Abstract
Deoxynivalenol (DON) contamination in food is a public health concern; however, the effect of DON exposure on immune disorders including allergies remains unclear. The aim of this study is to elucidate the effect of oral exposure to DON on pro-inflammatory and pro-pruritic responses in a mouse model of allergic dermatitis, which was generated by topical application of toluene-2,4-diisocyanate (TDI), a hapten that induces type-2 helper T cells. To evaluate acute exposure to DON, the mice were orally administered vehicle alone, 0.1 mg/kg DON, or 0.3 mg/kg DON 48, 24, and 1 h before the final challenge with TDI. To study subacute exposure, the mice were fed DON-contaminated rodent diet (0.3 ppm) during the experimental period. After the itch behavior and ear-swelling response were monitored, the serum, auricular lymph node, and skin tissue were collected for analyzing immunocyte differentiation, cytokine determination, and histological changes. Acute oral administration of DON significantly enhanced pro-inflammatory responses including ear-swelling response, immunocyte infiltration, and cytokine productions. Histological evaluation supported the occurrence of pro-inflammatory responses. In contrast, acute DON exposure only slightly increased itch behavior. Subacute oral exposure to DON significantly up-regulated the inflammatory responses, but showed almost no effect on pruritic response. In vitro evaluation in dendritic cells and keratinocytes indicated that DON pre-exposure induced a dose-dependent significant increase in cytokine production. Our results imply that both acute and subacute exposures to DON are associated with pro-inflammatory responses in cutaneous allergy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Contamination of mycotoxins in food and feed commodities is a serious problem worldwide; therefore, governments around the globe have strengthened countermeasures against mycotoxins through the development of detection methods and disease-resistant crop varieties and continuous review of safety standards. However, certain mycotoxins such as aflatoxins (e.g., aflatoxin B1), fumonisins, zearalenone, type B trichothecenes (e.g., deoxynivalenol; DON), type A trichothecenes (e.g., T-2 toxin; T-2), and ochratoxin A still pose a high risk of contamination in maize, wheat, and soybean in not only developing countries but also developed countries. Among these mycotoxins, the Fusarium mycotoxins DON and fumonisins are the most prevalent; they were detected in 64% and 60%, respectively, of 74,821 samples collected from 100 countries from 2008 to 2017 (Gruber-Dorninger et al. 2019). Particularly, DON showed the highest median concentration of 723 µg/kg (Gruber-Dorninger et al. 2019). Yan et al. (2020) showed that 100% of wheat samples and 99.83% of maize samples collected from China in 2017 contained an average of 165.87 and 175.30 μg/kg DON, respectively. Moreover, the DON contents in 3.63% of wheat and 2.97% of the maize samples were above the maximum limit of 1000 μg/kg. DON is usually detected at low levels (< 1 mg/kg) and occasionally at higher levels (5–20 mg/kg) in cereals intended for animal or human consumption (EFSA 2017). The global occurrence and toxicity of DON are currently considered a major food safety risk for both human and animals. Nevertheless, the toxicity of DON, particularly its involvement in immune disorders, remains unclear.
DON, also known as “vomitoxin,” is a group B trichothecene and is produced mainly by Fusarium graminearum and Fusarium culmorum. Cereal grains including wheat, barley, rye, maize, and oats are the predominant sources of DON exposure (Mishra et al. 2020). The major adverse effects of DON exposure in animals are gastroenteritis, growth inhibition, immunologic dysregulation, and impairment of reproductive function (Marin et al. 2013; Pestka 2010; Wang et al. 2014). The cardinal symptoms of DON-induced acute intoxication in humans are nausea, vomiting, diarrhea, abdominal pain, and headaches. (EFSA 2017). It is still unclear whether DON exposure contributes to the development of certain diseases such as allergy.
Oral administration of DON abnormally stimulates the immune system. Meky et al. (2001) demonstrated that DON induces potent effects on human lymphocyte cytokine production. Pierron et al. (2018) suggested that DON exhibits immune-modulatory properties, especially the ability to stimulate a specific antibody response during vaccination. Clark et al. (2015) highlighted the effects of acute intraperitoneal administration of DON with anorectic responses in mice; 1 mg/kg and 5 mg/kg DON significantly elevated the secretions of pro-inflammatory cytokines including IL-6, IL-1β, and TNF-α. Amuzie et al. (2009) corroborated the DON-induced cytokine upregulation by demonstrating the increased expression of several suppressors of cytokine signaling. It is hypothesized that these immunostimulatory activities of DON could induce the development of autoimmune diseases or allergic diseases; however, this theory requires further experimental support using animal models.
As an initial step to explore this hypothesis, the current study focused on the association between oral exposure to DON and the development of cutaneous allergy. A hapten-induced mouse model of allergic dermatitis and in vitro cytokine-release assay were used to elucidate the effect of oral exposure to DON on pro-inflammatory and pro-pruritic responses in cutaneous allergy.
Materials and methods
Animals and chemicals
Six-week-old female BALB/c mice were ordered from Japan SLC, Inc. (Shizuoka, Japan) and used to generate a mouse allergic dermatitis model. BALB/c mice were housed under a 12 h daily light cycle at a temperature of 22 ± 3 ℃ and humidity of 50% ± 20%. The mice were provided with food and water ad libitum. All aspects of this study were conducted in accordance with the Animal Care and Use Program of Azabu University (Approval No. 1910097).
Toluene-2,4-diisocyanate (TDI, CAS No. 584-84-9) was purchased from Tokyo Chemical Industry CO., LTD. (Tokyo, Japan). Deoxynivalenol (DON, CAS No. 51481-10-8) from Sigma-Aldrich Co., LLC. (Tokyo, Japan) was used for the acute-exposure protocol (Supplemental Fig. 1a) and in vitro experiments. For the subacute-exposure protocol (Supplemental Fig. 1 b), standard rodent diet (CE-2, CLEA Japan, Inc., Tokyo, Japan) and wheat grain samples (Minaminokaori, a Japanese hard red spring wheat) with 3 ppm of DON were mixed (Supplemental Fig. 2) to obtain a final DON concentration of 0.3 ppm. The control diet also contained the same amount of normal wheat. DON-contaminated wheat was generated by spray inoculation of two isolates of F. graminearum species complex and intense sprinkler precipitation in accordance with the method described by Yoshida et al. (2008).
A mouse model of allergic dermatitis
A mouse model of allergic dermatitis was generated by repetitive sensitization and challenge with TDI, an allergen that induces type-2 helper T cells (Th2), as described by Fukuyama et al. (2015). Briefly, the abdomens of female BALB/c mice were depilated with depilatory cream 1 day prior to the first sensitization. On day 1 through day 3, 5% TDI dissolved in acetone was topically applied onto the abdomen after tape stripping five times with adhesive tape. Three weeks after the first sensitization (day 22), the mice were re-sensitized with 0.5% TDI in acetone to boost the allergic reaction. Seven days after the re-sensitization, 0.5% TDI in acetone was applied onto both sides of the ear auricle and rostral back skin as a challenge. DON is occasionally detected at higher levels (5–20 mg/kg) in food intended for animal or human consumption. Therefore, in the acute-exposure study, the mice were orally administered vehicle alone or DON at 0.1 or 0.3 mg/kg b.w. (diluted in corn oil) 48, 24, and 1 h before (3 different timings in one setting) the final challenge with TDI to confirm the direct effect of DON exposure on the allergic reaction (Supplemental Fig. 1a). In the subacute-exposure study, a diet contaminated with a low dose of DON (0.3 ppm) was continuously fed to mice during the experimental period to mimic the actual exposure to DON (Supplemental Fig. 1b). Itch behavior (scratching bouts) was video monitored for 60 min immediately after the TDI challenge. Ear-swelling response was determined by calculating the difference in ear thickness before and 24 h after the TDI challenge. After measuring the ear-swelling response, the mice were euthanized under isoflurane anesthesia. Subsequently, the serum, auricular lymph node (LN), and skin samples were isolated for further analysis. Repeatability of the results was confirmed by means of another setting with limited number of animals (5 mice in 0.3 mg/kg DON), and the second setting denoted the same tendency of the main study.
Histopathological evaluation of ear auricle
A portion of the auricular samples was fixed in 10% formalin solution, embedded in paraffin wax, sectioned to 5 μm thickness, and stained with hematoxylin and eosin. A semi-quantitative histopathological evaluation for folliculitis, ulcer, edema, and cellular infiltration was performed in a blinded fashion using the following grading system: 0, within normal limits; 1, mild; 2, moderate; 3, severe. The total score of the lesions was used for statistical evaluation.
Analysis of LNs
Single-cell suspensions were prepared from the LNs removed from each mouse and were used for flow cytometry and cytokine analysis (Makino et al. 2019; Tajima et al. 2019; Watanabe et al. 2018). The total number of live cells was measured using Tali® Image-Based Cytometer (Thermo Fisher Scientific, Inc., Kanagawa, Japan). To avoid nonspecific binding in flow cytometric analysis, 1 × 106 cells were first incubated with 1 µg of mouse Fc Block (Miltenyi Biotec K.K., Tokyo, Japan) prior to incubation with monoclonal antibodies (FITC-conjugated anti-mouse CD3, PE-conjugated anti-mouse CD4, FITC-conjugated anti-mouse IgE, PE-conjugated anti-mouse CD19, FITC-conjugated anti-mouse CD11c, PE-conjugated anti-mouse CD40, Miltenyi Biotec K.K.). The cells were washed and analyzed using an EC800 flow cytometer (Sony Imaging Products & Solutions Inc., Tokyo, Japan); 10,000 events were collected from the disaggregated LN to analyze cell surface marker expression. For cytokine evaluation, single-cell suspensions of LNs (5 × 105 cells/well) were incubated with Dynabeads mouse T-Activator CD3/CD28 (Thermo Fisher Scientific, Inc.) for 24 h or 96 h. IL-4, IL-5, IL-9, IL-13, and IL-17 (R&D Systems, Minneapolis, MN) concentrations in the supernatant were evaluated using an enzyme-linked immunosorbent assay (ELISA). The optical density at 450 nm was measured using a microplate reader (iMark microplate reader, Bio-Rad Laboratories, Inc., Tokyo, Japan).
Cytokine determination in ear-skin tissue
Frozen ear-skin sample was homogenized in 500 µl of RPMI-1640 medium containing a protease inhibitor (Halt™ Protease Inhibitor Cocktail, Thermo Fisher Scientific, Inc.) using an electric homogenizer, as described by Tajiki-Nishino et al. (2018). Protein content was determined using the DC protein assay kit (Bio-Rad Laboratories, Inc.). The concentrations of IL-4, IL-13, IL-17, IL-33, and thymic stromal lymphopoietin (TSLP) were measured by ELISA, following the manufacturer’s instructions (R&D Systems).
Measurement of total IgE in serum
The total IgE level in the serum was determined using ELISA, according to the manufacturer’s protocol (Thermo Fisher Scientific Inc.).
Cytokine-release assay in murine bone marrow-derived dendritic cells (mBMDCs)
mBMDCs were obtained from the bone marrow of female BALB/c mice and generated by co-culturing with GM-CSF (PeproTech, Inc., Rocky Hill, NJ) for 8 days (Fukuyama et al. 2015; Lutz et al. 1999; Makino et al. 2019; Tajiki-Nishino et al. 2018; Tajima et al. 2019; Watanabe et al. 2018). Matured BMDCs (5 × 104 cells, containing > 90% CD11c-positive matured DCs.) were isolated and seeded in 200 μL of completed media in a 96-well plate. BMDCs were treated with 2.5, 5, or 10 μmol/L of DON for 24 h before being stimulated with the Toll-like receptor (TLR) 4 ligand lipopolysaccharide (LPS; Sigma-Aldrich Co., LLC.) at 1 μg/mL for an additional 24 h. TLR ligands such as LPS might mimic what is happening in/on the skin; however, we used LPS as they are a well-characterized stimulant for DCs and keratinocytes (Fukuyama et al. 2015; Lee et al. 2011; Watanabe et al. 2018). The concentrations of IL-12 and TNFα in the supernatant were quantified using ELISA. The phenotype of LPS-stimulated BMDCs was evaluated using monoclonal antibodies (FITC-conjugated anti-mouse CD11c, PE-conjugated anti-mouse CD86, Miltenyi Biotec K.K.) using a flow cytometer. The DON concentrations used in this experiment were determined based on a cytotoxicity test conducted prior to the experiments (data not shown). Cytotoxicity was not observed even at the highest concentrations. To verify the reproducibility of the experiments, a minimum of three independent experiments were performed using BMDCs from different mice.
Cytokine-release assay in THP-1 cell line (human monocytic leukemia)
THP-1 cells were obtained from the European Collection of Cell Cultures and cultured in RPMI-1640 medium (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) supplemented with 10% fetal calf serum (Sigma-Aldrich Co. LLC.) and penicillin–streptomycin (FUJIFILM Wako Pure Chemical Corporation). THP-1 cells (5 × 104 cells) were seeded in 200 μL of completed medium at 70% confluency in 96-well culture plates and treated for 24 h with 0.01, 0.1, or 1 μmol/L DON before being stimulated with LPS at 1 μg/mL for an additional 24 h. The concentrations of IL-1β, IL-8, and TNF-α in the supernatant were quantified using ELISA. The DON concentrations used in this experiment were determined based on a cytotoxicity test conducted prior to the experiments (data not shown). Cytotoxicity was not observed even at the highest concentrations. To verify the reproducibility of the experiments, a minimum of three independent experiments were performed using different passages of THP-1 cells.
Cytokine-release assay in PHK16-0b cell line (human epidermal keratinocyte line)
PHK16-0b cells were obtained from the Japanese Collection of Research Bioresources Cell Bank and cultured with KGM-Gold™ BulletKit™ (LONZA KK., Tokyo, Japan). The cells (1 × 104 cells/200 μL) were seeded in a 96-well culture plate and exposed for 48 h to DON at 2.5, 5, or 10 μmol/L before being stimulated with LPS at 1 μg/mL for an additional 24 h. A preliminary cytotoxicity test was used to determine the concentration of each test substance for in vitro studies. Even at the highest concentration, none of the test substances induced any signs of cytotoxicity (data not shown). Levels of IL-8 in supernatants were measured using ELISA.
Statistical analyses
Data are expressed as the mean ± 1 SEM. Analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test was used to evaluate the results of the in vivo acute-exposure study and in vitro cytokine-release assays. For the in vivo subacute-exposure study, Student’s t test was used to test the significance of differences between the values of two groups. The statistical significance was estimated at 5% and 1% levels of probability. The data were analyzed using Prism 8 (GraphPad Software, San Diego, CA, USA).
Results
Effect of acute exposure to DON on inflammatory and itch response in TDI-induced mouse model of allergic dermatitis
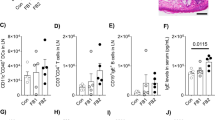
Initially, the direct effect of DON on the development of allergic dermatitis was examined via acute exposure (thrice) of the mice to DON 48, 24, and 1 h prior to the final challenge with TDI. Figure 2a presents the ear-swelling response, which reflects the inflammatory response induced by TDI and DON. Oral administration of DON 0.3 mg/kg significantly heightened the ear-swelling response compared with the TDI + vehicle control group (Fig. 1a, b). Upregulation of the inflammatory response by DON 0.3 mg/kg was supported by histopathological evaluation (Fig. 1b and Table 1). The DON 0.3 mg/kg group displayed a significantly higher extent of folliculitis, edema, and cellular infiltration compared with the TDI + vehicle control group. Scratching behavior was greater in the DON-exposure groups; however, there was no significant difference compared with the TDI + vehicle control group (Fig. 1c).
Pro-inflammatory and pro-pruritic responses to acute oral administration of DON in a TDI-induced mouse model of allergic dermatitis. The ear-swelling response (μm) (a) was significantly up-regulated by exposure to 0.3 mg/kg DON. Representative pictures of the ear auricles and microscopic features of ear tissues of the TDI + vehicle control group and the 0.3 mg/kg DON group (b). A slight upward trend in scratching behavior (c) was observed after the administration of DON; however, there was no significant change. A significant increase in dendritic cells (d), helper T cells (e), and IgE-positive B cells (f) was observed in the DON-exposure group. However, DON exposure had almost no impact on total serum IgE levels (ng/mL) (g). Bar = 100 μm. Each result is presented as the mean ± 1 SEM. n = 6 per group. *p < 0.05, **p < 0.01 (Dunnett’s multiple comparison test) vs. the TDI + vehicle control group. LN lymph node
Influence of acute exposure to DON on immunocyte infiltration, IgE level, and cytokine production after TDI challenge
To assess the influence of DON exposure on the local immune reaction, the number of dendritic cells (DCs), helper T cells, and IgE-positive B cells in the auricular lymph node was measured by flow cytometry. Acute oral exposure to DON dose dependently up-regulated the number of DCs, helper T cells, and IgE-positive B cells (Fig. 1d-f). Particularly, the immunocyte levels were significantly higher in the DON 0.3 mg/kg group than in the TDI + vehicle control group. Although the number of IgE-positive B cells in the DON 0.3 mg/kg group was significantly higher, DON exposure did not affect the total serum IgE level, compared with the control group (Fig. 1g). To elucidate whether DON affects T-cell function, we used ELISA to analyze IL-4, IL-5, IL-9, IL-13, and IL-17 production by T cells stimulated with CD3/CD28 antibody. All cytokines were increased with DON exposure in a dose-dependent manner, and a significant change was observed in DON 0.3 mg/kg exposure groups compared with TDI + vehicle control group (Fig. 2).
Cytokine production by activated T cells in the auricular LN after acute oral administration of DON in a TDI-induced male mouse model of allergic dermatitis. A significant increase in IL-4 (a), IL-5 (b), IL-9 (c), IL-13 (d), and IL-17 (e) was observed in the DON-exposure group. Each result is presented as the mean (pg/mL) ± 1 SEM. n = 6 per group. *p < 0.05, **p < 0.01 (Dunnett’s multiple comparison test) vs. the TDI + vehicle control group
Effect of acute exposure to DON on cytokine levels in allergic-dermatitis mouse model
The DON-treated mice exhibited significantly higher levels of IL-4, IL-3, IL-17, IL-33, and TSLP in the ear-skin homogenate 24 h after the TDI challenge compared with those treated with TDI + vehicle, indicating allergic inflammation (Table 2).
Effect of subacute oral exposure to DON on inflammatory and itch response in allergic-dermatitis mouse model
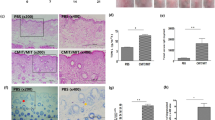
Corresponding to the results of the acute-exposure study, the ear-swelling response was significantly higher with subacute oral exposure to DON (0.3 ppm) than with TDI + vehicle (Fig. 3a); however, scratching behavior was not affected by DON (Fig. 3b). A comparable increase in the edema score was observed upon histological evaluation of the ear skin from mice treated orally with DON; however, folliculitis and cellular infiltration were not affected by DON exposure (Supplemental Table 1 and Supplemental Fig. 3). Analysis of the local immune response in auricular LNs revealed that oral treatment with DON, compared with TDI + vehicle, resulted in a significantly higher infiltration of helper T cells and IgE-positive B cells (Fig. 3d, e) but only a slightly higher number of DCs (Fig. 3c). Total IgE levels in the serum were not influenced by DON, as demonstrated in the acute-exposure experiment (Fig. 3f). DON treatment significantly increased the IL-5, IL-9, and IL-13 production by activated T cells, but did not affect IL-4 and IL-17 production (Supplemental Fig. 4). The DON-treated and vehicle control mice exhibited comparable levels of IL-4, IL-13, and IL-17 in the ear-skin tissue; however, the IL-33 and TSLP levels were significantly higher in the ear-skin tissue of DON-treated mice compared with the latter, consistent with the ear-swelling response, LN responses, and histological scores (Supplemental Table 2).
Subacute oral administration of low-dose DON (0.3 ppm) significantly exacerbated skin inflammation but not the itch behavior in a TDI-induced male mouse model of allergic dermatitis. Subacute exposure to DON significantly increased the ear-swelling response (μm) (a), but showed no impact on scratching behavior (b). In the auricular LN, a slight impact on the presence of dendritic cells (c) was noted; however, the number of helper T cells (d) and IgE-positive B cells (e) was significantly increased. DON exposure did not affect the total serum IgE levels (ng/mL) (f). Each result is presented as the mean ± 1 SEM. n = 8 per group. **p < 0.01 (Dunnett’s multiple comparison test) vs. the TDI + vehicle control group
Effect of DON pre-exposure on LPS-stimulated BMDCs
To assess whether exposure to DON affects allergic disease in vitro, we evaluated LPS-induced TNFα production in murine BMDCs. Production of TNFα in DON pre-treated BMDCs increased in a dose-dependent manner; these values were significantly different from those for the vehicle-alone control group (Fig. 4a). To confirm the enhanced effect of DON, we measured the co-stimulatory molecules of DCs by flow cytometry. CD11c and CD86 molecules were expressed in a dose-dependent manner (Fig. 4b), which is critical for DC function. These expressions were significantly different between cells treated with DON (2.5, 5, and 10 μmol/L) DON and those treated with the vehicle alone.
Effect of DON exposure on BMDC and human epidermal keratinocytes functions in vitro. Secretion of a TNFα (pg/mL) by LPS-induced BMDCs was significantly enhanced by pre-exposure to DON in a dose-dependent manner. b Up-regulation of LPS-induced expression of co-stimulatory molecules of BMDCs by pre-exposure to DON. c Pre-exposure to DON significantly induced LPS-stimulated IL-8 secretion by LPS-stimulated human epidermal keratinocytes. Each result is presented as the mean ± 1 SEM. n = 7 per group. *p < 0.05, **p < 0.01 (Dunnett’s multiple comparison test) vs. the vehicle-alone control group. BMDC bone marrow-derived dendritic cell
Impact of DON on human monocytic leukemia cell line (THP-1) in vitro
To corroborate the data from the in vivo and BMDC experiments, in vitro experiments were performed using a human monocytic leukemia cell line (THP-1). IL-1β, IL-8, and TNFα secretion by LPS-stimulated THP-1 cells was significantly enhanced by DON pre-exposure in a dose-dependent manner (Supplemental Fig. 5).
Effect of DON pre-exposure on IL-8 secretion by human epidermal keratinocytes in vitro
The DON pre-exposure groups displayed a significant dose-dependent increase in IL-8 secretion by human epidermal keratinocytes, indicating keratinocyte activation, compared to the vehicle control group (Fig. 4c).
Discussion
The present study aimed to elucidate the possible association between exposure to DON and development of cutaneous allergy. Our findings identified several crucial aspects: (1) in a mouse model of Th2-driven allergic dermatitis, acute oral treatment with DON resulted in a significant increase in inflammatory responses including ear-swelling responses, cellular infiltrations, and cytokine productions, but the itch behavior and total IgE levels in the serum were not significantly enhanced; (2) subacute oral exposure to a low dose of DON significantly exacerbated the inflammatory responses in the mouse model of allergic dermatitis; and (3) DON pre-treatment significantly up-regulated the cytokine production and maturation of BMDCs, as corroborated by enhanced cytokine secretion by THP-1 and keratinocytes that were pre-treated with DON.
Prevalence of cutaneous allergy including atopic dermatitis, allergic contact dermatitis, and psoriasis has increased dramatically over the last century among humans, as well as companion animals. Dermatitis is a skin inflammatory condition occurring in many forms including itch, eczema, dry red skin, rash, and swelling (Williams 2005). Among these types of cutaneous allergy, atopic dermatitis is one of the most frequent forms of dermatitis that occurs in infants (Rizk et al. 2019). Atopic dermatitis is also a common skin disease in dogs and cats. Its clinical, immunological, histological, and pathological features are highly similar between dogs and humans due to which canine atopic dermatitis has been suggested as an animal model for human atopic dermatitis (Mineshige et al. 2018). Environmental and lifestyle factors and increasing use of chemicals have often been suggested as contributors to the current situation (Watanabe et al. 2018). Thus, many studies have attempted to identify the risk factors that contribute to the development of cutaneous allergic diseases. Our previous study demonstrated that both endogenous and exogenous estrogens significantly exacerbated skin allergy in a mouse model of Th2-type allergic dermatitis (Watanabe et al. 2018). Tajiki-Nishino et al. (2018) also indicated that the estrogen disruptor Bisphenol A obviously augmented respiratory allergy in a mouse model of respiratory allergic inflammation. However, as contamination with these chemicals is under strict legal regulation by individual nations, the current increase in the incidence of allergy cannot be clearly explained. Therefore, the present study focuses on the mycotoxin DON as a broader environmental toxin.
DON occurs in grains and cereal products; it is often hazardous to humans and livestock (Tucker et al. 2019; Yan et al. 2020). Recently, Bol-Schoenmakers et al. (2016) reported that DON facilitates allergic sensitization to food proteins (whey) in mice including increased serum levels of whey-specific IgE, an acute allergic skin reaction upon allergen challenge, and production of Th2-type cytokines such as IL-5 and IL-13 in whey-restimulated splenocytes. Their data illustrated the possible contribution of DON contaminants in Th2-type allergic sensitization in humans, as well as companion animals. Abnormal Th2 immune responses are also found in atopic dermatitis and are considered a potent target for treatment (Werfel et al. 2016). We hypothesized that oral exposure to DON facilitates Th2-driven cutaneous allergy; thus, we attempted to elucidate the possible interaction between DON exposure and Th2-type cutaneous allergy.
Initially, we investigated the direct relationship between acute oral administration of DON and the development of Th2-type allergy in a highly reliable mouse model of TDI-induced allergic dermatitis, which has been widely used for evaluation of immunotoxicological effects of environmental chemicals including estrogen disruptors and Benzo[a]pyrene (Tajiki-Nishino et al. 2018; Tajima et al. 2019; Watanabe et al. 2018). The biggest advantage of this model is that inflammatory and itch responses can be simultaneously observed; thus, we monitored pro-inflammatory and pro-pruritic responses to DON exposure in this model. Our first in vivo experiment focused on the acute response to short-term oral exposure to DON at high doses. The results showed that DON exposure significantly induced a pro-inflammatory effect in a dose-dependent manner. The mouse model used in this study mainly represents the acute phase of Th2 immune responses in cutaneous allergy. Future studies may use an atopic dermatitis model representing the chronic phase to accurately determine whether DON affects serum IgE levels. Cytokine production (IL-4, IL-5, IL-13, IL-9, IL-17, IL-33, and TSLP) by stimulated T cells and skin tissue supported the observation of pro-inflammatory responses upon acute DON exposure. IL-9 has been shown to be released from Th9 cells in models of allergic inflammation; it plays an important role in mast-cell accumulation and activation (Sehra et al. 2015). Additionally, Th17 cells, a helper T-cell subset that secretes IL-17A, IL-17F, IL-22, IL-23, and TNF-α, play an important role in the development of allergic inflammation (Ahn et al. 2020; McGeachy and Cua 2008). Recent evidence has suggested that group 2 innate lymphoid cells (ILC2) and their effector cytokines including IL-4, IL-5, IL-9, IL-13, and IL-17 promote acute skin inflammation (Schwartz et al. 2019). Although the mouse model used in this study was a Th2-dominated allergy model, Th9 and Th17 immunoreactions were also influenced by DON exposure. Besides T-cell-derived cytokines, keratinocytes have been indicated to actively participate in the cutaneous immune response (Steinhoff et al. 2001); thus, we monitored IL-33 and TSLP as representative cytokines secreted by keratinocytes. The major targets of IL-33 in vivo are tissue-resident immune cells such as mast cells, ILC2, and regulatory T cells. Other cellular targets include Th2 cells, eosinophils, basophils, DCs, Th1 cells, CD8 + T cells, NK cells, iNKT cells, B cells, neutrophils, and macrophages. IL-33 is thus emerging as a crucial immune modulator with pleiotropic activities in type-2, type-1, and regulatory immune responses; it may play important roles in allergic and chronic inflammatory diseases (Cayrol and Girard 2014; Liew et al. 2016; Molofsky et al. 2015). Additionally, TSLP is an epithelial cell-derived cytokine synthesized in response to various stimuli, including protease allergens and microorganisms including viruses and bacteria. TSLP polarizes DCs to induce type-2 inflammation and directly expand and/or activate Th2 cells, ILC2, basophils, and other immune cells. TSLP is thus considered a master regulator of type-2 immune responses at the barrier surfaces of skin (Bell et al. 2013; Pandey et al. 2000). The present study suggested that significant enhancement of IL-33 and TSLP secretion via DON exposure might directly activate Th2 cells, ILC2, and other immune cells, resulting in the exacerbation of pro-inflammatory responses in cutaneous allergy.
DON is usually detected at low levels (< 1 mg/kg); therefore, in the second experiment, we examined whether subacute (more than 4 weeks) oral exposure to low-dose DON exacerbates cutaneous allergy in a mouse model of allergic dermatitis. The overall results correspond to the results from the acute-exposure study including IL-5, IL-9, and IL-13 secretions by stimulated T cells, IL-33, and TSLP levels in the skin tissue. These observations demonstrate that DON exacerbates Th2-type cutaneous allergy not only via acute exposure at a high dose but also via chronic exposure at a low dose. On the other hand, unlike acute exposure, exposure to low-dose DON did not affect IL-4 and IL-17 secretions by stimulated T cells; IL-4, IL-13, and IL-17 levels in the skin tissue, itch behavior, and total serum IgE levels. These findings from the subacute-exposure protocol simultaneously suggest that DON-induced allergic responses are dose-dependent and there might be a threshold level.
Finally, we examined the direct effect of DON exposure on the function of immune cells in vitro using mBMDCs, THP-1, and keratinocytes. DCs are pivotal in both the initiation and maintenance phase of allergic inflammatory diseases. Thus, we exposed mature BMDCs to DON for 24 h and stimulated them with LPS to examine the effect of DON on mature DCs. TNFα production was significantly up-regulated by DON at multiple doses, as corroborated by the increased expression of co-stimulatory molecules (CD11c and CD86) in DON pre-treatment groups. The active influence of DON on DC function was further confirmed by the significant elevation of IL-1β, IL-8, and TNFα secretion by THP-1 cells. THP-1, a human monocytic leukemia cell line, could replace DCs in in vitro skin sensitization tests (Ashikaga et al. 2006). Additionally, we observed a significant dose-dependent upregulation of IL-8 production by human keratinocytes following pre-treatment with DON. IL-8 can be secreted by any cell with TLR activation that is involved in the innate immune response; IL-8 is elevated in human patients with atopic dermatitis (Hatano et al. 1999; Kaburagi et al. 2001; Kimata and Lindley 1994).
Collectively, our findings demonstrate that both acute (high dose) and subacute (low dose) oral administration of DON is directly associated with pro-inflammatory responses in a mouse model of allergic dermatitis. Our results additionally demonstrate the ability of DON to significantly induce a cytokine response in DCs and keratinocytes in vitro. To our knowledge, this is the first study to illustrate the direct effect of DON on the development of cutaneous allergy. Next, we plan to explore the immunomodulatory effects of DON using actual allergens for humans, e.g., house dust mite, and other human-relevant models such as atopic dermatitis and allergic asthma.
References
Ahn K, Kim BE, Kim J, Leung DY (2020) Recent advances in atopic dermatitis. Curr Opin Immunol 66:14–21. https://doi.org/10.1016/j.coi.2020.02.007
Amuzie CJ, Shinozuka J, Pestka JJ (2009) Induction of suppressors of cytokine signaling by the trichothecene deoxynivalenol in the mouse. Toxicol Sci 111(2):277–287. https://doi.org/10.1093/toxsci/kfp150
Ashikaga T, Yoshida Y, Hirota M et al (2006) Development of an in vitro skin sensitization test using human cell lines: the human Cell Line Activation Test (h-CLAT). I. Optimization of the h-CLAT protocol. Toxicol In Vitro 20(5):767–773. https://doi.org/10.1016/j.tiv.2005.10.012
Bell BD, Kitajima M, Larson RP et al (2013) The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat Immunol 14(4):364–371. https://doi.org/10.1038/ni.2541
Bol-Schoenmakers M, Braber S, Akbari P et al (2016) The mycotoxin deoxynivalenol facilitates allergic sensitization to whey in mice. Mucosal Immunol 9(6):1477–1486. https://doi.org/10.1038/mi.2016.13
Cayrol C, Girard JP (2014) IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol 31:31–37. https://doi.org/10.1016/j.coi.2014.09.004
Clark ES, Flannery BM, Gardner EM, Pestka JJ (2015) High sensitivity of aged mice to deoxynivalenol (vomitoxin)-induced anorexia corresponds to elevated proinflammatory cytokine and satiety hormone responses. Toxins (Basel) 7(10):4199–4215. https://doi.org/10.3390/toxins7104199
EFSA (2017) Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA Journal 15:4718 (EFSA (ed))
Fukuyama T, Ehling S, Cook E, Baumer W (2015) Topically administered janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther 354(3):394–405. https://doi.org/10.1124/jpet.115.223784
Gruber-Dorninger C, Jenkins T, Schatzmayr G (2019) Global mycotoxin occurrence in feed: a ten-year survey. Toxins (Basel). https://doi.org/10.3390/toxins11070375
Hatano Y, Katagiri K, Takayasu S (1999) Increased levels in vivo of mRNAs for IL-8 and macrophage inflammatory protein-1 alpha (MIP-1 alpha), but not of RANTES mRNA in peripheral blood mononuclear cells of patients with atopic dermatitis (AD). Clin Exp Immunol 117(2):237–243
Kaburagi Y, Shimada Y, Nagaoka T, Hasegawa M, Takehara K, Sato S (2001) Enhanced production of CC-chemokines (RANTES, MCP-1, MIP-1alpha, MIP-1beta, and eotaxin) in patients with atopic dermatitis. Arch Dermatol Res 293(7):350–355
Kimata H, Lindley I (1994) Detection of plasma interleukin-8 in atopic dermatitis. Arch Dis Child 70(2):119–122
Lee KH, Cho KA, Kim JY et al (2011) Filaggrin knockdown and toll-like receptor 3 (TLR3) stimulation enhanced the production of thymic stromal lymphopoietin (TSLP) from epidermal layers. Exp Dermatol 20(2):149–151. https://doi.org/10.1111/j.1600-0625.2010.01203.x
Liew FY, Girard JP, Turnquist HR (2016) Interleukin-33 in health and disease. Nat Rev Immunol 16(11):676–689. https://doi.org/10.1038/nri.2016.95
Lutz MB, Kukutsch N, Ogilvie AL et al (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Method 223(1):77–92
Makino E, Fukuyama T, Watanabe Y et al (2019) Subacute oral administration of folic acid elicits anti-inflammatory response in a mouse model of allergic dermatitis. J Nutr Biochem 67:14–19. https://doi.org/10.1016/j.jnutbio.2019.01.009
Marin S, Ramos AJ, Cano-Sancho G, Sanchis V (2013) Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem Toxicol 60:218–237. https://doi.org/10.1016/j.fct.2013.07.047
McGeachy MJ, Cua DJ (2008) Th17 cell differentiation: the long and winding road. Immunity 28(4):445–453. https://doi.org/10.1016/j.immuni.2008.03.001
Meky FA, Hardie LJ, Evans SW, Wild CP (2001) Deoxynivalenol-induced immunomodulation of human lymphocyte proliferation and cytokine production. Food Chem Toxicol 39(8):827–836. https://doi.org/10.1016/s0278-6915(01)00029-1
Mineshige T, Kamiie J, Sugahara G, Shirota K (2018) A study on periostin involvement in the pathophysiology of canine atopic skin. J Vet Med Sci 80(1):103–111. https://doi.org/10.1292/jvms.17-0453
Mishra S, Srivastava S, Dewangan J, Divakar A, Kumar Rath S (2020) Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: a survey. Crit Rev Food Sci Nutr 60(8):1346–1374. https://doi.org/10.1080/10408398.2019.1571479
Molofsky AB, Savage AK, Locksley RM (2015) Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 42(6):1005–1019. https://doi.org/10.1016/j.immuni.2015.06.006
Pandey A, Ozaki K, Baumann H et al (2000) Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol 1(1):59–64. https://doi.org/10.1038/76923
Pestka JJ (2010) Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84(9):663–679. https://doi.org/10.1007/s00204-010-0579-8
Pierron A, Bracarense A, Cossalter AM et al (2018) Deepoxy-deoxynivalenol retains some immune-modulatory properties of the parent molecule deoxynivalenol in piglets. Arch Toxicol 92(11):3381–3389. https://doi.org/10.1007/s00204-018-2293-x
Rizk P, Rodenas M, De Benedetto A (2019) Allergen immunotherapy and atopic dermatitis: the good, the bad, and the unknown. Curr Allergy Asthma Rep 19(12):57. https://doi.org/10.1007/s11882-019-0893-z
Schwartz C, Moran T, Saunders SP et al (2019) Spontaneous atopic dermatitis in mice with a defective skin barrier is independent of ILC2 and mediated by IL-1beta. Allergy 74(10):1920–1933. https://doi.org/10.1111/all.13801
Sehra S, Yao W, Nguyen ET et al (2015) TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol 136(2):433–40.e1. https://doi.org/10.1016/j.jaci.2015.01.021
Steinhoff M, Brzoska T, Luger TA (2001) Keratinocytes in epidermal immune responses. Curr Opin Allergy Clin Immunol 1(5):469–476
Tajiki-Nishino R, Makino E, Watanabe Y, Tajima H, Ishimota M, Fukuyama T (2018) Oral administration of bisphenol A directly exacerbates allergic airway inflammation but not allergic skin inflammation in mice. Toxicol Sci. https://doi.org/10.1093/toxsci/kfy132
Tajima H, Tajiki-Nishino R, Watanabe Y, Fukuyama T (2019) Direct activation of aryl hydrocarbon receptor by benzo[a]pyrene elicits T-helper 2-driven proinflammatory responses in a mouse model of allergic dermatitis. J Appl Toxicol. https://doi.org/10.1002/jat.3782
Tucker JR, Badea A, Blagden R, Pleskach K, Tittlemier SA, Fernando WGD (2019) Deoxynivalenol-3-glucoside content is highly associated with deoxynivalenol levels in two-row barley genotypes of importance to canadian barley breeding programs. Toxins (Basel). https://doi.org/10.3390/toxins11060319
Wang Z, Wu Q, Kuca K, Dohnal V, Tian Z (2014) Deoxynivalenol: signaling pathways and human exposure risk assessment–an update. Arch Toxicol 88(11):1915–1928. https://doi.org/10.1007/s00204-014-1354-z
Watanabe Y, Makino E, Tajiki-Nishino R et al (2018) Involvement of estrogen receptor alpha in pro-pruritic and pro-inflammatory responses in a mouse model of allergic dermatitis. Toxicol Appl Pharmacol 355:226–237. https://doi.org/10.1016/j.taap.2018.07.008
Werfel T, Allam JP, Biedermann T et al (2016) Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol 138(2):336–349. https://doi.org/10.1016/j.jaci.2016.06.010
Williams HC (2005) Clinical practice. Atopic dermatitis. N Engl J Med 352(22):2314–2324. https://doi.org/10.1056/NEJMcp042803
Yan P, Liu Z, Liu S et al (2020) Natural occurrence of deoxynivalenol and its acetylated derivatives in chinese maize and wheat collected in 2017. Toxins (Basel). https://doi.org/10.3390/toxins12030200
Yoshida M, Nakajima T, Tonooka T (2008) Effect of nitrogen application at anthesis on Fusarium head blight and mycotoxin accumulation in breadmaking wheat in the western part of Japan. J Gen Plant Pathol 74:355–363. https://doi.org/10.1007/s10327-008-0109-1
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
A part of this study was conducted under the research project on “Toxic Compounds and Foodborne Pathogens for Food Safety Risk Management” funded by the Ministry of Agriculture, Forestry and Fisheries of Japan. A part of this study was also funded by a Grant-in-aid for scientific research of The Tojuro Iijima Foundation for Food Science and Technology.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the study conception and design. Material preparation, data collection, and analysis were performed by RA, TO, AM, NI, YT, AM, MK, SM, and TF. The first draft of the manuscript was written by RA and TF, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualization: TF. Methodology: RA, TO, AM, NI, YT, AM, MK, SM, and TF. Formal analysis and investigation: RA and TF. Writing–original draft preparation: RA and TF; writing–review and editing: RA, TO, AM, NI, YT, AM, MK, SM, and TF. Funding acquisition: MK, SM, and TF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no actual or potential competing financial interests.
Ethical approval
All aspects of in vivo study were conducted in accordance with the Animal Care and Use Program of Azabu University (Approval No. 1910097). The manuscript does not contain clinical studies or patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

204_2020_2875_MOESM2_ESM.jpg
Supplementary Fig 1 Experimental protocol used to sensitize and challenge BALB/c mice with TDI in acetone. Acute-exposure protocol (a); mice were orally administered vehicle alone, 0.1, or 0.3 mg/kg b.w. of DON (diluted in corn oil) 48, 24, and 1 h before final challenge with TDI. Subacute-exposure protocol (b); diet containing DON-contaminated wheat (0.3 ppm DON as the final concentration) or diet containing non-contaminated wheat was continuously fed to mice during the experimental period. DON = deoxynivalenol, TDI = toluene-2,4-diisocyanate. (JPG 616 kb)

204_2020_2875_MOESM3_ESM.jpg
Supplementary Fig 2 Representative HPLC chromatograms of 5 ppm DON standard (a) and wheat grain extract (b) (JPG 557 kb)

204_2020_2875_MOESM4_ESM.jpg
Supplementary Fig 3 Typical microscopic features of ear tissues, as observed via hematoxylin and eosin staining, in the TDI + vehicle control group and the 0.3 ppm DON group in the subacute-exposure study. Bar = 100 μm (JPG 996 kb)

204_2020_2875_MOESM5_ESM.jpg
Supplementary Fig 4 Cytokine production by activated T cells in the auricular LN after subacute oral administration of DON in a TDI-induced male mouse model of allergic dermatitis. A significant increase in IL-5 (b), IL-9 (c), and IL-13 (d) were observed in the DON-exposure group, whereas the production of IL-4 (a) and IL-17 (e) were not affected by DON exposure. Each result is presented as the mean (pg/mL) ± 1 SEM. n = 8 per group. *p < 0.05, **p < 0.01 (Dunnett’s multiple comparison test) vs. the TDI + vehicle control group. (JPG 638 kb)

204_2020_2875_MOESM6_ESM.jpg
Supplementary Fig 5 Pre-exposure to DON significantly counteracted LPS-stimulated (a) IL-1β, (b) IL-8 and (c) TNFα secretion by the THP-1 cell line (human monocytic leukemia). Each result is presented as the mean (pg/mL) ± 1 SEM. n = 7 per group. *p < 0.05, **p < 0.01 (Dunnett’s multiple comparison test) vs. the vehicle-alone control group (JPG 513 kb)
Rights and permissions
About this article
Cite this article
Aihara, R., Ookawara, T., Morimoto, A. et al. Acute and subacute oral administration of mycotoxin deoxynivalenol exacerbates the pro-inflammatory and pro-pruritic responses in a mouse model of allergic dermatitis. Arch Toxicol 94, 4197–4207 (2020). https://doi.org/10.1007/s00204-020-02875-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02875-3