Abstract
Contamination with fumonisins produced by Fusarium spp. is rapidly growing in both developing and developed countries. The purpose of this study was to determine whether oral exposure to fumonisin contributed to the development of allergic diseases. We initially examined the immunotoxic potential of short-term, oral administration of fumonisin B1 (FB1, 1 mg/kg) and fumonisin B2 (FB2, 1 mg/kg), both naturally occurring fumonisins, using a BALB/c mouse model of allergic contact dermatitis and Dermatophagoides farina-induced asthma. Using an NC/nga mouse model of atopic dermatitis (AD), we evaluated the adverse effects of subchronic oral exposure to low concentrations of FB2 (2 or 200 μg/kg). Finally, we explored the influence of FB2 on regulatory T cell proliferation and function in mesenteric lymph nodes after 1-week oral exposure to FB2 in BALB/c mice. Oral exposure to FB2 markedly exacerbated the symptoms of allergy, including skin thickness, histological evaluation, immunocyte proliferation, and proinflammatory cytokine production, although no change was observed following exposure to FB1. Furthermore, oral exposure to low concentrations of FB2 considerably exacerbated the AD scores, skin thickness, transepidermal water loss, histological features, and proinflammatory cytokine production. The aggravated allergic symptoms induced by oral exposure to FB2 could be attributed to the direct inhibition of IL-10 production by regulatory T cells in mesenteric lymph nodes. Our findings indicate that the recommended maximum fumonisin level should be reconsidered based on the potential for allergy development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Contamination of food and feed with mycotoxins, including aflatoxins, fumonisins, ochratoxin, zearalenone, and trichothecenes, is a global challenge (Danicke et al. 2023; Munoz-Solano and Gonzalez-Penas 2023). Among these mycotoxins, trichothecene mycotoxins, such as deoxynivalenol and fumonisin, are reportedly the most prevalent and can induce considerable adverse effects on human and animal health (Kulcsar et al. 2023). According to a recent epidemiological study, deoxynivalenol and fumonisin were detected in 64 and 60%, respectively, of the 74,821 samples collected from 100 countries between 2008 and 2017, and fumonisins exhibited the highest median concentration at 723 µg/kg (Gruber-Dorninger et al. 2019). Despite the high risk of exposure to trichothecene mycotoxins, detailed adverse effects, particularly those associated with the immune system, remain poorly understood. Therefore, the objective of the present study was to clarify the novel immunotoxic effects of fumonisins by focusing on their potential to induce allergic diseases.
Fumonisins were first isolated in 1988 and classified into four major groups: Fumonisin A, Fumonisin B, Fumonisin C, and Fumonisin P (Gelderblom et al. 1988). Considering the global exposure status of fumonisin, an estimated average daily intake of 5.8 and 6.7 μg/kg body weight (b.w.)/day has been reported in the Byzana and Centene regions, respectively, of the Republic of South Africa (van der Westhuizen et al. 2008). In Brazil, fumonisin exposure levels of 0.48 µg/kg b.w./day overall and 7.11 µg/kg b.w./day have been documented in those consuming corn as raw material (Caldas and Silva 2007). Moreover, exposure to high concentrations of fumonisin reportedly causes encephalomalacia in horses, pulmonary edema in pigs, neural tube defects in human fetuses, and esophageal cancer in humans (Alizadeh et al. 2012; Gordon et al. 2020; Missmer et al. 2006). Considering potential mechanisms through which fumonisin induces these toxic effects, the inhibition of the sphingolipid biosynthesis pathway has been suggested. Fumonisins possess similar chemical structures to sphingolipids sphinganine and sphingosine, thereby inhibiting ceramide synthase through competitive antagonism, causing accumulation and reduction of ceramide-related lipids (Wang et al. 1991). Among fumonisins, Fumonisin B is a secondary metabolite produced by Fusarium spp., such as Fusarium verticillioides and F. proliferatum. Fumonisin B comprises fumonisin B1 (FB1) and B2 (FB2), both of which exhibit a markedly similar inhibitory pattern on ceramide synthase and are most frequently detected in foods, often occurring together (Norred et al. 1997; Rheeder et al. 2002; World Health 2017).
Ceramide, produced by sphinganine in the ceramide synthesis pathway, is a major lipid component in the intercellular space of the stratum corneum. Recently, ceramide has been associated with the formation of the epidermal permeability barrier and several immune cells (Sofi et al. 2022). Depletion of the ceramide synthesis pathway was shown to influence immune modulative diseases, such as dermatitis, psoriasis, and inflammatory bowel disease (Komuro et al. 2022a, b; Wang et al. 1991). We postulate that inhibition of ceramide synthase by oral exposure to FB1 or FB2 could accelerate the progression of these immune-modulative diseases. Therefore, the main objective of the present study was to determine the adverse effects of oral exposure to FB1 and FB2 on the development of allergic diseases, including allergic contact dermatitis (ACD), allergic asthma, and atopic dermatitis (AD), using suitable mouse models.
Materials and methods
Animals
Seven-week-old female BALB/c mice (5–8 mice/group) were kindly provided by Japan SLC, lnc. (Shizuoka, Japan) and used to generate the ACD and allergic asthma models. Seven-week-old female NC/nga mice (n = 5 mice/group) were also provided by Japan SLC, lnc. and used to generate the AD model. A maximum of four mice were housed per cage under controlled conditions (lighting, 12 h light–dark cycle; temperature, 22 ± 3 °C; humidity, 55 ± 15%). The mice were provided a certified pellet diet and tap water ad libitum. All aspects of this study were conducted in accordance with the Animal Care and Use Program of Azabu University (Approval No. 220316-46).
Test chemicals
Fusarium fujikuroi IMF50102 was used as a producer of fumonisins (Yoshinari et al. 2022). A piece of mycelium from a 10-day-old culture of F. fujikuroi was introduced in 1.2 L of modified fumonis inducing medium, and incubated statically for 10 days at 25 °C. FB1 and FB2 were purified from the culture broth using a C18 cartridge, a strong anion exchanger cartridge and high performance liquid chromatography. FB1 and FB2 are occasionally detected at high levels (5–20 mg/kg) in food intended for animal or human consumption. Therefore, in the short-term exposure studies performed in ACD and asthma models, mice were orally administered FB1 or FB2 at 1 mg/kg b.w. (diluted in distilled water) 48, 24, and 1 h before (3 different timings in one setting) the final challenge with toluene-2,4-diisocyanate (TDI; CAS No. 584-84-9, FUJIFILM Wako Pure Chemical Corporation., Osaka, Japan) to confirm the direct effect of FB1 or FB2 exposure on the allergic reaction. For the studies involving the AD model, low concentrations (2 and 200 μg/kg b.w.) of FB2 were used based on the provisional maximum tolerable daily intake of FB2 (2 μg/kg b.w.) specified by The Food and Agriculture Organization/World Health Organization (World Health 2017; Zentai et al. 2019).
Murine model of ACD
To generate a mouse model of ACD, mice were subjected to repetitive sensitization and challenge with TDI, an allergen known to induce type 2 helper T cells (Th2), as described previously (Supplemental Fig. 1A) (Aihara et al. 2020; Makino et al. 2019; Watanabe et al. 2019). Briefly, 5% TDI dissolved in acetone was topically applied onto the depilated abdominal surface on day 1 through day 3, and re-sensitization with 0.5% acetone was performed 3 weeks after the first sensitization. One week after re-sensitization, 0.5% TDI was applied on both sides of the ear auricle as a challenge. Mice were orally administered vehicle alone, FB1, or FB2 at 48, 24, and 1 h before (3 different timings in one setting) the final TDI challenge to confirm the direct effect of FB1 and FB2 exposure on the allergic reaction (Supplemental Fig. 1A). The ear-swelling response was determined by calculating the difference in ear thickness before and 24 h after the TDI challenge. After measuring the ear-swelling response, serum, auricular lymph node (LN) and skin samples were isolated under isoflurane anesthesia for further analysis. The reproducibility of results was confirmed using another setting (6 mice in 1 mg/kg b.w. FB1 or FB2), and the second setting exhibited the same tendency as the main study.
Mouse model of allergic asthma
To generate a mouse model of allergic asthma, mice were subjected to repetitive intranasal sensitization and a house dust mite (Dermatophagoides farinae) extract challenge (1 mg/mL Derf; ITEA Inc., Tokyo, Japan), as described by Ookawara et al. (2021) with a slight modification (Supplemental Fig. 1B). Briefly, mice were intranasally sensitized with Derf (25 μg in 25 µL phosphate-buffered saline [PBS]; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) under isoflurane anesthesia on days 1, 8, and 15. One week after the last sensitization, mice were intranasally challenged with 5 μg Derf in 25 µL PBS under isoflurane anesthesia for three consecutive days (days 21–23 or 35–37). Mice were orally administered vehicle alone, FB1, or FB2 48, 24, and 1 h before (3 different timings in one setting) the final Derf challenge. Twenty-four hours after the final Derf challenge, mice were euthanized under isoflurane anesthesia. Subsequently, serum, bronchoalveolar lavage fluid (BALF), hilar LN, and lung samples were harvested for further analysis.
Mouse model of AD
To generate a mouse model of AD, NC/Nga mice were subjected to topical sensitization with 5% TDI and topical challenge with 0.5% TDI on depilated back skin and the ear auricle, based on a previously described method with minor modifications (Supplemental Fig. 1C) (Kishimoto et al. 2021). Daily oral administration in drinking water containing 2 or 200 μg/kg b.w. of FB2 or vehicle (water) was started on the first day of the experiment. Transepidermal water loss (TEWL), ear and back skin thickness, and clinical scores were monitored once weekly during the experimental period. TEWL was measured using VAPO SCAN (AS-VT100RS, ASCH JAPAN Co., LTD, Tokyo, Japan), and a clinical score from 0 to 4 was assigned as follows: no symptoms, 0; mild, 1; moderate, 2; severe, 3; and extreme, 4, as described by Fukuyama et al. (2018). The back skin, auricular LN, and serum samples were collected from each mouse 4 weeks after the first sensitization. The collected samples were processed for staphylococci count, histology, cytokine analysis, IgE detection, RNA analysis, and cell differentiation.
Histopathological assessment of skin samples
A portion of skin samples harvested from ACD and AD mice models was fixed in 10% formalin solution, embedded in paraffin wax, sectioned to 5 μm thickness, and stained with hematoxylin and eosin, or Gram stain. A semi-quantitative histopathological evaluation of hyperplasia of the epidermis, crust, cellular infiltration, necrosis, abscesses, and Gram-positive bacteria in the epidermis, dermis, and muscle layer was performed in a blinded fashion using the following grading system: 0, within normal limits; 1, mild; 2, moderate; 3, severe. The total score of lesions was used for statistical evaluation.
Flow cytometric analysis of LNs
Single-cell suspensions isolated from the auricular LN were prepared as described previously (Aihara et al. 2020; Ookawara et al. 2020), and the total number of cells was measured using a CellDrop™ Cell Counting system (DeNovix Inc., DE, USA). To avoid nonspecific binding in flow cytometric analysis, 1 × 106 cells were first incubated with 1 µg of mouse Fc Block (Miltenyi Biotec K.K., Tokyo, Japan) prior to incubation with monoclonal antibodies (anti-mouse CD3, anti-mouse CD4, anti-mouse CD11b, anti-mouse CD11c, anti-mouse CD19, anti-mouse MHC class II, DAPI [Miltenyi Biotec K.K.], and anti-mouse IgE [Sony Biotechnology Inc., Tokyo, Japan]). The cells were washed and analyzed using a BD FACSAria™ III cell sorter (BD Biosciences, Tokyo, Japan).
Measurement of total serum IgE
Total IgE levels in serum samples were determined using ELISA according to the manufacturer’s protocol (BD Biosciences). Optical density was measured using a microplate reader (iMark microplate reader, Bio-Rad Laboratories, Inc., Tokyo, Japan).
Cytokine release assay for LNs
Single-cell suspensions of LNs were used to examine cytokine release by T cells. Briefly, single-cell suspensions of LNs (5 × 105 cells/well) were incubated with Dynabeads mouse T-Activator CD3/CD28 (Thermo Fisher Scientific, Inc., Kanagawa, Japan) for 24 or 96 h. Subsequently, levels of interferon (IFN)-γ, interleukin (IL)-4, IL-5, IL-13, and tumor necrosis factor (TNF) α in the supernatant were evaluated using ELISA (DuoSet ELISA kit, R&D Systems, Minneapolis, MN, USA).
Determination of cytokine levels in skin or lung tissues
A portion of frozen tissue was homogenized, and the supernatant was used to determine the cytokine levels as described previously (Stover et al. 2016; Tajima et al. 2019). The protein content in the supernatant was measured using a dendritic cell protein assay kit (Bio-Rad Laboratories). Levels of IL-1β, -4, -13, and -33 were measured using ELISAs (R&D Systems), and the amount of each cytokine per milligram of protein was calculated.
Gene expression in skin tissues
Briefly, frozen tissue was homogenized using a bead beater homogenizer (μT-12, TAITEC CORPORATION, Saitama, Japan), and total RNA was extracted using NucleoSpin® RNA kit (TaKaRa Bio Inc., Shiga, Japan) according to the manufacturer’s instructions. Extracted total RNA (500 ng) was reverse transcribed using the PrimeScript™ RT Master Mix (TaKaRa Bio Inc.). Expression levels of β-actin, IL4, IL10, IL13, IL33, and transcription factor forkhead box protein P3 (Foxp3) were assessed using respective specific primers (Takara Bio Inc., see supplemental Table 1), PowerUp™ SYBR™ Green Master Mix (Thermo Fisher Scientific, Inc.), and a qPCR system (CFX Duet Real-Time PCR System, Bio-Rad Laboratories, Inc.). The expression of each target gene was normalized to that of β-actin.
BALF analysis
BALF was collected by cannulating the trachea and lavaging the lungs three times with 1 mL PBS containing 0.1% bovine serum albumin (Thermo Fisher Scientific, Inc.) (Tajiki-Nishino et al. 2018; Watanabe et al. 2019). The cell pellets of BALF were used for measuring the total number of live cells and eosinophil and neutrophil differentiation. The total number of live cells was measured using a CellDrop™ Cell Counting system. For flow cytometric analysis, 5 × 105 cells were first incubated with 1 µg of mouse Fc Block prior to incubation with monoclonal antibodies (anti-mouse CCR3, anti-mouse CD11c, anti-mouse CD11b, and anti-mouse Gr-1; BioLegend, Inc., CA, USA). Then, cells were washed and analyzed using a BD FACSAria™ III cell sorter.; a total of 10,000 events were collected from the disaggregated BALF to analyze cell surface marker expression.
Determination of regulatory T cells in mesenteric LN
We next examined the direct effects of oral exposure to FB2 on the proliferation and function of regulatory T cells in gut-associated lymphoid tissue. Accordingly, BALB/c mice were administered 1 mg/kg b.w. of FB2 for 3 days. Mice were then euthanized under isoflurane anesthesia, and mesenteric LNs were harvested (see Fig. 6A). Preparation of LN and measurement of the total number of live cells were performed using the above-described method. Regulatory T cells (Tregs) were stained using the Inside Stain Kit (Miltenyi Biotec K.K.) and monoclonal antibodies (anti-mouse CD4, anti-mouse CD25, and anti-mouse FoxP3; BioLegend, Inc.). Tregs were analyzed and sorted using a BD FACSAria™ III cell sorter; a total of 10,000 events were collected from the disaggregated LNs to analyze cell surface marker expression. Sorted Tregs (5 × 105 cells/well) were incubated with Dynabeads mouse T-Activator CD3/CD28 for 96 h. The IL-10 concentration in the supernatant was evaluated using ELISA (DuoSet ELISA kit, R&D Systems).
Direct influence of FB2 on cytokine release in THP-1 cell line (human monocytic leukemia), HaCaT cell line (human epidermal keratinocyte cell line), and BEAS-2B cell line (human bronchial epithelium)
HaCaT cells were obtained from Cell Lines Service GmbH (Eppelheim, Germany) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; FUJIFILM Wako Pure Chemical Corporation), supplemented with 10% fetal calf serum (FCS; Sigma-Aldrich Co. LLC., Tokyo, Japan) and penicillin–streptomycin (FUJIFILM Wako Pure Chemical Corporation). THP-1 cells were obtained from the European Collection of Cell Cultures (ECACC) and cultured in RPMI 1640 medium (DMEM, FUJIFILM Wako Pure Chemical Corporation) supplemented with 10% FCS and penicillin–streptomycin. The BEAS-2B cell line, procured from ECACC, was cultured in the bronchial epithelial cell growth media (BEGM™; Lonza Japan Ltd., Tokyo, Japan). Considering each cell line, cells (1 × 104 cells/200 μL) at 70% confluency were seeded in a 96-well culture plate and exposed to FB2 at 10, 100, and 1000 nmol/L for 24 h, followed by stimulation with LPS (1 μg/mL) or recombinant human TNFα/IFN-γ (PeproTech, Inc., Cranbury, NJ) for another 24 h. A preliminary cytotoxicity test was performed to determine the concentration of each test substance for in vitro investigations. Even at the highest concentration, none of the test substances induced signs of cytotoxicity (data not shown). Levels of IL-6 or -8 in supernatants were measured using ELISA (DuoSet ELISA kit, R&D Systems). A minimum of three independent experiments were performed to verify the reproducibility of experiments.
Statistical analyses
For estimating Tregs in mesenteric LNs, the Student’s t-test was used to determine significant differences between the values of two groups. Other in vivo and in vitro data are expressed as the mean ± standard error of the mean (SEM). To evaluate the results of in vivo experiments and in vitro staphylococcal studies, we performed analysis of variance (ANOVA), followed by Dunnett’s multiple comparison test. The statistical significance was estimated at 5 and 1% probability levels. Data were analyzed using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA).
Results
Oral exposure to FB2, but not FB1, significantly exacerbated the inflammatory response in a mouse model of TDI-induced allergic dermatitis
Oral exposure to high-concentration FB2 (1 mg/kg b.w.) significantly enhanced the TDI-induced ear swelling response (Fig. 1A); however, FB1 exposure had no such impact. The FB2-induced increased ear swelling was corroborated by histological features such as significant induction of inflammatory cell infiltration and edema in the dermis (Fig. 1B and Table 1). To determine the impact of FB2 exposure on the local immune reaction, we measured the number of CD11c+CD40+ DCs, CD3+CD4+ T cells, and CD19+IgE+ B cells in the auricular LNs using flow cytometry. Although FB2 exposure induced only a slight increase in immune cell proliferation (Fig. 1C–E), IgE levels in serum, IL-4 secretion by stimulated T cells, and IL-13 levels in skin tissue were significantly enhanced (Fig. 1F, G, J). The FB2 exposure group showed an upward trend without significant change in IL-13 secretion (in stimulated T cells) and IL-4 levels (in skin tissues) (Fig. 1H, I). To confirm the reproducibility of FB1 and FB2 effects in a mouse model of ACD, the same experiment was performed using different settings. Oral FB2 exposure significantly enhanced ear swelling, histological alterations, CD3+CD4+ T cells, CD19+IgE+ B cells, and IL-4 and IL-13 secretion by stimulated T cells, with only an increasing trend detected in terms of CD11c+CD40+ DCs and IgE in serum (Supplemental Fig. 2, Supplemental Table 2). The FB1 treatment group showed no significant change when compared with the control group.
Effects of oral administration of fumonisin B1 (FB1) and fumonisin B2 (FB2) in a mouse model of allergic contact dermatitis. Oral exposure to FB2 significantly increases the ear swelling response (A). Representative histological image of affected skin in each group (B). The number of CD11c+CD40+ dendritic cells (DCs) (C), CD3+CD4+ T cells (D), and CD19+IgE+ B cells (E) in the auricular lymph node (LN). Oral exposure to FB2 significantly increases serum levels of level of IgE (F). FB2 exposure upregulates interleukin (IL)-4 (G) and IL-13 (H) production in LNs and IL-4 (I) and IL-13 (J) levels in the skin. Bar = 100 μm. Each result is presented as the mean ± standard error of the mean (SEM)
Oral exposure to FB2, but not FB1, significantly exacerbated inflammatory responses in a mouse model of Derf-induced asthma
To determine whether oral administration of FB1 or FB2 can impact the type of allergy, a mouse model of Derf-induced asthma was established, and immune reactions were analyzed. Exposure to FB1 and FB2 did not alter cellular infiltration of BALF by eosinophils and neutrophils (Fig. 2A, B). Conversely, oral administration of FB2, but not by the FB1, significantly increased levels of IL-1β and IL-33 in lung tissues, CD11c+CD40+ DCs, CD3+CD4+ T cells, CD19+IgE+ B cells, IL-4, and IL-13 secretion by stimulated T cells (Fig. 2C–I).
Effects of oral administration of fumonisin B1 (FB1) and fumonisin B2 (FB2) in a mouse model of asthma. There is no change in eosinophils (A) and neutrophils (B) count in BALF. FB2 exposure significantly increases IL-1β (C) and IL-33 (D) levels in the lung tissue. The number of CD11c+CD40+ DCs (E), CD3+CD4+ T cells (F), CD19+IgE+ B cells (G), and production of IL-4 (H), IL-13 (I) in LNs are significantly increased in the FB2 treated group. Each result is presented as the mean ± standard error of the mean (SEM). BALF bronchoalveolar lavage fluid, DC dendritic cell, LN lymph node, IL interleukin
Chronic oral exposure to low-dose FB2 significantly and dose-dependently exacerbates AD symptoms and related immune reactions
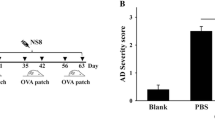
After establishing the direct impact of oral high-dose FB2 exposure on the development of allergic symptoms in ACD and asthma mice models, we next explored the effects of chronic oral, low-dose FB2 in a mouse model of AD. Compared with exposure to the vehicle, FB2 exposure at 2 and 200 μg significantly aggravated the AD score (Fig. 3A); this finding was corroborated by the significant alterations in histological characteristics, back skin, and ear skin thickness (Fig. 3B–D, Table 2). Compared with the vehicle control group, the FB2 exposure groups showed a significant increase in TEWL values (Fig. 3E). Analyzing the local immune responses in auricular LNs, we noted an increasing trend in the number of MHC class II+CD11c+CD11b+ DCs, CD3+CD4+ T cells, CD19+IgE+ B cells, and total IgE levels in serum; however, the increase was not statistically significant when compared with the AD control group (Fig. 4A–D). In contrast, secretion of typical proinflammatory cytokines, including IL-4, IL-5, and IL-13, was significantly enhanced following exposure to 200 μg/kg FB2 (Fig. 4E–H). Gene expression levels of T cell- or innate lymphoid cell (ILC)-dependent cytokines, such as IL-4, IL-10, IL-13, IL-33, and FoxP3, in the skin tissue were slightly increased in the FB2 exposure groups; however, the observed increase was not statically significant when compared with the AD control group (Fig. 5).
Impact of oral administration of low-dose FB2 in a mouse model of AD. AD scores are significantly elevated in both FB2 exposure groups (2 or 200 μg/kg) (A). Representative histological image of affected skin in each group (B). Oral exposure to FB2 increases back (C) and ear (D) skin thickness and TEWL (E). Bar = 100 μm. Each result is presented as the mean ± standard error of the mean (SEM). *p < 0.05, **p < 0.01 (Dunnett’s multiple comparison test) vs. AD control group. AD atopic dermatitis, FB2 fumonisin B2, TEWL transepidermal water loss
Impact of oral exposure to FB2 on local immune function in a mouse model of AD. No significant change can be observed in MHC II+CD11c+CD11b+ DCs (A), CD3+CD4+ T cells (B), and CD19+IgE+ B cells (C) in LN and serum IgE levels (D). FB2 administration significantly increases the production of IL-4 (E), IL-5 (F), IL-13 (G), and TNFα (H) from CD3/CD28-stimulated T cells in LNs. Each result is presented as the mean ± standard error of the mean (SEM). AD atopic dermatitis, FB2 fumonisin B2, IL interleukin, LN lymph node, TNFα tumor necrosis factor α
Impact of oral exposure to FB2 on gene expression in the skin of a mouse model of AD. There are no significant changes in gene expression of IL4 (A), IL10 (B), IL13 (C), IL33 (D), and FoxP3 (E) in the back skin tissues. Each result is presented as the mean ± standard error of the mean (SEM). AD atopic dermatitis, FB2 fumonisin B2, IL interleukin
Oral exposure to FB2 directly stimulated Tregs in mesenteric LNs and significantly enhanced IL-10 secretion
To examine the direct impact of oral FB2 exposure on gut immunity, particularly on immune tolerance, we focused on Tregs and IL-10 production (Fig. 5A). Oral exposure to FB2 did not impact Treg proliferation in mesenteric LNs. Compared with the vehicle control, FB2 exposure significantly enhanced Treg-mediated IL-10 secretion (Fig. 5B, C).
FB2 exposure did not directly impact inflammatory cytokine production by monocytes, epidermal keratinocytes, and bronchial epithelium
Finally, to demonstrate the direct interaction between FB2 exposure and inflammatory responses in local tissues and immune cells, we measured IL-6 or IL-8 secretion by stimulated THP-1, HaCaT cells, and BEAS-2B cells treated with FB2. We observed that FB2 treatment did not affect inflammatory cytokine production in examined cell lines (Supplemental Fig. 3).
Discussion
The prevalence of allergic diseases, including AD, allergic rhinitis, food allergy, and asthma, has increased over the recent decades, affecting approximately 20% of the global population and 30% of children worldwide (Laughter et al. 2021; Spergel and Paller 2003; Tham et al. 2023). The concept of atopic march was developed to describe the progression of atopic disorders from AD in infants to allergic rhinitis and asthma in children (Spergel and Paller 2003). Atopic march is an extremely common, pruritic, and difficult-to-treat disease, even in animals (Marsella and De Benedetto 2017), owing to its multifactorial nature. The symptoms of atopic march are known to be associated with living conditions, with indoor temperature, humidity, particles, and chemicals considered possible causative agents. As potential causative agents, we have focused on several mycotoxins that frequently contaminate human and animal food products. Previously, we examined deoxynivalenol (DON), a group B trichothecene produced mainly by F. graminearum and F. culmorum (Mishra et al. 2020). Our findings demonstrated that both short-term exposure to high-concentration (0.3 mg/kg b.w.) DON and chronic exposure to real-world DON contamination (0.3 ppm) in wheat grain samples could directly exacerbate proinflammatory responses in mice models of allergic dermatitis and Derf-induced asthma (Aihara et al. 2020; Ookawara et al. 2021). These were the first reports illustrating the direct association between mycotoxin exposure and allergy development. The structure of mycotoxins is complex, occasionally comprising several structural isomers. It should be noted that despite the similar origin, the toxicological effects of structural isomers could differ. In the present study, we focused on FB1 and FB2, both major groups of fumonisin, and compared their toxicological profiles in terms of their impact on the development of allergic diseases (Fig. 6).
Direct association between oral exposure to FB2 and regulatory T cells (Tregs) in mesentery LNs. Experimental protocol used in this study (A). Oral exposure to FB2 does not alter the number of Tregs (B), whereas IL-10 secretion is significantly reduced following FB2 exposure. Each result is presented as the mean ± standard error of the mean (SEM). FB2 fumonisin B2, IL interleukin, LNs lymph nodes, Tregs regulatory T cells
Initially, we analyzed the direct association between oral exposure to FB1 or FB2 and the inflammatory responses in mice models of ACD and asthma using a high-dose, short-term exposure protocol. In the ACD model, FB2 exposure considerably affected both cutaneous inflammatory responses and local immune reactions; these observations were further confirmed by repeating the study under different settings. TDI is a well-known chemical allergen that can generate the robust type 2 helper T cell (Th2)-associated allergic dermatitis, as represented by IL-4, IL-13, and IgE production (Aihara et al. 2020; Fukuyama et al. 2015; Kishimoto et al. 2021). Herein, FB2 exposure could significantly enhance all Th2-type reactions. A similar tendency was observed in the Derf-induced asthma model, demonstrating that FB2 exposure could enhance antigen-specific acquired immunity, as determined by upregulated levels of DCs, helper T cells, and IgE-positive B cells. Interestingly, FB2 exposure significantly increased levels of IL-33, mainly produced by damaged epithelium. Recent evidence indicates that allergens can induce M2a macrophages or airway epithelial cells to release IL-33, which in turn activate and polarize type 2 ILC (ILC2), eosinophils, and Th2 cells (Han et al. 2022; Murphy et al. 2023; Rossi et al. 2022). Stimulated ILC2 can secrete IL-4 and IL-13 regardless of allergen exposure, independently driving the differentiation of cDC2 precursors into a CD11b phenotype that fosters Th2 priming (Guilliams et al. 2014; Yang et al. 2023). The FB2 exposure group showed a significant increase in IL-33 levels, thereby suggesting that FB2 potentially affects not only the antigen-specific immune response but also antigen-independent allergic reactions.
Conversely, oral exposure to FB1 did not induce any statistically significant effects on allergy development. Cawood et al. (1994) have shown that the lowest levels of FB1 and FB2 binding hepatocytes and eliciting cytotoxicity were similar for the two analogs, and therefore the effective dose levels did not differ. Furthermore, Norred et al. (1997) have suggested that the naturally occurring fumonisins (B1, B2, B3, B4, C4) are approximately equipotent in their ability to disrupt sphingolipid metabolism and would exert similar degrees of toxicity if they also share similar toxicokinetic properties. We hypothesized that the exacerbated allergy development induced by fumonisins was mediated via the disruption of sphingolipid metabolism; however, if FB1 and FB2 are equipotent in their ability to disrupt sphingolipid metabolism, toxic properties must be similar. In the present study, although FB1 exposure resulted in a slightly increasing trend in immune cell infiltration into LNs and cytokine production, the magnitude was notably smaller than that induced by FB2. To the best of our knowledge, this is the first study to demonstrate the interaction between fumonisin exposure and allergy development and compare major groups FB1 and FB2. Thus, in the next step, we focused on issues related to FB2 rather than FB1.
In the ACD and asthma models, we performed short-term, high-dose (1 mg/kg) fumonisin exposure to clarify the direct effects of FB2 on allergy induction. Contamination with 1 mg/kg rarely occurs in real-world settings; therefore, we examined an anticipated risk with chronic exposure to the tolerable daily intake of FB2 (2 μg/kg b.w.) using a mouse model of AD as a model of chronic allergy. Compared with the AD control group, the 200 μg/kg FB2 exposure group exhibited significantly aggravated AD scores, skin thickness, histological characteristics, and cutaneous barrier function (TEWL). Moreover, exposure to the tolerable daily intake of 2 μg/kg FB2 significantly increased AD scores, histological characteristics, and TEWL. Given that daily exposure to the current tolerable daily intake level for more than 4 weeks could exacerbate allergy development, it is critical to reconsider the accepted level of FB2 and fumonisin. Exposure to FB2 did not significantly impact the proliferation of allergy-related immune cells such as DCs, helper T cells, and IgE-positive B cells and total IgE levels even at 200 μg/kg. In contrast, exposure to 200 μg/kg FB2 significantly increased cytokine production by T cells. Th2-associated cytokines, including IL-4, IL-5, and IL-13, play a key role in stimulating B cells to switch to IgE antibody production (Mosmann and Coffman 1989). Our data suggest that exposure to FB2 affects cellular function, such as cytokine release, rather than the proliferation of immune cells. Gene expression of cytokines in skin tissue showed an increasing trend; however, the amount of FB2 distributed in the skin might be insufficient for notably altering local tissue gene levels. Fumonisins are hydrophilic in nature, and their skin penetration level is reportedly lower than the detection limit (Boonen et al. 2012). In addition, our data revealed that FB2 exposure had no direct impact on cytokine production by keratinocytes or the bronchial epithelium. Therefore, we speculate that the indirect effects of FB2 could induce the breakdown of immune tolerance, thereby exacerbating allergy development. Recently, Komuro et al. (2022a) have demonstrated that the knockdown of UDP-glucose ceramide glucosyltransferase (UGCG) in T cells exacerbated the pathogenesis of colitis, which was accompanied by a decrease in Treg levels. Glycosphingolipids are ubiquitously distributed in the lipid microdomains of cell membranes, and the first step in ganglioside biosynthesis is the synthesis of glucosylceramide, which involves the UGCG-mediated transfer of a glucose residue to ceramide (Lahiri and Futerman 2007). We hypothesize that direct FB2-induced disrupted sphingolipid metabolism in Tregs of gut-associated lymphoid tissue may reduce Treg levels or function. Our results indicate that short-term FB2 exposure does not alter the number of Tregs but reduces the level of IL-10, thereby suggesting that FB2 functionally affects Tregs and inhibits IL-10 production. Naturally occurring CD25+CD4+FoxP3+ Tregs actively maintain immunological self-tolerance and homeostasis via the secretion of IL-10 (Rubtsov et al. 2008). IL-10-deficient mice were shown to exhibit enhanced development of airway allergic reactions, including IL-4, IL-5, IL-13, IgA, IgG1, IgE, eosinophilia, and lymphocyte recruitment to the airways (Justice et al. 2001). Downregulation IL-10 expression following FB2 exposure might be a critical factor underlying the exacerbated allergic symptoms documented in the present study.
Taken together, oral exposure to FB2, but not FB1, both naturally abundant fumonisins, can exacerbate cutaneous and airway allergic symptoms, and chronic administration, even at a tolerable daily intake dose, can worsen AD. Notably, FB2 can impact the function of Tregs, including inhibition of the IL-10 secretion, resulting in the hyperreactivity of Th2 cells and production of large quantities of Th2-type cytokines, thereby inducing abnormal local immunity and allergic reactions to antigen exposure (Fig. 7). The pathogenesis of atopic march remains poorly understood owing to its multifactorial origin, along with unknown factors such as mycotoxin contamination of daily food. Further investigations into the relationship between fumonisin exposure and allergy development are ongoing in our project.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Aihara R, Ookawara T, Morimoto A et al (2020) Acute and subacute oral administration of mycotoxin deoxynivalenol exacerbates the pro-inflammatory and pro-pruritic responses in a mouse model of allergic dermatitis. Arch Toxicol 94(12):4197–4207. https://doi.org/10.1007/s00204-020-02875-3
Alizadeh AM, Rohandel G, Roudbarmohammadi S et al (2012) Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in northeastern Iran. Asian Pac J Cancer Prev 13(6):2625–2628. https://doi.org/10.7314/apjcp.2012.13.6.2625
Boonen J, Malysheva SV, Taevernier L, Diana Di Mavungu J, De Saeger S, De Spiegeleer B (2012) Human skin penetration of selected model mycotoxins. Toxicology 301(1–3):21–32. https://doi.org/10.1016/j.tox.2012.06.012
Caldas ED, Silva AC (2007) Mycotoxins in corn-based food products consumed in Brazil: an exposure assessment for fumonisins. J Agric Food Chem 55(19):7974–7980. https://doi.org/10.1021/jf0712898
Cawood ME, Gelderblom WC, Alberts JF, Snyman SD (1994) Interaction of 14C-labelled fumonisin B mycotoxins with primary rat hepatocyte cultures. Food Chem Toxicol 32(7):627–632. https://doi.org/10.1016/0278-6915(94)90006-x
Danicke S, Carlson L, Heymann AK et al (2023) Inactivation of zearalenone (ZEN) and deoxynivalenol (DON) in complete feed for weaned piglets: efficacy of ZEN hydrolase ZenA and of sodium metabisulfite (SBS) as feed additives. Mycotoxin Res. https://doi.org/10.1007/s12550-023-00486-2
Fukuyama T, Ehling S, Cook E, Baumer W (2015) Topically administered janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther 354(3):394–405. https://doi.org/10.1124/jpet.115.223784
Fukuyama T, Martel BC, Linder KE, Ehling S, Ganchingco JR, Baumer W (2018) Hypochlorous acid is antipruritic and anti-inflammatory in a mouse model of atopic dermatitis. Clin Exp Allergy 48(1):78–88. https://doi.org/10.1111/cea.13045
Gelderblom WC, Jaskiewicz K, Marasas WF et al (1988) Fumonisins–novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol 54(7):1806–1811. https://doi.org/10.1128/aem.54.7.1806-1811.1988
Gordon RJ, Hungerford NL, Laycock B, Fletcher MT (2020) A review on Pimelea poisoning of livestock. Toxicon 186:46–57. https://doi.org/10.1016/j.toxicon.2020.07.023
Gruber-Dorninger C, Jenkins T, Schatzmayr G (2019) Global mycotoxin occurrence in feed: a ten-year survey. Toxins (Basel). https://doi.org/10.3390/toxins11070375
Guilliams M, Ginhoux F, Jakubzick C et al (2014) Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14(8):571–578. https://doi.org/10.1038/nri3712
Han M, Breckenridge HA, Kuo S et al (2022) M2 Macrophages promote IL-33 expression, ILC2 expansion and mucous metaplasia in response to early life rhinovirus infections. Front Immunol 13:952509. https://doi.org/10.3389/fimmu.2022.952509
Justice JP, Shibata Y, Sur S, Mustafa J, Fan M, Van Scott MR (2001) IL-10 gene knockout attenuates allergen-induced airway hyperresponsiveness in C57BL/6 mice. Am J Physiol Lung Cell Mol Physiol 280(2):L363–L368. https://doi.org/10.1152/ajplung.2001.280.2.L363
Kishimoto R, Kato N, Koike M, Iwashita N, Takagi Y, Fukuyama T (2021) Topical treatment with mastic (resin from Pistacia lentiscus) elicits anti-inflammatory and anti-pruritic responses by modulating keratinocyte activation in a mouse model of allergic dermatitis. Phytomedicine 91:153679. https://doi.org/10.1016/j.phymed.2021.153679
Komuro M, Nagane M, Endo R et al (2022a) Glucosylceramide in T cells regulates the pathology of inflammatory bowel disease. Biochem Biophys Res Commun 599:24–30. https://doi.org/10.1016/j.bbrc.2022.02.004
Komuro M, Nagane M, Fukuyama T et al (2022) Sphingomyelin maintains the cutaneous barrier via regulation of the STAT3 pathway. FASEB J 36(4):e22111. https://doi.org/10.1096/fj.202100721RR
Kulcsar S, Kovesi B, Balogh K et al (2023) The co-occurrence of T-2 toxin, deoxynivalenol, and fumonisin B1 activated the glutathione redox system in the EU-limiting doses in laying hens. Toxins (Basel). https://doi.org/10.3390/toxins15050305
Lahiri S, Futerman AH (2007) The metabolism and function of sphingolipids and glycosphingolipids. Cell Mol Life Sci 64(17):2270–2284. https://doi.org/10.1007/s00018-007-7076-0
Laughter MR, Maymone MBC, Mashayekhi S et al (2021) The global burden of atopic dermatitis: lessons from the global burden of disease study 1990–2017. Br J Dermatol 184(2):304–309. https://doi.org/10.1111/bjd.19580
Makino E, Fukuyama T, Watanabe Y et al (2019) Subacute oral administration of folic acid elicits anti-inflammatory response in a mouse model of allergic dermatitis. J Nutr Biochem 67:14–19. https://doi.org/10.1016/j.jnutbio.2019.01.009
Marsella R, De Benedetto A (2017) Atopic dermatitis in animals and people: an update and comparative review. Vet Sci. https://doi.org/10.3390/vetsci4030037
Mishra S, Srivastava S, Dewangan J, Divakar A, Kumar Rath S (2020) Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: a survey. Crit Rev Food Sci Nutr 60(8):1346–1374. https://doi.org/10.1080/10408398.2019.1571479
Missmer SA, Suarez L, Felkner M et al (2006) Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ Health Perspect 114(2):237–241. https://doi.org/10.1289/ehp.8221
Mosmann TR, Coffman RL (1989) TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7:145–173. https://doi.org/10.1146/annurev.iy.07.040189.001045
Munoz-Solano B, Gonzalez-Penas E (2023) Co-occurrence of mycotoxins in feed for cattle, pigs, poultry, and sheep in navarra, a region of Northern Spain. Toxins (Basel). https://doi.org/10.3390/toxins15030172
Murphy RC, Lai Y, Liu M et al (2023) Distinct epithelial-innate immune cell transcriptional circuits underlie airway hyperresponsiveness in asthma. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202209-1707OC
Norred WP, Plattner RD, Dombrink-Kurtzman MA, Meredith FI, Riley RT (1997) Mycotoxin-induced elevation of free sphingoid bases in precision-cut rat liver slices: specificity of the response and structure-activity relationships. Toxicol Appl Pharmacol 147(1):63–70. https://doi.org/10.1006/taap.1997.8272
Ookawara T, Aihara R, Morimoto A et al (2020) Acute and subacute oral toxicity of deoxynivalenol exposure in a Dermatophagoides farinae induced murine asthma model. Toxicol Sci. https://doi.org/10.1093/toxsci/kfaa168
Ookawara T, Aihara R, Morimoto A et al (2021) Acute and subacute oral toxicity of deoxynivalenol exposure in a dermatophagoides farinae-induced murine asthma model. Toxicol Sci 179(2):229–240. https://doi.org/10.1093/toxsci/kfaa168
Rheeder JP, Marasas WF, Vismer HF (2002) Production of fumonisin analogs by Fusarium species. Appl Environ Microbiol 68(5):2101–2105. https://doi.org/10.1128/AEM.68.5.2101-2105.2002
Rossi GA, Ballarini S, Salvati P, Sacco O, Colin AA (2022) Alarmins and innate lymphoid cells 2 activation: a common pathogenetic link connecting respiratory syncytial virus bronchiolitis and later wheezing/asthma? Pediatr Allergy Immunol 33(6):e13803. https://doi.org/10.1111/pai.13803
Rubtsov YP, Rasmussen JP, Chi EY et al (2008) Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28(4):546–558. https://doi.org/10.1016/j.immuni.2008.02.017
Sofi MH, Tian L, Schutt S et al (2022) Ceramide synthase 6 impacts T-cell allogeneic response and graft-versus-host disease through regulating N-RAS/ERK pathway. Leukemia 36(7):1907–1915. https://doi.org/10.1038/s41375-022-01581-6
Spergel JM, Paller AS (2003) Atopic dermatitis and the atopic march. J Allergy Clin Immunol 112(6 Suppl):S118–S127. https://doi.org/10.1016/j.jaci.2003.09.033
Stover K, Fukuyama T, Young AT et al (2016) Topically applied manganese-porphyrins BMX-001 and BMX-010 display a significant anti-inflammatory response in a mouse model of allergic dermatitis. Arch Dermatol Res 308(10):711–721. https://doi.org/10.1007/s00403-016-1693-0
Tajiki-Nishino R, Makino E, Watanabe Y, Tajima H, Ishimota M, Fukuyama T (2018) Oral administration of bisphenol a directly exacerbates allergic airway inflammation but not allergic skin inflammation in mice. Toxicol Sci 165(2):314–321. https://doi.org/10.1093/toxsci/kfy132
Tajima H, Tajiki-Nishino R, Watanabe Y, Fukuyama T (2019) Direct activation of aryl hydrocarbon receptor by benzo[a]pyrene elicits T-helper 2-driven proinflammatory responses in a mouse model of allergic dermatitis. J Appl Toxicol. https://doi.org/10.1002/jat.3782
Tham EH, Leung ASY, Yamamoto-Hanada K et al (2023) A systematic review of quality and consistency of clinical practice guidelines on the primary prevention of food allergy and atopic dermatitis. World Allergy Organ J 16(4):100770. https://doi.org/10.1016/j.waojou.2023.100770
van der Westhuizen L, Shephard GS, Rheeder JP, Somdyala NI, Marasas WF (2008) Sphingoid base levels in humans consuming fumonisin-contaminated maize in rural areas of the former Transkei, South Africa: a cross-sectional study. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25(11):1385–1391. https://doi.org/10.1080/02652030802226195
Wang E, Norred WP, Bacon CW, Riley RT, Merrill AH Jr (1991) Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J Biol Chem 266(22):14486–90
Watanabe Y, Tajiki-Nishino R, Tajima H, Fukuyama T (2019) Role of estrogen receptors alpha and beta in the development of allergic airway inflammation in mice: a possible involvement of interleukin 33 and eosinophils. Toxicology 411:93–100. https://doi.org/10.1016/j.tox.2018.11.002
World Health O (2017) Evaluation of certain contaminants in food. World Health Organ Tech Rep Ser 1002:1–166
Yang B, Wilkie H, Das M et al (2023) The IL-4Ralpha Q576R polymorphism is associated with increased severity of atopic dermatitis and exaggerates allergic skin inflammation in mice. J Allergy Clin Immunol 151(5):1296-1306 e7. https://doi.org/10.1016/j.jaci.2023.01.011
Yoshinari T, Watanabe M, Hara-Kudo Y (2022) Cross-genus inhibitory activity of polyoxins against aflatoxin production by Aspergillus parasiticus and fumonisin production by Fusarium fujikuroi. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnac048
Zentai A, Szeitzne-Szabo M, Mihucz G, Szeli N, Szabo A, Kovacs M (2019) Occurrence and risk assessment of fumonisin B(1) and B(2) mycotoxins in maize-based food products in hungary. Toxins (Basel). https://doi.org/10.3390/toxins11120709
Acknowledgements
We thank Editage (www.editage.jp) for English language editing.
Funding
A part of this study was funded by the grant-in-aid for scientific research of The Tojuro Iijima Foundation for Food Science and Technology.
Author information
Authors and Affiliations
Contributions
Conceptualization: TF. Methodology: MA, HY, AM, NI, YT, MN, TY, and TF. Formal analysis and investigation: MA and TF. Writing—original draft preparation: MA and TF. Writing—review and editing: MA, HY, AM, NI, YT, MN, TY, and TF. Funding acquisition: TF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no actual or potential competing financial interests.
Ethical approval
All aspects of in vivo study were conducted in accordance with the Animal Care and Use Program of Azabu University (Approval No. 220316-46).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
204_2023_3579_MOESM1_ESM.jpg
Supplementary file1 Supplemental Fig. 1 Experimental protocol used to generate mouse models of allergic contact dermatitis (A), asthma (B), and atopic dermatitis (C) (JPG 1615 KB)
204_2023_3579_MOESM2_ESM.jpg
Supplementary file2 Supplemental Fig. 2 Impact of oral exposure to FB1 and FB2 on the reproducibility of a mouse model of allergic contact dermatitis. Oral exposure to FB2 significantly increases the ear swelling response (A). Representative histological image of affected skin in each group (B). Number of CD11c+CD40+ DCs (C), CD3+CD4+ T cells (D), and CD19+IgE+ B cells (E) in the auricular LNs. There is no significant change in serum levels of IgE (F). FB2 exposure upregulated IL-4 (G) and IL-13 (H) production in LNs. Bar = 100 μm. Each result is presented as the mean ± standard error of the mean (SEM). FB1, fumonisin B1; FB2, fumonisin B2; DCs, dendritic cells; LNs, lymph nodes; IL, interleukin (JPG 1282 KB)
204_2023_3579_MOESM3_ESM.jpg
Supplementary file3 Supplemental Fig. 3 Exposure to fumonisin B2 (FB2) did not alter the cytokine production by stimulated THP-1 (A), human keratinocytes (HaCaT) (B), or human bronchial epithelium (BEAS-2B) (C). Each result is presented as the mean ± standard error of the mean (SEM) (JPG 629 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ando, M., Yamaguchi, H., Morimoto, A. et al. Chronic oral exposure to low-concentration fumonisin B2 significantly exacerbates the inflammatory responses of allergies in mice via inhibition of IL-10 release by regulatory T cells in gut-associated lymphoid tissue. Arch Toxicol 97, 2707–2719 (2023). https://doi.org/10.1007/s00204-023-03579-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-023-03579-0