Abstract
Sepsis is a life-threatening organ dysfunction resulting from inflammatory responses instigated by toxins secreted by bacteria. Immunomodulatory effect of clindamycin is earlier reported in a murine lipopolysaccharide (LPS)-induced sepsis model. There are no studies demonstrating the immunomodulatory effect of clindamycin in combination with ceftriaxone in a clinically relevant murine polymicrobial sepsis model induced by cecal ligation and puncture (CLP). Ceftriaxone is combined to control the bacterial growth. Following 3 h of CLP challenge, Swiss albino mice were administered vehicle, ceftriaxone alone (100 mg/kg, subcutaneously), and in combination with clindamycin at immunomodulatory dose (200 mg/kg, intraperitoneally). Survival was assessed for 5 days, and bacterial count and biochemical and physiological parameters were measured after 18 h of CLP challenge. Ceftriaxone alone caused significant reduction in bacterial count in blood, peritoneal fluid, lung, liver, and kidney homogenate which was not further substantially reduced by ceftriaxone and clindamycin combination. Day 5 survival was greatly improved by combination compared with ceftriaxone alone which was also evident through marked drop in blood glucose, total white blood cell (WBC) count, and body temperature. The combination group significantly mitigated the cytokine (tumor necrosis factor (TNF)-α and interleukin (IL)-6) and myeloperoxidase (MPO) levels in plasma, lung, liver, and kidney of CLP-challenged mice, which further helped in significantly suppressing the elevated levels of liver and kidney function parameters. Clindamycin at immunomodulatory dose in combination with ceftriaxone attenuated organ damage and improved survival of septic mice by suppressing infection, inflammatory responses, and oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a life-threatening systemic inflammatory condition resulting from severe bacterial infection and poor immunity (Song et al. 2019). This condition is primarily triggered by bacterial cell wall components or endotoxins released during infections that are responsible for secreting proinflammatory cytokines and reactive oxygen species (ROS) implicated in organ dysfunction and associated mortality (Nau and Eiffert 2002; Wibke et al. 2013; Burkovskiy et al. 2013).
The murine CLP model of sepsis is mostly used to evaluate the immunomodulatory activity of drugs as this model closely resembles the human condition of sepsis (Dejager et al. 2011). Several publications report the use of ceftriaxone and clindamycin combination in the murine CLP sepsis model to improve survival and control bacterial growth (Hollenberg et al. 2001, 2000; Barichello et al. 2007; Ritter et al. 2004). Ceftriaxone, a broad-spectrum cephalosporin antibiotic, is used as it is active against Gram-negative bacteria, while clindamycin is combined due to activity against aerobic Gram-positive and anaerobic bacteria. Despite broad coverage offered by both ceftriaxone and clindamycin in combination, the survival benefit was not significant in these studies (Hollenberg et al. 2001, 2000; Barichello et al. 2007; Ritter et al. 2004). The probable reasons could be inadequate drug exposures due to lower doses of either drugs or inability to control the inflammatory responses. It should be noted that clindamycin besides having antibacterial activity is also reported to possess immunomodulatory activity although at higher doses than currently used in the CLP model. Clindamycin at doses above 300 mg/kg by intraperitoneal route has been earlier reported to improve survival and reduce the proinflammatory cytokine in the LPS-induced sepsis model (Hirata et al. 2001). It is also reported to suppress the release of bacterial toxins that are responsible for sepsis and other secondary complications (Kishi et al. 1999; Böttcher et al. 2004). Since sepsis is a manifestation of inflammatory processes, the protective effect of immunomodulatory dose of clindamycin in combination with subprotective dose of ceftriaxone was evaluated in a murine CLP-induced polymicrobial sepsis model by monitoring the survival and inflammatory markers. Further antibacterial activity of ceftriaxone-clindamycin combination was also determined.
Materials and methods
Female Swiss albino mice (weighing 25–30 g) were obtained from Wockhardt’s animal breeding facility. They were housed under specific pathogen-free conditions at a constant temperature of 18–22 °C and humidity of 40–70% with a 12-h light/dark cycle. They were allowed free access to standard rodent diet and pure water. All experiments were performed as per guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Ethics Committee.
Induction of polymicrobial sepsis
Mice were anesthetized with intraperitoneal injection of co-mixed solution of ketamine (100 mg/kg) and xylazine (10 mg/kg). Under sterile conditions, a small mid-abdominal incision was made to expose the cecum. The distal portion of the cecum was completely ligated 1 cm from the end with a 3–0 silk suture, punctured twice with an 18-gauge needle and gently squeezed until small quantity of feces extruded through them. The cecum was then placed back in the abdominal cavity, and then the incision was closed with sutures. A 1-mL injection of sterile saline (0.9%) was administered subcutaneously to all mice after surgery. Sham control underwent abdominal incision and cecal exposure without ligation and puncture. After the procedure, mice had free access to water and feed. Survival was monitored for 5 days, while parameters such as bacterial count, body temperature, blood glucose, total WBC count, cytokines, MPO, glutathione (GSH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, and blood urea nitrogen (BUN) were measured after 18 h of CLP challenge.
Treatment groups
From the initial survival studies performed in CLP mice (data not shown), a subprotective dose of ceftriaxone (100 mg/kg, s.c.) providing protection in 37.5% of mice was determined. The survival study included a sham group, CLP mice treated with vehicle (saline), ceftriaxone (100 mg/kg, s.c.), clindamycin (200 mg/kg, i.p.), and both in combination. For estimation of biochemical and other parameters, groups included were sham control, CLP mice treated with vehicle (saline), ceftriaxone (100 mg/kg, s.c.), and ceftriaxone (100 mg/kg, s.c.) plus clindamycin (200 mg/kg, i.p.), all administered 3 h post CLP challenge.
Experimental protocol
For estimation of various parameters, 34 mice were included in each treatment group. Mice were made septic by CLP technique and 18 h later body temperature (n = 8) and blood glucose (n = 8) were measured. Subsequently, 6 mice were bled through the retro-orbital sinus in EDTA tubes for total WBC count, and other 6 mice were bled in heparinized tubes to obtain plasma for estimation of cytokines, MPO, and GSH. The remaining 6 mice were bled to obtain serum for estimation of ALT, AST, BUN, and creatinine. The lung, liver, and kidneys were harvested from CLP mice (n = 14), rinsed with saline, and weighed. Tissues collected from 6 mice were individually homogenized with chilled saline to obtain 20% homogenate and then centrifuged (15,000 rpm for 10 min at 4 °C) for cytokine measurements. Tissues from remaining mice (n = 8) were individually homogenized with 50 mM potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide, sonicated, and centrifuged to obtain supernatant for MPO estimation.
Measurements
Body temperature and blood glucose
Rectal temperature and blood glucose were measured using a digital thermometer (CareTouch®) and a calibrated Bayer Contour® TS glucometer, respectively.
Total WBC count in blood
Blood samples collected in EDTA were analyzed on an automatic blood cell counter (Sysmex hematology analyzer) for assessing the total WBC count.
Cytokine levels in plasma and tissue homogenates
TNF-α and IL-6 were estimated using commercially available enzyme-linked immunosorbent assays (ELISAs), according to manufacturer’s instruction (R&D Systems Inc., USA).
Plasma and tissue MPO activity and plasma GSH levels
MPO activity was determined by o-dianisidine method and GSH was estimated using Ellman’s reagent (5,5′-dithiobis (2-nitrobenzoic acid) or DTNB] with modification for 96-well plates as described previously (Patel et al. 2018). For MPO estimation, absorbance was recorded at 460 nm per minute for a period of 10 min, and for GSH estimation, the absorbance was read after 10-min incubation at 412 nm following addition of all reagents using a spectrophotometer (SpectraMax® Plus 384 microplate reader). One unit of MPO was defined as that degrading 1 μmol of hydrogen peroxide per minute at 25 °C. A molar extinction coefficient of 10,062 M−1 cm−1 of oxidized o-dianisidine was used for calculation. MPO activity was expressed in μM/min/mL of plasma and in U/g of tissue. The GSH concentration was estimated using a standard curve of l-glutathione (Sigma-Aldrich).
Biochemical parameters
Markers of kidney and liver damage such as BUN, creatinine, ALT, and AST were analyzed in fresh serum samples using a semi-automated chemistry analyzer (LabLife ChemMaster).
Bacterial clearance
In brief, mice were anesthetized with ketamine and xylazine solution after 18 h of CLP challenge. The blood samples were collected through the retro-orbital sinus in heparinized tubes, mixed, and placed on ice bath. Mice were later injected with 2 mL of sterile saline intraperitoneally, and the abdomen was gently massaged. The dorsal region of the mice was cleansed with 70% alcohol, and the abdomen was cut open to expose the peritoneal cavity to collect the peritoneal lavage fluid. The lung, liver, and kidneys were then harvested aseptically and homogenized with 3 times volume of tissue weight using sterile saline. Eppendorf containing 100 μL of peritoneal lavage fluids, blood, and tissue homogenates was kept on ice and serially diluted with sterile saline. Ten microliters of each diluted sample was placed on trypticase soy agar plates (BD Biosciences, San Diego, CA) and incubated at 37 °C for 24 h. The numbers of bacterial colonies were then counted and expressed as colony-forming units (CFU) per milliliter of blood or peritoneal lavage and CFU per gram of tissue.
Statistical analyses
The values in all figures are represented as mean ± SEM, except for the survival curve. Statistical analyses were done using GraphPad Prism software (version 5), where P < 0.05 was considered statistically significant. Percent survival between groups was compared using log rank test, while all other parameters were compared using one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test.
Results
Effect on survival, blood glucose, WBC count, and body temperature
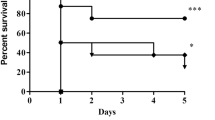
Significant improvement in survival was noticed when ceftriaxone (100 mg/kg) was combined with clindamycin at immunomodulatory dose (200 mg/kg), resulting in 5-day survival of 75% compared with 0% in the CLP control group, whereas the ceftriaxone and clindamycin alone group provided protection in 37.5% of mice. The high-dose clindamycin combination in our study provided better protection compared with combination of ceftriaxone with low-dose clindamycin as reported earlier (Hollenberg et al. 2001; Hollenberg et al. 2000; Barichello et al. 2007; Ritter et al. 2004). The CLP control group showed significant (P < 0.001) decrease in blood glucose (26.3 ± 3.4 mg/dL), WBC count (1.17 × 103/μL ± 0.1), and body temperature (92.8 ± 1.1 °F) compared with 99.0 ± 1.2 mg/dL, 6.07 × 103/μL ± 0.2, and 98.8 ± 0.3 °F in the sham group, respectively. Although ceftriaxone alone increased the glucose level and body temperature, the effect was more pronounced in the ceftriaxone and clindamycin combination groups. The combination significantly elevated the blood glucose (62.0 ± 8.4 mg/dL; P < 0.01), WBC count (3.25 × 103/μL ± 0.4; P < 0.01), and body temperature (97.1 ± 0.5 °F; P < 0.001) compared with the CLP control (Fig. 1).
Effect of ceftriaxone (CTX) + clindamycin (CLI) combination on survival, total WBC, blood glucose, and body temperature: Following 3 h of CLP, mice were administered vehicle, CTX (100 mg/kg, s.c.), CLI (200 mg/kg, i.p.), and CTX (100 mg/kg, s.c.) + CLI (200 mg/kg, i.p.). For survival effect, mice (n = 8) were observed for 5 days for mortality, and the statistical analysis was performed using the log rank test, whereas for blood glucose (n = 8), body temperature (n = 8), and total WBC count (n = 6), estimations were performed 18 h after CLP challenge. Each value is represented as mean ± SEM. ###P < 0.001 for significance versus the sham group and *P < 0.5, **P < 0.01, and ***P < 0.001 for significance versus CLP control
Effect on plasma parameters
Figure 2 demonstrates the effect of treatment groups on plasma cytokines, MPO, and GSH. As expected, the CLP group showed significant (P < 0.001) increase in TNF-α (121.5 ± 16.7 pg/mL), IL-6 (13,858.3 ± 1419.7 pg/mL), and MPO (647.9 ± 41.5 μM/min/mL) levels compared with the sham group (TNF-α, 8.9 ± 1.0 pg/mL; IL-6, 33.6 ± 8.7 pg/mL; and MPO, 72.7 ± 12.6 μM/min/mL). In contrast, there was significant (P < 0.001) decrease in the GSH level in the CLP group (1.04 ± 0.1 mg/mL) compared with the sham group (2.5 ± 0.4 mg/mL). Ceftriaxone alone demonstrated significant reduction in TNF-α and IL-6 levels but had no significant effect on the MPO and GSH. While, the combination group exhibited substantial (P < 0.001) drop in plasma TNF-α (25.0 ± 1.5 pg/mL), IL-6 (680.8 ± 141.9 pg/mL), and MPO (147.2 ± 18.3 μM/min/mL) and elevated the plasma GSH (2.16 ± 0.2 mg/mL) levels.
Effect of ceftriaxone (CTX) + clindamycin (CLI) combination on plasma IL-6, TNF-α, MPO, and GSH: Following 3 h of CLP, mice were administered vehicle, CTX (100 mg/kg, s.c.), or CTX (100 mg/kg, s.c.) + CLI (200 mg/kg, i.p.), and plasma cytokines, MPO, and GSH were estimated post 18 h of CLP challenge. The graphs depict values in mean ± SEM. ###P < 0.001 for significance versus the sham group and ***P < 0.001 for significance versus CLP control
Effect on lung parameters
The lung cytokine concentration in the sham control group was undetectable due to levels below quantification. The lung IL-6 and TNF-α levels for the CLP control group were 468.7 ± 105.4 pg/mL and 252.3 ± 48.9 pg/mL, respectively, while the lung MPO level was 63.1 ± 4.7 U/g, which was significantly (P < 0.001) higher than the sham group (8.7 ± 0.7 U/g). Ceftriaxone alone had no significant effect on lung cytokine and MPO levels, while the combination group significantly attenuated the IL-6 (126.2 ± 17.2 pg/mL), TNF-α (40.3 ± 15.6 pg/mL), and MPO (36.8 ± 5.4 U/g) levels compared with the CLP control group (Fig. 3).
Effect of ceftriaxone (CTX) + clindamycin (CLI) combination on lung IL-6, TNF-α, and MPO: Following 3 h of CLP, mice were administered vehicle, CTX (100 mg/kg, s.c.), or CTX (100 mg/kg, s.c.) + CLI (200 mg/kg, i.p.), and lung cytokine and MPO levels were estimated post 18 h of CLP challenge. The graphs depict values in mean ± SEM. ###P < 0.001 for significance versus the sham group and **P < 0.01 for significance versus CLP control
Effect on liver parameters
There were no detectable levels of IL-6 and TNF-α in the liver of the sham group. In the case of CLP control, the liver IL-6 and TNF-α concentrations were 610.6 ± 93.6 pg/mL and 294.2 ± 56.1 pg/mL, respectively. The liver MPO, serum ALT, and AST levels in the CLP group were 10.7 ± 1.2 U/g, 230.4 ± 12.9 IU/L, and 505.1 ± 20.6 IU/L, respectively, which were significantly (P < 0.001) higher than those of the sham group (MPO, 0.76 ± 0.2 U/g; ALT, 63.5 ± 7.9 IU/L; and AST, 134.8 ± 8.4 IU/L). Ceftriaxone alone showed significant decrease in liver cytokine levels. However, it had no significant effect on liver MPO, ALT, and AST levels. The combination group brought about significant decrease in IL-6 (62.0 ± 3.9 pg/ml), TNF-α (64.5 ± 4.6 pg/mL), MPO (5.2 ± 1.0 U/g), ALT (159.7 ± 6.7 IU/L), and AST (347.0 ± 27.8 IU/L) compared with the CLP control (Fig. 4).
Effect of ceftriaxone (CTX) +clindamycin (CLI) combination on liver IL-6, TNF-α, MPO, serum ALT, and AST: Following 3 h of CLP, mice were administered vehicle, CTX (100 mg/kg, s.c.), or CTX (100 mg/kg, s.c.) + CLI (200 mg/kg, i.p.), and liver cytokines, MPO, serum ALT, and AST were estimated post 18 h of CLP challenge. The graphs depict values in mean ± SEM. ###P < 0.001 for significance versus the sham group and *P < 0.05, **P < 0.01, and ***P < 0.001 for significance versus CLP control
Effect on kidney parameters
The increase in kidney IL-6, TNF-α, MPO, serum BUN, and creatinine was significant in the CLP control group measuring 647.4 ± 95.2 pg/mL, 84.3 ± 5.3 pg/mL, 34.9 ± 0.78 U/g, 94.7 ± 4.3 mg/dL, and 0.80 ± 0.04 mg/dL, respectively. Ceftriaxone alone showed significant reduction in kidney IL-6, TNF-α, serum BUN, and creatinine levels vs CLP control; however, it had no effect on kidney MPO activity. The combination group further suppressed the kidney IL-6 (65.6 ± 11.0 pg/mL; P < 0.001), TNF-α (35.9 ± 5.0 pg/mL; P < 0.001), MPO (26.0 ± 3.1 U/g; P < 0.001), BUN (31.1 ± 4.0 mg/dL; P < 0.001), and creatinine (0.53 ± 0.03 mg/dL; P < 0.01) (Fig. 5).
Effect of ceftriaxone (CTX) + clindamycin (CLI) combination on kidney IL-6, TNF-α, MPO, serum BUN, and creatinine: Following 3 h of CLP, mice were administered vehicle, CTX (100 mg/kg, s.c.), or CTX (100 mg/kg, s.c.) + CLI (200 mg/kg, i.p.), and kidney cytokines, MPO, serum BUN, and creatinine were estimated post 18 h of CLP challenge. The graphs depict values in mean ± SEM. ###P < 0.001 for significance versus the sham group and *P < 0.05, **P < 0.01, and ***P < 0.001 for significance versus CLP control
Effect on bacterial clearance
Both ceftriaxone alone and ceftriaxone in combination with clindamycin significantly reduced the bacterial count in blood, peritoneal lavage fluid, lung, liver, and kidney homogenates. Although statistically the antibacterial effect of combination appeared similar to the ceftriaxone alone group, the combination group did exhibit further reduction in bacterial count in blood, lung, liver, and kidney tissues suggesting some synergism between both the drugs (Fig. 6).
Effect of ceftriaxone (CTX) + clindamycin (CLI) combination on bacterial burden in blood, peritoneal lavage fluid, lung, liver, and kidney: Following 3 h of CLP, mice were administered vehicle, CTX (100 mg/kg, s.c.), or CTX (100 mg/kg, s.c.) + CLI (200 mg/kg, i.p.), and bacterial counting was performed in blood, peritoneal lavage fluid, lung, liver, and kidney homogenate after 18 h of CLP challenge. The graphs depict values in mean ± SEM. **P < 0.01 and ***P < 0.001 for significance versus CLP control
Discussion
Currently, there are no treatment options available to counteract the overwhelming inflammatory response encountered during sepsis which is the major factor responsible for health deterioration of such patients. The only treatment option for severe sepsis is timely administration of intravenous broad-spectrum antibiotics either alone or in combination with other antibiotics along with supportive treatment measures (Rello et al. 2017). Considering the role of immune responses in sepsis pathogenesis, research for treatment of sepsis is nowadays directed towards immunomodulatory agents (Delano and Ward 2016).
Ceftriaxone is one of the preferred antibiotics to treat sepsis caused by Gram-negative pathogens, whereas clindamycin is a lincosamide antibiotic primarily used in combination with aminoglycoside to treat dental, respiratory tract, skin, and soft tissue infection and peritonitis. As a result of its anti-inflammatory or immunomodulatory action, clindamycin has been reported to provide benefit in relieving the symptoms of dermatological conditions in humans (Pradhan et al. 2016). It is also reported to suppress the endotoxin released by Escherichia coli on treatment with ceftazidime in an in vitro assay employing THP-1 cells (Kishi et al. 1999). The present study aimed to assess the beneficial role of immunomodulatory effect of clindamycin in protecting the mice from lethal effect of polymicrobial sepsis induced by CLP. For this purpose, clindamycin was administered at an immunomodulatory dose identified from the previous LPS-induced sepsis model (Hirata et al. 2001). During an initial survival study in the CLP-induced sepsis model, clindamycin alone at the immunomodulatory dose did not offer survival benefit, possibly due to lack of activity against the enteric bacteria. Therefore, to evaluate the benefits of immunomodulatory action of clindamycin in sepsis, it was combined with a subprotective dose of ceftriaxone to retard the bacterial growth. As the clindamycin alone group showed no substantial effect on survival of CLP-challenged mice and also considering our objective of assessing the immunomodulatory activity of clindamycin that was possible only in combination with ceftriaxone, the clindamycin alone group was not included in subsequent studies.
Clindamycin due to activity against anaerobic bacteria was expected to provide synergistic antimicrobial activity with ceftriaxone. However, the assessment of bacterial load in blood, peritoneal fluid, and tissue homogenate suggested no statistically significant reduction in bacterial count by the combination group compared with ceftriaxone alone. Although not significant, the combination group showed little synergistic antibacterial effect in the blood, lung, liver, and kidney as observed through further lowering of bacterial count compared with the ceftriaxone group. Despite ceftriaxone having antibacterial activity, it was not able to provide much required survival benefit, while its combination with clindamycin offered improved survival. This demonstrates that combined immunomodulatory and antibacterial activity is a requisite for improved survival benefits in sepsis. It is commonly noted that the sepsis condition is accompanied with lowering of WBC count, body temperature, and blood glucose (Brooks et al. 2007). The CLP-challenged mice showed significant reduction in WBC count, blood glucose, and body temperature, while the treatment with combination considerably improved their levels. Earlier reports have demonstrated significant elevation in levels of proinflammatory cytokines (IL-6, TNF-α) and MPO (an indirect measure of neutrophil migration) in sepsis (Aziz et al. 2013; Schrijver et al. 2017). In our study also, we found significant rise in plasma and tissue levels of IL-6, TNF-α, and MPO in the CLP control group. Ceftriaxone was found to suppress the cytokine levels in plasma, liver, and kidney but not in lung tissue, while the treatment with combination brought about further reduction in cytokine levels in all the tissues including lung. Surprisingly, ceftriaxone had no effect on the augmented plasma and tissue MPO and low plasma GSH (endogenous antioxidant) levels. The antibiotic combination significantly suppressed the tissue MPO activity and improved the plasma GSH levels. GSH, an endogenous antioxidant, protects tissues against damage by reactive oxygen species and also helps in inhibiting the release of inflammatory cytokines (Villa et al. 2002). Excessive secretion of proinflammatory cytokines, reactive oxygen species, and myeloperoxidase is implicated in multiple organ dysfunctions via inflammation and oxidative stress (Ismail et al. 2020). Ceftriaxone-clindamycin combination significantly inhibited the levels of plasma and tissue cytokines and myeloperoxidase and also improved plasma GSH content; the cumulative effect of all resulted into lowering of liver and kidney damage as observed through recovery of elevated hepatic and renal function parameters in CLP mice.
The ceftriaxone-clindamycin combination group was found to demonstrate protective effect in sepsis; however, the absence of the clindamycin alone group in this study limited proper analysis of the results with respect to clindamycin contribution.
Overall, it can be concluded that the administration of clindamycin at the immunomodulatory dose in conjunction with ceftriaxone exhibited beneficial outcome in the murine sepsis model possibly by suppressing the bacterial growth, inflammatory response, neutrophil infiltration, and oxidative stress.
References
Aziz M, Jacob A, Yang WL, Matsuda A, Wang P (2013) Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol 93:329–342
Barichello T, Martins MR, Reinke A, Constantino LS, Machado RA, Valvassori SS, Moreira JCF, Quevedo J, Dal-Pizzol F (2007) Behavioral deficits in sepsis-surviving rats induced by cecal ligation and perforation. Braz J Med Biol Res 40:831–837
Böttcher T, Ren H, Goiny M, Gerber J, Lykkesfeldt J, Kuhnt U, Lotz M, Bunkowski S, Werner C, Schau I, Spreer A, Christen S, Nau R (2004) Clindamycin is neuroprotective in experimental Streptococcus pneumoniae meningitis compared with ceftriaxone. J Neurochem 91:1450–1460
Brooks HF, Osabutey CK, Moss RF, Andrews PLR, Davies DC (2007) Caecal ligation and puncture in the rat mimics the pathophysiological changes in human sepsis and causes multi-organ dysfunction. Metab Brain Dis 22:353–373
Burkovskiy I, Sardinha J, Zhou J, Lehmann C (2013) Cytokine release in sepsis. Adv Biosci Biotechnol 4:860–865
Dejager L, Pinheiro I, Dejonckheere E, Libert C (2011) Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19:198–208
Delano MJ, Ward PA (2016) Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest 126:23–31
Hirata N, Hiramatsu K, Kishi K, Yamasaki T, Ichimiya T, Nasu M (2001) Pretreatment of mice with clindamycin improves survival of endotoxic shock by modulating the release of inflammatory cytokines. Antimicrob Agents Chemother 45:2638–2642
Hollenberg SM, Broussard M, Osman J, Parrillo JE (2000) Increased microvascular reactivity and improved mortality in septic mice lacking inducible nitric oxide synthase. Circ Res 86:774–778
Hollenberg SM, Dumasius A, Easington C et al (2001) Characterization of a hyperdynamic murine model of resuscitated sepsis using echocardiography. Am J Respir Crit Care Med 164:891–895
Ismail HF, Didari T, Khan F et al (2020) A review on the protective effects of metformin in sepsis-induced organ failure. Cell J. https://doi.org/10.22074/cellj.2020.6286
Kishi K, Hirai K, Hiramatsu K, Yamasaki T, Nasu M (1999) Clindamycin suppresses endotoxin released by ceftazidime-treated Escherichia coli O55:B5 and subsequent production of tumor necrosis factor alpha and interleukin-1 beta. Antimicrob Agents Chemother 43:616–622
Nau R, Eiffert H (2002) Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 15:95–110
Patel A, Joseph J, Periasamy H et al (2018) Azithromycin in combination with ceftriaxone reduces systemic inflammation and provides survival benefit in a murine model of polymicrobial sepsis. Antimicrob Agents Chemother 62:e00752–e00718
Pradhan S, Madke B, Kabra P, Singh AL (2016) Anti-inflammatory and immunomodulatory effects of antibiotics and their use in dermatology. Indian J Dermatol 61:469–481
Rello J, Valenzuela-Sánchez F, Ruiz-Rodriguez M, Moyano S (2017) Sepsis: a review of advances in management. Adv Ther 34:2393–2411
Ritter C, Andrades M, Reinke A et al (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349
Schrijver IT, Kemperman H, Roest M, Kesecioglu J, de Lange DW (2017) Myeloperoxidase can differentiate between sepsis and non-infectious SIRS and predicts mortality in intensive care patients with SIRS. Intensive Care Med Exp 5:43
Song Y, Miao S, Li Y, Fu H (2019) Ulinastatin attenuates liver injury and inflammation in a cecal ligation and puncture induced sepsis mouse model. J Cell Biochem 120:417–424
Villa P, Saccani A, Sica A, Ghezzi P (2002) Glutathione protects mice from lethal sepsis by limiting inflammation and potentiating host defense. J Infect Dis 185:1115–1120
Wibke S, Jürgen B, Richard B (2013) Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediat Inflamm 2013:1–16. https://doi.org/10.1155/2013/165974
Acknowledgments
We thank the Wockhardt Research Centre, India, and Dr. Mahesh Patel for granting permission for performing these studies. We also thank Mr. Satish Damle and Mr. Bhushan Choudhari for assisting in sample analysis.
Funding
This work was self-funded.
Author information
Authors and Affiliations
Contributions
AP and SM conceived and designed the research. AP and HP conducted experiments. AP analyzed the data. AP and SM wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Study protocols were approved by Institutional Animal Ethics Committee of Wockhardt Research Centre, India, registered under Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, A.M., Periasamy, H. & Mokale, S.N. Immunomodulatory dose of clindamycin in combination with ceftriaxone improves survival and prevents organ damage in murine polymicrobial sepsis. Naunyn-Schmiedeberg's Arch Pharmacol 393, 1671–1679 (2020). https://doi.org/10.1007/s00210-020-01876-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01876-4