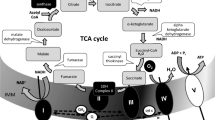
Abstract
Assessment of drug-induced mitochondrial dysfunctions is important in drug development as well as in the understanding of molecular mechanism of therapeutic or adverse effects of drugs. The aim of this study was to investigate the effects of three typical antipsychotics (APs) and seven atypical APs on mitochondrial bioenergetics. The effects of selected APs on citrate synthase, electron transport chain complexes (ETC), and mitochondrial complex I- or complex II-linked respiratory rate were measured using mitochondria isolated from pig brain. Complex I activity was decreased by chlorpromazine, haloperidol, zotepine, aripiprazole, quetiapine, risperidone, and clozapine. Complex II + III was significantly inhibited by zotepine, aripiprazole, quetiapine, and risperidone. Complex IV was inhibited by zotepine, chlorpromazine, and levomepromazine. Mitochondrial respiratory rate was significantly inhibited by all tested APs, except for olanzapine. Typical APs did not exhibit greater efficacy in altering mitochondrial function compared to atypical APs except for complex I inhibition by chlorpromazine and haloperidol. A comparison of the effects of APs on individual respiratory complexes and on the overall mitochondrial respiration has shown that mitochondrial functions may not fully reflect the disruption of complexes of ETC, which indicates AP-induced modulation of other mitochondrial proteins. Due to the complicated processes associated with mitochondrial activity, it is necessary to measure not only the effect of the drug on individual mitochondrial enzymes but also the respiration rate of the mitochondria or a similar complex process. The experimental approach used in the study can be applied to mitochondrial toxicity testing of newly developed drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antipsychotics (APs) are commonly used and widely prescribed medications for both psychiatric and non-psychiatric indications. The main molecular mechanism of action of APs, except for aripiprazole, is the blockade of dopamine D2 receptors. Antagonism of D2 receptors in mesolimbic dopamine pathway is responsible for treatment of positive symptoms of schizophrenia; however, the high and non-specific occupation of D2 receptors through the CNS exhibits a wide range of adverse effects, most remarkably extrapyramidal syndrome and hyperprolactinemia (Sangani and Saadabadi 2019). The effects of individual drugs depend on affinity for the affected neurotransmitter receptors and transporters.
The first generation of APs (typical) strongly antagonizes D2 receptors in cortical and striatal areas. Sedative typical APs, e.g., chlorpromazine and levomepromazine (syn. methotrimeprazine), affect dopaminergic and other mediator systems (cholinergic and histaminergic system) and have significant tranquilizing and anticholinergic effects (Church and Young 1983; Trabucchi et al. 1974). Haloperidol is characterized as a typical incisive AP and affects D2 receptors in ventral striatum with higher specificity. It exhibits distinctive side effects, and haloperidol-induced movement disorders include parkinsonism, dyskinesia, dystonia, and akathisia (Wirshing 2001).
The second generation of APs (atypical) have a higher affinity for diverse neurotransmitter systems (5-HT, dopamine, muscarinic, adrenergic, and histamine receptors) (Kapur and Seeman 2001; Seeman 2002). Serotonin and dopamine antagonist (SDA) class of APs is represented by risperidone and ziprasidone. Risperidone has a higher binding affinity to 5-HT2 receptors than it does to dopamine D2 receptors (Keegan 1994); ziprasidone displays partial agonism at 5-HT1A receptors and has a relatively high affinity for the H1 receptor (Nasrallah 2008). Multi-acting receptor-targeted antipsychotics (MARTA) act as antagonists on numerous neurotransmitter receptors such as dopamine (D1, D2, D3, or D4), serotonin (5-HT2A, 5-HT2C, 5-HT6, or 5-HT7), adrenergic receptors (α1 and α2), muscarinic receptors (M1, M2, M3, M4, and M5), and histamine H1 receptors (Nasrallah 2008). MARTA are represented by clozapine, olanzapine, zotepine, and quetiapine. Aripiprazole differs from other atypical APs by partial agonism at D2 receptors (Bolonna and Kerwin 2005), and it acts also as a partial 5-HT1A receptor agonist and 5-HT2A receptor antagonist. Although atypical APs are well tolerated, hyperglycemia, dyslipidemia, weight gain, and hypertension are risk factors of metabolic syndrome, which should be a concern in long-term antipsychotic treatment (Masand et al. 2005). Furthermore, QT interval prolongation, cardiac effects, seizures, etc. can accompany treatment with APs.
Disturbed brain bioenergetics, described as defect in oxidative phosphorylation (OXPHOS) in specific brain areas of patients with schizophrenia (Maurer et al. 2001), indicates the need to study the effects of antipsychotics on mitochondrial dysfunction. Treatment-responsive schizophrenia patients showed a significant reduction in the number of mitochondria at synapses in certain areas of the brain compared to treatment-resistant cases (Roberts 2017). Although the primary biochemical mechanisms of AP action are well described, their effect on mitochondrial dysfunction is not sufficiently recognized. Mitochondrial impairment appears to be related to some adverse effects of APs due to inhibition of the mitochondrial respiratory chain complexes. Haloperidol, chlorpromazine, and fluphenazine, classical APs, have long been reported to inhibit complex I in rat or mouse brain mitochondria more strongly than clozapine, an atypical AP (Balijepalli et al. 1999; Burkhardt et al. 1993; Prince et al. 1997). It has been suggested that reduced complex I activity may correlate with the extrapyramidal side effects of APs. The hypothesis that extrapyramidal side effects of APs may be caused by inhibition of mitochondrial electron transport chain (ETC) was supported by observing the different effects of haloperidol and chlorpromazine and atypical APs risperidone, zotepine, and clozapine on the activity of ETC complexes and citrate synthase (CS) activity in human brain tissue (Maurer and Möller 1997). However, the therapeutic and side effects of antipsychotics seem unrelated (Maurer and Volz 2001). The extrapyramidal side effects of APs appear to be caused by mitochondrial impairment, which is more noticeable for the typical APs than for the atypical APs (Casademont et al. 2007). Tardive dyskinesia correlated with the inhibition of the electron transport chain (ETC) complexes and the production of reactive oxygen species (ROS) during the antipsychotic treatment (Elkashef and Wyatt 1999; Goff et al. 1995).
The metabolic side effects of APs could be connected with alterations in mitochondrial homeostasis, which leads to an imbalance in the mitochondrial fusion/fission ratio and to an inefficient mitochondrial phenotype of muscle cells (Del Campo et al. 2018). For example, olanzapine induced a downregulation of genes involved in the mitochondrial enzymes of the ETC, as well as decreased enzyme activity, ATP synthesis, and oxygen consumption in blood cells of patients at elevated risk for metabolic syndrome (Scaini et al. 2018). The other effect of olanzapine was assessed on freshly isolated rat hepatocytes, and the results of the study showed that cytotoxicity of olanzapine leads to hepatotoxicity, which are mediated by mitochondrial potential collapse and oxidative stress (Eftekhari et al. 2016).
In recent years, the new atypical antipsychotics have opened new ways to therapy. While the effects of APs on the activity of individual mitochondrial enzymes are relatively well described, little is known about the effects of APs on complex mitochondrial processes such as respiratory rate. It is hypothesized that various APs modulate synaptic activity and cell energy metabolism, especially OXPHOS and activities of ETC complexes. To verify this hypothesis, we determined the effect of both older and new APs on mitochondrial energy metabolism. We investigated the in vitro effects of three typical APs (chlorpromazine, levomepromazine, and haloperidol) and seven atypical APs (risperidone, ziprasidone, zotepine, aripiprazole, clozapine, olanzapine, and quetiapine) on complex I- and complex II-linked mitochondrial respiration and activities of individual mitochondrial enzymes, CS, and ETC complexes I, II + III, and IV.
Materials and methods
Media and chemicals
The media used for isolation and preservation of mitochondria consisted of sucrose 0.32 mol/L and HEPES 4 mmol/L, buffered to pH 7.4. The Krebs-Henseleit buffer (KH buffer) was composed of 118 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4·7H2O, 25 mmol/L NaHCO3, and 11.1 mmol/L glucose (pH 7.4). Mitochondrial respiratory medium (MiR05) was applied in mitochondrial respiration measurements and consisted of sucrose 110 mmol/L, K+-lactobionate 60 mmol/L, taurine 20 mmol/L, MgCl2·6H2O 3 mmol/L, KH2PO4 10 mmol/L, EDTA 0.5 mmol/L, BSA essentially fatty acid free 1 g/L, and HEPES 20 mmol/L, adjusted to pH 7.1 with KOH (Pesta and Gnaiger 2012). Other media are specified in the chapters describing the measurement of enzyme activities. All chemicals were purchased from Sigma-Aldrich, Co. (St. Louis, MO, USA).
Isolation of brain mitochondria
Pig brains were obtained from a slaughterhouse and the mitochondria for the assays were isolated from brain cortex and prepared by a previously described method (Fišar and Hroudová 2016; Fišar et al. 2010; Pinna et al. 2003). Mitochondria were purified on a sucrose gradient and kept on ice until the assay. The freshly purified mitochondria were used for measurements of the mitochondrial oxygen consumption rate (Fišar and Hroudová 2016). The enzyme activity measurements were assayed with the frozen mitochondria (stored at − 70 °C). The Lowry method was applied in order to determine the concentration of proteins; bovine serum albumin was taken as a standard (Lowry et al. 1951).
Activity of citrate synthase and complexes of electron transport chain
The suspension of purified mitochondria with hypotonic buffer (25 mmol/L potassium phosphate and 5 mmol/L MgCl2, pH 7.2) was ultrasonicated three times in order to reach the highest activity of the enzymes. Prepared samples were incubated with particular drugs for 30 min at a temperature of 30 °C. The examined drugs were added at a concentration of 50 μmol/L. Every single measurement was compared with a control set of solvent instead of the drug; the total sample volume was 1 mL. The temperature during the measurement was 30 °C. All the measurement of enzyme activities was carried out spectrophotometrically using Uvicon XL spectrophotometer (SECOMAM, Alès, France).
Complex I (NADH dehydrogenase (ubiquinone), EC 1.6.5.3)
The activity of mitochondrial complex I was measured as the rotenone-sensitive rate of NADH oxidation at 340 nm. The oxido-reductive reaction was initiated by adding coenzyme Q1 (to a final concentration of 33 μmol/L) and NADH (1 mmol/L) and measured for 3 min (Folbergrová et al. 2010; Hroudova and Fisar 2010). The final protein concentration was higher than that used for other complexes (1 mg/mL) to achieve good quality of the measurement.
Complex II + III (succinate cytochrome c oxidoreductase, EC 1.8.3.1)
The activity of complex II + III was measured as the antimycin A-sensitive rate of cytochrome c reduction (Trounce et al. 1996). The reduction of cytochrome c was monitored by a spectrophotometer at 550 nm for 1 min. Succinate was used as a substrate. The final protein concentration was 500 μg/mL.
Complex IV (cytochrome c oxidase, EC 1.9.3.1)
The principle of the measurement of the cytochrome c oxidase (COX) activity was based on monitoring the decrease in absorbance during the oxidation of reduced cytochrome c at 550 nm for 3 min (Rustin et al. 1994). The final protein concentration was 2.2 μg/mL.
Citrate synthase (EC 2.3.3.1)
In the spectrophotometric measurement of CS activity, 5,5′-dithiobis-(2-nitrobenzoic) acid (DNTB) was observed for a color change. The reaction was started after the addition of oxaloacetate (0.5 mmol/L) and measured at 412 nm for 3 min (Srere 1969). The final protein concentration was 20 μg/mL.
Mitochondrial respiration
Mitochondrial respiration was measured as oxygen consumption rate at 37 °C using the O2k-Respirometer (Oroboros Instruments Corp, Innsbruck, Austria) equipped with Clark-type electrodes. Protocols of the experiments were designed on the basis of experience from previous studies (Fišar et al. 2016b; Hroudová and Fišar 2012; Pesta and Gnaiger 2012). The chambers of the oxygraph were filled by the respiration medium MiR05 (oxygen solubility factor = 0.92) to a final volume of 2 mL. The isolated mitochondria purified on a sucrose gradient were used; the final protein concentration was 0.05–0.20 mg/mL. The specific mitochondrial substrates 2 mmol/L malate, 5 mmol/L pyruvate, and 1 mmol/L ADP were utilized to assess the respiration rate of mitochondria linked to complex I; 0.5 μmol/L rotenone was used to inhibit complex I. For complex II-linked respiration, 1 mmol/L ADP, 0.5 μmol/L rotenone, and 10 mmol/L succinate were added to mitochondria; and 1.25 μg/mL antimycin A was used to inhibit complex III. Two simultaneous measurements were assessed—titration with drug and titration with solvent. The final drug concentrations were in the range 100–102 μmol/L.
Data analysis and statistics
Activities of mitochondrial enzymes were measured as the slope of time dependence of absorbance using LabPower Junior software (SECOMAM). Relative changes of enzyme activities induced by the drugs were determined assuming that the control sample activity was equal to 100%. High-resolution respirometry data were recorded and analyzed using DatLab software version 4.3 (Oroboros Instruments Corp, Innsbruck, Austria). Respiration rates were expressed as mass-specific oxygen flux (pmol O2 consumed per second per mg of protein in the sample).
The inhibition of respiration rate was analyzed using the four-parameter logistic regression with SigmaPlot software (Systat Software Inc., Richmond, CA, USA) to establish the half maximal inhibitory concentration (IC50), the Hill slope, and the residual activity at high drug concentration.
Thea Statistica data analysis software, version 13 (TIBCO Software Inc., Palo Alto, CA, USA) was used for statistical analyses. One-sample t tests for single means were used to determine whether enzyme activity in the sample with drug was significantly different from the control. The data are expressed as the mean ± standard deviation.
Results
The different AP structures seem to be responsible for their various therapeutic and side effects (Jafari et al. 2012). The structures of the compounds analyzed in the present study are shown in Table 1. Therapeutic plasma concentrations of the tested APs (Hiemke et al. 2018) are listed in Table 2.
Activity of CS and respiratory chain complexes
Activities of both CS and ETC complexes were evaluated after 30 min of incubation with 10 pharmacologically different antipsychotics at concentration 50 μmol/L. The most significantly affected was complex I (Fig. 1). Significant inhibitory effects were observed after the incubation with haloperidol, zotepine, aripiprazole, risperidone, clozapine, and quetiapine. Chlorpromazine completely blocked the reaction. Levomepromazine, ziprasidone, and olanzapine did not significantly inhibit complex I. Complex II + III activity was most strongly inhibited by zotepine. Significantly decreased complex II + III activity was found also for aripiprazole, quetiapine, and risperidone. Other tested APs did not significantly affect the complex II + III activity (Fig. 2). Activity of complex IV was significantly inhibited by zotepine, levomepromazine, and chlorpromazine. In contrast, little but significantly increased activity of complex IV was observed after incubation with quetiapine (Fig. 3). None of the tested drugs statistically significantly affected the CS activity (Fig. 4).
Effects of antipsychotics on respiratory chain complex I activity in brain mitochondria. The isolated mitochondria purified on a sucrose gradient were incubated with a drug at 30 °C for 30 min and enzyme kinetic was measured spectrophotometrically as described in the section “Materials and methods.” Relative activity is displayed (100% = activity of control sample without the drug). Values are means ± standard deviation of at least three independent measurements. Comparison between control and sample with drug was performed using the Wilcoxon matched pairs test (*p < 0.05, **p < 0.01, ***p < 0.001)
Effects of antipsychotics on respiratory chain complex II + III activity in brain mitochondria. The isolated mitochondria purified on a sucrose gradient were incubated with a drug at 30 °C for 30 min and enzyme kinetic was measured spectrophotometrically as described in the section “Materials and methods.” Relative activity is displayed (100% = activity of control sample without the drug). Values are means ± standard deviation of at least three independent measurements. Comparison between control and sample with drug was performed using the Wilcoxon matched pairs test (*p < 0.05, **p < 0.01)
Effects of antipsychotics on respiratory chain complex IV activity in brain mitochondria. The isolated mitochondria purified on a sucrose gradient were incubated with a drug at 30 °C for 30 min and enzyme kinetic was measured spectrophotometrically as described in the section “Materials and methods.” Relative activity is displayed (100% = activity of control sample without the drug). Values are means ± standard deviation of at least three independent measurements. Comparison between control and sample with drug was performed using the Wilcoxon matched pairs test (*p < 0.05, **p < 0.01)
Effects of antipsychotics on citrate synthase (CS) activity in brain mitochondria. The isolated mitochondria purified on a sucrose gradient were incubated with a drug at 30 °C for 30 min and enzyme kinetic was measured spectrophotometrically as described in the section “Materials and methods.” Relative activity is displayed (100% = activity of control sample without the drug). Values are means ± standard deviation of at least three independent measurements. Comparison between control and sample with drug was performed using the Wilcoxon matched pairs test (*p < 0.05, **p < 0.01)
Mitochondrial respiration
Inhibitory effects of tested antipsychotics on mitochondrial respiration rate are shown in Figs. 5 and 6, except for chlorpromazine, whose inhibitory curves were published in our previous work (Hroudová and Fišar 2012). The parameters characterizing the strength and type of inhibition are summarized in Tables 2 and 3.
Drug effect on complex I-linked mitochondrial respiration in isolated pig brain mitochondria. Dose-response curves are displayed as plots of the relative respiratory rate against the drug concentration. The samples were continuously stirred and incubated at 37 °C in two chambers: drug titration in one chamber and buffer titration in the second chamber were performed at 2–3 min intervals. Following subtraction of residual oxygen consumption from all respiratory rates, the time change in respiratory rate during titration was corrected and relative drug-induced changes in respiratory rate were determined, presuming that the relative respiratory rate equals to one at zero drug concentration. Points represent means from at least three independent measurements. Lines represent the best-fitted curves using the four-parameter logistic function. Half maximal inhibitory concentration (IC50), residual activity, and Hill slope were calculated (Table 2)
Drug effect on complex II-linked mitochondrial respiration in isolated pig brain mitochondria. Dose-response curves are displayed as plots of the relative respiratory rate against the drug concentration. The samples were continuously stirred and incubated at 37 °C in two chambers: drug titration in one chamber and buffer titration in the second chamber were performed at 2–3 min intervals. Following subtraction of residual oxygen consumption from all respiratory rates, the time change in respiratory rate during titration was corrected and relative drug-induced changes in respiratory rate were determined, presuming that the relative respiratory rate equals to one at zero drug concentration. Points represent means from at least three independent measurements. Lines represent the best-fitted curves using the four-parameter logistic function. Half maximal inhibitory concentration (IC50), residual activity, and Hill slope were calculated (Table 3)
Complex I-linked respiration was strongly inhibited by aripiprazole, zotepine, and haloperidol. These drugs were full inhibitors of mitochondrial respiration with IC50 13.1, 39.5, and 64.9 μmol/L, respectively. Risperidone and quetiapine were also found as full inhibitors, and the inhibitory effects were observed at higher concentrations (IC50 263 and 424 μmol/L, respectively). Other examined antipsychotics (ziprasidone, clozapine, and levomepromazine) were revealed as partial inhibitors of complex I-linked respiration with IC50 in the range 10−4 mol/L (Table 2). The AP/rotenone ratio represents the ratio of IC50 values determined for AP and for rotenone (Fišar et al. 2017) and approximately expresses the relative inhibitory capacity of the AP relative to rotenone; it should be noted that this ratio does not capture partial inhibition.
Full inhibition of complex II-linked respiration was observed after exposition by zotepine, quetiapine, and clozapine with IC50 107, 491, and 650 μmol/L, respectively. Other antipsychotics were partial inhibitors of complex II-linked respiration (Table 3). The AP/antimycin ratio represents the ratio of IC50 values determined for AP and for antimycin A and approximately expresses the relative inhibitory capacity of the AP relative to antimycin A.
Correlations
Correlation between relative complex I activity and complex I-linked respiration in isolated pig brain mitochondria after the addition of atypical AP at a final concentration of 50 μmol/L to the sample (Fig. 7) was found rather weak (r = 0.753, p = 0.051, N = 7).
Correlation between relative complex I activity and complex I-linked respiration in isolated pig brain mitochondria after the addition of antipsychotics at a final concentration of 50 μmol/L to the sample. The dotted line shows the correlation for atypical antipsychotics (r = 0.753, p = 0.051, N = 7)
No significant correlation was found between the therapeutic concentration of AP calculated as mean of lower and upper limit of the therapeutic reference range (Table 2) and the AP-induced inhibition of individual respiratory complexes (Figs. 1, 2, and 3), including the complex I activity at 50 μmol/L AP concentration (r = 0.280, p = 0.43, N = 10). Similarly, there was no statistically significant correlation between therapeutic concentration and IC50 for both the complex I-linked respiration (r = 0.369, p = 0.29, N = 10) and complex II-linked respiration (r = 0.457, p = 0.18, N = 10).
Discussion
The in vitro effects of pharmacologically different APs on mitochondrial energy metabolism were examined in isolated pig brain mitochondria. Activities of both CS and ETC complexes and mitochondrial respiration rate were studied with the aim of supporting the hypothesis that AP-induced mitochondrial dysfunction can result in impaired cell energy metabolism and likely in adverse effects of APs.
Antipsychotics and complex I activity
Seven of the 10 APs tested showed a significant inhibitory effect on the complex I. Chlorpromazine and haloperidol, conventional APs, have been found to be the strongest inhibitors of complex I (Fig. 1), which confirmed earlier findings that conventional APs are more potent inhibitors of complex I than atypical APs (Balijepalli et al. 1999; Burkhardt et al. 1993; Prince et al. 1997). However, this is not generally the case since levomepromazine showed less complex I inhibitory effects than some atypical APs. Our results closely correspond to an in vitro study in which effects of APs were examined in rat liver mitochondria (Modica-Napolitano et al. 2003). In that study, (1) the typical APs (chlorpromazine, haloperidol, and thioridazine) inhibited complex I enzyme activity more strongly than the atypical APs (risperidone, quetiapine, clozapine, and olanzapine) with the least inhibitory effect observed for olanzapine and clozapine, and (2) complex I-linked mitochondrial respiration was inhibited by chlorpromazine, haloperidol, risperidone, and quetiapine but not by clozapine, olanzapine, or thioridazine.
Complex I plays a decisive role in the control of OXPHOS and its altered activity could lead to impairment in cell energy metabolism and subsequently to changes in neuronal activity (Pathak and Davey 2008). Based on the observation that conventional antipsychotics with a higher risk of causing undesirable side effects are stronger inhibitors of the complex I than atypical antipsychotics, it has been suggested that the degree of complex I inhibition is likened, at least partially, to potency for the extrapyramidal effect, tardive dyskinesia included (Burkhardt et al. 1993; Maurer and Möller 1997; Maurer and Volz 2001; Modica-Napolitano et al. 2003). Our observation that levomepromazine, which causes side effects such as akathisia, does not significantly inhibit the complex I could be explained by the fact that some of the adverse effects of APs can be attributed to mitochondrial dysfunction and other direct changes in neurotransmitter systems.
Spectrophotometric measurement of a complex I activity refers to a high-resolution respirometry measurement of mitochondrial respiration. All significant inhibitors of complex I were full (haloperidol, risperidone, zotepine, aripiprazole, and quetiapine), or almost full (clozapine), inhibitors of complex I-linked respiration rate, confirming the causal association of inhibition of complex I activity and inhibition of the overall mitochondrial respiratory rate. However, correlation between complex I activity and complex I-linked respiration at high AP concentration (Fig. 7) indicates that association between complex I activity and complex I-linked respiration is not trivial.
Our results support another study, where chlorpromazine, haloperidol, risperidone, and clozapine affected ATP production (depending on the AP concentration), led to a significant increase in lactate production, led to a significant reduction in mitochondrial complex I activity, and lastly decreased oxygen consumption rates and mitochondrial membrane potential in rat ovarian theca cells (Elmorsy et al. 2017).
Antipsychotics and complex II + III activity
Significant inhibitors of complex II + III, zotepine and quetiapine, were full inhibitors of complex II-linked respiration rate. Olanzapine, which showed no inhibitory effects on the activities of the complexes I, II + III, and IV, also did not substantially inhibit the mitochondrial respiratory rate. Risperidone and aripiprazole inhibited complex II + III, however, were only partial inhibitors of complex II-linked respiration, indicating that the association of complex II + III inhibition with inhibition of mitochondrial respiration is less tight than that of complex I inhibition.
Antipsychotics and complex IV activity
Chlorpromazine, levomepromazine, and zotepine significantly inhibited the activity of complex IV. Other antipsychotic drugs tested showed no inhibitory effects on complex IV; quetiapine showed a stimulatory effect (Fig. 3). Our data are consistent with another study in which haloperidol, olanzapine, clozapine, and aripiprazole did not significantly affect the activity of complex IV (Streck et al. 2007) or haloperidol and olanzapine did not significantly affect changes in COX subunit expression in rat brains after chronic administration (Rice et al. 2014). Complex IV represented by cytochrome c oxidase is often considered as an endogenous metabolic marker of neuronal activity (Wong-Riley 1989). Chlorpromazine selectively and significantly inhibited the growth and proliferation of glioma cells by switching the expression of the COX4-1 regulatory subunit. Thus, chlorpromazine could be used in the future for treating chemoresistant glioma on the basis of antiproliferative activity relating to the inhibition of complex IV activity (Oliva et al. 2017).
We confirmed that chlorpromazine acts as an eminent inhibitor of mitochondrial complex I and complex IV (Figs. 1 and 3). However, both complex I- and complex II-linked mitochondrial respiration is only partially inhibited by chlorpromazine and at higher concentrations (Tables 2 and 3) (Hroudová and Fišar 2012). This can explain why the mitochondrial toxicity of chlorpromazine is not as high as one would expect from its effects on individual respiratory chain complexes. In contrast, zotepine exhibited inhibitory effects on individual ECT complexes and at the same time full inhibition of complex I- and complex II-linked respiration. There appears to be no simple correlation between the effects of drugs on individual ETC complexes and complex mitochondrial functions such as oxygen consumption rate or ATP production. We assume that this is due to the different drug-mitochondria interaction and different drug-induced compensatory or neuroprotective mechanisms (Robertson et al. 2019; Scatena et al. 2007), which can reduce the mitochondrial toxicity of drugs.
Antipsychotics and citrate synthase activity
CS is an enzyme localized in the mitochondrial matrix; it holds a regulatory function within cell energy metabolism. We did not find any significant AP-induced changes in CS activity (Fig. 4). In another study, aripiprazole enhanced the activity of CS, while clozapine and haloperidol did not affect CS activity in PC12 cells (Ota et al. 2012). Acute treatment with olanzapine inhibited CS activity in the cerebellum and prefrontal cortex, while the acute administration of olanzapine increased CS activity in the prefrontal cortex, hippocampus, and striatum in rats (Agostinho et al. 2011). Our in vitro study shows that the effects of APs on CS activity are small and are therefore probably not involved in the therapeutic or side effects of APs.
Possible mitochondria-related side effects of antipsychotics
Our observation that there is no significant correlation between the therapeutic concentration of APs and AP-induced inhibition of mitochondrial enzymes supports the hypothesis that the direct mitochondrial effects of APs are associated with their adverse rather than therapeutic effects.
Based on our data and already established facts about prolonged QTc intervals after treatment with APs (Leucht et al. 2013; Taylor 2003b), we noticed a relation between inhibition of complex I and prolongation of the QTc interval. AP-induced inhibition of complex I in our study (Fig. 1) may be associated with QTc interval prolongation (e.g., chlorpromazine, clozapine, haloperidol, risperidone, quetiapine, and ziprasidone) (Glassman and Bigger 2001; Haddad and Anderson 2002; Leucht et al. 2013; Spellmann et al. 2018; Taylor 2003a; Taylor 2003b; Vieweg 2003). In spite of this, ziprasidone, which causes the significant degree of drug-induced QTc interval prolongation, did not inhibit complex I activity, and aripiprazole, which effect did not differ from QTc prolongation with placebo (Leucht et al. 2013), inhibited complex I activity. Further basic research and consequent clinical trials are required to confirm that there is QTc interval prolongation as an adverse effect stemming from alterations in ETC enzyme activity.
Treatment with second-generation APs is accompanied with an increase in cardiovascular risk through insulin resistance, hypercholesterolemia, and accelerated weight gain. Altogether, these adverse effects might be called the metabolic syndrome. The metabolic side effects of APs could be connected with alterations in mitochondrial homeostasis, which leads to an imbalance in the mitochondrial fusion/fission ratio and to an inefficient mitochondrial phenotype of muscle cells (Del Campo et al. 2018). Olanzapine induced a downregulation of genes involved in the mitochondrial enzymes of the ETC, as well as decreased enzyme activity, ATP synthesis, and oxygen consumption in blood cells of patients at elevated risk for metabolic syndrome (Scaini et al. 2018). The other effect of olanzapine was assessed on freshly isolated rat hepatocytes, and the results of the study showed cytotoxicity of olanzapine leads to hepatotoxicity, which are mediated by mitochondrial potential collapse and oxidative stress (Eftekhari et al. 2016). Autophagy of mitochondria in the SH-SY5Y neuronal cell line in humans was observed after olanzapine exposition. Thus, olanzapine induced mitochondrial damage related to ROS production and extensive mitochondrial depolarization, provoking the autophagic clearance of dysfunctional mitochondria (Vucicevic et al. 2014).
Experimental approach
The suitability of using (1) pig brain mitochondria as a biological model, (2) mitochondrial isolation method and experimental protocol of mitochondrial respiration measurement, and (3) concentration of APs used in in vitro experiments may be discussed. (1) Pig is a relatively uncommon species for pharmacological studies, but pig brain mitochondria are successfully used in measuring the effect of drugs on mitochondrial respiration (Fišar and Hroudová 2016; Fišar et al. 2016b; Hroudová and Fišar 2012). It seems that pig mitochondria are more similar to human mitochondria than rodent mitochondria (Hroudova and Fisar 2010). (2) Different laboratories differ in both the method of preparing the mitochondria (the origin of the animal, the tissue, and the purification) and the method of measuring the oxygen consumption. To compare the mitochondrial effects of drugs, the effects of a known inhibitor (e.g., rotenone for complex I-linked respiration and antimycin A for complex II-linked respiration) should always be measured using the same experimental protocols (Fišar et al. 2017). Therefore, we also calculated the IC50 ratios AP/rotenone (Table 2) or AP/antimycin A (Table 3). (3) The use of examined APs at high (50 μmol/L) concentration for in vitro experiments could be relevant in a case of intraneuronal accumulation of some APs in the brain, e.g., zotepine reaches brain levels of the unchanged drug 20 to 30 times higher than serum levels (Noda et al. 1979).
Possibilities of structure-activity relationship studies
Structures of mitochondrial complexes and APs can contribute significantly to understanding the mechanisms associated with the regulation of cellular energy by drugs such as APs. Quantitative structure-activity relationship (QSAR) studies are widely used to develop new drugs and their toxicological analysis (Jafari et al. 2012). However, the QSAR model has not been used to study the interactions of APs with mitochondria and respiratory chain complexes, respectively.
Complex I is the largest enzyme of the mitochondrial respiratory chain consisting of 45 subunits arranged in hydrophilic and membrane domains. It has a key role in ATP production, is a major source of ROS, and its dysfunctions have been associated with aging and neurodegenerative disorders (Schapira 1998). Crystal structure of bacterial complex I has been determined (Efremov and Sazanov 2011); however, the atomic structure of mammalian complex I with sufficient resolution has only recently been achieved using single particle cryo-electron microscopy (Fiedorczuk et al. 2016; Fiedorczuk and Sazanov 2018). The structures of complexes II (Iverson 2013; Sun et al. 2005), III (Hunte et al. 2000; Hunte et al. 2008; Iwata et al. 1998), and IV (Tsukihara et al. 1995) were determined earlier by X-ray crystallography. The complexes I, III, and IV may form supercomplexes (respirasomes) whose function is still under investigation (Letts and Sazanov 2017; Sousa et al. 2018).
It is known that chlorpromazine acts as a potent inhibitor of mitochondrial complex I. In our study, complex I activity was strongly inhibited by haloperidol, which is structurally different from chlorpromazine. In contrast, complex I activity was not inhibited by levomepromazine, which is similar to chlorpromazine. The different effects of these typical APs could be related to chlorine on chlorpromazine. Similarly, no similar inhibitory effects on complex I were observed in structurally similar atypical APs, except for similar effects of clozapine and olanzapine. There was no association between the structure of APs and their effect on the activity of complex II + III. From the inhibitory effects of the tested APs on the activity of complex IV, only the possible role of chlorine and tricyclic structure in the inhibition by chlorpromazine or zotepine can be concluded. The observation that chlorpromazine is a full complex I inhibitor, but only a partial inhibitor of complex I-linked respiration in intact mitochondria, suggests the possible role of the lipid part of the inner mitochondrial membrane in AP effects.
We did not observe the effect of certain molecular structures of the tested APs on their mitochondrial effects, assessed by their effect on the overall respiratory rate. In addition, some APs fully inhibit the measured mitochondrial function, some partially, and the biphasic course of complex II-linked respiration was even observed for clozapine effect (Figs. 5 and 6). We assume that this is due to the complexity of the respiratory chain complex structures that consist of many subunits; it is known that dysfunction of some subunits is lethal for the function of the entire complex. Partial or full inhibition of mitochondrial respiration appears to be due to the cumulative effect of APs on the subunits of the individual complexes and possibly on the lipid part of the inner mitochondrial membrane. It can be hypothesized that while the therapeutic effects of APs are associated with their specific binding to neurotransmitter receptors and transporters, the mitochondrial side effects of APs are associated with AP-induced multiple allosteric regulation of various ETC components.
The structure-activity relationship of APs should be studied in terms of their effects on both individual mitochondrial complexes and supercomplexes. Obviously, recognizing the structural parts of the AP molecules responsible for their effect on mitochondrial functions would facilitate the development of new drugs. Although the possibilities and results of molecular modeling of AP interactions with mitochondrial complexes and supercomplexes are currently very uncertain and difficult to interpret, structure-activity relationship (SAR) studies on antipsychotics are essential to identify the potential modulatory sites not only on neurotransmitter receptors and transporters but also on mitochondrial proteins. We believe that in subsequent studies, attention should be paid to both SAR and the search for specific binding sites for APs in respiratory complexes using biochemical and biophysical methods.
Final notes
Schizophrenia frequently exhibits a neuroprogression and new APs should inhibit pathways of neurodegeneration, including mitochondrial dysfunction (Robertson et al. 2019). It is therefore necessary to study the mitochondrial effects of both existing and novel drugs. A comparison of the effects of antipsychotics on individual mitochondrial enzymes or enzyme complexes and on complex mitochondrial functions, such as oxygen consumption rate, has shown that complex mitochondrial functions may not fully reflect the disruption of individual components of ETC. It can be hypothesized that this is due both to the reversibility of AP-induced inhibition of ETC complexes and to the existence of feedbacks in the OXPHOS system leading to adaptive mitochondrial changes, similar to those described in vivo in platelets of patients with neurodegenerative disease (Fišar et al. 2016a; Fišar et al. 2019). Measurement of respiratory rate in mitochondria is much closer to the physiological situation and describes more suitably mitochondrial toxicity of drugs than measurement of inhibitory effects on individual ETC complexes. However, measuring the effects of drugs on individual complexes can help, at least in part, to clarify the drug-induced mitochondrial dysfunction mechanism.
There is a discussion as to whether atypical APs are better than typical and how correct and appropriate the division of APs is to typical and atypical. Independent Clinical Antipsychotic Trials on Intervention Effectiveness (CATIE) study (Lieberman et al. 2005) and meta-analyses (Leucht et al. 2013; Leucht et al. 2009) have shown that both first- and second-generation APs are not homogeneous classes and differ in both overall efficacy and efficacy for negative symptoms, in extrapyramidal side effect induction, weight gain induction, and sedation. In terms of inducing extrapyramidal side effects, all atypical APs are better than haloperidol, but this is not generally the case with low-potency typical APs. In addition, the use of some atypical APs is associated with greater weight gain or sedation than typical APs. The heterogeneous effects of typical and atypical APs on the activity of individual respiratory chain complexes as well as on the overall respiratory rate of mitochondria described in this paper confirm that even mitochondrial effects of APs cannot be used to classify/define typical and atypical APs. Thus, the view that the choice of AP should be individual was supported.
Conclusions
From the obtained data, APs affected both ETC mitochondrial enzyme activities and mitochondrial respiration. The typical APs did not show greater potency in alteration of mitochondrial functions, except for complex I inhibition by chlorpromazine and haloperidol, compared to the atypical APs. AP-induced mitochondrial dysfunctions can be expected to be associated with adverse effects of APs related to neurodegenerative processes induced by bioenergetic disruption. The diverse but largely inhibitory effects of both typical and atypical APs on complex I- or II-linked mitochondrial respiration confirm that the direct effects of APs on cell energy are associated with adverse effects of APs rather than their therapeutic effects. However, it can be considered that the possible neuroprotective effects of some APs, as well as the adaptive processes induced by the inhibition of ETC complexes, may result in greater or lesser compensation of the undesirable mitochondrial toxicity of APs.
The precise molecular mechanism by which various APs affect mitochondrial enzymes must be still determined. In further clinical research, it must specified whether AP-induced mitochondrial dysfunction is causally related to clinically manifested effects, such as extrapyramidal syndromes, QTc interval prolongation, or metabolic syndrome. Due to the complex processes associated with mitochondrial activity, it is necessary to measure not only the effect of test substances on individual ETC complexes but also on the total respiration rate of the mitochondria. The experimental methods used in the present study can be applied to mitochondrial toxicity testing of newly developed APs.
Abbreviations
- AP:
-
Antipsychotic
- COX:
-
Complex IV, cytochrome c oxidase
- CS:
-
Citrate synthase
- ETC:
-
Electron transport chain
- MARTA:
-
Multi-acting receptor targeted antipsychotics
- OXPHOS:
-
Oxidative phosphorylation
- ROS:
-
Reactive oxygen species
References
Agostinho FR, Réus GZ, Stringari RB, Ribeiro KF, Ferraro AK, Benedet J, Rochi N, Scaini G, Streck EL, Quevedo J (2011) Treatment with olanzapine, fluoxetine and olanzapine/fluoxetine alters citrate synthase activity in rat brain. Neurosci Lett 487:278–281. https://doi.org/10.1016/j.neulet.2010.10.037
Balijepalli S, Boyd MR, Ravindranath V (1999) Inhibition of mitochondrial complex I by haloperidol: the role of thiol oxidation. Neuropharmacology 38:567–577
Bolonna AA, Kerwin RW (2005) Partial agonism and schizophrenia. Br J Psychiatry 186:7–10. https://doi.org/10.1192/bjp.186.1.7
Burkhardt C, Kelly JP, Lim YH, Filley CM, Parker WD (1993) Neuroleptic medications inhibit complex I of the electron transport chain. Ann Neurol 33:512–517. https://doi.org/10.1002/ana.410330516
Casademont J, Garrabou G, Miró O, López S, Pons A, Bernardo M, Cardellach F (2007) Neuroleptic treatment effect on mitochondrial electron transport chain: peripheral blood mononuclear cells analysis in psychotic patients. J Clin Psychopharmacol 27:284–288. https://doi.org/10.1097/JCP.0b013e318054753e
Church MK, Young KD (1983) The characteristics of inhibition of histamine release from human lung fragments by sodium cromoglycate, salbutamol and chlorpromazine. Br J Pharmacol 78:671–679
Del Campo A, Bustos C, Mascayano C, Acuña-Castillo C, Troncoso R, Rojo LE (2018) Metabolic syndrome and antipsychotics: the role of mitochondrial fission/fusion imbalance. Front Endocrinol (Lausanne) 9:144. https://doi.org/10.3389/fendo.2018.00144
Efremov RG, Sazanov LA (2011) Structure of the membrane domain of respiratory complex I. Nature 476:414–420. https://doi.org/10.1038/nature10330
Eftekhari A, Azarmi Y, Parvizpur A, Eghbal MA (2016) Involvement of oxidative stress and mitochondrial/lysosomal cross-talk in olanzapine cytotoxicity in freshly isolated rat hepatocytes. Xenobiotica 46:369–378. https://doi.org/10.3109/00498254.2015.1078522
Elkashef AM, Wyatt RJ (1999) Tardive dyskinesia: possible involvement of free radicals and treatment with vitamin E. Schizophr Bull 25:731–740
Elmorsy E, Al-Ghafari A, Aggour AM, Mosad SM, Khan R, Amer S (2017) Effect of antipsychotics on mitochondrial bioenergetics of rat ovarian theca cells. Toxicol Lett 272:94–100. https://doi.org/10.1016/j.toxlet.2017.03.018
Fiedorczuk K, Sazanov LA (2018) Mammalian mitochondrial complex I structure and disease-causing mutations. Trends Cell Biol 28:835–867. https://doi.org/10.1016/j.tcb.2018.06.006
Fiedorczuk K, Letts JA, Degliesposti G, Kaszuba K, Skehel M, Sazanov LA (2016) Atomic structure of the entire mammalian mitochondrial complex I. Nature 538:406–410. https://doi.org/10.1038/nature19794
Fišar Z, Hroudová J (2016) Pig brain mitochondria as a biological model for study of mitochondrial respiration. Folia Biol (Praha) 62:15–25
Fišar Z, Hroudová J, Raboch J (2010) Inhibition of monoamine oxidase activity by antidepressants and mood stabilizers. Neuro Endocrinol Lett 31:645–656
Fišar Z et al (2016a) Mitochondrial respiration in the platelets of patients with Alzheimer's disease. Curr Alzheimer Res 13:930–941
Fišar Z, Hroudová J, Singh N, Kopřivová A, Macečková D (2016b) Effect of simvastatin, coenzyme Q10, resveratrol, acetylcysteine and acetylcarnitine on mitochondrial respiration. Folia Biol (Praha) 62:53–66
Fišar Z, Hroudová J, Singh N, Macečková D, Kopřivová A (2017) Protocols for high-resolution respirometry experiments to test the activity of electron transfer system of pig brain mitochondria. Indian J Biochem Biophys 54:258–272
Fišar Z, Jirák R, Zvěřová M, Setnička V, Habartová L, Hroudová J, Vaníčková Z, Raboch J (2019) Plasma amyloid beta levels and platelet mitochondrial respiration in patients with Alzheimer's disease. Clin Biochem. https://doi.org/10.1016/j.clinbiochem.2019.04.003
Folbergrová J, Ješina P, Haugvicová R, Lisý V, Houštěk J (2010) Sustained deficiency of mitochondrial complex I activity during long periods of survival after seizures induced in immature rats by homocysteic acid. Neurochem Int 56:394–403. https://doi.org/10.1016/j.neuint.2009.11.011
Glassman AH, Bigger JT (2001) Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry 158:1774–1782. https://doi.org/10.1176/appi.ajp.158.11.1774
Goff DC, Tsai G, Beal MF, Coyle JT (1995) Tardive dyskinesia and substrates of energy metabolism in CSF. Am J Psychiatry 152:1730–1736. https://doi.org/10.1176/ajp.152.12.1730
Haddad PM, Anderson IM (2002) Antipsychotic-related QTc prolongation, torsade de pointes and sudden death. Drugs 62:1649–1671
Hiemke C, Bergemann N, Clement H, Conca A, Deckert J, Domschke K, Eckermann G, Egberts K, Gerlach M, Greiner C, Gründer G, Haen E, Havemann-Reinecke U, Hefner G, Helmer R, Janssen G, Jaquenoud E, Laux G, Messer T, Mössner R, Müller M, Paulzen M, Pfuhlmann B, Riederer P, Saria A, Schoppek B, Schoretsanitis G, Schwarz M, Gracia M, Stegmann B, Steimer W, Stingl J, Uhr M, Ulrich S, Unterecker S, Waschgler R, Zernig G, Zurek G, Baumann P (2018) Consensus guidelines for therapeutic drug monitoring in Neuropsychopharmacology: update 2017. Pharmacopsychiatry 51:9–62. https://doi.org/10.1055/s-0043-116492
Hroudova J, Fisar Z (2010) Activities of respiratory chain complexes and citrate synthase influenced by pharmacologically different antidepressants and mood stabilizers. Neuro Endocrinol Lett 31:336–342
Hroudová J, Fišar Z (2012) In vitro inhibition of mitochondrial respiratory rate by antidepressants. Toxicol Lett 213:345–352. https://doi.org/10.1016/j.toxlet.2012.07.017
Hunte C, Koepke J, Lange C, Rossmanith T, Michel H (2000) Structure at 2.3 Å resolution of the cytochrome bc(1) complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure 8:669–684
Hunte C, Solmaz S, Palsdottir H, Wenz T (2008) A structural perspective on mechanism and function of the cytochrome bc (1) complex. Results Probl Cell Differ 45:253–278. https://doi.org/10.1007/400_2007_042
Iverson TM (2013) Catalytic mechanisms of complex II enzymes: a structural perspective. Biochim Biophys Acta 1827:648–657. https://doi.org/10.1016/j.bbabio.2012.09.008
Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK (1998) Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science 281:64–71
Jafari S, Fernandez-Enright F, Huang XF (2012) Structural contributions of antipsychotic drugs to their therapeutic profiles and metabolic side effects. J Neurochem 120:371–384. https://doi.org/10.1111/j.1471-4159.2011.07590.x
Kapur S, Seeman P (2001) Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: a new hypothesis. Am J Psychiatry 158:360–369. https://doi.org/10.1176/appi.ajp.158.3.360
Keegan D (1994) Risperidone: neurochemical, pharmacologic and clinical properties of a new antipsychotic drug. Can J Psychiatr 39:S46–S52
Letts JA, Sazanov LA (2017) Clarifying the supercomplex: the higher-order organization of the mitochondrial electron transport chain. Nat Struct Mol Biol 24:800–808. https://doi.org/10.1038/nsmb.3460
Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM (2009) Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373:31–41. https://doi.org/10.1016/S0140-6736(08)61764-X
Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962. https://doi.org/10.1016/S0140-6736(13)60733-3
Lieberman JA, Stroup TS, McEvoy J, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223. https://doi.org/10.1056/NEJMoa051688
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Masand PS, Culpepper L, Henderson D, Lee S, Littrell K, Newcomer JW, Rasgon N (2005) Metabolic and endocrine disturbances in psychiatric disorders: a multidisciplinary approach to appropriate atypical antipsychotic utilization. CNS spectrums 10(suppl14):11–15
Maurer I, Möller HJ (1997) Inhibition of complex I by neuroleptics in normal human brain cortex parallels the extrapyramidal toxicity of neuroleptics. Mol Cell Biochem 174:255–259
Maurer I, Volz HP (2001) Cell-mediated side effects of psychopharmacological treatment. Arzneimittelforschung 51:785–792. https://doi.org/10.1055/s-0031-1300116
Maurer I, Zierz S, Moller H (2001) Evidence for a mitochondrial oxidative phosphorylation defect in brains from patients with schizophrenia. Schizophr Res 48:125–136
Modica-Napolitano JS, Lagace CJ, Brennan WA, Aprille JR (2003) Differential effects of typical and atypical neuroleptics on mitochondrial function in vitro. Arch Pharm Res 26:951–959
Nasrallah HA (2008) Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry 13:27–35. https://doi.org/10.1038/sj.mp.4002066
Noda K, Suzuki A, Okui M, Noguchi H, Nishiura M, Nishiura N (1979) Pharmacokinetics and metabolism of 2-chloro-11-(2-dimethylaminoethoxy)-dibenzo[b,f]thiepine (zotepine) in rat, mouse, dog and man Arzneimittelforschung 29:1595–1600
Oliva CR, Zhang W, Langford C, Suto MJ, Griguer CE (2017) Repositioning chlorpromazine for treating chemoresistant glioma through the inhibition of cytochrome c oxidase bearing the COX4-1 regulatory subunit. Oncotarget 8:37568–37583. https://doi.org/10.18632/oncotarget.17247
Ota A, Nakashima A, Kaneko YS, Mori K, Nagasaki H, Takayanagi T, Itoh M, Kondo K, Nagatsu T, Ota M (2012) Effects of aripiprazole and clozapine on the treatment of glycolytic carbon in PC12 cells. J Neural Transm (Vienna) 119:1327–1342. https://doi.org/10.1007/s00702-012-0782-2
Pathak RU, Davey GP (2008) Complex I and energy thresholds in the brain. Biochim Biophys Acta 1777:777–782. https://doi.org/10.1016/j.bbabio.2008.05.443
Pesta D, Gnaiger E (2012) High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810:25–58. https://doi.org/10.1007/978-1-61779-382-0_3
Pinna G, Broedel O, Eravci M, Stoltenburg-Didinger G, Plueckhan H, Fuxius S, Meinhold H, Baumgartner A (2003) Thyroid hormones in the rat amygdala as common targets for antidepressant drugs, mood stabilizers, and sleep deprivation. Biol Psychiatry 54:1049–1059
Prince JA, Yassin MS, Oreland L (1997) Neuroleptic-induced mitochondrial enzyme alterations in the rat brain. J Pharmacol Exp Ther 280:261–267
Rice MW, Smith KL, Roberts RC, Perez-Costas E, Melendez-Ferro M (2014) Assessment of cytochrome C oxidase dysfunction in the substantia nigra/ventral tegmental area in schizophrenia. PLoS One 9:e100054. https://doi.org/10.1371/journal.pone.0100054
Roberts RC (2017) Postmortem studies on mitochondria in schizophrenia. Schizophr Res 187:17–25. https://doi.org/10.1016/j.schres.2017.01.056
Robertson OD, Coronado NG, Sethi R, Berk M, Dodd S (2019) Putative neuroprotective pharmacotherapies to target the staged progression of mental illness. Early Interv Psychiatry. https://doi.org/10.1111/eip.12775
Rustin P, Chretien D, Bourgeron T, Gerard B, Rotig A, Saudubray JM, Munnich A (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta: Int J Clin Chem 228:35–51
Sangani A, Saadabadi A (2019) Neuroleptic medications. In: StatPearls. Treasure Island (FL),
Scaini G, Quevedo J, Velligan D, Roberts DL, Raventos H, Walss-Bass C (2018) Second generation antipsychotic-induced mitochondrial alterations: implications for increased risk of metabolic syndrome in patients with schizophrenia. Eur Neuropsychopharmacol 28:369–380. https://doi.org/10.1016/j.euroneuro.2018.01.004
Scatena R, Bottoni P, Botta G, Martorana GE, Giardina B (2007) The role of mitochondria in pharmacotoxicology: a reevaluation of an old, newly emerging topic. Am J Phys Cell Phys 293:C12–C21. https://doi.org/10.1152/ajpcell.00314.2006
Schapira AH (1998) Human complex I defects in neurodegenerative diseases. Biochim Biophys Acta 1364:261–270
Seeman P (2002) Atypical antipsychotics: mechanism of action. Can J Psychiatr 47:27–38
Sousa JS, D'Imprima E, Vonck J (2018) Mitochondrial respiratory chain complexes. Subcell Biochem 87:167–227. https://doi.org/10.1007/978-981-10-7757-9_7
Spellmann I, Reinhard MA, Veverka D, Zill P, Obermeier M, Dehning S, Schennach R, Müller N, Möller HJ, Riedel M, Musil R (2018) QTc prolongation in short-term treatment of schizophrenia patients: effects of different antipsychotics and genetic factors. Eur Arch Psychiatry Clin Neurosci 268:383–390. https://doi.org/10.1007/s00406-018-0880-8
Srere (1969) Citrate synthase: [EC 4.1.3.7 citrate oxaloacetate-lyase (CoA acetylating). Methods Enzymol 13:3–11
Streck EL, Rezin GT, Barbosa LM, Assis LC, Grandi E, Quevedo J (2007) Effect of antipsychotics on succinate dehydrogenase and cytochrome oxidase activities in rat brain. Naunyn Schmiedeberg's Arch Pharmacol 376:127–133. https://doi.org/10.1007/s00210-007-0178-2
Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D, Bartlam M, Rao Z (2005) Crystal structure of mitochondrial respiratory membrane protein complex II. Cell 121:1043–1057. https://doi.org/10.1016/j.cell.2005.05.025
Taylor D (2003a) Ziprasidone in the management of schizophrenia: the QT interval issue in context. CNS Drugs 17:423–430
Taylor DM (2003b) Antipsychotics and QT prolongation. Acta Psychiatr Scand 107:85–95
Trabucchi M, Cheney D, Racagni G, Costa E (1974) Involvement of brain cholinergic mechanisms in the action of chlorpromazine. Nature 249:664–666
Trounce IA, Kim YL, Jun AS, Wallace DC (1996) Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264:484–509
Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S (1995) Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 Å. Science 269:1069–1074
Vieweg WV (2003) New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry 5:205–215
Vucicevic L, Misirkic-Marjanovic M, Paunovic V, Kravic-Stevovic T, Martinovic T, Ciric D, Maric N, Petricevic S, Harhaji-Trajkovic L, Bumbasirevic V, Trajkovic V (2014) Autophagy inhibition uncovers the neurotoxic action of the antipsychotic drug olanzapine. Autophagy 10:2362–2378. https://doi.org/10.4161/15548627.2014.984270
Wirshing WC (2001) Movement disorders associated with neuroleptic treatment. J Clin Psychiatry 62(Suppl 21):15–18
Wong-Riley MT (1989) Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci 12:94–101
Acknowledgments
This work was supported by the Czech Science Foundation (grant number 17-07585Y) and by Charles University Grant Agency (grant number 34119), Czech Republic. The authors thank Zdeněk Hanuš for his assistance.
Author information
Authors and Affiliations
Contributions
JH and ZF conceived and designed research. TC, YB, ML, and JH conducted experiments. TC, JH, and ZF analyzed data and wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cikánková, T., Fišar, Z., Bakhouche, Y. et al. In vitro effects of antipsychotics on mitochondrial respiration. Naunyn-Schmiedeberg's Arch Pharmacol 392, 1209–1223 (2019). https://doi.org/10.1007/s00210-019-01665-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01665-8