Abstract
Diabetes mellitus is an ever growing world-wide health problem. The patient has to stick to a firm life-long therapeutic regimen, otherwise diabetic complications will develop. Diabetic nephropathy (DN) is one of the most common diabetic complications and it requires careful medical attendance. Nilotinib hydrochloride is a protein tyrosine kinase inhibitor reported to have numerous therapeutic efficacies besides being an anticancer. In the current study, single I.P. streptozotocin (50 mg/kg) injection was used to induce type I diabetes mellitus in male Sprague–Dawley rats. After 8 weeks, significant deterioration of renal function with urinary excretion of nephrin, podocalyxin, and albumin was observed. Daily oral administration of nilotinib (20 mg/kg) for 8 weeks significantly improved signs of DN on all investigated scales. On a biochemical scale, kidney functions, albuminuria, urinary nephrin, podocalyxin excretion, and host oxidant/antioxidant balance significantly improved. Kidney content of nitric oxide, expression of toll-like receptors 4 and NF-κB/p65 activity significantly declined as well. On a histopathological scale, α-smooth muscle actin and nestin expression significantly declined. Meanwhile, area of fibrosis significantly declined as seen with significant reduction in accumulation of extracellular matrix components and kidney content of collagen. Ultimately, such improvements were accompanied by significant restoration of normal kidney physiology and function. In conclusion, nilotinib can hinder progression of DN through various mechanisms. Reduction of oxidative stress, enhancement of host antioxidant defense system, reduction of inflammation, angiogenesis, tissue hypoxia, and pro-fibrogenic biomarker expression can be implicated in the beneficial therapeutic outcome observed with nilotinib therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a group of metabolic diseases characterized mainly by hyperglycemia that results from either defect in insulin secretion or defect in insulin action, or both. Chronic hyperglycemia is associated with long-term damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels [4].
Diabetic nephropathy (DN) refers to a spectrum of renal diseases in diabetic patients. It has been classically defined as increased protein excretion in urine. Early stage is characterized by a small increase in urinary albumin excretion—microalbuminuria. More advanced disease is defined by the presence of macroalbuminuria or proteinuria. In most cases, proteinuria and decreased glomerular filtration rate occur in parallel [48]. Renal pathological changes associated with DN include thickness of glomerular basement membranes and progressive accumulation of extracellular matrix components (ECM), with change of podocytes’ ultra-structure and function [15], glomerular mesangial expansion, and tubulointerstitial fibrosis [41].
In the past, DN was progressive and irreversible. Despite improved prognosis over the last decades, DN remains a major health problem, and many patients still struggle and progress to end-stage renal diseases. With this in mind, the importance of control and management of DN has become inevitable. Multiple pathological mechanisms including increased oxidative stress, inflammation, and fibrosis have been reported to be involved in the pathogenesis of DN [12]. Therapies that can target critical checkpoints in the pathogenic pathway of DN progression can offer a new hope for management of such a diabetic complications.
Nilotinib hydrochloride is a highly potent tyrosine kinase inhibitor used for treatment of leukemia. It is a high-affinity aminopyrimidine-based ATP-competitive inhibitor that has been reported to decrease proliferation and viability of wild-type Bcr-Abl- and imatinib-resistant Bcr-Abl mutant-expressing cells by selectively inhibiting Bcr-Abl autophosphorylation [16]. It has been reported to potently inhibit the tyrosine kinase activity of ABL/BCR–ABL, as well as that of the platelet-derived growth factor (PDGFR), discoidin domain (DDR), and stem cell factor (KIT) receptors [32]. However, besides its anticancer effect, nilotinib has been reported to possess other beneficial pharmacological activities such as antifibrotic [3], anti-inflammatory [8], and antioxidant activities [13]. Intriguingly, nilotinib was reported to inhibit the progression of chronic kidney disease through combating inflammation and fibrosis [24]. However, the role of nilotinib in the management of DN has not been yet investigated. Therefore, the current study was designed to evaluate the protective therapeutic potential of nilotinib against experimentally induced DN and to delineate the possible underlying mechanisms.
Materials and methods
Animals
Fifty male Sprague–Dawley rats (8 weeks, 180–230 g) were purchased from the Urology and Nephrology Center–Experimental Animal Center, Mansoura, Egypt. The rats were housed in standard animal facility under controlled environmental conditions at room temperature 22 ± 2 °C and 12-h light–dark cycle and allowed access to food and water ad libitum. The experimental protocol complied with the ethical guidelines and the principles of care, use, and handling of experimental animals adopted by the Research Ethics Committee, Faculty of Pharmacy, Mansoura University, Egypt. The adopted guidelines were in accordance with the Guide for the Care and Use of Laboratory Animals [22].
Chemicals and reagents
Nilotinib hydrochloride, a generous gift from NOVARTIS pharmaceuticals (Basel, Switzerland), was suspended in 0.5 % carboxymethylcellulose (CMC) for oral administration. Streptozotocin (STZ) was purchased from Sigma Chemicals Co. (St. Louis, MO, USA) for induction of diabetes mellitus, and it was dissolved in citrate buffer (0.01 mol/L, pH 4.4) immediately before use. Hydroxyproline, p-dimethylaminobenzaldhyde, chloramine-T, n-propanol, and perchloric acid (70 %) were purchased from Sigma Chemicals Co. (St. Louis, MO, USA) for the assay of kidney hydroxyproline content.
Experimental protocol
Induction of diabetes mellitus and DN
Type I diabetes was induced as described by [14]. Briefly, diabetes mellitus was induced by a single intraperitoneal (I.P.) injection of streptozotocin (STZ) (50 mg/kg) freshly dissolved in citrate buffer (0.01 mol/L, pH 4.4) after fasting rats for 12 h. After 48 h, type I diabetes was confirmed by measuring the glucose concentrations in peripheral blood obtained from the tail vein using a glucometer (OneTouch® System; LifeScan, USA). Rats with blood glucose levels greater than 250 mg/dL were considered diabetic and were further kept for 8 weeks to ensure development of DN. Nilotinib treatment (20 mg/kg, orally) started after confirmation of type I diabetes mellitus.
Rats were randomly divided into four groups (12 rats/group), except for the diabetic control group (14 rats/group) as follows: group I, healthy normal control group received the vehicle (5 mL/kg, 0.5 % CMC, orally) for 8 weeks; group II, nilotinib control received nilotinib (20 mg/kg, orally) daily for 8 weeks; group III, diabetic control group received single I.P. STZ (50 mg/kg) and the vehicle (5 mL/kg, 0.5 % CMC, orally) for 8 weeks; and group IV, nilotinib treated group received single I.P. STZ (50 mg/kg) and nilotinib (20 mg/kg, orally) for 8 weeks after confirmation of type I diabetes mellitus.
After 8 weeks, the rats were sacrificed by an overdose of thiopental sodium (40 mg/kg). Blood samples were collected from the retro-orbital plexus using heparinized capillary hematocrit tubes. The serum was separated by centrifugation at 3000 rpm for 10 min, and it was used for immediate determination of biochemical parameters.
The animals’ body weights were recorded and the kidneys were harvested and weighed immediately for calculation of kidney/body weight index. The left kidneys were immediately immersed in liquid nitrogen and stored at −80 °C for preparation of tissue homogenate and estimation of kidney hydroxyproline and collagen content. The right kidneys were rinsed in ice-cold saline, slit longitudinally, and fixed in 10 % buffered formalin for subsequent histopathological and immunohistochemical examination.
Measurement of renal glomerular function, serum creatinine, and serum blood urea nitrogen (BUN)
Serum samples were immediately used for the estimation of BUN and creatinine using commercially available kits (Stanbio Co., Boerne, TX, USA). Test procedures were carried out according to the supplied manufacturer’s instructions.
Assessment of urinary albumin excretion, nephrin, and podocalyxin
Twenty-four hours before the end of the study period, rats in each group were individually housed in metabolic cages (Nalgene; Nalge Company, Rochester, NY, USA) for 24-h urine collection. The rats were allowed access to water and food ad libitum. Aliquots of the 24-h urine samples were used for estimation of urine content of albumin, nephrin, and podocalyxin using enzyme-linked immunosorbent assay (ELISA) kits (Exocell Inc., PA, USA). Test procedures were carried out according to the supplied manufacturer’s instructions.
Preparation of kidney homogenate for determination of kidney content of toll-like receptors 4 (TLR4), nitric oxide (NO), malondialdehyde (MDA), reduced glutathione (GSH) content, and superoxide dismutase (SOD) activity
Kidney homogenate was prepared according to de Cavanagh et al. [9]. Briefly, 100 mg of kidney tissue and 4 vol of 120 mM potassium chloride, 30 mM potassium phosphate, pH 7.4 buffer were sonicated for 1 min and then centrifuged at 3000 rpm at 4 °C for 10 min. The supernatant was referred to as kidney homogenate and it was used to estimate TLR4 levels using TLR4 ELISA kits (My Bio Source Inc., San Diego, CA, USA), NO levels as nitrite content, MDA levels, SOD activity, and reduced GSH content using Biodiagnostic kits (Giza, Egypt). Test procedures were carried out according to the supplied manufacturer’s instructions.
Assessment of renal NF-κB/p65 activity
For quantitative analysis of NF-κB/p65 activity, the NF-κB TransAM Activity Assay Kit (Abcam, USA) was used. For this assay, the nuclear extracts of renal tissues were prepared using the Nuclear Extraction Kit (Abcam, USA). Test procedures were carried out according to the supplied manufacturer’s instructions.
Estimation of kidney hydroxyproline and collagen content
One hundred milligrams of the isolated left kidney tissue was used for the estimation of kidney hydroxyproline content. The hydroxyproline assay was carried according to the previously reported methodology of Bergman and Loxley [6] using base-induced rather than acid-induced tissue dissolution. The absorbance of the reddish purple complex was measured at 550 nm. Kidney collagen content was calculated according to Hessien et al. [20] by multiplying the hydroxyproline concentration by 7.46, where this amino acid represents 13.4 % of collagen.
Histopathology and immunohistochemical analysis of α-smooth muscle actin (α-SMA) and nestin expression
The right kidneys were cut longitudinally and one half was fixed in 10 % buffered formalin and embedded in paraffin. Five-micrometer-thick sections were cut with a microtome and de-paraffinated with xylene by a specialized technician. Four sets of slides were prepared. At least two different sections were examined per kidney sample.
The first set was stained with hematoxylin and eosin (H&E) to assess histopathological changes. The second set was stained with Masson’s Trichrome stain for examination of collagen and other ECM accumulation and calculation of area and area percent of fibrosis. The third set of slides was stained with α-SMA immunoperoxidase stain (Sigma Chemicals Co., St. Louis, MO, USA) for evaluation of α-SMA expression. Mouse monoclonal anti-α-SMA antibody (Dako 1A4) was diluted in a ratio of 1:100 and was used as the primary antibody. The fourth set of slides was stained with anti-nestin antibody produced in rabbit N5413 (Sigma Chemicals Co., St. Louis, MO, USA) for evaluation of nestin expression.
For calculation of area and area percent of fibrosis, the data were collected using Leica Qwin 500 image analyzer computer system. The image analyzer was first calibrated automatically to convert the measurement units (pixels) produced by the image analyzer program into actual micrometer units. In each chosen field, the kidney tissue was enclosed inside the standard measuring frame and then the area to be measured was masked by a blue binary color. These measurements were done using total magnification power of ×400. Several readings were obtained for each specimen and their total area was summated.
Statistical analysis
Data are presented as the mean ± the standard error of the mean (SEM), and significance was calculated at p <0.05. The following statistical tests were used: one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparisons test for statistical comparison between parametric data and linear regression analysis for the best fitting line of all the standard points. Statistical calculations were carried out using Instat-3 computer program (Graph Pad Software Inc. v2. 04, San Diego, CA, USA).
Results
Effect of nilotinib on body weight, kidney weight, kidney/body weight index, and blood sugar following induction of DN
Type I diabetes was confirmed throughout the experimental period (Table 1). STZ (50 mg/kg, I.P.) significantly increased basal blood sugar in all the experimental groups in comparison to normal and nilotinib control groups. After 8 weeks, blood sugar in the diabetic control was significantly high in comparison to both normal and nilotinib control groups. After oral nilotinib treatment (20 mg/kg), blood sugar was significantly high in comparison to the normal control yet significantly low in comparison to the diabetic control (n = 12, p < 0.05) (Table 1).
Regarding body weight, kidney weight, and kidney/body weight index, there was no observed significant difference between the normal healthy control group and the nilotinib control group (n = 12, p < 0.05) (Table 1). Nilotinib treatment had a positive impact on animals’ body weights, kidney weights, and hence kidney/body weight index (Table 1). Diabetic control demonstrated a significant decline in body weight by approximately 39 % with a significant increase in kidney weights by approximately 16 % and subsequent increase in kidney/body weight index by approximately 52 % in comparison to both healthy normal and nilotinib controls (n = 14, p < 0.05) (Table 1). Daily oral nilotinib (20 mg/kg, orally) for 8 weeks significantly increased the animals’ body weights and decreased kidney weights in comparison to the diabetic control. Indeed, nilotinib treatment almost restored rats’ body and kidney weights to normal levels (n = 12, p < 0.05) (Table 1).
Effect of nilotinib on kidney functions following induction of DN
Serum creatinine and BUN levels
Diabetic control group revealed a significant increase in serum creatinine by approximately 11-fold and BUN by approximately 2-fold in comparison to the normal and nilotinib controls (n = 14, p < 0.05) (Table 2), suggestive for severe impairment of renal glomerular functions consistent with progression of DN. Daily oral nilotinib (20 mg/kg) for 8 weeks significantly reduced both serum creatinine by approximately 79 % and BUN by approximately 37 % in comparison to the diabetic control (n = 12, p < 0.05) (Table 2).
Albuminuria, urine nephrin, and podocalyxin contents
As observed in Fig. 1, unopposed type I diabetes mellitus for 8 weeks significantly increased urine content of albumin (approximately 6-fold), nephrin (approximately 7-fold), and podocalyxin (approximately 3.5-folds) in comparison to healthy normal control and nilotinib control groups (n = 14, p < 0.05). Daily oral nilotinib (20 mg/kg) for 8 weeks significantly reduced urine content of albumin, nephrin, and podocalyxin by approximately68, 68, and 45 %, respectively, in comparison to the diabetic control (n = 12, p < 0.05) (Fig. 1).
Effect of nilotinib administration (20 mg/kg, orally) for 8 weeks on albuminuria, nephrin, and podocalyxin following induction of diabetic nephropathy. Data are expressed as a function of mean ± SEM. STZ was dissolved in citrate buffer and administered in a single I.P. (50 mg/kg) dose. Eight weeks later, the animals were sacrificed. Twenty-four hours before sacrifice time, urine aliquots were collected and used for estimation of biochemical parameters. Statistical analysis was done using ANOVA followed by Tukey-Kramer’s test. *Significantly different in comparison to normal and nilotinib controls (n = 12, p < 0.05). #Significantly different in comparison to diabetic control (n = 14, p < 0.05)
Effect of nilotinib on oxidative stress biomarkers, inflammatory biomarkers, and cytokine expression following induction of DN
Kidney MDA content, SOD activity, reduced GSH content, and NO content
Eight weeks after diabetes induction, the kidney homogenate of the diabetic control rats revealed a significant increase in MDA and NO content by approximately 2- and 4-fold, respectively, in comparison to normal and nilotinib control groups (n = 14, p < 0.05) (Table 3). Meanwhile, there was a significant decline in kidney content of SOD and reduced GSH by approximately 3.5- and 1.2-fold, respectively, in comparison to both normal and nilotinib controls (n = 14, p < 0.05) (Table 3). Nilotinib treatment (20 mg/kg, orally) for 8 weeks significantly reduced kidney content of both MDA and NO by approximately 48 and 28 %, respectively, with significant elevation of kidney SOD activity and reduced GSH content by approximately 57 and 7 %, respectively, in comparison to the diabetic control group (n = 12, p < 0.05) (Table 3).
TLR4 expression and renal NF-κB/p65 activity
Significant elevation in kidney content of TLR4 and NF-κB/p65 activity was observed in the diabetic control group in comparison to healthy normal and nilotinib control groups (n = 14, p < 0.05), after 8 weeks of type I diabetes mellitus induction (Figs. 2 and 3). On the other hand, nilotinib treatment (20 mg/kg, orally) for 8 weeks significantly reduced the elevated kidney content of TLR4 and NF-κB/p65 activity in comparison to the diabetic control group (n = 12, p < 0.05) (Figs. 2 and 3).
Effect of nilotinib administration (20 mg/kg, orally) for 8 weeks on TLR4 expression following induction of diabetic nephropathy. Data are expressed as a function of mean ± SEM. STZ was dissolved in citrate buffer and administered in a single I.P. (50 mg/kg) dose. Eight weeks later, the animals were sacrificed and kidney homogenate was prepared and used for estimation of biochemical parameters. Statistical analysis was done using ANOVA followed by Tukey-Kramer’s test. *Significantly different in comparison to normal and nilotinib controls (n = 12, p < 0.05). #Significantly different in comparison to diabetic control (n = 14, p < 0.05)
Effect of nilotinib administration (20 mg/kg, orally) for 8 weeks on NF-κB/p65 activity following induction of diabetic nephropathy. Data are expressed as a function of mean ± SEM. STZ was dissolved in citrate buffer and administered in a single I.P. (50 mg/kg) dose. Eight weeks later, the animals were sacrificed and kidney homogenate was prepared and used for estimation of biochemical parameters. Statistical analysis was done using (ANOVA) followed by Tukey-Kramer’s test. *Significantly different in comparison to normal and nilotinib controls (n = 12, p < 0.05). #Significantly different in comparison to diabetic control (n = 14, p < 0.05)
Effect of nilotinib on kidney morphological changes following induction of DN
Kidney hydroxyproline and collagen content
Kidney content of hydroxyproline and collagen significantly increased by approximately 1.4-fold in the diabetic control after 8 weeks in comparison to normal control and nilotinib control groups (n = 14, p < 0.05) (Table 4). Eight weeks following daily oral nilotinib (20 mg/kg, orally) treatment, kidney content of both hydroxyproline and collagen significantly declined by approximately 17 % in comparison to diabetic control group (n = 12, p < 0.05) (Table 4).
Histopathological changes in kidney specimens
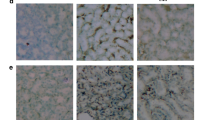
As demonstrated in Fig. 4a and b, histopathological examination of H&E-stained kidney specimens isolated from the normal healthy control and nilotinib control groups, respectively, revealed ideal kidney architecture with absence of any evidence of tissue injury or necro-inflammatory changes. Persistent unopposed diabetes for 8 weeks resulted in deleterious histopathological changes. The blood vessels in the renal cortex were congested and the glomeruli revealed irregularly dilated corpuscular space. Most of the proximal convoluted tubules became irregular with markedly degenerated sloughed lining cells and large casts in their widened lumina. The brush border was lost in most lumen of tubules (Fig. 4c). However, daily oral nilotinib (20 mg/kg) for 8 weeks resulted in significant tissue improvement and recovery. Renal corpuscles were more or less of normal appearance and most of proximal convoluted tubules appeared normal (Fig. 4d).
Kidney specimen stained with H&E from a normal healthy control group and b nilotinib control (20 mg/kg, orally) for 8 weeks reveals ideal kidney architecture with absence of any evidence of necro-inflammatory changes. c Diabetic control reveals deleterious histopathological changes with renal cortex showing congested blood vessels and irregularly dilated corpuscular space, marked degeneration of the proximal convoluted tubules, and loss of brush borders in the lumen of the tubules. d Nilotinib treated group (20 mg/kg, orally) for 8 weeks reveals significant tissue improvement and renal corpuscles and proximal convoluted tubules with normal appearance
Furthermore, histopathological examination of the kidney specimens of the healthy normal control group (Fig. 5a) and nilotinib control groups (Fig. 5b) after Masson’s Trichrome staining revealed the presence of a non-significant amount of fibrous tissue. On the other hand, the renal cortex of the diabetic control revealed the accumulation of a significant amount of fibrous tissue around the blood vessel and between the tubules with increased fibrous tissue accumulation around the glomeruli and in the interstitium (Fig. 5c). A significant increase in area and area percent of fibrosis by approximately 39- and 41-fold, respectively, was observed in a specimen isolated from diabetic control rats with DN in comparison to the normal control group (n = 14, p < 0.05) (Table 5). However, retraction of fibrosis, little amount fibrous tissue around renal corpuscles, and interstitial blood vessels with mild amount of interstitial fibrous tissue were detected in a kidney specimen from the nilotinib treated group (20 mg/kg, orally) for 8 weeks (Fig. 5d). Nilotinib (20 mg/kg, orally) for 8 weeks resulted in a significant reduction in area and area percent of fibrosis by approximately 40 % in comparison to the diabetic control group (n = 12, p < 0.05) (Table 5).
Kidney specimen stained with Masson’s Trichrome from a normal healthy control group and b nilotinib control (20 mg/kg, orally) for 8 weeks reveals no significant amount of fibrous tissue. c Diabetic control reveals massive fibrosis with accumulation of collagen and extracellular matrix components. d Nilotinib treated group (20 mg/kg, orally) for 8 weeks reveals retraction of fibrosis with significant reduction in area and area percent of fibrosis
α-SMA expression
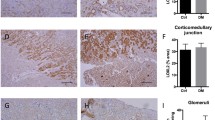
Following immunohistochemical analysis of kidney specimen isolated from the healthy normal control (Fig. 6a) and nilotinib control groups (Fig. 6b), α-SMA was detected in the muscle layer of the renal vessels. The renal corpuscle revealed few α-SMA positive cells and the tubules were all negative for α-SMA actin. However, positive α-SMA cells were detected in the renal cortex, around blood vessels, and in the cell fibrous bands between tubules in kidney specimen isolated from the diabetic control group (Fig. 6c). Significant elevation in area and area percent of α-SMA expression by approximately 2.4-fold was observed in diabetic animals in comparison to the healthy control and nilotinib control groups (n = 14, p < 0.05) (Table 5).
Kidney specimen stained with α-SMA immunoperoxidase from a normal healthy control group and b nilotinib control (20 mg/kg, orally ) for 8 weeks reveals few α-SMA positive cells in the muscle layer of renal vessels and renal corpuscle. c Diabetic control reveals α-SMA positive cells in the renal cortex, around the blood vessels, and in the cell fibrous band between tubules with a significant increase in α-SMA area and area percent expression. d Nilotinib treated group (20 mg/kg, orally) for 8 weeks reveals significant reduction of α-SMA positive cells with significant retraction in α-SMA area and area percent expression
Nilotinib administration resulted in detection of α-SMA in the renal cortex; the muscle layer of renal vessels and the renal corpuscle revealed few α-SMA positive cells (Table 5, Fig. 6d). Significant reduction in area and area percent of α-SMA expression by approximately 18 and 19 %, respectively, was observed in comparison to the diabetic control group (n = 12, p < 0.05) (Table 5).
Nestin expression
Renal cortex of diabetic group showed a significant increase in expression of nestin positive stained cells in renal corpuscle (Fig. 7d) compared to the healthy control (Fig. 7a), nilotinib control (Fig. 7b), and nilotinib treated group (20 mg/kg, orally) daily for 8 weeks (Fig. 7c).
Kidney specimen stained with anti-nestin antibody from a normal healthy control group and b nilotinib control (20 mg/kg, orally ) for 8 weeks reveals few positive nestin stained cells. c Diabetic control reveals a marked significant increase in nestin positive cells. d Nilotinib treated group (20 mg/kg, orally) for 8 weeks reveals a marked reduction in nestin positive cells
Discussion
Diabetes mellitus and its complications are the major cause of end-stage renal diseases [4]. The functional modifications allied to DN include an early increase in glomerular filtration rate, intraglomerular hypertension, subsequent proteinuria, and eventual loss of renal function [41].
Nilotinib, a second-generation tyrosine kinase inhibitor, is primarily used for the treatment of leukemia. However, results obtained from various studies provide strong evidence that nilotinib can exert additional beneficial pharmacological activities, which may be attributed to its additional abilities to inhibit PDGFR tyrosine kinase, transforming growth factor-β (TGF-β) [3], and mitogen-activated protein kinases [36].
The current study provides evidence on the beneficial outcomes of nilotinib administration in the management and control of the progression of DN. Nilotinib administration significantly improved glomerular filtration functions. Nilotinib reduced serum creatinine and blood urea nitrogen levels, and it decreased albumin, nephrin, and podocalyxin urinary excretion as well.
Proteinuria is the clinical hallmark of DN. Microalbuminuria is an early clinical marker of diabetic kidney disease resulting from damage of the glomerular filtration barrier, which comprises endothelial cells, glomerular basement membrane, and podocytes [18]. Podocytes are specialized kidney cells that form the basis of blood filtration in the glomerulus. The glomerular podocytes are the cells primarily responsible for the prevention of proteinuria in health [1], and podocalyxin is the integral membrane protein on the foot processes of kidney podocytes [18].
Podocyte injury has been considered to be one of the most important factors contributing to and underlying DN. Specialized cell junctions between podocyte foot processes, slit diaphragms, are thought to maintain a barrier that prevents the diffusion of larger proteins out of the blood [21, 37]. Indeed, an increase in foot-process width has been identified in patients with type I diabetes and microalbuminuria, and foot-process width has been shown to correlate directly with the urinary albumin excretion rate [5].
Nephrin is the trans-membrane protein that exists in the slit diaphragm itself and is thought to contribute to its zipper-like, extracellular structure. A correlation between changes in nephrin expression and proteinuria has been shown in several experimental models of glomerulonephritis [11, 30]. Nilotinib administration preserved podocyte integrity and slit-diaphragm structure with a marked decline in excretion of podocalyxin and nephrin, respectively, in the urine. In turn, nilotinib administration resulted in preservation of basement membrane structure and reduction of albumin loss in the urine in the current study.
TLRs are a family of receptors positioned as a first line of innate defense by recognizing pathogen-associated molecular patterns and endogenous signals of tissue injury. Tubular epithelial cells express TLRs, suggesting that these TLRs might contribute to the activation of immune responses in tubulointerstitial injury [49]. Activation of TLR4 on renal parenchymal cells has been reported to elicit the production of several cytokines and chemokines, which leads to propagation of subsequent inflammatory response [49]. Moreover, absence of TLR4 signaling has been reported to preserve podocyte integrity and to attenuate interstitial fibrosis in DN [31]. Interestingly, TLR4 was reported to mediate interstitial macrophage infiltration and subsequent production of pro-inflammatory cytokines and chemokines in DN through NF-κB activation [27].
The subsequent pro-inflammatory cascades have been reported to evoke oxidative stress by sensitizing infiltrating leukocytes, so that they respond with increased oxidant formation. In turn, the released reactive oxygen species (ROS) act as second messengers and increase the transcription of genes encoding cytokines, growth factors, and ECM proteins by activation of NF-κB, setting up a vicious cycle [7, 50]. These data suggest that oxidative-stress inflammatory-induced cytokine signaling is a pertinent target to promote therapeutic options for DN.
Evidences are accumulating regarding a potential anti-inflammatory effect of nilotinib in different experimental models [19, 24, 47]. Herein, nilotinib’s ability to reduce oxidative stress, boost host antioxidant defense mechanism and immune system, and reduce expression of TLR4 receptors and NF-κB/p65 activity may account for its capacity to reduce tissue injury and accumulation of inflammatory cells with preservation of kidney structure and ultimately improvement of kidney functions.
Nestin is a member of the intermediate filament protein family expressed in dividing cells during the early stages of development. Upon differentiation, nestin becomes down-regulated. Interestingly, its expression is re-induced in the adult during pathological situations [35]. Much evidence has shown that nestin expression in vascular endothelial cells is associated with proliferation and angiogenesis [17, 45]. In non-cancerous diseases, nestin has been reported to play important roles in wound healing in various tissues [34]. Accumulation of inflammatory infiltrate and development of fibrosis increase the resistance of the tissue to blood flow and delivery of oxygen, resulting in hypoxia. Under these circumstances, angiogenesis switch occurs leading to up-regulation of pro-angiogenic factors responsible for vascular remodeling and neovessel formation [39, 44].
Vascular endothelial growth factor (VEGF) is considered a modulator involved in angiogenesis [26], and it has been reported to be involved in nestin expression [34]. However, VEGF is one factor that is widely accepted to promote angiogenesis, and vasoactive autacoids, such as NO, also promote angiogenesis. Nitric oxide has been reported to promote new blood vessel formation. NO increases cGMP and activates protein kinase G, which phosphorylates vasodilator-stimulated phosphoprotein, promoting VEGF secretion [40, 44].
Therefore, it can be concluded that if VEGF is involved in nestin expression and promotion of angiogenesis, NO-induced increase in VEGF expression would in turn increase nestin expression as observed with the results of the current study. Progression of DN and kidney fibrosis increases tissue hypoxia with increased resistance to regular blood flow which would in turn increase NO production and nestin expression. These two events were significantly reversed with nilotinib treatment according to the results observed in the current study. Indeed, previous studies demonstrated an inhibitory effect of nilotinib on angiogenesis [10] as well as NO production [33].
Persistent injury leads to a wounding response, typified by fibrogenesis. A key feature of the cellular response to injury is the appearance of a population of specialized cells known as myofibroblasts which play a key role in the synthesis of ECM components [42]. Nilotinib administration significantly reduced kidney α-SMA expression which is suggestive for its ability to inhibit myofibroblast infiltration and collagen and ECM accumulation during the progression of DN with retraction of fibrosis and induction of tissue healing. In line with this, nilotinib has been reported to attenuate tubulointerstitial fibrosis in part by suppression of macrophage recruitment and concomitant down-regulation of pro-inflammatory cytokine expression [24].
Nilotinib has also been reported to reduce the up-regulation of several fibrosis-related genes, collagen type I, fibronectin, TGF-β, and PDGF in animal model of renal fibrosis with reflection on total collagen content. Nilotinib’s ability to inhibit PDGFRs, macrophage accumulation, and subsequent cytokine production has been reported to result in less vigorous fibrotic and inflammatory responses [24]. Dual inhibition of c-Abl and PDGF receptor by nilotinib and its significant inhibitory activity towards discoidin domain receptor (DDR1 and DDR2) have been proposed as a mechanism for its antifibrotic activity in some fibrotic disorders [10, 28, 46].
Interestingly, the effect of tyrosine kinase inhibitors on blood sugar is controversial. While there are some reports that tyrosine kinase inhibitors can reverse type I diabetes mellitus [29], improve glycemic control in type II diabetes mellitus [2], and can be proposed as novel drugs for the treatment of diabetes [38], there are some other reports concerning its ability to exacerbate diabetes mellitus in humans by decreasing secretion of endogenous insulin [23] and to induce mild and reversible hyperglycemia in type II diabetic patients [25, 43].
However, in the current study, nilotinib at the investigated dose (20 mg/kg) for 8 weeks did not interfere with blood sugar levels. Basal and sacrifice blood sugar levels were not significantly different in nilotinib control group. Meanwhile, treating diabetic animals with nilotinib for 8 weeks resulted in a significant reduction in sacrifice blood sugar in comparison to the diabetic control. However, blood sugar was still significantly high in comparison to healthy and nilotinib control groups.
In conclusion, nilotinib can be proposed as a promising drug for the management of DN. Positive therapeutic potential of nilotinib was evident in its ability to improve renal functions and to reduce nephrin, podocalyxin, and albumin excretion in the urine with concomitant preservation of morphological characteristics of the kidney. Significant structural and functional kidney improvements were evident. Nilotinib therapeutic potential is mediated through its ability to simultaneously target several key checkpoints and various intracellular signaling pathways implicated in the pathogenic pathway of DN including oxidative stress, NO production, angiogenesis, TLR4 expression, nestin and α-SMA expression, ECM accumulation, and NF-κB/p65 activity.
References
Abrahamson DR, Wang R (2003) Development of the glomerular capillary and its basement membrane. In: Vize P, Woolf A, Bard J (eds) The kidney; from normal development to congenital disease. Academic Press, San Diego, pp 221–243
Agostino NM, Chinchilli VM, Koszyk-Szewczyk AM, Gingrich R, Sivik JM, Drabick JJ (2011) Effect of the tyrosine kinase inhibitors (sunitinib, sorafenib, dasatinib, and imatinib) on blood glucose levels in diabetic and non-diabetic patients in general clinical practice. J Oncol Pharm Pract 17:197–202
Akhmetshina A, Dees C, Pileckyte M, Maurer B, Axmann R, Jüngel A, Zwerina J, Gay S, Schett G, Distler O, Distler JHW (2008) Dual inhibition of c-abl and PDGF receptor signaling by dasatinib and nilotinib for the treatment of dermal fibrosis. FASEB J 22:2214–2222
Arora S (2010) Renal function in diabetic nephropathy. J Diabetes 1:48–56
Berg UB, Torbjornsdotter TB, Jaremko G, Thalme B (1998) Kidney morphological changes in relation to long-term renal function and metabolic control in adolescents with IDDM. Diabetologia 41:1047–1056
Bergman L, Loxley R (1963) Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem 35:1961–1965
Davis C, Nick H, Agarwal A (2001) Manganese superoxide dismutase attenuates cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol 12:2683–2690
Day E, Waters B, Spiegel K, Alnadaf T, Manley PW, Buchdunger E, Walker C, Jarai G (2008) Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib. Eur J Pharmacol 599:44–53
de Cavanagh EM, Inserra F, Ferder L, Fraga CG (2000) Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am J Physiol Regul Integr Comp Physiol 278:572–577
Distler JHW, Distler O (2009) Tyrosine kinase inhibitors for the treatment of fibrotic diseases such as systemic sclerosis: towards molecular targeted therapies. Ann Rheum Dis 69:48–51
Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G (2003) Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes 52:1023–1030
Duran-Salgado MB, Rubio-Guerra AF (2014) Diabetic nephropathy and inflammation. J Diabetes 5:393–398
El Jamali A, Valente AJ, Lechleiter JD, Gamez MJ, Pearson DW, Nauseef WM, Clark RA (2008) Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic Biol Med 44:868–681
Elsherbiny NM, Abd El Galil KH, Gabr MM, Al-Gayyar MM, Eissa LA, El-Shishtawy MM (2012) Reno-protective effect of NECA in diabetic nephropathy: implication of IL-18 and ICAM-1. Eur Cytokine Netw 23:78–86
Fang J, Wei H, Sun Y, Zhang X, Liu W, Chang Q, Wang R, Gong Y (2013) Regulation of podocalyxin expression in the kidney of streptozotocin-induced diabetic rats with Chinese herbs (Yishen capsule). BMC Complement Altern Med 13:76–86
Golemovic M, Verstovsek S, Giles F, Cortes J, Manshouri T, Manley PW, Mestan J, Dugan M, Alland L, Griffin JD, Arlinghaus RB, Sun T, Kantarjian H, Beran M (2005) AMN107, a novel aminopyrimidine inhibitor of Bcr-Abl, has in vitro activity against imatinib-resistant chronic myeloid leukaemia. Clin Cancer Res 11:494–4947
Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA (2009) Proliferation of immature tumor vessels is a novel marker of clinical progression in prostate cancer. Cancer Res 69:4708–4715
Hara M, Yamagata K, Tomino Y, Saito A, Hirayama Y, Ogasawara S, Kurosawa H, Sekine S, Yan K (2012) Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia 55:2913–2919
Hebron ML, Lonskaya I, Olopade P, Selby ST, Pagan F, Moussa CE (2014) Tyrosine kinase inhibition regulates early systemic immune changes and modulates the neuroimmune response in α-synucleinopathy. J Clin Cell Immunol 30:5–259
Hessien MH, El-Sharkawi IM, El-Barbary AA, El-Beltagy DM, Snyder N (2010) Non-invasive index of liver fibrosis induced by alcohol, thioacetamide and schistosomal infection in mice. BMC Gastroenterol 1:10–53
Ijpelaar DH, Schulz A, Koop K, Schlesener M, Bruijn JA, Kerjaschki D, Kreutz R, de Heer E (2008) Glomerular hypertrophy precedes albuminuria and segmental loss of podoplanin in podocytes in Munich-Wistar-Fromter rats. Am J Physiol Renal Physiol 294(4):758–767
ILAR (Institute of Laboratory Animal Resources). Guide for the Care and Use of Laboratory Animals 1985 NIH Publication No. 86-23. National Academy Press, Washington, D.C
Ito Y, Miyamoto T, Chong Y, Maki T, Akashi K, Kamimura T (2013) Nilotinib exacerbates diabetes mellitus by decreasing secretion of endogenous insulin. Int J Hematol 9:135–138
Iyoda M, Shibata T, Hirai Y, Kuno Y, Akizawa T (2011) Nilotinib attenuates renal injury and prolongs survival in chronic kidney disease. J Am Soc Nephrol 22:1486–1496
Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, Goh YT, Rosti G, Nakamae H, Gallagher NJ, Hoenekopp A, Blakesley RE, Larson RA, Hughes TP (2011) Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol 12:841–851
Kim BK, Kim SE, Shim JH, Woo DH, Gil JE, Kim SK, Kim JH (2006) Neurogenic effect of vascular endothelial growth factor during germ layer formation of human embryonic stem cells. FEBS Lett 580:5869–5874
Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS, Chan KW, Lai KN, Tang SC (2012) Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol 23:86–102
Liu Y, Wang Z, Kwong SQ, Lui EL, Friedman SL, Li FR, Lam RW, Zhang GC, Zhang H, Ye T (2011) Inhibition of PDGF, TGF-β, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist nilotinib. J Hepatol 55:612–625
Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, Zhu S, Weiss A, Bluestone JA (2008) Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A 105:18895–18900
Luimula P, Ahola H, Wang SX, Solin ML, Aaltonen P, Tikkanen I, Kerjaschki D, Holthofer H (2000) Nephrin in experimental glomerular disease. Kidney Int 58:1461–1468
Ma J, Chadban SJ, Zhao CY, Chen X, Kwan T, Panchapakesan U, Pollock CA, Wu H (2014) TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS One 9, e97985
Manley PW, Drueckes P, Fendrich G, Furet P, Liebetanz J, Martiny-Baron G, Mestan J, Trappe J, Wartmann M, Fabbro D (2010) Extended kinase profile and properties of the protein kinase inhibitor nilotinib. Biochim Biophys Acta 1804:445–453
Manley PW, Stiefl N, Cowan-Jacob SW, Kaufman S, Mestan J, Wartmann M, Wiesmann M, Woodman R, Gallagher N (2010) Structural resemblances and comparisons of the relative pharmacological properties of imatinib and nilotinib. Bioorg Med Chem 18:6977–6986
Matsuda Y, Hagio M, Ishiwata T (2013) Nestin: a novel angiogenesis marker and possible target for tumor angiogenesis. J Gastroenterol 19:42–48
Michalczyk K, Ziman M (2005) Nestin structure and predicted function in cellular cytoskeletal organisation. Histol Histopathol 20:665–671
Ocuin LM, Zeng S, Cavnar MJ, Sorenson EC, Bamboat ZM, Greer JB, Kim TS, Popow R, DeMatteo RP (2012) Nilotinib protects the murine liver from ischemia/reperfusion injury. J Hepatol 57:766–773
Pavenstadt H, Kriz W, Kretzler M (2003) Cell biology of the glomerular podocyte. Physiol Rev 83:253–307
Prada PO, Saad MJ (2013) Tyrosine kinase inhibitors as novel drugs for the treatment of diabetes. Expert Opin Investig Drugs 22:751–763
Pugh CW, Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9:677–684
Pyriochou A, Beis D, Koika V, Potytarchou C, Papadimitriou E, Zhou Z, Papapetropoulos A (2006) Soluble guanylyl cyclase activation promotes angiogenesis. J Pharmacol Exp Ther 319:663–671
Rohilla A, Tiwari S, Rohilla S, Kushnoor A (2011) Diabetic nephropathy: pathogenesis, prevention and treatment. Eur J Exp Biol 1:72–80
Roy SG, Nozaki Y, Phan SH (2001) Regulation of alpha-smooth muscle actin gene expression in myofibroblast differentiation from rat lung fibroblasts. Int J Biochem Cell Biol 33:723–734
Saglio G, Larson RA, Hughes TP, Issaragrisil S, Turkina AG, Marin D, Zanichelli M, Shibayama H, Kalaycio ME, Rigal-Huguet F, Gallagher NJ, Kayath M, Zheng M, Kantarjian HM, Hochhaus A (2010) Efficacy and safety of nilotinib in chronic phase (CP) chronic myeloid leukemia (CML) patients (Pts) with type 2 diabetes in the ENESTnd trial. Blood 116:3430
Said E, Said SA, Gameil NM, Ammar EM (2013) Modulation of thioacetamide-induced liver fibrosis/cirrhosis by sildenafil treatment. Can J Physiol Pharmacol 91:1055–1063
Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H (2010) The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem Cytochem 58:721–730
Van Steensel L, Paridaens D, Schrijver B, Dingjan GM, van Daele PL, van Hagen PM, van den Bosch WA, Drexhage HA, Hooijkaas H, Dik WA (2009) Imatinib mesylate and AMN107 inhibit PDGF-signaling in orbital fibroblasts: a potential treatment for Graves’ ophthalmopathy. Invest Ophthalmol Vis Sci 50:3091–3098
Wong J, Smith LB, Magun EA, Engstrom T, Kelley-Howard K, Jandhyala DM, Thorpe CM, Magun BE, Wood LJ (2013) Small molecule kinase inhibitors block the ZAK-dependent inflammatory effects of doxorubicin cancer. Biol Ther 14:56–63
Zelmanovitz T, Gerchman F, Balthazar AP, Thomazelli FC, Matos JD, Canani LH (2009) Diabetic nephropathy. Diabetol Metab Syndr 1:10
Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB (2008) TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol 19:923–932
Ziyadeh FN, Wolf G (2008) Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 4:39–45
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elsherbiny, N.M., El-Sherbiny, M. & Said, E. Amelioration of experimentally induced diabetic nephropathy and renal damage by nilotinib. J Physiol Biochem 71, 635–648 (2015). https://doi.org/10.1007/s13105-015-0428-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-015-0428-6