After 30 min of absolute ethanol administration, the animals were euthanized with lidocaine (10 mg/kg, i.p.) and sodium thiopental overdose (150 mg/kg, i.p.) to remove their stomachs. The percentage of the injured gastric glandular face was determined using a computerized planimetry program (ImageJ®), and the ulcerated area was expressed as a percentage of the total gastric body area.
Gastric lesions induced by acidified ethanol in mice
The animals were divided and pre-treated with vehicle (distilled water + 2% tween 80), hecogenin acetate in three different doses (5, 10, and 20 mg/kg p.o.) or carbenoxolone (100 mg/kg p.o.) 60 min before oral administration of the ulcerogenic agent: ethanol solution (60%) and HCl (0.3 M)) in a volume of 0.2 ml per animal. After 1 h of administration of acidified ethanol, the animals were euthanized with lidocaine (10 mg/kg, i.p.) and sodium thiopental overdose (150 mg/kg, i.p.) to remove their stomachs. The percentage of the injured gastric glandular face was determined using a computerized planimetry program (ImageJ®), and the ulcerated area was expressed as a percentage of the total gastric body area.
Gastric lesions induced by ischemia and reperfusion in Wistar rats
The animals were divided into and were treated orally with the vehicle (distilled water + 2% tween 80), N-acetylcysteine (NAC 200 mg/kg), and hecogenin acetate in three different doses (5, 10, and 20 mg/kg). After 30 min, the animals were anaesthetized with ketamine and xylazine (100 mg/kg and 5.0 mg/kg i.p. respectively). The animals were submitted to 30 min of ischemia induced by occlusion of the celiac artery by a microvascular “clamp” and followed by a reperfusion of 1. Next, the animals were euthanized with lidocaine (10 mg/kg, i.p.) and sodium thiopental overdose (150 mg/kg, i.p.), their stomachs removed, and the area of gastric lesions measured by computerized planimetry program (ImageJ®).
Acetic acid-induced gastric ulcers in Wistar rats
Animals were divided and previously anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (5.0 mg/kg, i.p.). The animals were submitted to a surgical process for gastric ulcer induction using acetic acid (80%) as an inducing agent. The injured area was delimited using an 8-mm diameter and 2-cm high glass tube in contact with the serosa of the stomach. Acetic acid (70 μL) was added and remained in contact with the serosa of the stomach for 1 min. After, the excess acetic acid was removed, and the stomach was accommodated in the abdominal cavity and the abdominal region sutured.
The treatment took place from the first day after induction of the ulcer and continued until the 7th day. The two different doses of hecogenin acetate (5 and 10 mg/kg p.o.), vehicle or cimetidine (200 mg/kg p.o.) were administered orally. On day 8, the animals were euthanized with lidocaine (10 mg/kg, i.p.) and sodium thiopental overdose (150 mg/kg, i.p.) to remove their stomachs. The volume of the lesion was measured (with the aid of a pachymeter) by length × height × depth (mm3). The stomachs of the sham group were added for histology and collagen.
Histological analysis of gastric lesions induced by acetic acid
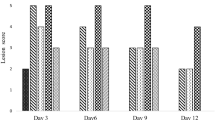
After the evaluations performed in the previous protocol, the glandular area of the stomachs was removed, fixed in buffered formalin (10%, pH between 6.8 and 7.4), and maintained for 24 h. After fixation, the samples were transferred to a 70% alcohol solution. Then, the samples were embedded in paraffin. The paraffin blocks were cut (thickness of 5 μm) and fixed on histological slides. The materials were stained with hematoxylin and eosin (H&E) for microscopic evaluation. The slides were examined with the aid of an optical microscope with a coupled camera to obtain photomicrographs with 4 × , 10 × , and 40 × magnification. The microscopic evaluation of the gastric lesions was availed for scores semi-quantitatively, using a scale of 0–3 (0 = negative; 1 = weak; 2 = moderate; 3 = strong). Each histological section was examined evaluating epithelial erosion (score: 0–3), edema in the submucous (score: 0–3), hemorrhagic damage (score: 0–3), and presence of inflammatory cells (score: 0–3), resulting in a maximum total score of 12.
Measurement of collagen deposition on gastric mucosa
The paraffin embolized gastric lesions from the gastric ulcer protocol induced by acetic acid were cut into a 5 µm. The sections were fixed on histological slides, dewaxed with xylol, hydrated with alcohols, and stained with a solution of picrosirius (Direct Red 80 and 1.3% picric acid) for 60 min, after the slides were mounted with Balsam from Canada. The slides were examined with the aid of an optical microscope with a coupled camera to obtain photomicrographs with 4 × , 10 × , and 40 × magnification. The collagen fibers were analyzed by ImageJ software (1.43) according to their color in the RGB system (red, green, blue) and were expressed as percentage (%) of collagen fibers using the deconvolution system.
Investigation of signs of toxicity for seven days of treatment with hecogenin acetate in Wistar rats
Signs of toxicity of daily exposure of Wistar rats to hecogenin acetate at doses of 5 and 10 mg/kg for 7 days followed by treatment of the animals were subjected to the acetic acid-induced gastric ulcer protocol. For evaluation of weight gain, the weight of the animals was measured every 2 days. On the 8th day, the animals were anesthetized with an association of ketamine and xylazine (50 mg/kg and 5.0 mg/kg i.m., respectively), blood was collected through the vena cava and centrifuged, and the respective serum was taken for evaluation of biochemical parameters. After euthanasia, the vital organs of the animals were macroscopically removed and weighed.
Statistical analysis
The results were expressed as mean ± standard error of the mean (EPM). Statistical evaluation was performed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. To compare three or more groups in independent models, the non-parametric Kruskal Wallis test was performed followed by Dunn post-test. All statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software Inc., La Jolla, CA, USA). P < 0.05, 0.01, or 0.001 was considered to be statistically significant.
Results
Effect of hecogenin acetate on gastric lesions induced by absolute ethanol in Swiss mice
Oral administration of absolute ethanol was able to produce a large ulcerated area in the stomach of pre-treated animals, causing severe damage to the gastric mucosa. The area of injury expressed as a percentage in relation to the total area of the stomach of the control group was 4.21 ± 0.70. Hecogenin acetate (HA) reduced the areas of injury at doses of 2.5 mg/kg (0.23 ± 0.05), 5 mg/kg (1.63 ± 0.43), and 10 mg/kg (1.37 ± 0.27) with 94.5% protection percentage, 61.2%, and 67.3%, respectively, when compared to the group treated with vehicle. The group pre-treated with carbenoxolone (100 mg/kg) presented a reduced injury area (1.16 ± 0.17) in 72.2% (Fig. 1).
Effect of hecogenin acetate (2.5; 5 and 10 mg/kg, v.o.), vehicle, or carbenoxolone (100 mg/kg, v.o.) on gastric lesions induced by absolute ethanol in mice. Data expressed as mean ± E.P.M. (ANOVA one way followed by Tukey's post-test) showing significance with *p < 0.05 and ***p < 0.001 when compared to the vehicle
Effect of hecogenin acetate on gastric lesions induced by acidified ethanol in Swiss mice
Oral administration of acidified ethanol was able to produce a large ulcerated area in the stomach of pre-treated animals, causing severe damage to the gastric mucosa. The area of injury expressed as a percentage in relation to the total area of the stomach of the control group was 4.97 ± 0.53. Hecogenin acetate (HA) reduced the injury areas in doses of 5 mg/kg (3.00 ± 0.50), 10 mg/kg (0.51 ± 0.33), and 20 mg/kg (2.84 ± 0.88) with 39.6% of protection, 89.7%, and 57.1%, respectively, when compared to the group treated with vehicle. The pre-treated group with carbenoxolone (100 mg/kg) presented a reduced injury area (0.79 ± 0.12) in 84%. Among the doses, there was a significant difference with HA 5 and 10 mg/kg; carbenoxolone 200 mg/kg and HA 20 mg/kg; and HA 5 mg/kg and carbenoxolone 200 mg/kg (Fig. 2).
Effect of hecogenin acetate (5, 10, and 20 mg/kg, v.o.), vehicle, or carbenoxolone (100 mg/kg, v.o.) on gastric lesions induced by acidified ethanol in mice. Data expressed as mean ± E.P.M. (ANOVA one way followed by Tukey post-test) showing significance with *p < 0.05 and ****p < 0.001 when compared to the vehicle; +p < 0.05 when compared to the dose of 5 mg/kg; §p < 0.05 when compared to the dose of 20 mg/kg; #p < 0.05 when compared to carbenoxolone 100 mg/kg
Effect of hecogenin acetate on gastric lesions induced by ischemia and reperfusion in Wistar rats
In this model, the area of injury expressed as a percentage in relation to the total body area of the stomach of the vehicle group was 9.93 ± 2.47. Hecogenin acetate showed reduction in the area of injury in the doses of 5 mg/kg (1.50 ± 0.66), 10 mg/kg (2.80 ± 1.53), and 20 mg/kg (0.77 ± 0.23) with a reduction in the area of injury of 84.8%, 71.7%, and 92.2%, respectively, when compared to the vehicle group. The pre-treated group with N-acetylcysteine (200 mg/kg) presented antiulcerogenic activity (2.14 ± 0.81) with a 78% reduction in the area of injury (Fig. 3).
Effect of hecogenin acetate (5, 10 and 20 mg/kg, v.o.) and N-acetylcysteine (NAC) at a dose of 200 mg/kg on ischemically induced gastric lesions and reperfusion in rats. Data expressed as mean ± E.P.M. (ANOVA one way followed by Tukey’s post-test) showing significance with *p < 0.05 and p < 0.005 when compared with the vehicle
Effect of hecogenin acetate on acetic acid-induced gastric ulcers in Wistar rats
In this model, the area of injury expressed as a percentage in relation to the total area of the stomach of the vehicle group was 61.53 ± 15.47 in the area of gastric injury induced by acetic acid. It was demonstrated that hecogenin acetate at doses of 5 mg/kg (17.22 ± 4.95) and 10 mg/kg (16.60 ± 6.74) in oral treatment for seven consecutive days in Wistar rats was able to significantly decrease the volume of chronic ulcerated gastric injury in 72.02% and 73.02%, respectively, compared to the vehicle group, as well as cimetidine at a dose of 200 mg/kg (8.84 ± 5.03), which reduced the volume of the lesion by 85.62% demonstrating a healing effect (Fig. 4).
Effect of hecogenin acetate (5 and 10 mg/kg) and cimetidine (200 mg/kg) on acetic acid-induced gastric ulcer. Data expressed as mean ± E.P.M. (ANOVA one way followed by Tukey’s post-test) showing significance with *p < 0.05 when compared with the vehicle. Histological analysis of the effect of hecogenin acetate on gastric lesions induced by 80% acetic acid
The stomachs of rats of the Sham group, animals that did not receive treatment and did not undergo the process of induction of acetic acid ulcer, presented normal architecture of the gastric tissues: mucous, submucous, muscular, and serous, as shown in Fig. 5A. Animals that received only the vehicle and suffered the process of inducing acetic acid ulcer at 80% had a pronounced epithelial damage with loss of superficial cells, strong submucous edema, and presence of inflammatory cells, with practically no repair in the induced ulcer (Fig. 5B). Animals treated with hecogenin acetate at doses of 5 and 10 mg/kg (Fig. 5C and D) or cimetidine (Fig. 5E) had, to some extent, a significant repair of the epithelial tissue architecture and of the secretory glands when compared to the vehicle and, especially, the repair of the induced ulcer. The 10 mg/kg dose and the cimetidine showed significant differences in the histological scores when compared to the vehicle, especially in relation to submucous edema and hemorrhage (Table 1). Thus, the animals that received the treatments (hecogenin acetate or cimetidine) showed a reduction in the size of the ulcerated area, with the lesion almost completely covered by repaired epithelial tissue, few inflammatory cells, and a very visible secretory glandular reconstruction. This shows that the treatment was able to treat the indicators of the gastric lesion, with significant differences when compared to the vehicle (Fig. 5A–E; Table 1).
Effect of hecogenin acetate on the deposition of collagen in gastric lesions induced by 80% acetic acid
Regarding collagen deposition (Figs. 6 and 7), it can be observed that the rats of the sham group had their collagen fibers physiologically present (8.26 ± 0.95), and in the vehicle group, it can be observed that the collagen practically does not become present (0.37 ± 0.10). The group treated with hecogenin acetate at a dose of 5 mg/kg (2.23 ± 0.48), 10 mg/kg (5.16 ± 0.92), and cimetidine 200 mg/kg (8.14 ± 0.98) for 7 days presented with a considerable increase in the collagen fibers when compared to the vehicle group (Figs. 6 and 7), in particular the dose of 10 mg/kg, as it had a significant increase in the percentage of collagen fibers when compared to the vehicle, showing its participation in the healing through the collagen fibers pathway. Among the doses, there was a significant difference with HA 5 and cimetidine 200 mg/kg.
Effect of hecogenin acetate, in different doses, and cimetidine on collagen fiber deposition (%) in the gastric lesion induced by acetic acid 80% in rats. Data expressed as mean ± E.P.M. (ANOVA one way followed by Tukey’s post-test) showing significance with §§p < 0.001 when compared to Sham; *p < 0.05 and ****p < 0. 0.001 when compared to vehicle; +++p < 0.001 when compared to 5 mg/kg dose
Investigation of signs of toxicity for seven days of treatment with hecogenin acetate in Wistar rats
There were no deaths reported while the animals were being treated with hecogenin acetate (5 and 10 mg/kg, v.o.), as well as their respective controls. Biochemical parameters were made whose results are described in the Table 2. It can be observed that most of the parameters described in the table did not have significant changes when compared to the vehicle, except for creatinine, in which the dose of 10 mg/kg (0.56 ± 0.04 mg/dL) had a significant difference when compared to the vehicle (0.34 ± 0.02 mg/dL). Regarding the weight gain (Table 3), there was no significant weight gain when compared to the vehicle.
Regarding the absolute weight of the organs of the animals treated with HA 5 and 10 mg/kg and Cimetidine 200 mg/kg (Table 4), it can be observed that there was no significant difference when compared to the vehicle, in most organs, except for dose of 5 mg/kg and cimetidine, in the weight of the spleen. Regarding the weight of the liver, there was a significant difference between cimetidine 200 mg/kg and the vehicle.
Discussion
Peptic ulcer is still one of the most common gastrointestinal diseases, resulting in loss of quality of life, loss of work, and excessive medical expenses, where current therapies are based on H2 receptor antagonists, proton pump inhibitors (PPIs), and drugs that provide mucosal defense; however, such therapies do not change the recurrence rates of ulcers, where the cause may be multifactorial (Kumar et al. 2019; Malfertheiner and Schulz 2020).
Ethanol causes an imbalance between oxidizing and antioxidant agents in the gastric mucosa. This imbalance causes bleeding from ruptured blood vessels (Ribeiro-Junior et al. 2015). It is also an agent that causes a decrease in the physiological defense of the gastric mucosa as it causes a decrease in mucus production, local blood flow, secretion of bicarbonate, endogenous prostaglandin, and glutathione levels. Ethanol is also capable of boosting the mechanisms that are considered aggressive factors that can cause ulceration, in addition to causing increased histamine release, calcium ion inflow, free radical generation, and leukotriene synthesis (Possenti et al. 2011).
In acidified ethanol, HCl gives a more aggressive power to ethanol; it infiltrates instantaneously in the gastric mucosa and causes lesions to the membrane, leading to cell exfoliation, erosion and the appearance of ulcers, in which the mechanism of ulcer induction occurs by reactive oxygen species (Ewald et al. 2015). We can observe that hecogenin acetate was able to reduce the injury, having an anti-ulcerogenic effect, in the experimental model of absolute ethanol and acidified ethanol in a significant way when compared to the vehicle.
Some showed that hecogenin at doses 15, 30, 60, and 90 mg/kg decreased gastric injury, showing antiulcerogenic activity in the experimental model of absolute and acidified ethanol in mice. This suggests that the addition of acetate in hecogenin improved the effect of antiulcerogenic activity in experimental ethanol models, since hecogenin acetate showed antiulcerogenic activity in doses lower than 15 mg/kg and 30 mg/kg of absolute and acidified ethanol. This shows that the acetylation of hecogenin can make the molecule more potent than hecogenin, since it had antiulcerogenic activity at lower doses. However, more studies should be done to relate the structure and activity of the molecule (Cerqueira et al. 2012).
Regarding ischemia and reperfusion, the reperfusion lesion is a term used to describe the functional and structural changes that become evident during flow restoration after an ischemic period. In addition to ischemia reversal, re-establishing blood flow can cause several deleterious effects, such as irreversibly injured cell necrosis, marked cell edema, and non-uniform restoration of flow to all portions of the tissue (Evora et al. 1996).
Hecogenin acetate had significant antiulcerogenic effects at 5, 10, and 20 mg/kg in the model of gastric lesions induced by ischemia and reperfusion in rats, showing that consequently it can have an effect on the decrease of oxygen free radical generation after periods of ischemia and reperfusion, because episodes of ischemia–reperfusion cause an increase in free radical activity, with consequent irreversible loss of vascular integrity, tissue injury, fibroblast activation and immune system activation (Kayser et al. 2006).
Consolidated 40 years ago, the experimental acetic acid model has been widely used to investigate the effect and mechanism of drugs on ulcer healing. It is a reliable and reproducible model, and the ulcer is quite similar to human, as well as the healing process. Acetic acid in high concentration can directly injure the gastric wall and lead to gastric ulcer. When in contact with the serosa of the stomach, acetic acid damages the epithelial cells and the submucosal vessels, causing inflammation of the mucosa (Li et al. 2018).
Ulcer healing is a complex process that requires both mucosal filling and tissue reconstruction under the mucosa and is intrinsically related to hormones secreted into the gastrointestinal tract and cytokines that can regulate gastrointestinal motility affecting gastric acid and pepsin secretion; some cell growth factors related to gastric acid secretion, migration cellular differentiation, cell proliferation and formation of extracellular matrix, and new vessels can collaborate in the process of gastric ulcer healing, in addition to other factors that may be directly linked to this positive effect, such as the elevation of PGE2 levels, which opens more doors for investigation into the actions of hecogenin acetate (Mussy et al. 2014; Boeing et al. 2021).
Hecogenin acetate demonstrated healing effect by decreasing the volume of chronic ulcerated gastric injury significantly, as well as demonstrated in histological photomicrographs representative of gastric tissue. These findings corroborate with other authors (Ercan et al. 2019), in which it shows that ruscogenin, a steroidal sapogenin, structurally similar with hecogenin, has a therapeutic effect on ulcers induced by acetic acid, since hecogenin acetate is considered an acetylated sapogenin (Gama et al. 2013; Carvalho et al. 2017).
In addition, some studies show that the acetylation of molecules can modify pharmacokinetics. The roxatidine acetate has an active metabolite, roxatidine, and that both are antagonists of histamine H2 receptors, as well as both promote a decrease in gastric acid secretion, and that studies have already been carried out in patients with peptic ulcer for such proof with significant results (Murdoch and Mctavish, 1991). Such a study may open the possibility that hecogenin is an active metabolite of hecogenin acetate and that both hecogenin and hecogenin acetate have healing activity, because the hecogenin reduced gastric lesions induced by acetic acid (Cerqueira et al. 2012). However, further studies should be done to prove the possibilities of the mechanism of action of hecogenin acetate and whether it actually has such an active metabolite.
The healing process begins with the inflammation of the area, with the arrival of cells such as macrophages, neutrophils, and other immune cells, which will promote the removal of possible infectious agents and drain cellular debris in the injured area. Subsequently, there is a constant decrease in the presence of these inflammatory cells, and a series of signaling cascades are activated to stimulate the production of fibroblasts and tissue re-epithelialization (Daemi et al. 2019; Farahpour et al. 2020). And the last phase is collagen synthesis, because collagen is the most abundant protein in connective tissue in the healing phase. The various differences in its chemical composition determine its biological functions. Type I collagen is the most frequent, it is synthesized by fibroblasts, and it is more predominant in bones and tendons. Type III is most commonly found in soft tissues such as blood vessels, dermis, and fascia (Campos et al. 2007).
For the purposes of percentages of collagen, the present work showed that there was an increase of collagen in the all studied doses. The ruscogenin, a steroidal sapogenin, promoted the presence of different types of collagen in an acetic acid-induced gastric ulcer model. Since hecogenin acetate is an acetylated steroidal sapogenin, its action in the healing process becomes plausible with the presence and increase of collagen in gastric ulcers induced by acetic acid (Ercan et al. 2019).
Through this experimental model, possible signs of toxicity can also be investigated by animals being exposed to the test substance for seven days in a row. In the present study, a significant difference in creatinine can be observed. Creatinine (2-amino-1-methyl-5H-imidazole-4-one) is one of the components of human blood and urine. It is a final product of creatine phosphate metabolism in muscles and provides energy to muscle tissues. Creatinine determination is an important clinical measure for the evaluation of renal dysfunction, thyroid dysfunction and muscle damage (Pundir et al. 2019).
Some findings show that hecogenin acetate is within the reference standards of values in such a rat lineage, Thus, it can be inferred that the acetylation of hecogenin is possibly a way to maintain such a molecule in the biochemical standards, because ruscogenin, in its respective doses, had the creatinine of Wistar rats increased when compared with the reference value of creatinine in rats (Rattus norvegicus lineage Wistar) (Lima et al. 2014; Lu et al. 2014). Therefore, the acetate hecogenin showed the promising molecule for the antiulcerogenic and healing activity.
Conclusion
Considering the study carried out, it can be concluded that hecogenin acetate has antiulcerogenic activity, reducing the percentage of ulcerated area in the acute model induced by absolute ethanol and acidified ethanol. It has a healing activity because it reduces the volume of the ulcer lesion and shows an effect on the deposition of collagen fibers, which showed no signs of toxicity relative to seven days of treatment. These data showed that hecogenin acetate showed gastroprotective and healing activity at lower doses than its molecule without chemical modifications (hecogenin), making it a promising candidate in the development of new drugs and exploring new possibilities regarding chemical modifications in unstable substances.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Boeing T et al (2021). Gastric healing effect of p-coumaric acid isolated from Baccharis dracunculifolia DC on animal model. Naunyn-Schmiedeberg's Arch Pharmacol 394: 49-57. https://doi.org/10.1007/s00210-020-01928-9
Campos ACL, Borges-Branco A, Groth AK (2007) Cicatrização de feridas. Arq Bras Cir Dig 20:51–58. https://doi.org/10.1590/S0102-67202007000100010
Carvalho YMBG et al (2017) Inclusion complex between b-cyclodextrin and hecogenin acetate produces superior analgesic effect in animal models for orofacial pain. Biomed Pharmacother 93:754–762. https://doi.org/10.1016/j.biopha.2017.06.091
Cerqueira GS et al (2012) Effects of hecogenin and its possible mechanism of action on experimental models of gastric ulcer in mice. Eur J Pharmacol 683:260–269. https://doi.org/10.1016/j.ejphar.2012.02.043
Cerqueira, GS (2012). Efeitos farmacológicos e possíveis mecanismos de ação da hecogenina em modelos animais de lesão gástrica. Thesis (Doctorate in Farmacology) Universidade Federal do Ceará – UFC, Brazil
Cripps AL, Bluden G (1978) A quantitative gas-liquid chromatographic method for the estimation of hecogenin and tigogenin in the leaves, juice and sapogenin concentrates of agave sisalana. Steroids 31:661–669. https://doi.org/10.1016/S0039-128X(78)80006-3
Daemi A et al (2019) Topical administration of hydroethanolic extract of Lawsonia inermis (henna) accelerates excisional wound healing process by reducing tissue inflammation and amplifying glucose uptake. Kaohsiung J Med Sci 35:24–32. https://doi.org/10.1002/kjm2.12005
Ercan G et al (2019) Potent therapeutic effects of ruscogenin on gastric ulcer established by acetic acid. Asian J Surg 19:1–12. https://doi.org/10.1016/j.asjsur.2019.07.001
Evora PRB et al (1996) Lesão de Isquemia-Reperfusão. Aspectos Fisiopatológicos e a Importância da Função Endotelial. Arq Bras Cardiol 66:239–245
Ewald BT et al (2015) Atividade gastroprotetora do extrato etanólico de Pavonia alnifolia A.St.- Hil. Rev Bras Plantas Med 17:392–397. https://doi.org/10.1590/1983-084X/13_054
Fazalda A, Quraisiah A, Azlina MFN (2018) Antiulcer effect of honey in nonsteroidal anti-inflammatory drugs induced gastric ulcer model in rats: a systematic review. Evid Based Complementary Altern Med 2018:1–12. https://doi.org/10.1155/2018/7515692
Farahpour MR et al (2020) Accelerative effect of topical Zataria multiflora essential oil against infected wound model by modulating inflammation, angiogenesis, and collagen biosynthesis. Pharm Biol 59:1–10. https://doi.org/10.1080/13880209.2020.1861029
Gama KB et al (2013) Evidence for the involvement of descending pain-inhibitory mechanisms in the antinociceptive effect of hecogenin acetate. J Nat Prod 76:559–563. https://doi.org/10.1021/np3007342
Ingawale DK, Patel SS (2016) Anti-inflammatory potential of hecogenin in experimental animals: possible involvement of inflammatory cytokines and myeloperoxidase. Drug Res 66:644–656. https://doi.org/10.1055/s-0042-113184
Kaiser S et al (2010) Estudo da relação estrutura-atividade de saponinas hemolíticas e/ou imunoadjuvantes mediante uso de análise multivariada. Rev Bras Farmacogn 20:300–309. https://doi.org/10.1590/S0102-695X2010000300003
Kayser C et al (2006) Tratamento de pacientes com úlceras isquêmicas secundárias à esclerose sistêmica com N-acetilcisteína endovenosa. Rev Bras Reumatol 46:148–152. https://doi.org/10.1590/S0482-50042006000200012
Kumar A, Ashwlayan V, Verma M (2019). Diagnostic approach & pharmacological treatment regimen of Peptic Ulcer Disease. Phar Pharm Res Open Acc J 1:01–12. https://doi.org/10.30881/pproaj.00001
Kuna L et al (2019) Peptic ulcer disease: a brief review of conventional therapy and herbal treatment options. J Clin Med 8:1–19. https://doi.org/10.3390/jcm8020179
Li Q et al (2018) Activity of Brucea javanica oil emulsion against gastric ulcers in rodents. Asian J Pharm Sci 13:279–288. https://doi.org/10.1016/j.ajps.2017.12.005
Lima CM et al (2014) Valores de referência hematológicos e bioquímicos de ratos (Rattus novergicus linhagem Wistar) provenientes do biotério da Universidade Tiradentes. Scientia Plena 10:1–9
Lu HJ et al (2014) Ruscogenin ameliorates diabetic nephropathy by its anti-inflammatory and antifibrotic effects in streptozotocin-induced diabetic rat. BMC Complement Altern Med 14:1–12. https://doi.org/10.1186/1472-6882-14-110
Malfertheiner P, Schulz C (2020) Peptic ulcer: chapter closed? Dig Dis 38:112–116. https://doi.org/10.1159/000505367
Murdoch D, Mctavish D (1991) Roxatidine acetate a review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic potential in peptic ulcer disease and related disorders. Drugs 42(240):260. https://doi.org/10.2165/00003495-199142020-00006
Mussy JHA et al (2014). Cicatrização de ferimentos incisionais em ratos submetidos a alimentação com carne suína Rev Par Med 28:9-18
Nehra AK et al (2018) Proton pump inhibitors: review of emerging concerns. Mayo Clin Proc 93:240–246. https://doi.org/10.1016/j.mayocp.2017.10.022
Périco LL et al (2020) Systematic analysis of monoterpenes: advances and challenges in the treatment of peptic ulcer diseases. Biomolecules 10:1–18. https://doi.org/10.3390/biom10020265
Possenti A et al (2011) Atividade antiulcerogênica e mecanismo de ação de alimento fermentado à base de trigo e soja utilizado como alimento funcional. Ged Gastroenterol Endosc Dig 30:125–130
Pundir CS, Kumar P, Jaiwal R (2019) Biosensing methods for determination of creatinine: a review. Biosens Bioelectron 126:707–724. https://doi.org/10.1016/j.bios.2018.11.031
Quintans JSS et al (2014) Evidence for the involvement of spinal cord-inhibitory and cytokines modulatory mechanisms in the anti-hyperalgesic effect of hecogenin acetate, a steroidal sapogenin-acetylated, in mice. Molecules 19:8303–8316. https://doi.org/10.3390/molecules19068303
Ribeiro-Junior JA et al (2015) Gastroprotective effect of geopropolis from melipona scutellarisis dependent on production of nitric oxide and prostaglandin. Evid Based Complementary Altern Med 2015:1–5
Rosa RL (2013). Avaliação do potencial gastroprotetor e antiúlcera das sementes da Eugenia involucrata dc. (cereja do mato) e Artocarpus heterophyllus lam. (jaca) em roedores. Dissertation (Master in Pharmaceutical Sciences). Universidade do Vale do Itajaí – UNIVALI, Brazil
Tarasconi A et al. (2021). Perforated and bleeding peptic ulcer: WSES guidelines. World J Emerg Surg 15:1–24. https://doi.org/10.1186/s13017-019-0283-9
Xing M et al (2020) Human-like collagen promotes the healing of acetic acid-induced gastric ulcers in rats by regulating NOS and growth factors. Food Funct 11:4123–4137. https://doi.org/10.1039/D0FO00288G
Zullo MAT et al (1984) Efeito de diferentes condições de fermentação sobre o teor e composição da fração de sapogeninas do suco de sisal. Bragantia 2:479–486. https://doi.org/10.1590/S0006-87051984000200018
Zullo MAT et al (1989) Sapogeninas esteroídicas em sisal. Bragantia 48:21–25. https://doi.org/10.1590/S0006-87051989000100003
Funding
This work was supported by the Coordination of Improvement of Higher Education Personnel (CAPES) and Research Support Foundation of the State of Piauí (FAPEPI).
Author information
Authors and Affiliations
Contributions
AJCS, FVS, AFSCV, and RCMO conceived and designed research. AJCS. BPSN, DSC, MCS, CESC, FVS, JAN, and AFSCV conducted experiments. JSSQ and LJQJ contributed new reagents or analytical tools. AJCS, DSC, PHMN, and RCMO analyzed data and prepared figures. AJCS and RCMO wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of ARRIVE Guidelines for animal research, and approval was granted by the Ethics Committee for Animal Use of Federal University of Piauí – CEUA/UFPI (2018/516).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sousa, A.J.C., de Sousa Neto, B.P., da Costa, D.S. et al. Antiulcerogenic and healing activity of hecogenin acetate in rodents. Naunyn-Schmiedeberg's Arch Pharmacol 396, 759–769 (2023). https://doi.org/10.1007/s00210-022-02341-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02341-0