Abstract
The recent resolution of G-protein-coupled receptor (GPCR) structures in complex with Na+ bound to an allosteric modulatory site has renewed interest of the regulation of GPCRs by ions. Here, we summarise key data on ion modulation of GPCRs, obtained in pharmacological, crystallographic, mutagenesis and molecular modelling studies. We show that ion modulation is a highly complex process, involving not only cations but also, rather neglected until now, anions. Pharmacotherapeutic and toxicological aspects are discussed. We provide a mathematical framework for the analysis of ion effects. Finally, we discuss open questions in the field and future research directions. Most importantly, the in vivo relevance of the modulation of GPCR function by monovalent ions must be clarified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many G-protein-coupled receptors (GPCRs) exhibit sensitivity to modulation by monovalent cations, e.g. sodium ions (Neve 1991; Martin et al. 1999; Schetz 2005; Ericksen et al. 2009; Schnell and Seifert 2010). Table 1 summarises representative studies for Gs-, Gi- and Gq-coupled receptors. Most studies were performed with cell or organ membranes. When working with cell membranes, the experimentally given ion concentration is the same for the intra- and extracellular side of the analysed receptor, while this is not the case when working with intact cells. In the latter case, Na+ is the predominant cation extracellularly, while K+ dominates intracellularly. Initially, the analysis of the effects of monovalent on GPCRs in cell membranes was conducted without an a priori mechanistic hypothesis but rather with the intention to “optimise” experimental conditions for radioligand binding studies and G-protein assays, i.e. high-affinity GTPase assays and [35S]GTPγS-binding assays. With regard to GTPase and [35S]GTPγS-binding assays, the major goal was to enlarge the signal-to-noise window between “basal” G-protein activity and agonist-stimulated G-protein activity.
The functional studies on ion effects are very heterogeneous in terms of the systems studied, parameters analysed and results obtained. Particularly, the rank order of efficacy/potency of cations is highly system-dependent, and in some cases, anions have more influence than cations, pointing to a highly complex modulation of GPCRs by ions (Table 1). Because of the complexity of the effects of monovalent cations, for a long time, it was difficult to frame them into an overarching pharmacological concept, although the differential effects of various salts clearly indicated that ion effects exhibit some degree of specificity (see examples in Table 1).
The differential effects of various salts on receptor/G-protein coupling suggested some sort of “binding site” for ions on signalling proteins, a hypothesis that developed post hoc based on experimental data. In the late 1980s and early 1990s, the concept of constitutive GPCR activity was developed. This concept assumes that receptors exist in an inactive (R) and an active (R*) state and that they isomerize between these two states (Lefkowitz et al. 1993). The equilibrium between these two states is different for any given receptor, and when agonist-independent (constitutive) R to R* isomerization is sufficiently high, measurable basal G-protein- and effector activation emerges (Seifert and Wenzel-Seifert 2002). This basal G-protein and effector activity can be reduced by inverse agonists that stabilise the R state. Furthermore, it was noted for several receptors including β2-adrenoceptor and chemoattractant receptors that in addition to inverse agonists, Na+ can effectively reduce constitutive GPCR activity (see examples in Table 1) giving rise to the concept that Na+ acts as universal allosteric inverse agonist (Seifert and Wenzel-Seifert 2001). This pharmacological concept was confirmed by the recent elegant studies showing crystal structures of GPCRs bound to Na+ (Liu et al. 2012; Fenalti et al. 2014; Miller-Gallacher et al. 2014; Katritch et al. 2014). The crystal structures show that the Na+ binds in an allosteric binding site near the highly conserved Asp2.50 (Liu et al. 2012; Fenalti et al. 2014; Miller-Gallacher et al. 2014; Katritch et al. 2014). Based on the simulation data available in literature, so far it can be assumed that the monovalent cations bind to the allosteric binding site by coming from the extracellular side (Selent et al. 2010; Yuan et al. 2013; Wittmann et al. 2014b). In this binding trajectory, the cation has to pass the orthosteric ligand binding site (Selent et al. 2010; Yuan et al. 2013; Wittmann et al. 2014b). An entry of the monovalent cation coming from the intracellular side has not been described until now and is unlikely because the intracellular side of the receptor is, compared with the extracellular side, more positively charged. For example, according to the amino acid sequence, the extracellular domains of the hH4R exhibit an elementary net charge of −5, whereas the intracellular domains exhibit an elementary net charge of +22 (Brunskole et al. 2011). Furthermore, it can be assumed that the binding trajectory for monovalent anions to GPCRs starts from the intracellular side of the receptor, anions ultimately binding between the transmembrane domains at the intracellular side of the hH4R (Wittmann et al. 2014b).
Molecular modelling approach
Based on crystal structures, e.g., the crystal structures of the human H1 receptor (Shimamura et al. 2011), human D3 receptor (Chien et al. 2010), human adenosine A2A receptor (A2AR) (Liu et al. 2012) or human δ-opioid receptor (DOPR) (Fenalti et al. 2014), homology models of the GPCR of interest can be modelled (Wittmann et al. 2014b). After docking of monovalent ions to the allosteric binding site, their interactions with the GPCR can be studied (Wittmann et al. 2014b). However, GPCRs, embedded in a lipid bilayer are not rigid structures. Rather they show a distinct flexibility and undergo dynamic processes. Thus, it is state of the art to perform molecular dynamic (MD) simulations with the GPCR embedded in a lipid bilayer and surrounded by water molecules and ions in order to observe its dynamics on a molecular level (Selent et al. 2010; Yuan et al. 2013; Wittmann et al. 2014b). These simulations are of special interest, because they allow observing the binding pathways from ions or the binding and unbinding of water from outside of the GPCR into an allosteric binding site. Another important technique is to illustrate an energetical term, e.g. an interaction energy, by a hypersurface (Wittmann et al. 2014b). Although such hypersurfaces represent intersections, often only considering two coordinates or two courses of interest, valuable information can be obtained, e.g. preferred binding areas with similar energy can be identified.
In the following, the methods used within this study are described briefly: The simulations were performed according to protocols, described previously (Wittmann et al. 2014b). Briefly, the homology model of the inactive hH3R was constructed using the crystal of the hH1R (Shimamura et al. 2011) as template, using establishes protocols (Wittmann et al. 2014b). Internal water molecules were included and the monovalent cation (Li+, Na+, K+, Rb+, Cs+) was docked manually into its allosteric binding site according to Fenalti et al. (2014), as described (Wittmann et al. 2014b). The resulting receptor models were placed manually in a POPC lipid bilayer, intra- and extracellular water molecules and an appropriate number of cations and Cl−, solved in the extra- and intracellular water were added, as described (Wittmann et al. 2014b). For the monovalent cations, appropriate force field parameters (Joung and Cheatham 2008) were used. Subsequent to a 5-ns equilibration phase for each system (force constants 250 kJ/(mol nm2) for the first 2.5 ns and 100 kJ/(mol nm2) for the second 2.5 ns were put onto the backbone atoms of the TM domains of hH3R), the 50 ns productive phases were performed. Each MD simulation was performed at least three times using different seed values (Wittmann et al. 2014b). During the 50-ns productive simulation, the overall conformation of the hH3R and the cation in its allosteric binding site remained stable. It should be noted that the aim of the present simulations was to predict the preferred area of the cation in its allosteric binding site of hH3R. For this aim, the simulation time of 50 ns is appropriate. It was not the aim to study the whole binding pathway of the cation from the extracellular water into the allosteric binding site of hH3R. The standard Gibbs energy of solvation of the cation for the transfer from the orthosteric to the allosteric binding site (ΔΔG o) was calculated for each cation using a protocol, described previously (Wittmann et al. 2014b). Each simulation was performed for 9 ns and was repeated two times, using a slightly different starting position of the cation in the orthosteric or allosteric binding site.
Impact of crystal structures and molecular modelling onto understanding of ion sensitivity on a molecular level
For many years, an allosteric binding site near the highly conserved Asp2.50 was suggested as binding site for the monovalent cations (Neve et al. 1991; Ceresa and Limbird 1994). This hypothesis was proven in mutagenesis studies by exchanging the aspartate for asparagines (Neve et al. 1991; Ceresa and Limbird 1994; Schetz and Sibley 2001; Schnell and Seifert 2010). The next milestone was the solution of the crystal structures of the A2AR (Liu et al. 2012) and of the DOPR (Fenalti et al. 2014), with a sodium ion bound in this allosteric binding site. The crystal structures revealed insights into the interaction of the Na+ with the amino side chains in the allosteric site, especially Asp2.50 and Ser3.39 (Liu et al. 2012; Fenalti et al. 2014). However, also the coordination of the ion by water molecules was shown to be of relevance (Liu et al. 2012). Although mutagenesis studies and crystal structures provided important insights into ion-sensitivity of GPCRs, the picture on a molecular level has to be completed by molecular modelling studies, especially MD simulations, because with this technique, the dynamics of different processes can be studied quite well (Selent et al. 2010; Yuan et al. 2013; Shang et al. 2014; Wittmann et al. 2014b). For the dopamine D2 receptor (D2R) and the μ-opioid receptor (MOPR), the complete binding of a sodium ion from the extracellular side of the receptor via the orthosteric into the allosteric binding site was shown, giving insights into the entry of a monovalent cation and the time scale of the entire binding process (Selent et al. 2010; Yuan et al. 2013). However, crystallographic studies have not yet addressed the question how anions affect GPCR function. Last but not least, structure-activity relationships for cations have not yet been examined in the crystallographic studies.
Movement of monovalent cations and anions to their binding sites
It was shown by MD simulations at the D2R (Selent et al. 2010) and opioid receptors (Yuan et al. 2013; Shang et al. 2014) and hH4R (Wittmann et al. 2014b) that a Na+ can bind from the extracellular side into the allosteric binding site of the receptor. The overlay of snapshots at different time steps of a MD simulation of hH4R shows the flexibility of the most important amino side chains as well as the preferred areas for Na+ and Cl− (Fig. 1a). A comparison of the simulation results suggests that the cation is caught by negatively charged glutamate or aspartate at the extracellular surface of the receptor (Fig. 1a (I)). However, a comparison of the amino acid sequences, forming the extracellular surface of GPCRs, suggests that negatively charged amino acids are located in different areas on the extracellular surface of various receptors. Thus, individual entry pathways for different GPCRs are likely. In case of hH4R, Glu160 catches the Na+ whereas Glu163 undergoes a conformational change and guides the Na+ into the orthosteric binding site (Wittmann et al. 2014b). As illustrated, this part of the process is very fast, while the cation remains for a longer period in the orthosteric binding site (Fig. 1a (IIa, IIb); Fig. 1b). Because of the additional negatively charged Glu5.46, the Na+ also binds sporadically there (Fig. 1a (IIa)). However, the preferred binding area in the orthosteric binding site is near Asp3.32 (Fig. 1a (IIb)). Afterwards, the Na+ again binds very fast into the allosteric binding site (Fig. 1a (III)), where it remains stable again for a longer period of time. In hH4R, a monovalent cation (Na+) and monovalent anion (Cl−) were observed to bind simultaneously to the allosteric binding site or the intracellular part between the TM domains, respectively. Whereas one and the same Na+ ion is stable in the allosteric binding site, a strong fluctuation of the Cl− regarding the distance to Arg3.50 was observed (Fig. 1a (IV)). These distinct pair configurations remain stable for about 0.5 ns. However, due to the strong fluctuation of the Cl−, a Cl− was observed to be in close contact to Arg3.50 during the complete simulation time. Similar to the Na+, the Cl− is caught by positively charged amino acids at the intracellular surface of the receptor. At hH4R, these are predominantly Arg6.29 and Arg6.32 (Fig. 1a (V)).
Movement of monovalent cations and anions to their binding site. a Binding pathway of Na+ and Cl− to hH4R as determined by MD simulations (Wittmann et al. 2014b). b Time course of the distance of Na+ to Asp2.50 and Cl− to Arg3.50 at hH4R, observed during MD simulations
Signature of the allosteric cation binding site on a molecular level
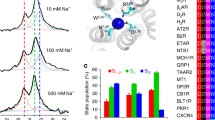
In biophysical and physiological processes, ion-specific effects play an important role because biological systems are affected when salt concentrations are varied or distinct ions are replaced (Lo Nostro and Ninham 2012). Because of the differences in size, interaction properties (e.g. Coulomb- or Lennard-Jones interaction energies) and hydration shell of monovalent cations (Table 2), those cations exhibit different physicochemical properties and were ordered in the so-called Hofmeister (1888) series. Additionally, anion and cation specificity was described for several biological systems, e.g. proteins or even enzymatic activity by experimental techniques and molecular simulations (Collins et al. 2007; Yang et al. 2010; Friedman 2011; Rembert et al. 2012; Lo Nostro and Ninham 2012; Stepankova et al. 2013). Thus, differences in interaction of monovalent cations with GPCRs in the allosteric binding site are expected. To elucidate these differences on a molecular level, different molecular modelling techniques can be used. The interaction energy at an intersecting plane through the channel between the ortho- and allosteric binding site of a representative GPCR, the human histamine H3-receptor (hH3R) shows that there are two preferred areas for cations, first, around the highly conserved Asp3.32 of the orthosteric and second, around the highly conserved Asp2.50 around the allosteric binding site (Figs. 2 and 3).
Signature of the allosteric cation binding site on a molecular level. a Interaction energy (Coulomb- and Lennard-Jones) surface of a cation (Li+, Na+, K+, Rb+ and Cs+) within the area of the orthosteric site, allosteric site and the binding channel of hH3R; o position of the Asp3.32 in the orthosteric binding site, a position of the Asp2.50 in the allosteric binding site; purple and dark blue, energetically preferred areas for a monovalent cation; yellow and orange, energetically disfavoured areas for a monovalent cation. b Preferred areas for the cation (Li+, Na+, K+, Rb+ and Cs+) around the orthosteric and allosteric binding site based on an overlay of snapshots of a molecular dynamic simulation of the corresponding cation in the hH3R. Red, position of the carboxy oxygens of Asp3.32 and Asp2.50; grey, position of the oxygens of water molecules (H2O). c Overlay of Li+, Na+, K+, Rb+ or Cs+ in the allosteric binding site of hH3R; shown are representative snapshots of MD simulations; most important amino acids are shown as sticks; representative water molecules between the orthosteric and allosteric binding site are shown as wireframe surface. d estimation of the change in standard Gibbs energy of solvation of the cation for the transfer from the orthosteric to the allosteric binding site (ΔΔG o). All data shown within this figure were obtained by calculations as described previously (Wittmann et al. 2014a, b).
Sequence alignment of different GPCRs, regarding amino acids being important for sensitivity to monovalent cations and anions. Asterisk, highly conserved amino acids according to Ballesteros-Weinstein nomenclature; yellow boxes, highly conserved cysteine residues, forming a disulphide bridge between the E2-loop and the transmembrane domain III; green boxes, most important amino acids forming the sodium binding channel between the orthosteric and the allosteric binding site; red boxes, negatively charged amino acids, observed in MD simulations to catch at Na+ at the extracellular surface and to guide the cation into the orthosteric binding site (Wittmann et al. 2014b; Selent et al. 2010; Yuan et al. 2013); red bold letters, negatively charged amino acids at the extracellular surface which could be involved in catching monovalent cations; cyan boxes, positively charged amino acids, observed in MD simulations to interact with monovalent anions at the intracellar part between the transmembrane domains of a GPCR (Wittmann et al. 2014b); blue bold letters, positively charged amino acids at the intracellular surface between the transmembrane domains of a GPCR which could be involved in interaction with monovalent anions; grey boxes, amino acids which may be responsible for differences in Na+ sensitivity between hH3R and hH4R (Wittmann et al. 2014b). (UniProtKB accession codes: human α2A adrenergic receptor (hADA2AR), P08913; human dopamine D2 receptor (hD2R), P14416; human histamine H1 receptor (hH1R), P35367; human histamine H3 receptor (hH3R), Q9Y5N1; human histamine H4 receptor (hH4R), Q9H3N8; mouse μ-type opioid receptor (mOPRM1R), P42866; human δ-type opioid receptor (hOPRD1), P41143; human adenosine A2A receptor (hAA2AR), P29274; human neuropeptide Y receptor type 2 (hNPY2R), P49146; human neuropeptide Y receptor type 4 (hNPY4R), P50391; human N-formyl peptide receptor (hFPR1), P21462; human chemokine receptor type 4 (hCXCR4), P61073; human cannabinoid receptor 1 (hCB1R), P21554; human cannabinoid receptor 2 (hCB2R), P34972)
We considered Na+, Ka+, Li+, Cs+ and Rb+. While only Na+ and K+ physiologically modulate GPCR function, the latter three cations provide extremely valuable tools for the analysis of ion effects on GPCRs because they differ from each other in physicochemical properties (Table 2). Moreover, Li+ is unique in this series because this cation is highly effective in the treatment of manic-depressive (bipolar) disorders (Beaulieu et al. 2009; Marlinge et al. 2014; Tselnicker et al. 2014) and there is recent interest in Li+ as drug for the treatment of neurodegenerative diseases (Forlenza et al. 2014). Although the concentrations at which the Li+ affects GPCR function in vitro are much higher than the therapeutic plasma concentrations in patients (∼1 mM) (Grandjean and Aubry 2009), it cannot be excluded that Li+ interacts with GPCRs in vivo. One limitation of the studies conducted with Li+ on GPCRs in vitro is the fact that Li+ was only studied alone but not together with Na+ or K+ (Gierschik et al. 1991; Seifert 2001). With increasing size of the cation in the series Li+ → Na+ → K+ → Rb+ → Cs+, the energetically favoured area decreases (Fig. 2a). Additionally, in the same series, the transition of the cation through the connecting channel becomes energetically more and more disfavoured (Fig. 2a). It is well known that also water molecules are an important key player in interaction of a cation with the allosteric binding site (Liu et al. 2012; Katritch et al. 2014). Furthermore, previous modelling studies showed that water molecules connect the orthosteric and the allosteric binding site (Fig. 2b, c) (Wittmann et al. 2014b). The interaction of a cation with a GPCR is a dynamic process. The preferred areas of water molecules and cations based on snapshots of MD simulations around the orthosteric and allosteric as well as the connecting channel of the hH3R are shown for the series Li+ → Na+ → K+ → Rb+ → Cs+ (Fig. 2b). In both cases, the cation bound in the orthosteric or allosteric site and water molecules are found in the cation binding channel (Fig. 2b). While the preferred binding area of the analysed cations is very similar for the orthosteric binding site, there are obvious differences in the preferred binding for Li+ and Na+ on the one hand and K+, Rb+ and Cs+ on the other hand: While Li+ and Na+ bind deeper into the allosteric pocket, K+, Rb+ and Cs+ bind preferably at the area between the binding channel and the upper part of the allosteric binding site (Fig. 2b, c). Here, the hydration by water molecules or coordination by amino acid side chains and the preferred hydration number (Table 2) play a role. These differences lead to distinct signatures of cation binding in the allosteric site. Consequently, binding of different monovalent cations to the allosteric site may result in distinct interaction networks within the receptor, and these different interaction patterns may result in different receptor conformations. This hypothesis is supported by the highly heterogeneous cation effects in various systems (Table 1). Thus, like for conventional GPCR ligands, GPCR-specific structure-activity relationships for various cations are emerging.
The cation signatures shown (Fig. 2a, b), may be similar between aminergic GPCRs, but different to GPCRs with no aspartate at position 3.32 (Fig. 3), e.g. the A2AR, because for the latter receptor, the preferred region for cation binding in the orthosteric binding site is missing. Furthermore, within the family of aminergic GPCRs more subtle differences in amino acids forming the binding channel are present (Fig. 3): for example, the hH3R is highly Na+ sensitive (Schnell and Seifert 2010), whereas the closely related human histamine H4-receptor (hH4R) shows no sensitivity regarding sodium ions (Schneider et al. 2009), although Na+ probably binds to the allosteric binding site (Schneider et al. 2009). Molecular modelling studies suggest that a reason for this may be the differences in amino acid sequence between hH3R and hH4R at positions 3.40 and 7.42 (Wittmann et al. 2014b). Additionally, differences in the change in standard Gibbs energy for the transfer of a monovalent cation from the orthosteric to the allosteric binding site (ΔΔG o) have to be considered, dependent of the nature of the monovalent cation. In the series Li+ → Na+ → K+ → Rb+ → Cs+, the standard Gibbs energy increases (Fig. 2d). Based on this data, it can be suggested that there is a rising preference for binding of a monovalent cation in the series Cs+ → Rb+ → K+ → Na+ → Li+. This trend regarding the basal GTP hydrolysis was also shown previously by experimental studies at hH3R for K+ → Na+ → Li+ (Schnell and Seifert 2010). The standard Gibbs energy for the transfer from the orthosteric to the allosteric site strongly depends on the nature of the monovalent cation. Furthermore, taking into account the high similarity of the allosteric binding site between different GPCRs (Fig. 3), this trend may be detected for other GPCRs, considering the heterogeneous cation effects in various systems. The calculations suggest that the binding of Li+ to the allosteric binding site is energetically strongly favoured compared with Na+ (Fig. 2d). This observation may be of relevance for the therapeutic effect of Li+, e.g. in therapy of bipolar disorders (Beaulieu et al. 2009; Marlinge et al. 2014; Tselnicker et al. 2014). In this context, it may be interesting to study the binding of Li+ in the allosteric binding site at different GPCRs by MD simulations or even to obtain crystal structures.
Impact of monovalent ions onto GPCRs—a structural perspective
As shown exemplarily for the hH3R by molecular modelling studies, monovalent cations can bind stably into the orthosteric and allosteric binding site of a GPCR (Wittmann et al. 2014b) (Fig. 2c). Furthermore, MD simulations revealed that monovalent anions preferably bind between the intracellular parts of the transmembrane (TM) helices (Wittmann et al. 2014a). However, in contrast to cations in the allosteric site, a specific anion binding site was not yet identified in crystallographic studies. In case of hH3R, monovalent anions bind closely to Arg3.50, Arg6.29 and Arg6.32 (Wittmann et al. 2014a) (Fig. 1a). The analysis of the percentage of conservation of positively charged amino acids located at the intracellular surface between the TM domains suggests that Arg3.50, Lys/Arg6.29 and Lys/Arg6.32 (Fig. 3) are promising candidates for an anion binding site. Moreover, due to the complete conservation of Arg3.50 in GPCRs, this amino acid may represent the anion binding site, whereas the other positively charged amino acids in this region, which are not fully conserved, e.g. Lys/Arg6.29 and Lys/Arg6.32 may guide the anion to the proposed allosteric anion binding site in a GPCR-specific manner (Fig. 3).
The binding of anions in the intracellular part of the GPCR may have two different consequences: First, the anion may keep TM VI and TM II closely together by electrostatic interaction so that opening of the intracellular part of the receptor—equivalent to receptor activation—is suppressed. Thus, anions bound in the intracellular part of the GPCR may stabilise its inactive state. Second, the binding of the anion may block binding of the G protein to the GPCR in the active state. Thus, it may be worthwhile to analyse crystal structures of GPCRs with regard to anion binding. As illustrated by the scheme (Figs. 1a and 4) and as shown by MD simulations, a monovalent cation and monovalent anion can bind simultaneously to the allosteric binding site and the intracellular part between the TM domains, respectively. The minimum distance between both monovalent ions is in the ∼16–25-Å range (Wittmann et al. 2014a). Clearly, this distance is too large for a direct electrostatic interaction. However, binding of the cation and anion may result in changes of the interaction network and consequently influence each other, e.g. result in a more favoured stabilization of the inactive receptor. In support for an anion binding site in GPCRs are data showing that in several cases, anions exhibit a much more pronounced effect on receptor/G-protein coupling than cations (Table 1). However, to this end anions have not yet been systematically analysed at GPCRs. By analogy to cations, anions with different physiochemical properties (Cl−, Br−, I−) (Table 2) can be used as pharmacological tools to analyse anion binding sites. It is possible that some of the CNS effects observed in bromide intoxication (James et al. 1997; Baird-Heinz et al. 2012) are the result of altered GPCR function. The assumed interactions of anions with GPCRs at the intracellular side are intriguing in the light of the fact that anions can also modulate the function of G-proteins (Higashijima et al. 1987). Thus, it is conceivable that anions act as allosteric modulators of receptor/G-protein coupling. However, there were only very few follow-up studies on the seminal work of Higashijima and colleagues (Seifert 2001).
Impact of monovalent cations and anions on GPCR function: possible pharmacological interventions. This scheme illustrates the influence of monovalent cations and anions onto a GPCR in the inactive (R) and active (R*) state and onto the Gα subunit of a G-protein. Monovalent cations, e.g. Na+ were shown to bind into the orthosteric and allosteric site of several GPCRs (Selent et al. 2010; Liu et al. 2012; Yuan et al. 2013; Fenalti et al. 2014; Wittmann et al. 2014b), while monovalent anions were detected in MD simulations to bind in the intracellular part of the R and active R* state of GPCRs, e.g. the hH3R (Wittmann et al. 2014a). Additionally, the scheme demonstrates that also an interaction of monovalent cations or anions with the Gα subunit of a G-protein has to be considered. The 7TM-topology of GPCRs and the globular shape of Gα as evident from X-ray crystallography is intentionally not depicted here to focus on the monovalent ion binding sites in these proteins
While it will be very difficult to achieve exquisite specificity in the effects of ions for a given receptor, the development of allosteric modulators, either positively or negatively modulating ion-receptor interaction, is feasible. With regard to cation entry to the orthosteric site, it should actually be quite easy to obtain such modulators because cation entry sites at the extracellular domains of GPCRs are very heterogeneous (Fig. 3), offering an unique allosteric opportunity for achieving receptor selectivity. Likewise, the intracellular domains, where the tentative anion binding site is located (Fig. 1a), is highly heterogeneous. However, since this domain is located at the intracellular site of the membrane, allosteric modulators must be more hydrophobic to reach this target. The same considerations apply to modulators of anion interactions with G-proteins. In principle, G-proteins are less suitable targets than GPCRs because they couple to many GPCRs, increasing the probability of side effects.
Monovalent ion mediated impact on binding and signalling—a mathematical description
In order to understand the influence of ligand binding onto signal transduction by GPCRs, the concept of an equilibrium between inactive R and active receptors R* was developed (Seifert and Wenzel-Seifert 2002). Assuming, efficacy is proportional to the concentration of the active receptor–G-protein complex, an analytical description of the receptor function becomes possible. A first extension of this quantitative model dealing with the impact of sodium ions on GTP hydrolysis induced by the hH3R was established (Wittmann et al. 2014a). Experimental evidence for an impact of anions on the GTP hydrolysis was shown for some GPCRs including the hH3R (Schnell and Seifert 2010). In order to establish a quantitative description of the influence of monovalent cations and anions on the efficacy an extension of the classical model regarding the GPCR-ion interaction is presented in Fig. 5a. The resulting equation for the efficacy E, shown in Fig. 5b, is a complex equation, including all equilibrium constants. Because the concentration of cations C is present in the enumerator and denominator, an increase in concentration of cations can lead to an increase as well as a decrease in efficacy. In contrast, an increase of the anion concentration always leads to a decrease in efficacy (Fig. 5b).
Mathematical model to describe the sensitivity of a GPCR to monovalent cations or anions. a Schematic presentation of the different binding processes; R inactive receptor without any ligand or ion, R* active receptor without any ligand or ion, C monovalent cation, CR ortho inactive receptor with a monovalent cation in the orthosteric binding site, CR allo inactive receptor with a monovalent cation in the allosteric binding site, A monovalent anion, CR ortho A inactive receptor with the monovalent cation in the orthosteric binding site and the monovalent anion between the intracellular transmembrane domains, CR allo A inactive receptor with the monovalent cation in the allosteric binding site and the monovalent anion between the intracellular transmembrane domains, RA inactive receptor with the monovalent anion between the intracellular transmembrane domains, R*A active receptor with the monovalent anion between the intracellular transmembrane domains, CR* ortho active receptor with the monovalent cation in the orthosteric binding site, CR* ortho A active receptor with the monovalent cation in the orthosteric binding site and the monovalent anion between the intracellular transmembrane domains; grey shaded, these equilibriums are not considered in the calculations under (b). b For the equilibriums, described under (a), the corresponding mass action laws and conservation of matter are given; considering the approximation that each active receptor-ion complex without an anion between the intracellular part of the TM domains (R*, CR*ortho) interacts with a G-protein, the efficacy can be calculated as described (Wittmann et al. 2014a); the given efficacy E represents the absolute efficacy of G-protein activation and is not calculated relative to a full agonist; f represents a proportional factor (Wittmann et al. 2014a); the binding of agonists to the GPCR, as already described (Wittmann et al. 2014a) is not included within the mathematical model shown here; the mentioned terms for all species represent the molar concentrations
In general, the quantitative analysis of the impact of monovalent cations and anions onto GPCRs, as already performed for the hH3R (Wittmann et al. 2014a), and consequently the calculation of the corresponding equilibrium constants will provide a more detailed insight into the interaction between ions and GPCRs on a molecular level. However, the quantitative treatment of the experimental data in order to evaluate binding constants regarding the different cation and anion complexes requires large experimental data sets with high precision.
Remaining questions and future directions
The combination of pharmacological, mutagenesis, molecular modelling and crystallography techniques has provided significant insights into the high complexity of the interactions of monovalent cations and anions with GPCRs. However, there are still many open questions: MD simulations indicate that monovalent ions, like Li+ and Na+ compared with K+, Rb+ and Cs+, bind into different parts of the allosteric binding sites of GPCRs, resulting in structural differences in the allosteric site. Thus, more crystal structures of different GPCRs showing sensitivity to monovalent cations in experimental studies should be solved, paying special attention to binding of Li+ because of its pharmacological relevance. As exemplarily shown for the hH3R, MD simulations provide hints that anions bind preferably between the intracellular parts of the TM domains in the inactive as well as in the active state of a GPCR. Whereas MD studies showed that the cation binding area in the allosteric site of a GPCR is more focused, the anion binding site is much wider. Although no single amino acid could be identified to be important for anion binding until know, there are some strong candidates, e.g. Arg3.50 and positively charged amino acids at the positions 6.29 or 6.32 that can be probed by mutagenesis. Additionally, crystallographic studies should examine possible anion binding sites in the intracellular GPCR portions between the TM domains. Furthermore, synergistic cooperative effects of cation and anion binding sites in GPCRs need to be studied. As an important next step towards understanding the effects of anions on GPCRs, it will be necessary to systematically analyse various anions in the presence of a fixed cation. Based on the highly heterogeneous effects of cations and anions on various GPCRs, it is reasonable to assume that any given GPCR interacts with cations and anions differently. Future studies should also examine various anions and cations in combination, an aspect that has not yet been fully recognised. In vitro studies will remain the backbone of future research in monovalent ion research because precise manipulation of cation and anion concentrations in intact cells is hardly possible. One should be aware of the fact that the variations of ion concentrations under pathophysiological in vivo conditions are not as large as under experimental in vitro conditions. Thus, one should be careful by transferring experimental results to pathophysiological conditions. However, despite this discrepancy, such experimental studies substantially increase our understanding of the impact of monovalent cations and anions on GPCRs on a molecular level.
It should be emphasised that both extra- and intracellular monovalent ion concentrations are not static. Specifically, both hyperosmolarity and hypoosmolarity syndromes are known (May and Jordan 2011; Lombardo et al. 2013). Overall, very little research has been performed so far on the impact of changes of extracellular monovalent ion concentrations on GPCR function in intact cells. In one notable study, it was observed that the constitutive activity of the luteinizing hormone receptor in transfected COS-7 cells as assessed by cAMP accumulation was markedly enhanced by removal of NaCl from the extracellular medium, sucrose serving as isotonic substitute (Cetani et al. 1996). Similar studies can be readily performed for any given GPCR, and we anticipate that such studies will reveal substantial differences in the monovalent ion modulation of GPCRs. During the past 20 years, such studies were probably not performed because they were perceived as “descriptive”, but in light of the recently resolved GPCR crystal structures in complex with Na+, such studies will gain mechanistic momentum. Moreover, intracellular Na+ and Cl− concentrations are dynamically regulated by multiple mechanisms and change in numerous diseases including cardiovascular diseases and pulmonary diseases (Coppini et al. 2013; Simon Bulley and Jaggar 2014). Furthermore, GPCRs can activate Na+ entry into cells (Krautwurst et al. 1992). Hence, it is conceivable that changes in extra- and intracellular monovalent ion concentrations modulate GPCR functions in a subtle manner in health and disease.
References
Baird-Heinz HE, Van Schoick AL, Pelsor FR, Ranivand L, Hungerford LL (2012) A systematic review of the safety of potassium bromide in dogs. J Am Vet Med Assoc 240:705–715
Beaulieu J-M, Gainetdinov RR, Caron MG (2009) Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol 49:327–347
Brunskole I, Strasser A, Seifert R, Buschauer A (2011) Role of the second and third extracellular loops of the histamine H4 receptor in receptor activation. Naunyn Schmiedeberg’s Arch Pharmacol 384:301–317
Ceresa BP, Limbird LE (1994) Mutation of an aspartate residue highly conserved among G-protein-coupled receptors results in nonreciprocal disruption of α2-adrenergic receptor-G-protein interactions. J Biol Chem 269:29557–29564
Cetani F, Tonacchera M, Vassart G (1996) Differential effects on NaCl concentration on the constitutive activity of the thyrotropin and the luteinizing hormone/chorionic gonadotropin receptors. FEBS Lett 378:27–31
Chang RS, Synder SH (1980) Histamine H1-receptor binding sites in guinea pig brain membranes: regulation of agonist interactions by guanine nucleotides and cations. J Neurochem 34:916–922
Chien EY, Liu W, Zhao Q, Katritch V, Han GW, Hanson MA, Shi L, Newman AH, Javitch JA, Cherezov V, Stevens RC (2010) Structure of the human dopamine D3 receptor in complex with D2/D3 selective antagonist. Science 330:1019–1095
Collins KD, Neilson GW, Endergy JE (2007) Ions in water: characterizing the forces that control chemical processes and biological structure. Biophys Chem 128:95–104
Connolly TM, Limbird LE (1983) The influence of Na+ on the alpha 2-adrenergic receptor system of human platelets. A method for removal of extraplatelet Na+. Effect of Na+ removal on aggregation, secretion, and cAMP accumulations. J Biol Chem 258:3907–3912
Coppini R, Ferrantini C, Mazzoni L, Sartiani L, Olivotto I, Poggesi C, Carbai E, Mugelli A (2013) Regulation of intracellular Na+ in health and disease: pathophysiological mechanism and implications for treatment. Glob Cardio / Sci Pract 3:222–242
Costa T, Lang J, Gless C, Herz A (1990) Spontaneous association between opioid receptors and GTP-binding regulatory proteins in native membranes: specific regulation by antagonists and sodium ions. Mol Pharmacol 37:383–394
Ericksen SS, Cummings DF, Weinstein H, Schetz JA (2009) Ligand selectivity of D2 dopamine receptors is modulated by changes in local dynamics produced by sodium binding. J Pharmacol Exp Ther 328:40–54
Fenalti G, Giguere PM, Katritch V, Huang X-P, Thompson AA, Cherezov V, Roth BL, Stevens RC (2014) Molecular control of δ-opioid receptor signaling. Nature 506:191–196
Forlenza OV, De-Paula VJR, Diniz BSO (2014) Neuroprotective effects of lithium: implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem Neurosci 5:443–450
Friedman R (2011) Ions and the protein surface revisited: extensive molecular dynamic simulations and analysis of protein structures in alkali-chloride solutions. J Phys Chem B 115:921–9223
Gibson WJ, Roques TW, Young JM (1994) Modulation of antagonist binding to histamine H1-receptors by sodium ions and by 2-amino-2-hydroxmethyl-propan-1,3-diol HCl. Br J Pharmacol 111:1262–1268
Gierschik P, Sidiropoulos D, Steisslinger M, Jakobs KH (1989) Na+ regulation of formyl peptide receptor-mediated signal transduction in HL60 cells. Evidence that the cation prevents activation of the G-protein by unoccupied receptors. Eur J Pharmacol (Mol Pharmacol) 172:481–492
Gierschik P, Moghtader R, Straub C, Dieterich K, Jakobs KH (1991) Signal amplification in HL-60 granulocytes: evidence that the chemotactic peptide receptor catalytically activates guanine-nucleotide-binding regulatory proteins in native plasma membranes. Eur J Biochem 197:725–732
Grandjean EM, Aubry JM (2009) Lithium: updated human knowledge using an evidence-based approach. Part II: clinical pharmacology and therapeutic monitoring. CNS Drugs 23:331–349
Higashijima T, Ferguson KM, Sternweis PC (1987) Regulation of hormone-sensitive GTP-dependent regulatory proteins by chloride. J Biol Chem 262:3597–3602
Hofmeister F (1888) Zur Lehre der Wirkung der Salze. Arch Exp Pathol Pharmakol (Leipzig) 24:247–260
James LP, Farrar HC, Griebel ML, Bates SR (1997) Bromism: intoxication from a rare anticonvulsant therapy. Pediatr Emerg Care 13:268–270
Joung IS, Cheatham EE (2008) Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J Phys Chem B 112:9020–9041
Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC (2014) Allosteric sodium in class A GPCR signalling. Trends Biochem Sci 39:233–244
Kleemann P, Papa D, Vigil-Cruzs, Seifert R (2008) Functional reconstitution of the human chemokine receptor CXCR4 with Gi/Go-proteins in Sf9 insect cells. Naunyn Schmiedebergs Arch Pharmacol 378:261–274
Krautwurst D, Seifert R, Hescheler J, Schultz G (1992) Formyl peptides and ATP stimulate Ca2+ and Na+ inward currents through non-selective cation channels via G-proteins in dibutyryl cyclic AMP-differentiated HL-60 cells. Involvement of Ca2+ and Na+ in the activation of beta-glucuronidase release and superoxide production. Biochem J 288(Pt 3):1025–1035
Lefkowitz RJ, Cotecchia S, Samama P, Costa T (1993) Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol Sci 14:303–307
Limbird LE, Speck JL, Smith SK (1982) Sodium ion modulates agonist and antagonist interaction with the human platelet alpha 2-adrenergic receptor in membrane and solubilized preparations. Mol Pharmacol 21:609–617
Liu W, Chun E, Thompson AA, Chubukov P, Xu F, Katritch V, Han GW, Roth CB, Heitman LH, Ijzerman AP, Cherezov V, Stevens RC (2012) Structural basis for allosteric regulation of GPCRs by sodium ions. Science 337:232–235
Lo Nostro P, Ninham BW (2012) Hofmeister phenomena: an update on ion specificity in biology. Chem Rev 112:2286–2322
Lombardo F, Maggini M, Gruden G, Bruno G (2013) Temporal trend in hospitalizations for acute diabetic complications: a nationwide study, Italy 2001–2010. PLoS One 8:e63675
Mähler J, Persson I (2012) A study of the hydration of the alkali metal ions in aqueous solution. Inorg Chem 51:425–438
Marlinge E, Bellivier F, Houenou J (2014) White matter alterations in bipolar disorder: potential for drug discovery and development. Bipolar Disord 16:97–112
Martin S, Botto JM, Vincent JP, Mazella J (1999) Pivotal role of an aspartate residue in sodium sensitivity and coupling to G proteins of neurotensin receptor. Mol Pharmacol 55:210–215
May M, Jordan J (2011) The osmopressor response to water drinking. Am J Physiol Regul Integr Comp Physiol 300:R40–R46
Miller-Gallacher JL, Nehme R, Warne T, Edwards PC, Schertler GFX, Leslie AGW, Tate CG (2014) The 2.1 Å resolution structure of cyanopindolol-bound β1-adrenoceptor identifies an intramembrane Na+ ion that stabilises the ligand-free receptor. PLoS ONE 9:e92727
Motulsky HJ, Insel PA (1983) Influence of sodium on the alpha 2-adrenergic receptor system of human platelets. Role for intraplatelet sodium in receptor binding. J Biol Chem 258:3913–3919
Neve K (1991) Regulation of dopamine D2 receptors by sodium and pH. Mol Pharmacol 39:570–578
Neve KA, Cox BA, Henningsen RA, Spanoyannis A, Neve RL (1991) Pivotial role for aspartate-80 in the regulation of dopamine D2 receptor affinity for drugs and inhibition of adenylyl cyclase. Mol Pharmacol 39:733–739
Nickl K, Gardner EE, Geiger S, Heilmann J, Seifert R (2008) Differential coupling of the human cannabinoid receptors hCB1R and hCB2R to the G protein Gαi2Gβ1γ2. Neurosci Lett 447:68–72
Pacheco MA, Ward SJ, Childers SR (1994) Differential requirements of sodium for coupling of cannabinoid receptors to adenylyl cyclase in rat brain membranes. J Neurochem 62:1773–1782
Pop N, Igel P, Brennauer A, Cabrele C, Bernhardt GN, Seifert R, Buschauer A (2011) Functional reconstitution of human neuropeptide Y (NPY) Y2 and Y4 receptors in Sf9 insect cells. J Recept Signal Transduct Res 31:271–285
Rembert KB, Paterova J, Heyda J, Hilty C, Jungwirth P, Cremer PS (2012) Molecular mechanism of ion-specific effects on proteins. J Am Chem Soc 134:10039–10046
Schetz JA (2005) Allosteric modulation of dopamine receptor. Mini Rev Med Chem 5:555–561
Schetz JA, Sibley DR (2001) The binding-site crevice of the D4 dopamine receptor is coupled to three distinct sites of allosteric modulation. J Pharmacol Exp Ther 296:359–363
Schneider EH, Schnell D, Papa D, Seifert R (2009) High constitutive activity and a G-protein-independent high-affinity state of the human histamine H4-receptor. Biochemistry 48:1424–1438
Schnell D, Seifert R (2010) Modulation of histamine H3 receptor function by monovalent ions. Neurosci Lett 472:114–118
Seifert R (2001) Monovalent anions differentially modulate coupling of the β2-adrenoceptor to Gsα splice variants. J Pharmacol Exp Ther 298:840–847
Seifert R, Wenzel-Seifert K (2001) Unmasking different constitutive activity of four chemoattractant receptors using Na+ as universal stabilizer of the inactive (R) state. Receptors Channels 7:357–369
Seifert R, Wenzel-Seifert K (2002) Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild type receptors. Naunyn Schmiedeberg’s Arch Pharmacol 366:381–416
Selent J, Sanz F, Pastor M, De Fabritiis G (2010) Induced effects of sodium ions on dopaminergic G-protein coupled receptors. PLOS Comput Chem 6:e10000884
Selley DE, Cao C-C, Liu Q, Childers SR (2000) Effects of sodium on agonist efficacy for G-protein activation in μ-opioid receptor-transfected CHO cells and rat thalamus. Br J Pharmacol 130:987–996
Shang Y, LeRouzic V, Schneider S, Bisignano P, Pasternak GW, Filizola M (2014) Mechanistic insight into the allosteric modulation of opioid receptors by sodium ions. Biochemistry 53:5140–5149
Shimamura T, Shiroishi M, Weyand S, Tsujimoto H, Graeme W, Katritch V, Abagyan R, Cherezov V, Liu W, Han GW, Kobayahi T, Stevens RS, Iwata S (2011) Structure of the human histamine H1 receptor complex with doxepin. Nature 475:65–70
Simon Bulley SB, Jaggar JH (2014) Cl channels in smooth muscle cells. Pflugers Arch 466:861–872
Soper AK, Weckström K (2006) Ion solvation and water structure in potassium halide aqueous solutions. Biophys Chem 124:180–191
Stepankova V, Paterova J, Damborsky J, Jungwirth P, Chaloupkova R, Heyda J (2013) Cation-specific effects on enzymatic catalysis driven by interactions at the tunnel mouth. J Phys Chem B 117:6394–6402
Treherne JM, Stern JS, Flack WJ, Young JM (1991) Inhibition by cations of antagonist binding to histamine H1-receptors: differential effect of sodium ions on the binding of two radioligands. Br J Pharmacol 103:1745–1751
Tselnicker JF, Tsemakhovich V, Rishal I, Kahanovitch U, Dessauer CW, Dascal N (2014) Dual regulation of G proteins and the G-protein-activated K+ channels by lithium. PNAS 111:5018–5023
Varma S, Rempe SB (2006) Coordination numbers of alkali metal ions in aqueous solutions. Biophys Chem 124:192–199
Vosahlikova M, Jurkiewicz P, Roubalova L, Hof M, Svoboda P (2014) High- and low-affinity sites for sodium in δ-OR-Gi1α (Cys351-Ile351) fusion protein stably expressed in HEK293 cells; functional significance and correlation with biophysica state of the plasma membrane. Naunyn Schmiedebergs Arch Pharmacol 387:487–502
Watanabe M, George SR, Seeman P (1985) Regulation of anterior pituitary D2 dopamine receptors by magnesium and sodium ions. J Neurochem 45:1842–1849
Wenzel-Seifert K, Hurt CM, Seifert R (1998) High constitutive activity of the human formyl peptide receptor. J Biol Chem 273:24181–24189
Wittmann H-J, Seifert R, Strasser A (2014a) Mathematical analysis of the sodium sensitivity of the human histamine H3 receptor. In silico Pharmacol 2:1–14
Wittmann H-J, Seifert R, Strasser A (2014b) Sodium binding to hH3R and hH4R—a molecular modelling study. J Mol Model. doi:10.1007/s00894-014-2394-2
Yang Z, Liu X-J, Chen C, Hallings PJ (2010) Hofmeister effects on activity and stability of alkaline phosphatase. Biochim Biophys Acta, Proteins Proteomics 1804:821–828
Yuan S, Vogel H, Filipek S (2013) The role of water and sodium ions in the activation of the μ-opioid receptor. Angew Chem Int Ed 52:10112–10115
Acknowledgments
We acknowledge the collaboration with Dr. D. Schnell, in the hH3R project. This work was supported by grants of the Deutsche Forschungsgemeinschaft (GRK 1910, GRK 1441, SFB 587) and the European Union (COST programme BM0806 (H4R network)).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strasser, A., Wittmann, HJ., Schneider, E.H. et al. Modulation of GPCRs by monovalent cations and anions. Naunyn-Schmiedeberg's Arch Pharmacol 388, 363–380 (2015). https://doi.org/10.1007/s00210-014-1073-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-014-1073-2