Abstract
Lupeol, a dietary triterpene present in many fruits and medicinal plants, has been reported to possess many pharmacological properties including cancer-preventive and anti-cancer effects in vitro and in vivo. Here, we investigated the anti-cancer efficacy and adjuvant chemotherapy action of lupeol in gastric cancer (GC) cells (SGC7901 and BGC823) and explored the underlying mechanisms. Cells were treated with lupeol and/or 5-fluorouracil (5-Fu) and subjected to cell viability, colony formation, apoptosis, western blot, semiquantitative RT-PCR, and xenograft tumorigenicity assay. Our results showed that lupeol and 5-Fu inhibited the proliferation of SGC7901 and BGC823 cells, and combination treatment with lupeol and 5-Fu resulted in a combination index < 1, indicating a synergistic effect. Co-treatment with lupeol and 5-Fu induced apoptosis through up-regulating the expressions of Bax and p53 and down-regulating the expressions of survivin and Bcl-2. Furthermore, co-treatment displayed more efficient inhibition of tumor weight and volume on BGC823 xenograft mouse model than single-agent treatment with 5-Fu or lupeol. Taken together, our findings highlight that lupeol sensitizes GC to 5-Fu treatment, and combination treatment with lupeol and 5-Fu would be a promising therapeutic strategy for human GC treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fourth most frequent type of cancer and the second leading cause of cancer-related death around the world (Siegel et al. 2012). The only potentially curative treatment for GC is surgery, but in patients with unresectable disease, chemotherapy is the main course of treatment (Cervantes et al. 2008). Unfortunately, the rapid development of new chemotherapeutic agents have done little to improve the prognosis of advanced and recurrent GC, with the overall 5-year survival rates ranging from 5 to 15 % (Luo et al. 2010). Therefore, there is an urgent need to identify novel therapeutic agents to treat patients with advanced GC.
5-Fluorouracil (5-Fu) is a widely used chemotherapeutic agent in gastrointestinal malignancies, including GC. Although most patients display chemosensitivity to 5-Fu at the beginning of therapy, fast acquired resistance and severe adverse effects have limited its application in cancer treatment (Longley et al. 2003). Nowadays, combination chemotherapy has been considered as a more effective treatment strategy. For instance, oxymatrine or resveratrol combined with 5-Fu cannot only improve the response rates but also reduce the seriousness of the side effects (Liu et al., 2015a; Santandreu et al. 2011). Despite these improvements, novel chemotherapeutic regimens and new chemo-sensitizers are urgently needed.
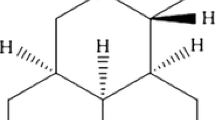
Lupeol [Lup-20(29)-en-3b-ol] (Fig. 1), a dietary triterpene, found in several medicinal plants and various fruits, such as olive, mango, grapes, figs, and vegetables, is used for treatment of lots of ailments worldwide (Imam et al. 2007; Saleem 2009; You et al. 2003). Extensive research over the past decades have revealed various important pharmacological activities of lupeol (Chaturvedi et al., 2008; Ardiansyah et al. 2012; Liu et al. 2013; Tarapore et al. 2010; Saleem et al. 2009), and our previous research also suggested that lupeol inhibited cell proliferation and induced cell apoptosis of human pancreatic cancer, gallbladder carcinoma, and osteosarcoma in a dose-dependent manner (Liu et al., 2015c; Liu et al. 2016; Liu et al., 2015d). However, there is no any investigation about the chemo-sensitization effect of lupeol until now.
In the present study, we utilized the human GC SGC7901 and BGC823 cells and BGC823 xenograft mouse model to explore the possible chemo-sensitization effect of lupeol to potentiate the anti-tumor effect of 5-Fu in vitro and in vivo and found that these two agents act synergistically anti-cancer activity. These findings may provide a new therapeutic strategy to achieve anti-cancer synergism.
Materials and methods
Reagents and antibodies
Lupeol and 5-Fu were purchased from Sigma-Aldrich (St. Louis, MO, USA), and a stock solution of lupeol (30 mmol/L) was prepared by resuspension in warm alcohol and dilution in dimethyl sulfoxide (DMSO) at 1:1 ratio. 5-Fu was dissolved in DMSO to a final storage concentration of 100 mg/mL. Lupeol and 5-Fu solution were sterilized through 0.22-μm filter for use in subsequent experiments and stored in –20 °C.
Materials used included 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma Chemical Company, St. Louis, CA); Apoptosis Detection Kit (MultiSciences Biotech, Shanghai, China); Hoechst 33342 Staining Assay Kit (Molecular Probes, Beyotime Institute of Biotechnology, Shanghai, China); TRIzol Reagent (Invitrogen, Carlsbad, CA); glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies (Kangchen Bio-tech, Shanghai, China); and survivin, Bax, Bcl-2, p53, and horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin G (IgG) and sheep anti-rabbit IgG antibodies (Cell Signaling Technology, Danvers, MA).
Cell lines and culture
The human GC cell lines (SGC7901 and BGC823) were obtained from the Shanghai Cell Institute Country Cell Bank (Shanghai, China). The cells were maintained in RPMI-1640 medium (Gibco, Gaithersburg, MD, USA); supplemented with 10 % fetal bovine serum (Gibco, USA), 100 mg/mL streptomycin, and 100 u/mL penicillin (HyClone, USA); and incubated in a humidified atmosphere with 5 % CO2 at 37 °C. The cells were kept in an exponential growth phase during experiments.
Cell viability assay
Cell viability was measured using the MTT assay and colony formation test. For MTT assay, SGC7901 and BGC823 cells were seeded at 1 × 104 cells/well into 96-well plates and cultured overnight, then exposed to 5-Fu (0, 2, 5, 10, 25, 100, and 200 μg/mL), lupeol (7.5, 15, and 30 μM), or 5-Fu + lupeol for 48 h. Untreated cells served as controls. Thereafter, 20 μL of MTT solution (5 mg/mL) was added to each well and the cells were incubated for a further 4 h at 37 °C. After removal of the culture medium, cell was lysed in 200 μL of DMSO and the optical density (OD) was measured at 490 nm using a microplate reader. The change of cell viability was calculated using the formula (1 − average absorbance of treated group/average absorbance of control group) × 100 %.
For colony formation test, SGC7901 and BGC823 (800 cells/well) cells were plated into 6-well plates and cultured at 37 °C with 5 % CO2. The medium was replaced with fresh culture media every 2 days. After 10 days, the plates were fixed with 4 % paraformaldehyde at 4 °C for 15 min and stained using Giemsa for 30 min. Then, the number of stained colonies that contained 50 cells was manually counted. Proliferation potential was assessed as relative colony formation rate (%) = number of colonies in the treatment group/number of colonies in the control group × 100 %.
Evaluation for combination index
After detection of the single and combination inhibitory effects of 5-Fu and lupeol, the combination index (CI) was calculated according to the method of Chou (Chou 2010) using CalcuSyn software program. The value of CI is a quantitative measure of the degree of interaction between different drugs. CI < 1 indicates synergism, CI = 1 denotes additive effects, and CI > 1 denotes antagonism.
Evaluation of cell apoptosis
Cell apoptosis was detected as previously described (Liu et al. 2015b). BGC823 cells were treated with different concentrations of 5-Fu and/or lupeol for 48 h, harvested, and prepared for flow cytometry analysis (Becton Dickinson, Franklin Lakes, NJ, USA).
Hoechst 33342 staining was used to confirm the alterations of nucleus morphology of BGC823 cells after 5-Fu, lupeol, or 5-Fu + lupeol treatment. Briefly, after incubation for 48 h, cells were stained with 10 μg/mL Hoechst 33342 at 37 °C in the dark for 15 min, then washed with phosphate-buffered saline (PBS), and observed using an inverted fluorescence microscope.
Western blot analysis
Western blot analysis was performed as described previously (Liu et al., 2015c; Liu et al. 2016). Proteins (40 μg) were separated by SDS-PAGE (8, 10, or 12 %) and then transferred to polyvinylidene difluoride membrane (Beyotime Institute of Biotechnology). The membranes were incubated with primary antibodies overnight at 4 °C and then with appropriate secondary antibodies conjugated to horseradish peroxidase for 1 h at room temperature. Signals were visualized by ECL chemiluminescence. The GAPDH expression was used as reference band. The protein expression rate was semiquantified using Image J software.
Semiquantitative real-time polymerase chain reaction analysis
For gene expression analysis, BGC823 cells (1 × 105 cells/well in a 24-well plate) were grown for 24 h and subsequently treated with 5-Fu, lupeol, or 5-Fu + lupeol for 48 h. Total RNA was extracted with TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions, and first-strand complementary DNA was synthesized from 2 μg of total RNA using Prime Script Reverse Transcriptase (TaKaRa, Shiga, Japan). The polymerase chain reaction (PCR) primer and regimen are as follows: 5′-CAGATTTGAATCGCGGGACCC-3′, 5′-CCAAGTCTGGCTCGTTCTCAG-3′ for survivin (206 bp, 38 cycles); 5′-GGCCCACCAGCTCTGAGCAGA-3′, 5′-GCCACGTGGGCGGTCCCAAAGT-3′ for Bax (479 bp, 42 cycles); 5′-GTGGAGGAGCTCTTCAGGGA-3′, 5′-AGGCACCCAGGGTGATGCAA-3′ for Bcl-2 (304 bp, 42 cycles); 5′-CAGCCAAGTCTGTGACTTGCACGTAC-3′, 5′-CTATGTCGAAAAGTG TTTCTGTCATC-3′ for p53 (396 bp, 38 cycles); and 5′-GGAGTCCTGTGGCATCCACG-3′, 5′-CTAGAAGCATTTGCGGTGGA-3′ for β-actin (322 bp, 30 cycles). The PCR conditions included denaturation at 94 °C for 1 min, annealing at 56 °C for 1 min, and extension at 72 °C for 2 min. Samples were separated in 20-g/L agarose gel and visualized with ethidium bromide staining under ultraviolet light.
Animal experiment
Animal studies were carried out as we previously described (Liu et al. 2015d). Exponentially growing BGC823 cells (5 × 106) were suspended in 200 μL PBS and subcutaneously injected into the right axillary fossa of each nude mouse. On day 6, a total of 32 nude mice whose tumors were similar in size (6–8 mm in diameter) were chosen and equal numbers were assigned to four groups (n = 8 per group). Group I was given sterile physiological saline via intraperitoneal injection every other day, group II was given 10 mg/kg 5-Fu via intraperitoneal injection every other day, group III was injected with 30 mg/kg lupeol intraperitoneally every other day, and group IV was given 10 mg/kg 5-Fu + 30 mg/kg lupeol. After 2 weeks of drug administration, all mice were killed on day 28, and tumors were dissected and weighed. The tumor volume and inhibition ratio were detected as previously described (Liu et al., 2015a).
Then, formaldehyde-fixed, paraffin-embedded tissue blocks were prepared from xenograft tissue and cut into serial sections (4 μm) for hematoxylin-eosin (H&E) staining. And, the apoptosis of paraffin-embedded tumor sections was detected using a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay kit according to manufacturer’s instructions. The animal studies were approved by the Wujiang No. 1 People’s Hospital Ethics Committee, and the principles of laboratory animal care were followed in all animal experiments.
Statistical analysis
All experiments presented here derived from at least three independent experiments. All data are expressed as mean ± standard deviation (SD) and analyzed by the SPSS 17.0 software. Comparisons among different groups were performed using one-way analysis of variance, and the P value less than 0.05 was considered statistically significant.
Results
Lupeol inhibited the growth of human GC cells and enhanced 5-Fu efficacy in vitro
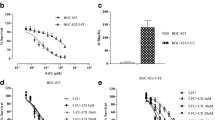
Firstly, we evaluated the potential role of 5-Fu and lupeol on the cell growth of SGC7901 and BGC823 cells using MTT assay. As shown in Fig. 2a, BGC823 cells performed more sensitive than SGC7901 cells to 5-Fu treatment. When the concentration exceeded 25 μg/mL, SGC7901 and BGC823 cells exhibited a resistance trend to 5-Fu treatment. However, lupeol inhibited SGC7901 and BGC823 cell proliferation in a dose-dependent manner (Fig. 2b, c). When cells were treated simultaneously with 5-Fu (2 and 5 μg/mL) and lupeol (7.5, 15, and 30 μM), a stronger inhibitory effect on cell proliferation was derived than single-agent treatment (Fig. 2b, c). To further verify the synergistic effects of 5-Fu and lupeol, the CalcuSyn software was used to analyze the cell viability inhibition effects of the single and combined treatment. The CI value for 5-Fu + lupeol treatment was 0.76 ± 0.08 for SGC7901 cells and 0.67 ± 0.11 for BGC823 cells under the applied dosages (Fig. 2b, c). For BGC823 cells, the combination of 5-Fu (2 μg/mL) and lupeol (7.5 μM) displayed the best synergistic inhibition capacity, which was selected for further investigations.
Lupeol synergistically enhanced the anti-proliferative effect of 5-Fu in GC cells. a–c Cell viability was measured by MTT assay after 5-Fu and/or lupeol treatment in SGC7901 and BGC823 cells and combination index (CI) value was analyzed using CalcuSyn software. d–f Colony formation of GC cells after 5-Fu and/or lupeol treatment. *P < 0.05 versus the control group, **P < 0.05 versus 5-Fu or lupeol alone group
Then, we performed colony formation test to explore the effect of 5-Fu and lupeol on GC cell tumorigenicity. We found that single-agent treatment with 5-Fu or lupeol decreased both the size and number of colonies, and the combination of both agents can further attenuate the colony-forming ability compared with single-drug therapy (Fig. 2d, f).
Lupeol enhanced 5-Fu-induced apoptosis in human GC cells
Hoechst 33342 staining was employed to reveal the morphological changes in the apoptotic cells. Results showed that BGC823 cells treated with 5-Fu or lupeol alone exhibited morphological features of early apoptotic cells, such as bright nuclear condensation or fragments, whereas in 5-Fu + lupeol group, apoptotic bodies began to appear and the number of late apoptotic cells increased (Fig. 3c, d). We further quantified the apoptosis of BGC823 cells by flow cytometry assay. As shown in Fig. 3a, b, either 5-Fu or lupeol could induce apoptosis of BGC823 cells, and combination treatment could achieve the greatest apoptosis rate (P < 0.05 vs 5-Fu or lupeol alone). These data suggested that lupeol enhanced 5-Fu-induced apoptosis in human GC cells.
Combination treatment with lupeol- and 5-Fu-induced apoptosis in GC cells. a,b Cell apoptosis was detected by annexin V–fluorescein isothiocyanate (FITC)/propidium iodide (PI) binding assay in BGC823 cells. c,d The nuclei were stained by Hoechst 33342 and the percentage of apoptosis cells was calculated as apoptosis index (AI; %). *P < 0.05 versus the control group, **P < 0.05 versus 5-Fu or lupeol alone group
Lupeol potentiated the anti-tumor activity of 5-Fu through promoting the apoptotic signaling pathway
To investigate the possible molecular mechanisms responsible for 5-Fu- and lupeol-induced apoptosis in BGC823 cells, we evaluated the changes of protein and messenger RNA (mRNA) levels of survivin, Bax, Bcl-2, and p53 by western blot and semiquantitative real-time (RT)-PCR analysis, respectively. Western blot analysis showed the expressions of Bax and p53 were up-regulated, while the expressions of survivin and Bcl-2 were down-regulated in the cells treated with 5-Fu and/or lupeol. This finding was confirmed in mRNA levels by RT-PCR analysis (Fig. 4c, d). It is important to note that these effects were more pronounced when 5-Fu and lupeol were used together than when each was used alone.
Lupeol potentiated the anti-tumor activity of 5-Fu through promoting the apoptotic signaling pathway. a Expressions of survivin, Bax, Bcl-2, and p53 were analyzed by western blot assay. An up-regulation in the levels of Bax and p53, and a down-regulation of survivin and Bcl-2, occurred in BGC823 cells after treatment with 5-Fu and/or lupeol for 48 h. b Band density ratio of survivin, Bax, Bcl-2, and p53 to GAPDH was shown as means ± standard deviation (SD). c An up-regulation in the mRNA levels of Bax and p53, and a down-regulation of survivin and Bcl-2, occurred in BGC823 cells after treatment with 5-Fu and/or lupeol for 48 h. d The band density ratio of survivin, Bax, Bcl-2, and p53 to β-actin was shown as means ± SD. For one experiment, three assays were carried out but only one set of gel is shown. *P < 0.01 versus 5-Fu or lupeol alone group
Lupeol treatment improved the anti-tumor effect of 5-Fu in vivo
Further, we studied the effects of lupeol and 5-Fu on GC cell growth in vivo. The xenograft model was established in BALB/c nude mice following subcutaneous transplantation of BGC823 cells. The results showed that treatment with 5-Fu (10 mg/kg) or lupeol (30 mg/kg) alone had little effect on the weight and volume of GC xenograft tumors, whereas the combination of both agents resulted in a significant reduction in GC tumor growth (Fig. 5a). The mean tumor weights in different groups were also calculated, and results showed that the tumor weight inhibition rates for 5-Fu, lupeol, and 5-Fu + lupeol treatment groups were 27.47, 37.94, and 73.79 %, respectively (Fig. 5c). The trends of tumor volume inhibition in different groups were consistent with tumor weight inhibition (Fig. 5d).
Combination treatment with 5-Fu and lupeol inhibited tumorigenicity in vivo. a The BGC823 cells were subcutaneously injected into mouse right axillary fossa to establish xenograft models, and mice were treated with control (sterile physiological saline), 5-Fu (10 mg/kg), lupeol (30 mg/kg), or 5-Fu + lupeol. Four representatives of eight tumors in each group were shown. b H&E staining analyses of the pathological features of the tumors from the four groups. c, d The final tumor weight and volume in each group after treatment. b, e The apoptotic index as determined by the percentage of TUNEL-stained nuclei was calculated. All data are expressed as means ± SD. *P < 0.01 versus 5-Fu or lupeol alone group
We next examined the subcutaneous tumor tissues by H&E staining. The tumor tissue from control mice showed compact tumor cells with blue-purple nuclei and pink cytoplasm. In 5-Fu or lupeol treatment group, the tumor cells were sparse and separated from each other. In 5-Fu + lupeol treatment group, the structure of the tumor tissue was more seriously damaged than the single-agent treatment group, and the nuclei were polygonal and lightly stained (Fig. 5c). TUNEL staining of tumor sections was performed to detect tumor apoptosis in vivo. Co-treatment with 5-Fu and lupeol significantly increased the apoptotic index, as determined by the percentage of TUNEL-stained nuclei (P < 0.01 vs 5-Fu or lupeol alone; Fig. 5e).
Discussion
Because of the limited efficiency of single-agent chemotherapy in the treatment of human cancers, many combination therapies, by using effective chemo-sensitizers to augment the response rate of existing anti-cancer drug and simultaneously overcome their resistance, have been applied in the clinic recently (Santandreu et al. 2011; Kong et al. 2015). Increasing studies have demonstrated that the extracts of Chinese traditional medicines, such as oxymatrine, curcumin, and gypenosides, could act as chemo-sensitizer to enhance the efficacy of 5-Fu in human cancer cells (Liu et al., 2015b; Shakibaei et al. 2013; Kong et al. 2015). However, whether lupeol can become a good chemo-sensitizer to amplify the effectiveness of chemotherapy in clinic is not clear. In this study, we found that lupeol displays a splendid chemo-sensitization effect to potentiate the 5-Fu-induced cell growth inhibition in vitro and in vivo. To the best of our knowledge, the present study is the first preclinical research that assesses the chemo-sensitization effect of lupeol and the anti-tumor effect of using 5-Fu and lupeol in combination.
Uncontrolled cell proliferation is a key aspect of tumorigenesis, and inhibiting proliferation can achieve growth arrest in tumor cells. Cell apoptosis is an autonomous cell death process, and it is reported that the deregulation of apoptosis is hallmark of all cancer cells (Kim et al. 2010). Clearly, an agent which could efficiently inhibit the proliferation and induce apoptosis of cancer cells would be a hopeful candidate to suppress cancer progression and thus could reduce mortality. Our previous studies have shown that lupeol possesses this capability to inhibit proliferation as well as induce apoptosis in human pancreatic cancer cells through AKT/ERK pathways (Liu et al., 2015c) and induces apoptosis of human osteosarcoma cells through PI3K/AKT/mTOR pathway (Liu et al. 2016). In our present study, the MTT assay and colony formation test demonstrated that the cell viabilities of SGC7901 and BGC823 decreased significantly after co-treated with low doses of 5-Fu and lupeol in comparison to either single treatment. The results from Annexin V-FITC/PI staining suggested that 5-Fu and lupeol in combination effectively induced BGC823 cell apoptosis compared to 5-Fu or lupeol alone.
Cell apoptosis is induced and controlled by many complicated factors, such as blockage of cell cycle and expression changes of correlative apoptosis genes. Previous studies have shown that the Bcl-2 family related genes and survivin gene function as key regulators in cell apoptosis (Mita et al., 2008; Richardson et al. 2008). The Bcl-2 family members can be classified into the following three subfamilies: pro-apoptotic members such as Bax, Bad, Bid, and Bcl-Xs; anti-apoptotic members such as Bcl-2 and Bcl-xL; and the BH3-only members such as Bim and Bad (Cotter et al. 2009). Most therapeutic agents act by blocking the function of anti-apoptotic Bcl-2 proteins or by enhancing the activities of pro-apoptotic proteins such as Bax. Once the brake provided by anti-apoptotic Bcl-2 members is removed, the pro-apoptotic protein Bax oligomerizes at the mitochondrial membrane leading to release of apoptogenic factors into the cytosol, an event initiating a deadly proteolytic cascade (Wei et al. 2004; Song et al. 2007). Survivin belongs to the anti-apoptotic protein family and is a good marker for most cancer cells (Marconi et al. 2007). Various strategies to target survivin in cancer treatment are currently under investigation with promising results (Altieri et al. 2006). P53 is a multifunctional protein with multiple modifications and biochemical properties. It has the ability to bind to specific DNA sequences and functions as a potent transcription factor that trans-represses and trans-activates specific genes involved in controlling cell apoptosis and cycle (Prives et al. 1999). Recent research indicated that common anti-cancer drugs can perform their functions by modulating Bcl-2 protein expression, whereas this modulation seems ultimately to lead to the presence of p53 (Coutts et al. 2006). In this study, we found that lupeol in combination with 5-Fu could up-regulate the expressions of Bax and p53 and down-regulate the expressions of survivin and Bcl-2, either in mRNA level (detected by semiquantitative RT-PCR) or in the protein level (detected by western blot analysis). These data strongly support that the inhibitory of lupeol in combination with 5-Fu is associated with their ability to induce apoptosis by regulating the transcription and translation of survivin, Bax, Bcl-2, and p53 genes.
In addition, in our in vivo study, we found that both 5-Fu and lupeol could induce tumor inhibition, especially when combination treatment was applied. More importantly, H&E staining analyses of the tumors from mice treated with lupeol or 5-Fu revealed morphological feature characteristic of apoptotic cells, and the structure of the tumor tissue was more seriously damaged in 5-Fu + lupeol treatment group. Also, co-treatment with 5-Fu and lupeol significantly increased the apoptosis of tumor cells, as determined by the TUNEL staining, which was consistent with our findings in vitro.
In conclusion, our present investigation confirms that co-treatment with lupeol and 5-Fu could synergistically inhibit human GC cell proliferation, induce cell apoptosis, and reduce tumorigenicity in vitro and in vivo. The synergistic effects of lupeol and 5-Fu may be associated with the down-regulation of survivin and Bcl-2 expressions and up-regulation of Bax and p53 expressions. Thus, we propose that lupeol may be a promising adjuvant chemotherapy agent in treatment of human GC.
Change history
13 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00210-021-02141-y
References
Altieri DC (2006) The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol 18(06):609–615
Ardiansyah YE, Shirakawa H, Hata K, Hiwatashi K, Ohinata K, Goto T, Komai M (2012) Lupeol supplementation improves blood pressure and lipid metabolism parameters in stroke-prone spontaneously hypertensive rats. Biosci Biotechnol Biochem 76(1):183–185
Cervantes A, Rosello S, Roda D, Rodriguez-Braun E (2008) The treatment of advanced gastric cancer: current strategies and future perspectives. Ann Oncol 19(Suppl 5):v103–v107
Chaturvedi PK, Bhui K, Shukla Y (2008) Lupeol: connotations for chemoprevention. Cancer Lett 263(1):1–13
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70(2):440–446
Cotter TG (2009) Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer 9(7):501–507
Coutts AS, La Thangue N (2006) The p53 response during DNA damage: impact of transcriptional cofactors. Biochem Soc Symp 73:181–189
Imam S, Azhar I, Hasan MM, Ali MS, Ahmed SW (2007) Two triterpenes lupanone and lupeol isolated and identified from Tamarindus indica linn. Pak J Pharm Sci 20(2):125–127
Kim JH, Choi YW, Park C, Jin CY, Lee YJ, Park DJ, Kim SG, Kim GY, Chou IW, Hwang WD, Jeong YK, Kim SK, Choi YH (2010) Apoptosis induction of human leukemia U937 cells by gomisin N, a dibenzocyclooctadiene lignan, isolated from Schizandra chinensis Baill. Food Chem Toxicol 48(3):807–813
Kong LL, Wang XB, Zhang K, Yuan WJ, Yang QW, Fan JP, Wang P, Liu QH (2015) Gypenosides synergistically enhances the anti-tumor effect of 5-fluorouracil on colorectal cancer in vitro and in vivo: a role for oxidative stress-mediated DNA damage and p53 activation. PLoS one 10(9):e0137888
Liu F, He Y, Liang Y, Wen LJ, Zhu WM, Wu Y, Zhao LX, Li YS, Mao XL, Liu HY (2013) Pi3-kinase inhibition synergistically promoted the anti-tumor effect of lupeol in hepatocellular carcinoma. Cancer Cell Int 13(1):108
Liu Y, Bi T, Wang G, Dai W, Wu G, Qian L, Gao Q, Shen G (2015a) Lupeol inhibits proliferation and induces apoptosis of human pancreatic cancer PCNA-1 cells through AKT/ERK pathways. Naunyn Schmiedeberg’s Arch Pharmacol 388(3):295–304
Liu Y, Bi T, Dai W, Wang G, Qian L, Shen G, Gao Q (2015b) Lupeol induces apoptosis and cell cycle arrest of human osteosarcoma cells through PI3K/AKT/mTOR pathway. Technol Cancer Res T. doi:10.1177/1533034615609014
Liu Y, Bi T, Dai W, Wang G, Qian L, Gao Q, Shen G (2015c) Oxymatrine synergistically enhances the inhibitory effect of 5-fluorouracil on hepatocellular carcinoma in vitro and in vivo. Tumor biol. doi:10.1007/s13277-015-4642-1
Liu Y, Bi T, Dai W, Wang G, Qian L, Gao Q, Shen G (2015d) Effects of oxymatrine on the proliferation and apoptosis of human hepatoma carcinoma cells. Technol Cancer Res T. doi: 10.1177/1533034615587616
Liu Y, Bi T, Shen G, Li Z, Wu G, Wang Z, Qian L, Gao Q (2016) Lupeol induces apoptosis and inhibits invasion in gallbladder carcinoma GBC-SD cells by suppression of EGFR/MMP-9 signaling pathway. Cytotechnology 68:123–133
Longley DB, Harkin DP, Johnston PG (2003) 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3(5):330–338
Luo HY, Wei W, Shi YX, Chen XQ, Li YH, Wang F, Qiu MZ, Li FH, Yan SL, Zeng MS, Huang P, Xu RH (2010) Cetuximab enhances the effect of oxaliplatin on hypoxic gastric cancer cell lines. Oncol Rep 23(6):1735–1745
Marconi A, Dallaglio K, Lotti R, Vaschieri C, Truzzi F, Fantini F, Pincelli C (2007) Survivin identifies keratinocyte stem cells and is downregulated by anti-beta1 integrin during anoikis. Stem Cells 25(01):149–155
Mita AC, Mita MM, Nawrocki ST, Giles FJ (2008) Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res 14(16):5000–5005
Prives C, Hall PA (1999) The p53 pathway. J Pathol 187(1):112–126
Richardson A, Kaye SB (2008) Pharmacological inhibition of the Bcl-2 family of apoptosis regulators as cancer therapy. Curr Mol Pharmacol 1(3):244–254
Saleem M (2009) Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett 285(2):109–115
Saleem M, Murtaza I, Tarapore R, Suh Y, Adhami VM, Johnson JJ, Siddiqui IA, Khan N, Asim M, Hafeez BB, Shekhani MT, Li B, Mukhtar H (2009) Lupeol inhibits proliferation of human prostate cancer cells by targeting beta-catenin signaling. Carcinogenesis 30(5):808–817
Santandreu FM, Valle A, Oliver J, Roca P (2011) Resveratrol potentiates the cytotoxic oxidative stress induced by chemotherapy in human colon cancer cells. Cell Physiol Biochem 28(2):219–228
Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A (2013) Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-kappaB and Src protein kinase signaling pathways. PLoS one 8(2):e57218
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics 2012. CA Cancer J Clin 62(1):10–29
Song MQ, Zhu JS, Chen JL, Wang L, Da W, Zhu L, Zhang WP (2007) Synergistic effect of oxymatrine and angiogenesis inhibitor NM-3 on modulating apoptosis in human gastric cancer cells. World J Gastroenterol 13(12):1788–1793
Tarapore RS, Siddiqui IA, Saleem M, Adhami VM, Spiegelman VS, Mukhtar H (2010) Specific targeting of Wnt/β-catenin signaling in human melanoma cells by a dietary triterpene lupeol. Carcinogenesis 31(10):1844–1853
Wei MC (2004) Bcl-2-related genes in lymphoid neoplasia. Int J Hematol 80(03):205–209
You YJ, Nam NH, Kim Y, Bae KH, Ahn BZ (2003) Antiangiogenic activity of lupeol from Bombax ceiba. Phytother Res 17(4):341–344
Acknowledgments
This study was supported by the Program for Young Scientist in Science and Education of Suzhou City (No. KJXW2014053) and the Program for Young Scientist in Science and Education of Wujiang District (Nos. WWK201415 and WWK201516).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Liu, Y., Bi, T., Dai, W. et al. RETRACTED ARTICLE: Lupeol enhances inhibitory effect of 5-fluorouracil on human gastric carcinoma cells. Naunyn-Schmiedeberg's Arch Pharmacol 389, 477–484 (2016). https://doi.org/10.1007/s00210-016-1221-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-016-1221-y