Abstract
Downregulation of endothelial connexins has been shown to result in impaired angiogenesis. Isoprenaline is known to upregulate Cx43 in cardiomyocytes. Effects of isoprenaline on endothelial connexins are unknown. We wanted to investigate whether isoprenaline might induce upregulation of connexins Cx37, Cx40, or Cx43 in human endothelial cells and whether it may promote angiogenesis. Human umbilical vein endothelial cells (HUVECs) were cultured until confluence (5 days) and subsequently seeded in Matrigel in vitro angiogenesis assays for 18 h. During the entire cell culture and angiogenesis period, cells were treated with vehicle or isoprenaline (100 nM). Finally, the resulting angiogenetic network was investigated (immuno)histologically. Moreover, expression of Cx37, Cx40, and Cx43 was determined by Western blot. In addition, we measured functional intercellular gap junction coupling by dye injection using patch clamp technique. Isoprenaline resulted in significantly enhanced expression of endothelial Cx43 and to a lower degree of Cx40 and Cx37. The number of coupling cells was significantly increased. Regarding angiogenesis, we observed significantly enhanced formation of branches and a higher complexity of the tube networks with more branches/length. Isoprenaline increases endothelial connexin expression and intercellular coupling and promotes tube formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In our previous investigations, we have shown that nicotine can reduce endothelial connexin expression and thereby reduces endothelial intercellular gap junction communication and as a consequence reduces angiogenesis, in particular reduces the number of branches per length (Haussig et al. 2008; Gärtner et al. 2012; Duerrschmidt et al. 2012). Because of these findings, we made up the hypothesis that an increase in endothelial connexin expression might be associated with enhanced angiogenesis or with enhanced number of branches/length. Since other findings showed that isoprenaline treatment can upregulate connexin expression in cardiomyocytes (Salameh et al. 2006, 2009) and since beta-adrenoceptors are present in endothelial cells (Ferro et al. 1999), we had the idea that in endothelial cells, isoprenaline might also increase connexin expression. According to the results, which showed that downregulation of connexins led to impaired angiogenesis (Gärtner et al. 2012), we further hypothetized that isoprenaline treatment might stimulate angiogenesis. An indirect evidence that this could be true comes from the observation that propranolol can inhibit angiogenesis in vitro (Lamy et al. 2010).
Methods
The study was approved by the local Institutional Ethical Committee and conformed to the declaration of Helsinki.
Cell culture (2D culture)
Each experimental condition was repeated six times, i.e., with six different human umbilical vein endothelial cell (HUVEC) cultures. HUVECs were isolated from fresh umbilical cords using collagenase IV as previously described (Morawietz et al. 1999). In order to minimize variations of primary cultures, the isolated endothelial cells from different umbilical cords were pooled and cultured in medium I (M199 with Earle’s balanced salt solution with l-glutamine and Hepes (Lonza, Verviers, Belgium) supplemented with 10 % fetal calf serum, Biochrom (FCS, Berlin, Germany), and 100,000 U L−1 penicillin/100 mg L−1 streptomycin (Sigma, Steinheim, Germany)). After 3 h, medium I was replaced by medium II (medium I with supplemental 16.7 g/L endothelial cell growth supplement (c.c. pro, Oberdorla, Germany) at 37 °C and 5 % CO2. For one Petri dish of 6-cm diameter, 1.5 Mio cells were seeded. Every 48 h, medium was replaced. After reaching subconfluence (after 5 days), cells were used for the angiogenesis assay (see below).
Treatment of cells
After the 5-day pre-cultivation period, cells were treated with drug or vehicle. In order to simulate a subacute beta-adrenergic stimulation, we applied 100 nmol/L isoprenaline (Sigma, Taufkirchen, Germany) for the entire period of 20 h of the angiogenesis assay. HUVECs were immediately incubated with isoprenaline at the final concentrations in medium II after isolation. Nearly confluent cells (after 5 ± 0.5 days) were trypsinized, and angiogenesis-assay was performed still in the presence of isoprenaline in medium. Control cells were treated with the vehicle H2O.
Angiogenesis assay
Testing for HUVECs, tube formation was performed in a chamber (A = 0.8 cm2) containing 100 μL Matrigel (In vitro angiogenesis Assay ECM625, Millipore, USA) according to the manufacturer’s protocol. Briefly, 100μL Matrigel solution were added to one chamber of the Lab-Tek II Chamber Slide system (Lab-Tek, Naperville, USA) and allowed to solidify and polymerize at 37 °C and 5 % CO2 for 1 h.
Nearly confluent HUVECs were stained 30 min with 5 μL 1 % FITC-lectin (from Ulex europaeus UEA I, Sigma, Steinheim, Germany) dissolved in medium I, than trypsinized, centrifuged at 1,200 rpm for 10 min and resuspended in medium II. Finally, 200 μL medium II containing 200,000 cells were layered on top of the Matrigel. For drug experiments, either the isoprenaline (100 nmol/L) or vehicle (H2O) was added to medium II.
After 20-h incubation at 37 °C and 5 % CO2, cells were stained with Hoechst 33258 (bisBenzimide, 0.1 mg/mL in dH2O, Molecular probes, Eugene, USA) for 10 min and mounted with DakoCytomation Fluorescent Mounting medium (Dako, Hamburg, Germany). Tube formation was inspected using a Zeiss Axiolab fluorescence microscope (Zeiss, Jena, Germany) and a commercial image analysis system (Zeiss Vision).
We evaluated 25 pictures per object plate (magnification ×200; for n = 6 experiments/series, i.e., each series is represented by 150 pictures) in blinded manner, and the number of cells, number of branches, average length of the capillary branches, and number of branches to the length of capillaries ratio and the pattern were assessed using Zeiss Axio Vision Software 4.7. For pattern assessment, pictures were assigned points according to the following scale: 0 = single cells; 1 = cells start to grow and orientate; 2 = cells form tubes, but there are no branches; 3 = cells form tubes and there are branches; 4 = nearly complex network; and 5 = complex network. Additional phase contrast pictures were taken (magnification ×100).
Extraction of proteins from Matrigel
Isolation of proteins from HUVECs in angiogenesis assay on Matrigel required a special protocol. After 20 h of growth, medium was removed and Matrigel was washed with PBS (containing Ca2+ and Mg2+). Thereafter, Matrigel was scraped off the glass slide into ice-cold 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)-buffer (30 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM β-mercaptoethanol, 1 % (w/v) CHAPS, with addition of protease and phosphatase inhibitors: 100 mM sodium orthovanadate, aprotinin (1 mg/mL), leupeptin (1 mg/mL), and pepstatin A (1 mg/mL)). After an overnight incubation at 4 °C, samples were centrifuged at 23,300 rpm for 60 min. Supernatants were concentrated using a vacuum concentrator, stored frozen, and later used for Western blot experiments.
Western blotting
Electrophoresis was performed on one-dimensional 12 % sodium dodecyl sulfate (SDS)-polyacrylamide gel for cell culture samples and on 8 % SDS-polyacrylamide gel for Matrigel samples. Afterward, proteins were transferred to a PVDF membrane (Roth, Karlsruhe, Germany) using a commercial wet blot system (Bio-Rad Laboratories, Hercules, USA). The membrane was blocked with either 5 % fat-free milk powder or 2 % bovine serum albumin (BSA, FractionV, Sigma, Steinheim, Germany) dissolved in Tris-buffered saline with Tween-20 (TBST; 50 mM Tris, 0.5 mM NaCl, pH 7.4, 0.1 % Tween 20). Membranes were incubated overnight at 4 °C with the primary antibodies diluted either in 1 % nonfat milk in TBST or 2 % BSA in TBST followed by incubation with horseradish peroxidase-conjugated secondary antibodies for 2–3 h at room temperature. Dilutions of the primary antibodies were as follows: anti-Cx37 (Biotrend, Cologne, Germany) 1:500, anti-Cx40 (Santa Cruz, Heidelberg, Germany) 1:1,000, anti-Cx43 (Sigma, Steinheim, Germany) 1:2,000, anti-NCX1 (Abcam, Cambridge, UK) 1:1,000, caveolin-1 (Abcam, Cambridge, UK) 1:1,000, and GAPDH (HyTest Ltd, Turku, Finland) 1:1,0000. Dilution of secondary antibodies was 1:5,000. For detection of the specific chemiluminescent signal, either Uptilight HRP Blot Chemiluminescent Substrate (Interchim, Montlucon Cedex, France) or SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, USA) was used according to the manufacturer’s instructions, and membranes were exposed to Kodak XOmat AR film.
The results were evaluated using Quantity One Software (Bio-Rad Laboratories, Hercules, USA) and expressed as factor of change from control values and normalized to the respective GAPDH-signal for cell culture samples or NCX1 signal for Matrigel samples.
Dye coupling
For dye transfer experiments, cells were grown on a coverslip and also treated with isoprenaline for 20 h. Then, they were transferred to a 1-mL organ bath superfused with Tyrode’s solution (135 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 0.33 mM NaH2PO4, 10 mM HEPES, and 10 mM glucose, pH 7.4) at 37 °C. Cells were patched using glass pipettes of 5–6 MΩ filled with “intracellular” solution (140 mM KCl, 4 mM MgCl2, 0.06 mM CaCl2, 5 mM EGTA, 3.1 mM Na2ATP, 5 mM Na2 creatine phosphate, 10 mM HEPES, pH 7.1, and 0.1 % Lucifer Yellow (Sigma, Steinheim, Germany)). After break-in and establishing the whole cell configuration, cells were injected with Lucifer Yellow, and the dye transfer in the adjacent cells was measured 5 min after dye injection. The number of communicating cells was measured using computer-assisted image analysis.
Immunohistochemistry
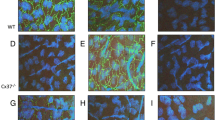
After 20h growth of cells into the Matrigel, Matrigel was washed with PBS for three times, fixed with formalin for 10 min, washed three times for 5 min with PBS and permeabilized for 30 min with Triton-X 100 (1 % in PBS). After that, Matrigel was incubated with 0.1 % BSA (dissolved in PBS) for 20 min. Then, Matrigels were incubated with the primary antibody at a dilution of 1:100 overnight: anti-Cx43 (Sigma, Steinheim, Germany). Matrigel was washed three times with 0.1 % BSA solution and incubated with the secondary antibody (1:100, 1 h, anti-rabbit-IgG conjugated with AlexaFluor488; Sigma, Steinheim, Germany). Matrigel was then washed with 0.1 % BSA solution, and nuclei were stained with DAPI (Roche Diagnostics, Mannheim, Germany) for 1 min. Matrigel was then embedded in Fluorescent Mounting medium (Dako, Hamburg, Germany). Connexin distribution was analyzed using a Zeiss Axiolab fluorescence microscope (Zeiss, Jena, Germany) and a commercial image analysis system (Zeiss Axio Vision, Zeiss, Jena, Germany).
Statistics
The data of each experimental series are shown as mean ± SEM. Statistical analysis was performed with Student’s t test and ANOVA procedure by Bonferroni method (SigmaStat, Jandel Scientific, San Rafael, CA), respectively. Differences were considered statistically significant if p < 0.05.
Results
We found that isoprenaline treatment (20 h, 100 nmol/L) significantly upregulated all three endothelial connexins. However, this effect was most prominent for Cx43 and lowest for Cx37 (Fig. 1). Caveolin-1, which is involved in connexin transport to the cell membrane, was also found to be upregulated.
Next, we investigated whether this isoprenaline-induced upregulation of connexins might result in a functionally improved coupling. Therefore, we submitted the cells after treatment to an assay for metabolic coupling. Lucifer Yellow was injected in one cell, and 5 min later, we counted the number of coupled cells, which indeed was significantly enhanced by the isoprenaline treatment (Fig. 2) indicating improved metabolic intercellular gap junction coupling.
Next, we investigated the effects of 20-h treatment with 100 nmol/L isoprenaline on angiogenesis. We found that isoprenaline resulted in an enhanced number of branches and increased number branches/length ratio. Length of the tubes and number of cells were not affected (Figs. 3 and 4).
Matrigel assay was performed in the presence of vehicle (H20; control) or 100 nmol/L isoprenaline for 20 h. Cells were stained with lectin (FITC-conjugated, green fluorescence), and nuclei are stained with Hoechst 33258 (blue fluorescence) (see Fig. 3). Treatment with isoprenaline (Iso) resulted in increased angiogenesis seen by a higher number of branches, a more complex overall pattern, and an enhanced branches/average length of tubes ratio as related to control treatment (Ctrl). Data are given as mean ± S.E.M. Significant changes versus control treatment are indicated by an asterisk (p < 0.05)
In further experiments, we wanted to know whether the enhanced expression of Cx43, as the predominantly upregulated connexin, can be seen in all cells of the tubes or whether there might be a preferred localization. After treatment of cells with isoprenaline (20-h Matrigel assay), we investigated the distribution of Cx43 in the tubes using immunohistochemistry. Cx43 appeared mostly at branching points (Fig. 5) and sprouts of new tubes (Fig. 6) in untreated and in isoprenaline treated cultures. Thus, while the overall expression of Cx43 was enhanced under isoprenaline (Figs. 1 and 7), the pattern of its distribution remained similar.
Discussion
These findings demonstrate an angiogenetic effect of isoprenaline associated with enhanced expression of endothelial connexins and intercellular gap junction coupling. It has been described that endothelial cells possess β-adrenoceptors of the β1- and the β2-subtype, which are involved in release of nitric oxide (β1-adrenoceptors; Ferro et al. 1999) and in endothelial cell growth (β2-adrenoceptors; Sexl et al. 1995). An induction of angiogenesis via β2-adrenoceptors has been described in the failing rat heart using a model of β2-adrenoceptor overexpression (Rengo et al. 2012). In line with this, others found decreased neo-angiogenesis in β2-adrenoceptor-deficient mice using a chronic hind limb ischemia model (Ciccarelli et al. 2011). However, these findings contrast with the observation of another group, who stated that the β2-adrenoceptor-blocker propranolol, but not carvedilol, promoted angiogenesis in the mouse aortic ring assay (Stati et al. 2014), so that these authors concluded that the pro-angiogenetic effect of the beta-blocker propranolol may be independent from the β-adrenoceptor. Interestingly, it is clinically known that hemangiomas can be treated with propranolol (Laranjo et al. 2014; Sagi et al 2014), but that this treatment fails in about 10 %, the reason of which remains unclear (Phillips et al. 2014). However, these clinical observations would support the idea that β-adrenergic stimulation could promote angiogenesis and that β-blockade could inhibit this, although the latter is still a matter of debate and there are conflicting results (Stati et al. 2014). Our data also indicate a pro-angiogenetic effect of β-adrenergic stimulation using isoprenaline. Interestingly, this was associated with enhanced intercellular coupling and with elevated expression of endothelial gap junction proteins.
Regarding endothelial connexins, it is well known that endothelial cells express Cx37, Cx40, and Cx43. However, while much is known about the regulation of cardiac and neuronal connexins, only little is known about the regulation of expression of endothelial connexins (Salameh and Dhein 2005; Hervé and Dhein 2010). In particular, a possible effect of β-adrenergic stimulation on endothelial connexin expression has not been investigated yet.
Endothelial cells express three connexin isoforms, Cx37, Cx40, and Cx43 (Burnier et al. 2009), which all are involved in angiogenesis: Specific knockdown of each of these three connexins led to reduced branch formation in an in vitro angiogenesis assay (Gärtner et al. 2012). The gap junction channels formed by the connexins allow intercellular signaling by the passage of small molecules and, besides endothelial-endothelial cell coupling, also are involved in myo-endothelial coupling (Burnier et al. 2009; de Wit et al. 2008). However, the specific role for each of these connexins in the endothelial cell remains widely unclear. It has been shown that Cx40 knockout in mice results in hypertension (de Wit et al. 2000) and that reduced endothelial Cx43 may be associated with hypotension and bradycardia in genetic mouse model (Liao et al. 2001), although others did not observe this effect of a Cx43 deletion (Theis et al. 2001). Our new data seems to indicate that endothelial Cx43 is the preferred target of isoprenaline, as upregulation of Cx43 was higher than that of the other two isoforms. The dye injection experiments show that the enhanced endothelial connexin expression results in enhanced coupling. However, we cannot state whether this enhanced coupling is due to enhanced formation of Cx43, Cx40, Cx37, or heteromeric channels.
To the best of our knowledge, this is the first report that isoprenaline leads to upregulation of endothelial connexins and enhanced endothelial intercellular gap junction communication. Interestingly, we found that caveolin-1 was also upregulated. This protein is known to colocate with Cx43 in endothelial cells (Looft-Wilson et al. 2012), and it was shown in keratinocytes that it is involved in Cx43 incorporation in the membrane and that it regulates gap junction intercellular communication (GJIC) (Langlois et al. 2008).
The next question would be whether the association of enhanced connexin expression increased coupling and angiogenetic effects is more than a simple association. Our previous finding that downregulation of endothelial connexins and reduction of GJIC impaired angiogenesis (Gärtner et al. 2012) would support the idea that upregulation of connexins and increase in GJIC could promote angiogenesis. In addition, we also could show that a direct uncoupling of endothelial gap junctions using palmitoleic acid resulted in impaired angiogenesis (Gärtner et al. 2012). However, it needs to be stressed that this is only indirect evidence. An experimental approach to prove the hypothesis that the increased GJIC under isoprenaline results in angiogenesis would be to treat the cells with isoprenaline together with simultaneous inhibition of endothelial connexin synthesis, e.g., by siRNA. This is, however, technically difficult, and moreover, interference between the knockdown technology and the drug never can be fully excluded. Thus, this was beyond the scope of the present investigation and will be investigated in future studies. However, the present findings, the previous observations with reduced GJIC, and the clinical observations of anti-angiogenetic effects of β-blockade are in favor of the hypothesis that the increase in GJIC is somehow involved in the angiogenetic effect of isoprenaline seen here. In addition, a causal relationship between endothelial GJIC becomes evident from the observation that in Cx37−/−/Cx40−/− mice pups die early from severe vascular malformations and hemangiomas (Simon and McWhorter 2002).
A point worth mentioning is that in the present experimental setup, the tubes grew into a matrix, while in most in vivo situations, sprouting capillaries will grow into tissue. The latter requires, besides communication among the endothelial cells, a reduction of the tissue intercellular communication and adherence in order to allow the outgrowth of the new vessel, a process which is regulated by ephrin ligands and ephrin receptor interplay (Ishii et al. 2011). Thus, our present study cannot be uncritically transferred to the more complex in vivo situation.
Although isoprenaline is known to act as a full agonist at beta-adrenoceptors with pKi of 6.4–7.0 and we used a considerably low concentration 100 nmol/L, future experiments using beta-blockers should be carried out to clarify whether isoprenaline action is solely attributable to beta-adrenoceptor activation and to which beta-adrenoceptor subtype. However, we and others have already shown that isoprenaline and adenylyl cyclase activation can enhance connexin 43 expression in cardiomyocytes (Salameh and Dhein 2011) and transfected HeLa cells and that the isoprenaline effect can be blocked by propranolol (Salameh et al. 2003, 2009; Xia et al. 2009).
Another aspect is the question whether the observed effect of isoprenaline on angiogenesis might be physiologically meaningful. β-Adrenergic stimulation physiologically is observed during physical exercise in the muscles, heart, lung, and other organs. Physical exercise results in increased oxygen demand, and thus, a mechanism which would, in the case of repeated physical exercise, result in increased angiogenesis appears to be an appropriate physiological regulatory mechanism. However, the present investigation can only give the idea for further investigation on β-adrenergic angiogenesis in physical exercise, as a possible mechanism additional to the well-known hypoxia-induced angiogenesis.
References
Burnier L, Fontana P, Angelillo-Scherrer A, Kwak BR (2009) Intercellular communication in atherosclerosis. Physiology (Bethesda) 24:36–44
Ciccarelli M, Sorriento D, Cipolletta E, Santulli G, Fusco A, Zhou RH, Eckhart AD, Peppel K, Koch WJ, Trimarco B, Iaccarino G (2011) Impaired neoangiogenesis in β2-adrenoceptor gene-deficient mice: restoration by intravascular human β2-adrenoceptor gene transfer and role of NFκB and CREB transcription factors. Br J Pharmacol 162(3):712–721
de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U (2000) Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res 86:649–655
de Wit C, Boettcher M, Schmidt VJ (2008) Signaling across myoendothelial gap junctions–fact or fiction? Cell Commun Adhes 15(3):231–245
Duerrschmidt N, Hagen A, Gaertner C, Wermke A, Nowicki M, Spanel-Borowski K, Stepan H, Mohr FW, Dhein S (2012) Nicotine effects on human endothelial intercellular communication via α4β2 and α3β2 nicotinic acetylcholine receptor subtypes. Naunyn Schmiedeberg’s Arch Pharmacol 385(6):621–632
Ferro A, Queen LR, Priest RM, Xu B, Ritter JM, Poston L, Ward JP (1999) Activation of nitric oxide synthase by beta 2-adrenoceptors in human umbilical vein endothelium in vitro. Br J Pharmacol 126(8):1872–1880
Gärtner C, Ziegelhöffer B, Kostelka M, Stepan H, Mohr FW, Dhein S (2012) Knock-down of endothelial connexins impairs angiogenesis. Pharmacol Res 65(3):347–357
Haussig S, Schubert A, Mohr FW, Dhein S (2008) Sub-chronic nicotine exposure induces intercellular communication failure and differential down-regulation of connexins in cultured human endothelial cells. Atherosclerosis 196(1):210–218
Hervé JC, Dhein S (2010) Peptides targeting gap junctional structures. Curr Pharm Des 16(28):3056–3070
Ishii M, Mueller I, Nakajima T, Pasquale EB, Ogawa K (2011) EphB signaling inhibits gap junctional intercellular communication and synchronized contraction in cultured cardiomyocytes. Basic Res Cardiol 106(6):1057–1068
Lamy S, Lachambre MP, Lord-Dufour S, Béliveau R (2010) Propranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration, and differentiation of endothelial cells. Vasc Pharmacol 53(5–6):200–208
Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW (2008) Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol Biol Cell 19(3):912–928
Laranjo S, Costa G, Paramés F, Freitas I, Martins JD, Trigo C, Pinto FF (2014) The role of propranolol in the treatment of infantile hemangioma. Rev Port Cardiol 33(5):289–295
Liao Y, Day KH, Damon DN, Duling BR (2001) Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci U S A 98:9989–9994
Looft-Wilson RC, Billaud M, Johnstone SR, Straub AC, Isakson BE (2012) Interaction between nitric oxide signaling and gap junctions: effects on vascular function. Biochim Biophys Acta 1818(8):1895–1902
Morawietz H, Rueckschloss U, Niemann B, Duerrschmidt N, Galle J, Hakim K, Zerkowski HR, Sawamura T, Holtz J (1999) Angiotensin II induces LOX-1, the human endothelial receptor for oxidized low-density lipoprotein. Circulation 100:899–902
Phillips RJ, Lokmic Z, Crock CM, Penington A (2014) Infantile haemangiomas that failed treatment with propranolol: clinical and histopathological features. J Paediatr Child Health 50(8):619–625
Rengo G, Zincarelli C, Femminella GD, Liccardo D, Pagano G, de Lucia C, Altobelli GG, Cimini V, Ruggiero D, Perrone-Filardi P, Gao E, Ferrara N, Lymperopoulos A, Koch WJ, Leosco D (2012) Myocardial β(2) -adrenoceptor gene delivery promotes coordinated cardiac adaptive remodelling and angiogenesis in heart failure. Br J Pharmacol 166(8):2348–2361
Sagi L, Zvulunov A, Lapidoth M, Ben Amitai D (2014) Efficacy and safety of propranolol for the treatment of infantile hemangioma: a presentation of ninety-nine cases. Dermatology 228(2):136–144
Salameh A, Dhein S (2005) Pharmacology of gap junctions. New pharmacological targets for treatment of arrhythmia, seizure and cancer? Biochim Biophys Acta 1719(1–2):36–58
Salameh A, Dhein S (2011) Adrenergic control of cardiac gap junction function and expression. Naunyn Schmiedeberg’s Arch Pharmacol 383(4):331–346
Salameh A, Polontchouk L, Dhein S, Hagendorff A, Pfeiffer D (2003) Chronic regulation of the expression of the gap junction protein connexin 43 in transfected HeLa cells. Naunyn Schmiedeberg’s Arch Pharmacol 368(1):33–40
Salameh A, Frenzel C, Boldt A, Rassler B, Glawe I, Schulte J, Mühlberg K, Zimmer HG, Pfeiffer D, Dhein S (2006) Subchronic alpha- and beta-adrenergic regulation of cardiac gap junction protein expression. FASEB J 20(2):365–367
Salameh A, Krautblatter S, Karl S, Blanke K, Gomez DR, Dhein S, Pfeiffer D, Janousek J (2009) The signal transduction cascade regulating the expression of the gap junction protein connexin43 by beta-adrenoceptors. Br J Pharmacol 158(1):198–208
Sexl V, Mancusi G, Baumgartner-Parzer S, Schütz W, Freissmuth M (1995) Stimulation of human umbilical vein endothelial cell proliferation by A2-adenosine and beta 2-adrenoceptors. Br J Pharmacol 114(8):1577–1586
Simon AM, McWhorter AR (2002) Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol 251:206–220
Stati T, Musumeci M, Maccari S, Massimi A, Corritore E, Strimpakos G, Pelosi E, Catalano L, Marano G (2014) β-Blockers promote angiogenesis in the mouse aortic ring assay. J Cardiovasc Pharmacol 64(1):21–27
Theis M, de Wit C, Schlaeger TM, Eckardt D, Krüger O, Döring B, Risau W, Deutsch U, Pohl U, Willecke K (2001) Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis 29(1):1–13
Xia Y, Gong KZ, Xu M, Zhang YY, Guo JH, Song Y, Zhang P (2009) Regulation of gap-junction protein connexin 43 by beta-adrenergic receptor stimulation in rat cardiomyocytes. Acta Pharmacol Sin 30(7):928–934
Author information
Authors and Affiliations
Corresponding author
Additional information
Stefan Dhein and Christiane Gaertner contributed equally.
Rights and permissions
About this article
Cite this article
Dhein, S., Gaertner, C., Georgieff, C. et al. Effects of isoprenaline on endothelial connexins and angiogenesis in a human endothelial cell culture system. Naunyn-Schmiedeberg's Arch Pharmacol 388, 101–108 (2015). https://doi.org/10.1007/s00210-014-1059-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-014-1059-0