Abstract
Androgens produce nongenomic effects in several cells by different mechanisms, including ion channel modulation. Adenohypophyseal cells express several K+ channels, including voltage and Ca2+-dependent K+ (BK) channels, which might be the target of androgens to modulate cellular action potentials and hormonal secretion. Androgen effects were studied in GH3 cells (from anterior pituitary rat tumor) by means of the patch-clamp technique. Cells were continuously perfused with saline solution, in the absence or presence of the androgens studied, while applying 40 mV pulses of 400 ms from a holding potential of −60 mV in whole-cell configuration with nystatin-perforated patches. Androgens reversibly blocked noninactivating K+ currents in a concentration-dependent manner without a latency period and with an order of efficacy of: 5β-dihydrotestosterone (DHT)>testosterone>5α-DHT. RT-PCR showed two isoforms of the α-pore forming subunits of BK channels. These channels are responsible for one third of the noninactivating current, according to the blockade of paxilline, a selective BK antagonist. Androgens seem to directly interact with BK channels since they were blocked in excised inside-out patches and independent of the whole-cell configuration and the NO-cGMP-dependent pathway. Testosterone, but not 5α- or 5β-DHT, increased BK currents in HEK-293 cells overexpressing the short isoform, suggesting a cellular selectivity based on the α-subunits. The effect on noninactivating currents may be responsible for the decrease of spontaneous action potential frequency. Long-term cellular incubation with testosterone did not modify noninactivating currents density in GH3 cells. It is remarkable that 5β-DHT, a reductase metabolite with weak androgenic activity, was the most efficient blocker.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sex hormones modulate the function of hypothalamic-pituitary-adrenal and gonadal axes, acting on different cells of the hypothalamus and adenohypophysis regulating hormonal secretion.
In males, overall, androgens produce negative feedback on the pituitary gland. This effect varies depending on the particular type of cell (Tobin and Canny 1998; Viau and Meaney 2004) and the species studied (Gunzel-Apel et al. 2009; Martin et al. 2006). Regarding the cellular secretion, the metabolism of testosterone to estradiol (Finkelstein et al. 1991a; Finkelstein et al. 1991b; Pitteloud et al. 2007; Rochira et al. 2006), by aromatization (Roselli et al. 1997), or to 5α-dihydrotestosterone (DHT) (Martin et al. 2006), a reduced nonaromatizable derivate (Denef 1983; Lephart 1993), may also be important.
The reported effects of testosterone on pituitary cells are mainly produced by genomic mechanisms, though other mechanisms may be involved. Adenohypophysis cells express several ion channels, including voltage-dependent K+ channels. Of this large family, large-conductance voltage- and Ca2+-activated K+ (BK) channels are critical for cellular repolarization, spontaneous excitability at resting conditions, and for the modulation of the Ca2+ influx necessary for cellular secretion (Stojilkovic et al. 2010). This contribution was shown in female mice deficient for the BK channel pore-forming α-subunit whose corticotrope secretion was inhibited during stimulation, but not under basal conditions (Brunton et al. 2007).
Ion channels are one of the proposed molecular targets for the nongenomic effect of androgens in several cell types and tissues (Michels and Hoppe 2008). They have been widely studied due to existing sex differences in the physiology and pathology of the cardiovascular system, such as in the heart (Furukawa and Kurokawa 2007) and in several vascular smooth muscles androgens regulate voltage-dependent K+ channels (Kelly and Jones 2013; Perusquia and Stallone 2010).
The reported acute regulation of androgens on voltage-dependent K+ channels led us to hypothesize that androgens may also affect adenohypophysis cell function via the modulation of activity of these channels, especially the BK which plays a critical role in modulating Ca2+ channel permeability and cellular secretion. Rat pituitary prolactinoma GH3 and HEK-293 cells (for transient transfection of BK channels) were used to perform electrophysiological studies by means of different configurations of the patch-clamp technique.
Material and methods
Experimental procedure for electrophysiological studies
The study was performed using GH3 cells provided by Dr. F. Barros (Departamento de Bioquímica y Biología Molecular, Universidad de Oviedo, Spain), and HEK-293 cells from Dr. C. Rodriguez and Dr. V. Martin (Departamento de Morfología y Biología Celular, Universidad de Oviedo, Spain). GH3 cells were grown at 37 °C and 5 % CO2 in Dulbecco’s modified Eagle’s medium/Ham’s F12 nutrient (Sigma-Aldrich ) mixture (1:1, v/v) supplemented with 15 % horse serum and 2.5 % fetal bovine serum (PAA, Austria) (Barros et al. 1991). HEK-293 cells were grown in Dulbecco’s modified Eagle’s medium-high glucose (Biowest), supplemented with 10 % fetal bovine serum. One hundred units of penicillin per milliliter and 0.1 mg of streptomycin per milliliter (Biowest SAS, Nuaillé, France) were added to both media.
Recordings in GH3 cells were performed 1–4 days after the passage of the cells and in HEK-293 cells 24–48 h after transient transfection, which was carried out 24 h after passage, with plasmid encoding the BK pore forming α-subunit. Microelectrodes created from borosilicate glass (Drummond Scientific, PA) and coated with bees wax were used. Electrode resistances were 1.5 to 2.5 MΩ when filled with the pipette solutions and immersed in the bath. The reference electrode was a silver-silver chloride wire within an agar bridge (4 % agar in 200 mM KCl). No corrections for junction potentials were used. Membrane currents were recorded using whole-cell (conventional and perforated by nystatin 0.25 mg/ml) and inside-out patches of the patch-clamp technique (Hamill et al. 1981), with an Axopatch 1D amplifier controlled by PULSE software (HEKA Elektronik, Lambrecht, Germany) and an ITC-16 computer interface (Instrutech, Port Washington, NY, USA). Currents were digitized at 10 kHz and filtered at 3 kHz and the action potential at 2 kHz. Capacitative and leak currents were subtracted by means of the P/4 protocol.
Current–voltage relation was performed by applying 400 ms voltage steps of 10 mV every 3 s from a holding membrane potential of −60 mV. To determine the effect of the androgens (testosterone, 5α-, and 5β-dihydrotestosterone), tetraethylammonium (TEA), and paxilline, 400 ms voltage steps of 40 mV were applied every 15 s, from a holding potential of −60 mV, and also to −30 mV for the androgens. Once the steady state of blockade was reached for each concentration, in some experiments, a current–voltage curve was performed to study the voltage dependence on the effect of these agents; otherwise, the drug was washed out from the perfusion solution.
For whole-cell configuration extracellular Na+ solution contained (millimolar (mM)): NaCl 160, KCl 3, CaCl2 2, MgCl2 1, HEPES 10, and glucose 10, pH adjusted to 7.2 with NaOH. Pipette solution (intracellular solution) for conventional whole-cell consisted of (mM): KCl 150, MgCl2 2, HEPES 10, and CaCl2 0.01, pH 7.2, and for nystatin, perforated patches consisted of (mM): KCl 65, K2SO4 35, NaCl 10, MgCl21, and HEPES 10, pH 7.2, with KOH.
Perfusion solutions were continuously applied to the experimental chamber (0.2–0.3 ml) by gravity at approximately 1 ml/min. Testosterone, 5α-, and 5β-DHT in whole-cell configurations (conventional or by nystatin-perforated patch-clamp) were added directly to perfusion solutions while voltage pulses of 400 ms at 40 mV were applied every 15 s, from a holding potential of −60 mV. The effect of paxilline (10 to 300 nM) was studied by continuous perfusion of the cells by whole-cell mode nystatin-perforated patch-clamp. In addition, the effect of androgens was studied after the steady-state with paxilline (300 nM) was reached. The effect of NO modifier agents, Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME; 10 μM) and sodium nitroprusside (10 and 100 μM), and methylene blue (10 μM), or the cGMP analog 8-Br cGMP (10 μM), on voltage-dependent K+ currents was studied by adding these drugs 20–30 min before the perfusion of the androgens.
For inside-out excided patches, the solution in the bath contained (mM): KCl 150, CaCl2 4.198, EGTA 5, and HEPES 10, and in the electrode (mM): KCl 150, CaCl2 0.01, MgCl2 2, and HEPES 10, pH adjusted to 7.2 with KOH, in both cases. The cells were continuously perfused while a depolarization voltage of 40 mV was applied. The drugs assayed were perfused after a stable control of at least 12 min, and then observed in the presence of the drug for 12 min, before washout of the drugs, when possible. The right configuration was confirmed by the rapid suppression of BK channel activity chelating the internal Ca2+ with EGTA.
Current-clamp recordings were performed to study the effect of androgens on spontaneous action potentials of GH3 cells, perforating the seal with nystatin, in the same extracellular solution, previously described, and using the same amplifier and devices. All experiments were performed at room temperature (22–25 °C).
HEK-293 cells were transiently transfected with plasmids containing green fluorescent protein (GFP) and the BK α subunit. Transfected cells were identified by the green color of GFP when exposed to blue light using fluorescence based optics (Nikon). Transfection was performed using the jetPEI reagent (Polyplus transfection, Illkirch France). Plasmids were donated by Dr. Martin Kholer (Merck RL, NJ, USA).
A group of GH3 cells were incubated with testosterone (30 or 100 μM) for 24 or 48 h to estimate the density of outward current, applying pulses of 60 mV from a holding potential of −60 mV. The controls were GH3 cells of the same passage incubated with the same concentration of dimethyl sulfoxide (DMSO) used as solvent for the androgens. The currents were recorded alternating cells incubated with testosterone and control.
RT-PCR analysis of RNA extracted from GH3 cells
For these experiments, total RNA was isolated from GH3 cell cultures and rat tissues as previously described (Chomczynski and Sacchi 1987) and then reverse transcribed using random hexamers as primers and SuperScriptTM reverse transcriptase (Invitrogen, Carlsbad, CA) following the instructions of the manufacturer. For each sample a negative control was prepared without transcriptase.
Target cDNAs were amplified by PCR using Taq DNA polymerase (Biotools) and pairs of specific primers for each gene product, 5′-GCCTGTCATGATGACGTC-3′and 5′-GCTGTCATCAAACTGCATAG-3′ for α subunit, 5′-CCAGGAATCCACCTGTCACT-3′ and 5′-CAGAGAGGGACCTGTTGAGC-3′ for β1 subunit, and 5′-CAATACAGGACTCTTTCGAG-3′and 5′-TTATGGTCGGAACTAACGACG-3′ for 18S rRNA, used as an internal control for relative RT-PCR.
Reactions were performed in a thermocycler (MyCycler, BioRad) with an initial 4 min denaturation step at 95 °C followed by 35 cycles (of 95 °C for 15 s, 55 °C for 30 s and 72 °C for 30 s) in the case of the α and β1 subunits coding genes and twenty cycles for 18S rRNA. Amplified products were resolved by electrophoresis in a 1.2 % agarose gel in TBE buffer (Tris 89 mM, ethylenediaminetetraacetic acid 2 mM, boric acid 89 mM, pH 8.3). DNA bands were visualized under UV after ethidium bromide staining and photographed using a Wilber Lourmat Photodocumentation system. The size of the specific bands matched with the predicted length of the amplicons.
Drugs
The following drugs were used: testosterone (17β-hydroxy-4-androsten-3-one), 5α-dihydrotestosterone (17β-hydroxy-5α-androstan-3-one), 5β-dihydrotestosterone (17β-hydroxy-5β-androstan-3-one), L-NAME, 8-bromoguanosine 3′,5′-cyclic monophosphate sodium salt (8-Br-cGMP), TEA, paxilline, nystatin (mycostatin), and ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid from Sigma-Aldrich, sodium nitroprusside and methylene blue from Merck (Darmstadt, Germany).
Testosterone, 5α-, 5β-DHT, and paxilline were dissolved in DMSO. The solvent was ineffective at concentrations ≤0.1 %, the maximum concentration used. The rest of the drugs were dissolved in purified water.
Analysis of data
Current amplitudes of voltage-dependent K+ channels were measured averaging 30 ms at the end of the 400 ms pulses with PULSEFIT software (HEKA Elektronik, Lambrecht, Germany). Single-channel analysis was carried out using Clampfit 10.0.4.36 (Molecular Devices Corporation, CA, USA) and the action potentials using AxoGraph X Version 1.4.4 software (John Clements, CA, USA). The graphs and examples were plotted using IGOR software Version 6.0 (Wavemetrics, Lake Oswego, OR, USA). The effect of the androgens was referred to as the fraction blocked: (I control − I drug)/I control. In a group of experiments, BK current was abolished by the incubation with a selective blocker, paxilline 300 nM, and the fraction blocked by the androgens was calculated taking into account paxilline-insensitive current as the control current.
Inhibitory concentration (IC)50 was described by the Hill equation of the form: Fraction Blocked = Maximal Block/[1+(IC50/[Drug])n], where IC50 is the concentration that produces 50 % of the maximal block and n is the apparent Hill coefficient.
The probability of a channel being open (P open) was given by: P open = t o/N × T, where t o is the total time that the channel was observed in the open state, T is the total observation time, and N is the number of channels in the patch. The outward current density was expressed in pA/pF.
The results were expressed as the value of the mean ± standard error of the mean (SEM) of at least five experiments in equal numbers of cells. Pearson correlation coefficient was used to indicate the relationship between the fraction blocked by paxilline and by the androgens on outward currents. Statistical significance was determined using the Student’s t test, with a cutoff value of p ≤ 0.05.
A one-way between-groups analysis of variance (ANOVA) with post hoc tests, Tukey’s honest significance test (HSD), was conducted to explore the impact of different androgens on the fraction outward currents blocked in the presence of paxilline and on the opening of BK channels in inside-out excided membrane patches in GH3 cells.
Results
Effect of androgens on whole-cell outward currents in GH3 cells
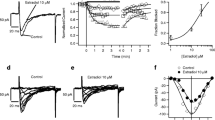
In whole-cells, using perforated patches with nystatin, with a holding potential of −60 mV and applying pulses at 40 mV, testosterone (3 to 200 μM), 5α- (3 to 200 μM), and 5β-DHT (1 to 100 μM) reversibly blocked outward currents in a concentration-dependent manner (Fig. 1a–c). This effect had no observable latency period. The IC50’s were in μM: 24.22 ± 1.77, 6.71 ± 0.68 and 11.13 ± 2.44, respectively, for testosterone, 5α- and 5β-DHT, with a time constant of blockade at the concentration of 30 μM of 24.46 ± 3.17, 34.65 ± 3.25 and 28.19 ± 3.04 s, and at 100 μM of 23.69 ± 1.79, 31.59 ± 3.87 and 20.04 ± 1.54 s, respectively.
Representative membrane currents (a) and (b) time-course blockade and recovery, after washout, of voltage-dependent K+ current in whole-cell (nystatin perforated patches) in response to 400 ms voltage steps to 40 mV every 15 s from −60 mV holding potential, in the absence (control) and in the presence of testosterone, 5α- or 5β-dihydrotestosterone (DHT; 100 μM) when the current amplitude remained unchanged after applying several pulses. (c) Fraction blocked of the current, the lines plot to the data with a Hill equation with an n value close to one. Each point represents the mean ± SEM for at least seven different cells
The incubation with androgens for 4 min, without the application of depolarization pulses, produced a similar blocked fraction to that produced when continuous pulses of 40 mV every 15 s were applied while perfusing the androgens.
The block of outward K+ currents by androgens occurred at all voltages (Fig. 2a–c). The fraction blocked was similar at a holding potential of −30 mV, at which some inactivating current are suppressed, when applying pulses at 40 mV, and when the outward current was studied in conventional whole-cell configuration, where intracellular cytoplasm is diluted (Fig. 3).
Mean I–V relationship of (a) testosterone, (b) 5α-, and (c) 5β-DHT (100 μM) on voltage-dependent opening of outward current in GH3 cells, normalized to cell capacitance (pA/pF), and its representative membrane currents (a, b, and c, respectively) in whole-cell nystatin-perforated patches in response to 400 ms voltage steps of 10 mV from −60 to 60 mV, every 3 s, and −60 mV holding potential, in the absence (control) and the presence of androgens. Values represent the mean ± SEM of at least seven different experiments.*p ≤ 0.05 and **p ≤ 0.01, for paired data by means of the Student’s t test
Effect of TEA (30 and 100 mM) and paxilline (10 nM to 3 μM) on outward currents in GH3 cells
The contribution of K+ channels to the outward current was studied by extracellular administration of TEA, a nonselective K+ channel blocker, or paxilline, a selective BK channel blocker (Sanchez and McManus 1996).
TEA (30 and 100 mM) blocked, in a concentration-dependent manner, GH3-elicited outward current. The blocked fraction was 0.43 ± 0.03 (N = 6) and 0.83 ± 0.11, respectively (N = 9; results not shown).
The fraction blocked by paxilline (10 nM to 3 μM) varied from cell to cell. The mean fraction blocked was similar for 10 to 300 nM, and superior for 1 and 3 μM with lower variability (Fig. 4a).
Fraction of voltage-dependent K+ current blocked in GH3 cells by paxilline (10 nM to 3 μM), representing the effect on each cell (open circles) and the average for each concentration (open squares) (a) and by testosterone, 5α- and 5β-DHT (30 μM), in the presence of paxilline 300 nM (b); vertical lines represent the SEM for at least six different experiments in each case. (c) Linear correlation between the fraction blocked by paxilline (300 nM) and the blockade elicited by testosterone (30 μM) on the remaining current after the exposure to paxilline
Effect of paxilline (300 nM) preincubation on androgens-elicited outward current blockade in GH3 cells
Paxilline, 300 nM, was used to establish the fraction of inhibition of voltage-dependent K+ currents by androgens independent of BK channels. For this, the effect of the androgens was studied after a steady-state of the blockade by previous incubation with paxilline was reached. In this case, paxilline (300 nM)-insensitive current was used as reference to calculate the fraction blocked by the androgens. The androgens blocked voltage dependent K+ current in the presence of paxilline (300 μM). One-way ANOVA showed a statistically significant difference in the fraction blocked between drugs (F (2, 24) = 3.89, p = 0.034). The effect size, calculated using eta squared, was 0.24 and was considered as a large effect according to Cohen’s classification (Cohen 1988). Post hoc comparisons using the Tukey HSD test indicated that the mean effect for 5β-DHT was significantly different from testosterone (p = 0.028); 5α-DHT did not differ significantly from either testosterone or 5β-DHT (Fig. 4b).
The effect of testosterone (30 μM) was weaker than in the absence of paxilline and showed a significant inverse linear correlation between the fraction blocked for paxilline (previous to the addition of testosterone) and the fraction blocked by testosterone on paxilline-insensitive currents (R, −0.826, p = 0.003, N = 10; Fig. 4c). The fraction blocked by paxilline was not significantly correlated with the effect of 5α- or 5β-DHT (30 μM) on paxilline-insensitive current.
Determination of BK channels in GH3 cells by RT-PCR
RT-PCR experiments were carried out to determine the BK channel mRNA profile. This showed that BK α-pore forming, but not β1 subunit, is expressed in GH3 cells. In contrast, strong expression of both subunits was observed in rat colon tissues, which were used as a positive control. In the case of the α subunit, two different alternative spliced transcripts were detected due to inclusion of the STREX exon in the largest one (Fig. 5).
Effect of androgens on BK channels in excided inside-out patches in GH3 cells
To determine the direct effect of androgens on BK channels, androgens were studied in single-channel recordings in an inside-out configuration with symmetrical KCl solutions and an internal CaCl2 of 1 μM (Fig. 6a, b). Single-channel activity was suppressed by EGTA perfused to the intracellular face of the channel (results not shown). Only recordings sensitive to EGTA were taken into account to analyze the effect of the androgens. Testosterone (30 μM) significantly (p < 0.05) decreased the P open of the channel, without differences in the mean opening of the channel, 0.44 ± 0.05 ms in the control and 0.44 ± 0.04 ms in the presence of testosterone. The ratio of testosterone P open/control P open was 0.76 ± 0.07 (N = 19). Similarly, for 5α-DHT (30 μM), the average openings of the channel were, respectively, for the control and in the presence of 5α-DHT, 1.14 ± 0.24 vs 1.21 ± 0.29 ms and a ratio of P open of 0.79 ± 0.17 (N = 8), and for 5β-DHT (30 μM), the average opening of the channel was, respectively, 1.11 ± 0.56 vs 0.97 ± 0.48 and a ratio of P open of 0.52 ± 0.20 (N = 8). 5α- and 5β-DHT significantly decreased the P open of BK channels (p < 0.05 and p < 0.01, respectively). One-way ANOVA detected a significant difference in the magnitude of the ratios (androgen P open/control P open) between groups (F (2, 30) = 4.91, p = 0.014). The effect size, calculated using eta squared, was 0.25 and was considered as a large effect according to Cohen’s classification. Post hoc comparisons using the Tukey HSD test indicated that the mean effect for 5β-DHT was significantly different from testosterone (p = 0.021); 5α-DHT did not differ significantly from either testosterone or 5β-DHT (Fig. 6c).
Effect of testosterone (30 μM), after 10 min of incubation, in excised inside-out membrane patches, during continuous 40 mV depolarization in 1 μM internal calcium (a and b), and (c) ratio of androgen P open/control P open of BK channels for testosterone, 5α-, or 5β-dihydrotestosterone (DHT). Each point represents the mean ± SEM for at least six different cells. One-way ANOVA detected differences between groups, being significant, according to post hoc tests, the effect of 5β-DHT by comparison with testosterone, *p < 0.05
Effect of androgens on BK α-pore forming subunit transiently expressed in HEK-293 cells
HEK-293 cells transiently transfected with BK α were used to study the effect of androgens on these channels in nystatin-perforated whole-cell configuration. Testosterone (30 μM) elicited a reversible increase of BK α current (Fig. 7a, b), while 5α- and 5β-DHT (30 μM) blocked the channels. The current modified fraction was 1.39 ± 0.23 (N = 5), 0.26 ± 0.07 (N = 5), and 0.38 ± 0.08 (N = 5), respectively. A significant difference exists in the fraction of current modified by androgens with respect to the control, in the absence of these agents, but not between 5α- and 5β-DHT (Fig. 7c).
Time-course (a) and recordings (b) of testosterone (30 μM) increase of BK current and recovery on HEK-293, after washout, of BK currents in whole-cell (nystatin perforated patches) in response to 400 ms voltage steps to 40 mV every 15 s from −60 mV holding potential, in the absence (control) and the presence of testosterone (30 μM) when the current amplitude remained unchanged after applying several pulses. (c) Current modified fraction by testosterone, 5α-, and 5β-dihydrotestosterone (DHT; 30 μM). Values represent the mean ± SEM for at least five different cells.*p ≤ 0.05, by comparing the effect of the androgens with respect to BK current in the absence of these compounds, for paired data by means of the Student’s t test
Effect of nitric oxide (NO) modifying agents on androgen-elicited effect on outward currents in GH3 cells
NO has been implicated as mediator of androgen-elicited opening of BK channels in vascular smooth muscle (Fernandes et al. 2012). To study its role in androgen-elicited blockade in GH3 cells, the cells were incubated for 30 min with L-NAME (10 μM), sodium nitroprusside (10 μM), methylene blue (10 μM), or 8-Br-cGMP (10 μM).These drugs did not modify noninactivating currents nor did they modify androgen-elicited blockade in nystatin perforated patches, when pulses were applied to 40 mV from a holding potential of −60 mV (results not shown).
Effect of GH3 cells long-term incubation with testosterone (30 and 100 μM) on their outward current
The incubation of GH3 culture cells with 30 or 100 μM testosterone for 24 or 48 h did not significantly modify the density of BK current expressed as pA obtained at 60 mV from a holding potential of −60 mV divided by the capacitance in pF. Density of BK currents after 24 h incubation with testosterone was: 153.86 ± 24.83, 154.91 ± 35.93, and 141.88 ± 21.91 pA/pF; and for 48 h, 87.98 ± 10.91, 88.43 ± 14.34, and 98.03 ± 25.57 pA/pF, in both cases, respectively, for control (testosterone free) cells, testosterone 30 μM, and testosterone 100 μM (results not shown).
Effect of androgens on spontaneous action potentials of GH3 cells at resting conditions
GH3 cells presented spontaneous action potentials at resting conditions in current-clamp recordings with an average of 0.55 ± 0.09 Hz. The depolarization was produced at 0.52 ± 0.09 mV/ms, with a width of 245.95 ± 42.37 ms, and the maximum repolarization was −50.62 ± 2.41 mV (N = 21).
Testosterone (30 μM), and qualitatively similar 5α- and 5β-DHT (10 to 100 μM), decreased the frequency of spontaneous action potentials in GH3 cells, without an apparent latency period (Fig. 8a, b). In some cells, an increase in the frequency of action potentials was observed during the first 1–3 min of exposure to testosterone, followed by a decrease or suppression of activity (Fig. 8b). The slope (measured in mV per ms) and the magnitude of depolarization and the width of the action potentials were also decreased. The effects were concentration-dependent, and in some cells, a partial reversal of the effects was observed (results not shown).
Paxilline (10 nM) blocked spontaneous action potential activity, preceded by cellular depolarization and an increase in its activity, in association with a decrease in the slope and the width of the action potentials (Fig. 8c).
Discussion
These results demonstrate that androgens block voltage-dependent K+ currents, including the BK channels of which α-pore forming subunits are expressed in GH3 cells. These effects are associated with a decrease in spontaneous action potentials.
Nystatin perforated-patch recordings (Horn and Marty 1988) were mainly used in the whole-cell configuration of the patch clamp technique to allow the study of ion channel modulation by intracellular mediators and current-clamp recordings of spontaneous action potential.
Testosterone and the nonaromatizable metabolites, 5α- and 5β-DHT, blocked outward potassium currents without an apparent latency period, reaching the steady-state in approximately 3 min. Therefore, it is unlikely that the effect of testosterone could be due to its conversion by aromatases or reductases. The blockade was completely reversed after the washout of these compounds.
The kinetics of the effect is compatible with a nongenomic response of androgens, being qualitatively similar for the 3 compounds. But the fraction blocked was not related to the androgenic potency nor with the androgen-receptor affinity, which should be stronger for 5α-DHT (Wilson and French 1976) than for the β isomer, which has weaker androgenic activity (Balthazart et al. 1984). However, in GH3 cells, 5α-DHT was less effective at blocking outward currents than 5β-DHT, 25 % and 50 % of blockade, respectively.
The IC50’s for the studied androgens were in the micromolar range, similar to several in vitro assays (Perusquia and Stallone 2010). The blockade of the channel was produced in the absence of depolarizing pulses, suggesting that they bind to the resting state of the channels.
GH3 cells expressed several outward potassium channels. Some are noninactivating K+ currents, which may include Kv1.5 (Takimoto et al. 1993) and the BK channels (Lang and Ritchie 1987). This current was measured at the end of the 400 ms depolarization pulses where channels with short time constants of inactivation are not present. The current was almost completely removed by the nonselective K+ channel blocker TEA and partially blocked by paxilline, a selective BK channel antagonist (Sanchez and McManus 1996), with wide variability in the blocked fraction from cell to cell. This is in agreement with the variations in the expression rate and the density of the BK channels during the cell cycle (Ouadid-Ahidouch et al. 2004). It seems that 30 nM paxilline might be enough to block BK channels in GH3 cells, since there are no differences in the average effect observed up to 300 nM. Higher concentrations might not be selective and also block noninactivation currents other than the BK.
Considering the fraction blocked by paxilline (a selective antagonist of BK channels) as the BK current, it can be assumed that these channels are functionally present in GH3 cells, representing one third of the whole outward current measured at the end of the pulse. This supports the findings of the RT-PCR, where two splicing variants of the α-pore forming subunit were observed without the β1 subunit, a regulatory subunit of BK channel.
To characterize the contribution of BK channel blockade on the effect of the studied androgens, these were functionally suppressed by paxilline. A concentration of 300 nM was chosen since it causes faster blockade than lower concentrations, apparently without losing selectivity. The fraction blocked by testosterone on the paxilline-insensitive current was weaker than that of the total noninactivated current and negatively correlated to the previous blockade by paxilline. This suggests that a significant part of the effect of testosterone is on BK channels, given that when these channels are functionally absent the magnitude of the blockade is lower. However, there is no correlation between the blockade elicited by paxilline and 5α- or 5β-DHT. This suggests, especially for 5β-DHT, an additional binding site on paxilline-insensitive currents.
For a more precise characterization of the effect of androgens on BK channels, they were assayed isolating the channels using excised inside-out patch recordings in GH3 cells and whole-cell configurations in HEK-293 cells overexpressing the short length human BK α-pore forming subunit. In inside-out patches of GH3 cells the 3 androgens studied decreased the P open of BK channels, without modification in the mean open time. The decrease in the P open by the androgens is independent of the mean open time of the channels in the control.
The inside-out effect means that androgens directly interacted and blocked the BK channels, suggesting that the effect is produced in the absence of intracellular mediators, as was suggested by the similar magnitude of blockade observed in nystatin-perforated or conventional whole-cell patch-clamp. This configuration allows the diffusion of intracellular constituents making it difficult for intracellular mediators to elicit the effect (Akaike and Harata 1994). The blockade seems independent of potential modifications in Ca2+ channel permeability, as reported for testosterone in L-type Ca2+ channels when inducing vasodilatory effects (Kelly and Jones 2013), given that a holding potential of −30 mV inactivates these channels but, at the same time, did not modify the fraction blocked compared to that at −60 mV.
However, the interaction of testosterone with short length BK α in HEK-293 did increase the current, while 5α- and 5β-DHT blocked the channel. The qualitative difference shown by testosterone is not explained by the side of the membrane to which the androgen was applied, external for whole-cell and internal for inside-out, since in both configurations, the channel was blocked. This may be related to the different α-subunits present in both cells (two in GH3, that might form hetero-tetramers of their α-pore forming subunits, and one in HEK-293) or the type of cell studied which may differ in the binding sites of the androgens to allow the effect on the channel. It has been reported that androgens might selectively produce nongenomic effects via the presence of target proteins and/or the coupling of transducer mechanisms in the different tissues (Fakler and Adelman 2008; Frye et al. 2008; Hu and Zhang 2012; Torres et al. 2007). Similar to the effect on BK α overexpressed in HEK-293 cells, androgens activated BK channels in smooth muscle of several species and tissues via NO- and cGMP-dependent pathways (Deenadayalu et al. 2011; Deenadayalu et al. 2001). This transduction pathway did not qualitatively nor quantitatively modify the inhibition of voltage-dependent K+ currents by androgens in GH3 cells. The incubation with an NO donor (sodium nitroprusside), an inhibitor of NO synthesis (L-NAME), an inhibitor of guanylyl cyclase (methylene blue), or a cGMP analog (8-Br cGMP), did not modify the fraction blocked by the studied androgens. This suggests the existence of different coupling mechanisms which might be related to tissue selectivity (Lin et al. 2006).
Sex hormones regulate K+ channel expression (Jamali et al. 2003; Ohno et al. 2009) and degradation (Korovkina et al. 2004), an effect less studied in androgens. In order to study this regulation of expression, GH3 cells were long-term incubated with supraphysiological concentrations of testosterone, 30 and 100 μM, for 24 or 48 h. These treatments neither significantly increased (compatible with an increase in expression) nor decreased (compatible with repression or proteolytic degradation) the density of outward noninactivating currents, expressed in pA/pF.
Voltage-dependent K+ channels play an important role on the repolarization of spontaneous action potentials and the maintenance of the membrane potential in GH3 cells. The ion channels that constitute the macroscopic noninactivating current or their individual contribution have not been completely characterized. The existence of a selective antagonist for each channel, except for the BK, has not been characterized either, making it difficult to study the contribution of a particular ion channel to the spontaneous action potentials and secretion. The role of BK channels has been pharmacologically characterized. They contribute to stopping the action potentials and limiting Ca2+ influx (Stojilkovic et al. 2010). This might have effects on hormone secretion, since the frequency of action potentials is relevant to this regulation.
The concentration of the studied androgens blocked voltage-dependent K+ currents and, in the same extracellular solution, modified the action potential in GH3 cells in a similar way. They decreased or suppressed the frequency of action potentials, which was preceded by an initial increase during the first minutes of exposure. Furthermore, they decreased the slope and the magnitude of voltage depolarization and shortened the width of action potentials. Differences existed in the sensitivity between cells and in the effect on voltage repolarization, which was not modified or caused depolarization.
The availability of paxilline, a selective BK channel blocker, allows a functional characterization of the effect of these channels on the spontaneous action potentials. Paxilline at a concentration close to the IC 50 (Sanchez and McManus 1996) depolarized the cells and decreased the amplitude of action potentials. These effects were initially associated with an increase in the frequency of action potentials. These effects are similar to that elicited by testosterone, 5α-, and 5β-DHT on the action potentials in some cells. The suppression of this effect did not allow the quantification of the response to these compounds. This suggests that depending on the component of the outward current, paxilline-sensitive (blocking BK channels) or paxilline-insensitive (not related to BK channels), androgens might elicit a different phenotype, depolarizing the cells or not, respectively. It seems that BK channels, in addition to the small contribution to the delayed K+ current in GH3 cells, are of functional importance to the modulation of action potentials and on Ca2+ permeability critical for the excitation-secretion coupling (Turner et al. 2011). This may be related to the colocalization of Ca2+ channels and the BK, forming macrocomplexes and allowing a rapid functional interaction (Berkefeld et al. 2006; Fakler and Adelman 2008).
The hypothalamic-pituitary-gonadal axis is an interconnected system of stimulatory and inhibitory mechanisms. Elevated prolactin often leads to mild hypogonadism and is usually associated with decreased testosterone levels (Carter et al. 1978), though it is unclear if this relationship is causal (Carani et al. 1996), and androgens might suppress hypothalamic-pituitary function (Seminara 2006). According to our results androgens might directly modulate adenohypophysis secretion, independently of the effect described on the hypothalamus (Tang 1991).
The results give evidence of an involvement of androgens on the regulation of BK channels and on action potential activity that might modulate adenohypophysis secretion.
Of interest is the fact that 5β-DHT showed a higher efficacy than testosterone or 5α-DHT, suggesting a potential biological role for β-reductases converting testosterone into a compound with weak or no androgenic effect that may modulate ion channels and cellular secretion.
References
Akaike N, Harata N (1994) Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn J Physiol 44(5):433–473
Balthazart J, Schumacher M, Malacarne G (1984) Relative potencies of testosterone and 5 alpha-dihydrotestosterone on crowing and cloacal gland growth in the Japanese quail (Coturnix coturnix japonica). J Endocrinol 100(1):19–23
Barros F, Delgado LM, Macia C, de la Pena P (1991) Effects of hypothalamic peptides on electrical activity and membrane currents of 'patch perforated' clamped GH3 anterior pituitary cells. FEBS Lett 279(1):33–37
Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B (2006) BKCa-Cav channel complexes mediate rapid and localized Ca2+−activated K+ signaling. Science 314(5799):615–620
Brunton PJ, Sausbier M, Wietzorrek G, Sausbier U, Knaus HG, Russell JA, Ruth P, Shipston MJ (2007) Hypothalamic-pituitary-adrenal axis hyporesponsiveness to restraint stress in mice deficient for large-conductance calcium- and voltage-activated potassium (BK) channels. Endocrinology 148(11):5496–5506
Carani C, Granata AR, Fustini MF, Marrama P (1996) Prolactin and testosterone: their role in male sexual function. Int J Androl 19(1):48–54
Carter JN, Tyson JE, Tolis G, Van Vliet S, Faiman C, Friesen HG (1978) Prolactin-screening tumors and hypogonadism in 22 men. N Engl J Med 299(16):847–852
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159
Cohen J (1988) Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates, Hillsdale
Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ (2001) Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol 281(4):H1720–H1727
Deenadayalu V, Puttabyatappa Y, Liu AT, Stallone JN, White RE (2011) Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase. Am J Physiol Heart Circ Physiol 302(1):H115–H123
Denef C (1983) 5 alpha-dihydrotestosterone formation and its functional significance in rat anterior pituitary, subpopulations of gonadotrophs and cell cultures. J Steroid Biochem 19(1A):235–239
Fakler B, Adelman JP (2008) Control of K(Ca) channels by calcium nano/microdomains. Neuron 59(6):873–881
Fernandes VS, Barahona MV, Recio P, Martinez-Saenz A, Ribeiro AS, Contreras C, Martinez AC, Bustamante S, Carballido J, Garcia-Sacristan A, Prieto D, Hernandez M (2012) Mechanisms involved in testosterone-induced relaxation to the pig urinary bladder neck. Steroids 77(5):394–402
Finkelstein JS, O’Dea LS, Whitcomb RW, Crowley WF Jr (1991a) Sex steroid control of gonadotropin secretion in the human male. II. Effects of estradiol administration in normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab 73(3):621–628
Finkelstein JS, Whitcomb RW, O’Dea LS, Longcope C, Schoenfeld DA, Crowley WF Jr (1991b) Sex steroid control of gonadotropin secretion in the human male. I. Effects of testosterone administration in normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab 73(3):609–620
Frye CA, Edinger K, Sumida K (2008) Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology 33(5):1049–1061
Furukawa T, Kurokawa J (2007) Regulation of cardiac ion channels via non-genomic action of sex steroid hormones: implication for the gender difference in cardiac arrhythmias. Pharmacol Ther 115(1):106–115
Gunzel-Apel AR, Seefeldt A, Eschricht FM, Urhausen C, Kramer S, Mischke R, Hoppen HO, Beyerbach M, Koivisto M, Dieleman SJ (2009) Effects of gonadectomy on prolactin and LH secretion and the pituitary-thyroid axis in male dogs. Theriogenology 71(5):746–753
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391(2):85–100
Horn R, Marty A (1988) Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol 92(2):145–159
Hu XQ, Zhang L (2012) Function and regulation of large conductance Ca(2+)-activated K+ channel in vascular smooth muscle cells. Drug Discov Today 17(17–18):974–987
Jamali K, Naylor BR, Kelly MJ, Ronnekleiv OK (2003) Effect of 17beta-estradiol on mRNA expression of large- conductance, voltage-dependent, and calcium-activated potassium channel alpha and beta subunits in guinea pig. Endocrine 20(3):227–237
Kelly DM, Jones TH (2013) Testosterone: a vascular hormone in health and disease. J Endocrinol 217(3):R47–R71
Korovkina VP, Brainard AM, Ismail P, Schmidt TJ, England SK (2004) Estradiol binding to maxi-K channels induces their down-regulation via proteasomal degradation. J Biol Chem 279(2):1217–1223
Lang DG, Ritchie AK (1987) Large and small conductance calcium-activated potassium channels in the GH3 anterior pituitary cell line. Pflugers Arch 410(6):614–622
Lephart ED (1993) Pituitary and brain 5alpha-reductase messenger RNA levels in control, castrated, and dihydrotestosterone-treated rats. Mol Cell Neurosci 4(6):526–531
Lin MT, Hessinger DA, Pearce WJ, Longo LD (2006) Modulation of BK channel calcium affinity by differential phosphorylation in developing ovine basilar artery myocytes. Am J Physiol Heart Circ Physiol 291(2):H732–H740
Martin LJ, Siliart B, Dumon HJ, Nguyen P (2006) Spontaneous hormonal variations in male cats following gonadectomy. J Feline Med Surg 8(5):309–314
Michels G, Hoppe UC (2008) Rapid actions of androgens. Front Neuroendocrinol 29(2):182–198
Ohno A, Ohya S, Yamamura H, Imaizumi Y (2009) Gender difference in BK channel expression in amygdala complex of rat brain. Biochem Biophys Res Commun 378(4):867–871
Ouadid-Ahidouch H, Roudbaraki M, Ahidouch A, Delcourt P, Prevarskaya N (2004) Cell-cycle-dependent expression of the large Ca2+−activated K+ channels in breast cancer cells. Biochem Biophys Res Commun 316(1):244–251
Perusquia M, Stallone JN (2010) Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am J Physiol Heart Circ Physiol 298(5):H1301–H1307
Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W (2007) Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest 117(2):457–463
Rochira V, Zirilli L, Genazzani AD, Balestrieri A, Aranda C, Fabre B, Antunez P, Diazzi C, Carani C, Maffei L (2006) Hypothalamic-pituitary-gonadal axis in two men with aromatase deficiency: evidence that circulating estrogens are required at the hypothalamic level for the integrity of gonadotropin negative feedback. Eur J Endocrinol 155(4):513–522
Roselli CE, Abdelgadir SE, Resko JA (1997) Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull 44(4):351–357
Sanchez M, McManus OB (1996) Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35(7):963–968
Seminara SB (2006) Mechanisms of disease: the first kiss-a crucial role for kisspeptin-1 and its receptor, G-protein-coupled receptor 54, in puberty and reproduction. Nat Clin Pract Endocrinol Metab 2(6):328–334
Stojilkovic SS, Tabak J, Bertram R (2010) Ion channels and signaling in the pituitary gland. Endocr Rev 31(6):845–915
Takimoto K, Fomina AF, Gealy R, Trimmer JS, Levitan ES (1993) Dexamethasone rapidly induces Kv1.5 K+ channel gene transcription and expression in clonal pituitary cells. Neuron 11(2):359–369
Tang F (1991) Endocrine control of hypothalamic and pituitary met-enkephalin and beta-endorphin contents. Neuroendocrinology 53(Suppl 1):68–76
Tobin VA, Canny BJ (1998) The regulation of gonadotropin-releasing hormone-induced calcium signals in male rat gonadotrophs by testosterone is mediated by dihydrotestosterone. Endocrinology 139(3):1038–1045
Torres YP, Morera FJ, Carvacho I, Latorre R (2007) A marriage of convenience: beta-subunits and voltage-dependent K+ channels. J Biol Chem 282(34):24485–24489
Turner RW, Anderson D, Zamponi GW (2011) Signaling complexes of voltage-gated calcium channels. Channels (Austin) 5(5):440–448
Viau V, Meaney MJ (2004) Testosterone-dependent variations in plasma and intrapituitary corticosteroid binding globulin and stress hypothalamic-pituitary-adrenal activity in the male rat. J Endocrinol 181(2):223–231
Wilson EM, French FS (1976) Binding properties of androgen receptors. Evidence for identical receptors in rat testis, epididymis, and prostate. J Biol Chem 251(18):5620–5629
Acknowledgments
The work was supported by grants from the University of Oviedo (SV-UNOV-09-MA and SV-UNOV-10-MA2), Spain, and Merck RL, Rahway, NJ, USA. Usama Bilal was recipient of a Collaborative Student Scholarships in University Departments for the academic year 2010–2011, from the Ministerio de Educación, Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lorena Suárez and Usama Bilal contributed equally to this work.
Rights and permissions
About this article
Cite this article
Suárez, L., Bilal, U., Bordallo, J. et al. Androgens block outward potassium currents and decrease spontaneous action potentials in GH3 cells. Naunyn-Schmiedeberg's Arch Pharmacol 388, 67–78 (2015). https://doi.org/10.1007/s00210-014-1057-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-014-1057-2