Abstract
The aim of this study was to examine the effect of tocilizumab, an interleukin-6 (IL-6) inhibitor on streptozotocin-induced diabetic nephropathy. Male Sprague-Dawley rats (n = 36) were distributed into six groups and treated for 4 weeks. Groups 1, 3, 5 received either saline, tocilizumab (2 mg/kg), or tocilizumab (8 mg/kg) injection intraperitoneally (i.p.), every 2 weeks, respectively. Groups 2, 4, 6 were rendered diabetic by a single i.p. injection of streptozotocin (65 mg/kg) and were treated as in groups 1, 3, 5, respectively. Biochemical parameters were measured in plasma, urine, and kidneys. In the untreated diabetic group, there was a significant decrease in body weight, polyuria, and increased kidney weight. There was increased urinary albumin/creatinine ratio (UACR) and N-acetyl-β-D-glucosaminidase (NAG)/creatinine ratio (UNCR). Streptozotocin also induced a significant increase in creatinine clearance. In addition, diabetes was associated with increased oxidative stress [reduced renal glutathione reductase (GR), superoxide dismutase (SOD), catalase activities, and increased malondialdhyde (MDA)] and increased plasma tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and nitric oxide (NO) concentrations. Kidneys from streptozotocin-treated rats showed marked vacuolation of the proximal tubular epithelium with focal tubular necrosis and the glomeruli showing increase in mesangial cells. Tocilizumab significantly mitigated the increase in UACR and UNCR, renal MDA, plasma TNF-α, IL-6 and NO levels, and the decrease in renal SOD and catalase activities in diabetic rats. Tocilizumab did not significantly improve creatinine clearance; however, it attenuated the histopathological changes induced by streptozotocin. This study shows that tocilizumab was able to ameliorate some of the changes seen in streptozotocin-induced early diabetic nephropathy in rats. This is mainly due to its anti-inflammatory and antioxidative effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic nephropathy (DN) is a leading cause of end-stage renal disease (ESRD), which affects 15–25% of type 1 diabetes mellitus (DM) patients and 30–40% of type 2 DM patients (Wang et al. 2018). Current management of DN relies on lifestyle changes, glycemic and lipid control, and blockade of the renin-angiotensin aldosterone system (Fouli and Gnudi 2018). Kodera et al. (2011) have mentioned numerous factors that can contribute to the development of diabetic nephropathy: glomerular hyperfiltration in early-stage nephropathy, oxidative stress, accumulation of advanced glycation end products (AGEs), activation of protein kinase C, acceleration of the polyol pathway, and transforming growth factor-β (TGF-β) overexpression. In addition, there is evidence that the inflammatory process is involved in the pathogenesis of diabetic nephropathy (Kodera et al. 2014).
Serum levels of proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), were shown to be increased in human and experimental animal models of diabetic nephropathy (Shikano et al. 2000; Moriwaki et al. 2007). Interleukin 6 (IL-6) is a multifunctional cytokine that has both pro- and anti-inflammatory properties (Yang et al. 2012). Clinical and experimental studies suggest that IL-6 contributes to renal injury in glomerulonephritis and other forms of renal disease (Jones et al. 2015).
Tocilizumab is a recombinant humanized anti-IL-6R monoclonal antibody that blocks IL-6-mediated signaling pathway by binding to both IL-6R (Jones et al. 2015; Kim et al. 2015). It is clinically useful in the treatment of several inflammatory diseases (Sheppard et al. 2017).
To determine the role of IL-6 in the pathogenesis of diabetic nephropathy, the aim of this study was to examine the effect of tocilizumab, on streptozotocin-induced early diabetic nephropathy.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats were provided from the small animal house, Sultan Qaboos University, and were housed in a room at a temperature of 22 ± 2 °C, relative humidity of about 60%, with a 12-h light–dark cycle, and fed standard diet and tap water. All experimental designs were approved by the Medical Research Committee, College of Medicine and Health Sciences, Sultan Qaboos University. All procedures involving animals and their care were carried out in accordance with the guidelines of the Animal Ethical Committee of Sultan Qaboos University and international laws and policies (EEC Council directives 2010/63/EU, 22 September, 2010 and NIH Guide for the Care and Use of Laboratory Animals, NIH Publications, 8th edition, 2011).
Induction of diabetes
Rats were rendered diabetic by an intraperitoneal injection of streptozotocin (65 mg/kg) dissolved in citrate buffer. Seventy-two hours after streptozotocin injection, a drop of blood was taken from the tail vein and random blood glucose level was measured by using a blood glucose monitoring system “One Touch® Horizon™” (Life Scan, Inc., Milpitas, CA, USA), and diabetic rats were confirmed to have blood glucose level > 20 mmol/L. Once rendered diabetic, rats were allocated into groups 2, 4, and 6.
Experimental design
Male Sprague-Dawley (SD) rats (n = 36) were randomly distributed into six equal groups and treated as follows for 4 weeks:
-
Group 1: Control: received saline injection intraperitoneally (i.p.), every 2 weeks.
-
Group 2: Diabetic: received saline i.p. every 2 weeks.
-
Group 3: Tocilizumab (2 mg/kg): received tocilizumab (2 mg/kg i.p. every 2 weeks).
-
Group 4: Diabetic + tocilizumab (2 mg/kg): received tocilizumab (2 mg/kg i.p. every 2 weeks).
-
Group 5: Tocilizumab (8 mg/kg): received tocilizumab (8 mg/kg i.p. every 2 weeks).
-
Group 6: Diabetic + tocilizumab (8 mg/kg): received tocilizumab (8 mg/kg i.p. every 2 weeks).
Doses of streptozotocin and tocilizumab were selected according to previously published work (Abdelrahman and Al Suleimani 2008; Kodera et al. 2014; Abdelrahman et al. 2018).
Rats were weighed at the beginning, every week and end of the experiment. Every week, the animals were placed in metabolic cages, and the amount of urine voided during the 24 h was measured. Collected urine was stored deep frozen (− 80 °C) after its volume had been recorded.
Biochemical measurements
At the end of the experiment, rats were sacrificed and blood was collected from the abdominal aorta of each animal and centrifuged at 4 °C to separate plasma. The plasma collected was frozen at − 80 °C pending analysis. Kidneys were removed, weighed, and then a part was dipped in liquid nitrogen and stored at a temperature of − 80 °C, pending analysis, and another part was prepared for histopathology. Homogenization of kidneys was done in cold PBS buffer by ULTRA-TURRAX homogenizer in ice. After this, homogenate was centrifuged for 10 min in a micro centrifuge, at 5000 rpm and temperature at 4 °C, and supernatant was collected for analysis.
Plasma creatinine as well as urine creatinine and albumin were measured using fully automated chemistry analyzer BS-120, MINDRAY, Shenzhen Mindray Bio-Medical Electronics Co (Shenzhen, China). Plasma tumor necrosis factor-alpha (TNF-α) was measured by Bender Med systems Gm BH Elisa kit (Vienna, Austria), and interleukin-6 (IL-6) was measured by ELISA kit (Thermo Fisher Scientific, Waltham, MA, USA). Renal glutathione reductase (GR), superoxide dismutase (SOD), catalase, and malondialdhyde (MDA) were measured by a colorimetric assay kit (BioVision, Milpitas, CA, USA). Urinary N-acetyl-β-D-glucosaminidase (NAG) was measured by colorimetric Assay Kit (Cusabio, Wuhan, Hubei Province, P.R. China). Total nitric oxide was measured by ELISA kit (R&D Systems, Minneapolis, MN, USA). Creatinine clearance (mL/min/100 g body weight) was calculated by the following formula and adjusted for body weight:
Histopathology
Renal tissue samples were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histological examination using light microscopy. The Masson trichrome stain was used for demonstration of fibrosis. The glass slides were histopathologically examined for glomerular changes, tubular changes, fibrosis, and blood vessel changes.
Drugs
Streptozotocin from Sigma-Aldrich (St. Louis, MO, USA). Tocilizumab (Actemra®) made for F. Hoffmann-La Roche Ltd., (Basel, Switzerland) By Chugai Pharma Manufacturing Co., Ltd., (Utsunomiya-city, Japan).
Statistical analysis
Data were expressed as means ± SEM and were analyzed with GraphPad Prism Version 5.03 for Windows software (Graphpad Software Inc., San Diego, USA). Comparisons between the groups were performed by one-way analysis of variance (ANOVA), followed by Tukey’s comparisons. P values < 0.05 were considered significant.
Results
Effect of tocilizumab on physiological parameters in streptozotocin-induced DN in rats
Table 1 shows that there was a significant reduction in body weight and increased water intake, urine output, and relative kidney weight in streptozotocin-induced diabetic rats when compared with the control group. Tocilizumab alone did not significantly affect any of the above parameters except it reduced body weight which reached statistical level with the lower dose. Tocilizumab did not significantly affect the above parameters, but the higher dose significantly reduced urine output in streptozotocin-treated rats.
Effect of tocilizumab on parameters of kidney function in streptozotocin-induced DN in rats
Table 2 shows that there was a significant increase in creatinine clearance and decreased plasma creatinine levels in streptozotocin-induced diabetic rats when compared with the control group. Tocilizumab had no significant effects on the above parameters in either normoglycemic or diabetic rats.
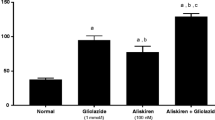
Table 3 and Fig. 1 show that there was a significant increase in urinary albumin/creatinine ratio and NAG/creatinine ratio in streptozotocin-induced diabetic rats when compared with the control group. Tocilizumab significantly reduced streptozotocin-induced increase in the above parameters. Tocilizumab alone did not significantly affect any of the above parameters.
Effect of tocilizumab on plasma total nitric oxide in streptozotocin-induced DN in rats
Table 2 shows that there was a significant increase in plasma total nitric oxide. Tocilizumab significantly reversed streptozotocin-induced effect. Both doses of tocilizumab alone did not significantly affect plasma total NO.
Effect of tocilizumab on oxidative stress marker in streptozotocin-induced DN in rats
Figure 2 shows that there was a significant decrease in renal GR, SOD, and catalase activities and increased malondialdhyde levels in streptozotocin-induced diabetic rats when compared with the control group. Tocilizumab significantly reversed streptozotocin-induced effect in SOD, catalase, MDA, and NO but not GR. Tocilizumab alone did not significantly affect any of the above parameters.
Effect of tocilizumab on inflammatory markers in streptozotocin-induced diabetic nephropathy in rats
Figure 3 shows that there was a significant increase in plasma TNF-α and IL-6 levels in streptozotocin-induced diabetic rats when compared with the control group. Tocilizumab (8 mg/kg) significantly reversed streptozotocin-induced effect. Both doses of tocilizumab alone did not significantly affect TNF-α or IL-6 levels.
Effect of tocilizumab on renal histopathological changes in streptozotocin-induced diabetic nephropathy in rats
Histologic examination of the animals from control group and group treated with tocilizumab (2 mg/kg and 8 mg/kg) showed normal glomeruli and tubules (Fig. 4a, c, e, respectively). Examination of animals from streptozotocin-treated group showed marked vacuolation of the proximal tubular epithelium with focal tubular necrosis. Most of the glomeruli show mild increase in mesangial cells (Fig. 4b). Examination of slides from animals treated with streptozotocin plus tocilizumab (2 mg/kg) showed decrease of the vacuolation of the tubular cells with regeneration of the necrotic tubular epithelium with mild decrease in the mesangial cellularity (Fig. 4d). Examination of the slides from animals treated with streptozotocin and tocilizumab (8 mg/kg) showed few tubules with regenerating epithelium and nearly normal cellularity of the glomeruli (Fig. 4f).
Histopathologic pictures of the kidneys from a normal control showing normal glomeruli (green arrow) and tubules (black arrow), b animals treated with streptozotocin showing extensive vacuolation of the tubular epithelium (black arrow) with some tubular damage and necrosis (green arrow). The glomeruli show mild increase in mesangial cellularity (arrow head), c animals treated with tocilizumab (2 mg/kg) showing normal glomeruli (green arrow) and tubules (black arrow), d animals treated with streptozotocin plus tocilizumab (2 mg/kg) show some tubules with vacuolation of the cells (black arrow) and few tubules with early regeneration of the lining cells (green arrow). The glomeruli show slight increase in mesangial cellularity (arrow heads), e animals treated with tocilizumab (8 mg/kg) show normal glomeruli (arrow heads) and tubules (arrows), f animals treated with streptozotocin plus tocilizumab (8 mg/kg) show few tubules with regenerating epithelium (arrows) and nearly normal glomeruli (arrow head)
Discussion
DN is one of the most common microvascular complications of DM (Hu et al. 2018) and is a major cause of morbidity and mortality in diabetic patients (Moriwaki et al. 2007). Inflammatory pathways are known to play a key role in the development and progression of DN (Fouli and Gnudi 2018). There is evidence that IL-6 contributes to renal injury in different forms of renal disease (Jones et al. 2015). We examined the role of the IL-6 in the pathogenesis of renal dysfunction in streptozotocin-induced early stage DM. In the present study, there was increased kidney weight, creatinine clearance, and urinary albumin/creatinine ratio in the diabetic groups when compared to control indicating renal hypertrophy, glomerular hyperfiltration, and albuminuria. This is in accordance with previous reports that showed that streptozotocin-induced early stage DM was associated with increased kidney weight, albuminuria, and glomerular hyperfilteration (Kodera et al. 2011; Kodera et al. 2014). Glomerular hyperfiltration is present in early-stage DN as it causes albuminuria and initiates the sclerotic process of glomeruli, resulting in fibrosis and irreversible renal failure (Hou et al. 2017). Albuminuria in early DN may result from altered glomerular filtration and/or altered processing of filtered albumin by the proximal tubule (Russo et al. 2009). In addition, there was increased NAG/creatinine ratio in the diabetic group. NAG is a hydrolytic lysosomal enzyme found predominantly in proximal tubule that has been demonstrated as a useful marker of renal tubular impairment (Fiseha and Tamir 2016). These findings were confirmed by histopathology where examination of kidneys from streptozotocin-treated group showed marked vacuolation of the proximal tubular epithelium with focal tubular necrosis and increased mesangial cells in most of the glomeruli. In addition, there was increased oxidative stress markers [reduced renal glutathione reductase (GR), superoxide dismutase (SOD), catalase and increased malondialdhyde (MDA)] and inflammatory markers [plasma IL-6 and TNF-α levels] in the diabetic groups. It was previously shown that oxidative stress and apoptosis contribute to the pathogenesis of hyperglycemia-induced renal injury and DN (Jaikumkao et al. 2017). There was also an increase in plasma total NO in the diabetic groups when compared to control. In agreement with our results, previous reports showed that NO is increased in early stage of streptozotocin-induced DN (Joshi and Woodman 2012). Increased NO production might likely contribute to the hyperfiltration and microalbuminuria at this early stage of DN (Tessari 2015). Overproduction of NO leads also to formation of peroxynitrite radicals and leads to cellular injury by formation of lipid peroxidation and reactive oxygen species (Abdelrahman et al. 2018).
In the present study, tocilizumab (8 mg/kg) significantly reduced polyuria (− 16.9%) but did not change blood glucose level or creatinine clearance. The exact mechanism is not clear, but it is possible that this is due to attenuation of renal tubular injury as shown by reduction in urinary NAG levels and histopathology. Tocilizumab did not significantly affect relative kidney weight or creatinine clearance. This suggests that tocilizumab did not improve glomerular hyperfiltration and renal hypertrophy. On the contrary, tocilizumab reduced urinary albumin/creatinine and NAG/creatinine ratios. This shows that tocilizumab improved albuminuria and reduced renal tubular injury. The protective effect of tocilizumab was confirmed by histopathology where tocilizumab attenuated the renal histopathological changes induced by streptozotocin. This protective effect may be due to reduced oxidative stress as indicated by increased SOD and catalase and decreased MDA and inflammation as indicated by reduced plasma TNF-α and IL-6 levels. In addition, tocilizumab also reduced total plasma NO levels that may lead to decrease formation of peroxynitrite radicals and less cellular injury. In accordance with our results, Wu et al. (2018) showed that in type 2 diabetic mice (db/db), tocilizumab reduced urine albumin, insulin resistance, glomerular mesangial matrix accumulation, inflammatory response, and oxidative stress. In our study, tocilizumab reduced serum IL-6 levels. In accordance with our results, tocilizumab reduced plasma and spinal cord tissue IL-6 levels in a model of spinal cord ischemia reperfusion in rabbits (Karatas et al. 2018). On the contrary, tocilizumab increased serum IL-6 level in db/db mice (Wu et al. 2018) and in clinical studies (Nishimoto et al. 2008; Kobayashi et al. 2015; Kleveland et al. 2018). However, changes in serum IL-6 levels after tocilizumab therapy were not significant in patients with rheumatoid arthritis (Choi et al. 2018; Diaz-Torne et al. 2018). Nishimoto et al. (2008) showed that serum IL-6 levels after tocilizumab administration differed greatly between various diseases and suggested that this reflect different levels of endogenous IL-6 production. The reason for the decrease in serum IL-6 in our study is not clear but it may be due to a decrease in the production of IL-6 from the kidneys after attenuation of renal injury. Suzuki et al. (1995) suggested that IL-6 may be associated with mesangial proliferation and renal damage in DN. In addition, increased IL-6 was shown to contribute to Ang II-induced chronic kidney damage in mice (Zhang et al. 2012). On the contrary, Yang et al. (2012) showed that interleukin 6 did not play a significant role in the development of renal fibrosis. In mercuric chloride-induced acute kidney injury in mice, IL-6 was shown to mediate both an injurious inflammatory response and a protective response that ameliorates injury and maintains renal function (Nechemia-Arbely et al. 2008). Furthermore, in patients with acute kidney injury, high plasma levels of IL-6 and other cytokines were predictive of high mortality (Simmons et al. 2004).
In conclusion, in a model of early stage DN, tocilizumab, an IL-6 inhibitor, did not improve glomerular hyperfiltration but reduced albuminuria and tubular injury possibly through reducing oxidative stress and inflammation.
References
Abdelrahman AM, Al Suleimani YM (2008) Four-week administration of nimesulide, a cyclooxygenase-2 inhibitor, improves endothelial dysfunction in the hindlimb vasculature of streptozotocin-induced diabetic rats. Arch Pharm Res 31:1584–1589
Abdelrahman AM, Al Suleimani YM, Ashique M, Manoj P, Ali BH (2018) Effect of infliximab and tocilizumab on fructose-induced hyperinsulinemia and hypertension in rats. Biomed Pharmacother 105:182–186
Choi IA, Lee SJ, Park W, Park SH, Shim SC, Baek HJ, Yoo DH, Kim HA, Lee SK, Lee YJ, Park YE, Cha HS, Lee EY, Lee EB, Song YW (2018) Effects of tocilizumab therapy on serum interleukin-33 and interleukin-6 levels in patients with rheumatoid arthritis. Arch Rheumatol 33:389–394
Diaz-Torne C, Ortiz MDA, Moya P, Hernandez MV, Reina D, Castellvi I, De Agustin JJ, Fuente D, Corominas H, Sanmarti R, Zamora C, Cantó E, Vidal S (2018) The combination of IL-6 and its soluble receptor is associated with the response of rheumatoid arthritis patients to tocilizumab. Semin Arthritis Rheum 47:757–764
Fiseha T, Tamir Z. (2016) Urinary markers of tubular injury in early diabetic nephropathy. Int J Nephrol ID 4647685, 10 https://doi.org/10.1155/2016/4647685
Fouli GE, Gnudi L (2018) The future: experimental therapies for renal disease in diabetes. Nephron 26:1–5
Hou B, Qiang G, Zhao Y, Yang X, Chen X, Yan Y, Wang X, Liu C, Zhang L, Du G (2017) Salvianolic acid a protects against diabetic nephropathy through ameliorating glomerular endothelial dysfunction via inhibiting AGE-RAGE signaling. Cell Physiol Biochem 44:2378–2394
Hu X, Liu W, Yan Y, Liu H, Huang Q, Xiao Y, Gong Z, Du J (2018, 2018) Vitamin D protects against diabetic nephropathy: evidence-based effectiveness and mechanism. Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2018.09.037
Jaikumkao K, Pongchaidecha A, Chatsudthipong V, Chattipakorn SC, Chattipakorn N, Lungkaphin A (2017) The roles of sodium-glucose cotransporter 2 inhibitors in preventing kidney injury in diabetes. Biomed Pharmacother 94:176–187
Jones SA, Fraser DJ, Fielding CA, Jones GW (2015) Interleukin-6 in renal disease and therapy. Nephrol Dial Transplant 30:564–574
Joshi A, Woodman OL (2012) Increased nitric oxide activity compensates for increased oxidative stress to maintain endothelial function in rat aorta in early type 1 diabetes. Naunyn Schmiedeberg's Arch Pharmacol 385:1083–1094
Karatas Y, Erdi MF, Kaya B, Keskin F, Cüce G, Kılınc I, Uyar M, Izci EK, Kalkan E (2018) Neuroprotective effects of tocilizumab on experimentally-induced spinal cord ischemia-reperfusion injury. World Neurosurg 124:e208–e213. https://doi.org/10.1016/j.wneu.2018.12.069
Kim GW, Lee NR, Pi RH, Lim YS, Lee YM, Lee JM, Jeong HS, Chung SH (2015) IL-6 inhibitors for treatment of rheumatoid arthritis: past, present and future. Arch Pharm Res 38:575–584
Kleveland O, Ueland T, Kunszt G, Bratlie M, Yndestad A, Broch K, Holte E, Ryan L, Amundsen BH, Bendz B, Aakhus S, Espevik T, Halvorsen B, Mollnes TE, Wiseth R, Gullestad L, Aukrust P, Damås JK (2018) Interleukin-6 receptor inhibition with tocilizumab induces a selective and substantial increase in plasma IP-10 and MIP-1β in non-ST-elevation myocardial infarction. Int J Cardiol 271:1–7
Kobayashi T, Ito S, Kobayashi D, Kojima A, Shimada A, Narita I, Murasawa A, Nakazono K, Yoshie (2015) Interleukin-6 receptor inhibitor tocilizumab ameliorates periodontal inflammation in patients with rheumatoid arthritis and periodontitis as well as tumor necrosis factor inhibitors. Clin Exp Dent Res 13:63–73
Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota D, Sato C, Ogawa D, Makino H, Matavelli LC, Zatz R, Siragy HM (2011) Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 54:965–978
Kodera R, Shikata K, Takatsuka T, Oda K, Miyamoto S, Kajitani N, Hirota D, Ono T, Usui HK, Makino H (2014) Dipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetes. Biochem Biophys Res Commun 443:828–833
Moriwaki Y, Inokuchi T, Yamamoto A, Ka T, Tsutsumi Z, Takahashi S, Yamamoto T (2007) Effect of TNF-a inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol 44:215–218
Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, Axelrod JH (2008) IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 19:1106–1115
Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T (2008) Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112:3959–3564
Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D (2009) Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol 20:489–494
Sheppard M, Laskou F, Stapleton PP, Hadavi S, Dasgupta B (2017) Tocilizumab (Actemra). Hum Vaccin Immunother 13:1972–1988
Shikano M, Sobajima H, Yoshikawa H, Toba T, Kushimoto H, Katsumata H, Tomita M, Kawashima S (2000) Usefulness of a highly sensitive urinary and serum IL-6 assay in patients with diabetic nephropathy. Nephron 85:81–85
Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K, Spratt D, Shyr Y, Ikizler TA, PICARD Study Group (2004) Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int 65:1357–1365
Suzuki D, Miyazaki M, Naka R, Koji T, Yagame M, Jinde K, Endoh M, Nomoto Y, Sakai H (1995) In situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes 44:1233–1238
Tessari P (2015) Nitric oxide in the normal kidney and in patients with diabetic nephropathy. J Nephrol 28:257–268
Wang D, Zhang G, Chen X, Wei T, Liu C, Chen C, Gong Y, Wei Q (2018) Sitagliptin ameliorates diabetic nephropathy by blocking TGF-β1/Smad signaling pathway. Int J Mol Med 41:2784–2792
Wu R, Liu X, Yin J, Wu H, Cai X, Wang N, Qian Y, Wang F (2018) IL-6 receptor blockade ameliorates diabetic nephropathy via inhibiting inflammasome in mice. Metabolism 83:18–24
Yang J, Chen J, Yan J, Zhang L, Chen G, He L, Wang Y (2012) Effect of interleukin 6 deficiency on renal interstitial fibrosis. PLos ONE 7(12):e52415. https://doi.org/10.1371/journal.pone.0052415
Zhang W, Wang W, Yu H, Zhang Y, Dai Y, Ning C, Tao L, Sun H, Kellems RE, Blackburn MR, Xia Y (2012) Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension. 59:136–144
Funding
Supported by a grant from Sultan Qaboos University (IG/MED//PHAR/16/02).
Author information
Authors and Affiliations
Contributions
A.A.; Y.S.; B.A.: conceived and designed research, participated in the interpretation of the results, writing and review of the manuscript.
A.S.: conducted histopathology.
M.A.; P.M.: conducted the experiments and analysis of data.
Corresponding author
Ethics declarations
All experimental designs were approved by the Medical Research Committee, College of Medicine and Health Sciences, Sultan Qaboos University. All procedures involving animals and their care were carried out in accordance with the guidelines of the Animal Ethical Committee of Sultan Qaboos University and international laws and policies (EEC Council directives 2010/63/EU, 22 September, 2010 and NIH Guide for the Care and Use of Laboratory Animals, NIH Publications, 8th edition, 2011).
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelrahman, A.M., Al Suleimani, Y., Shalaby, A. et al. Effect of tocilizumab, an interleukin-6 inhibitor, on early stage streptozotocin-induced diabetic nephropathy in rats. Naunyn-Schmiedeberg's Arch Pharmacol 392, 1005–1013 (2019). https://doi.org/10.1007/s00210-019-01655-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01655-w