Abstract
Glyphosate-based herbicides (GBHs) are the most globally used herbicides raising the risk of environmental exposition. Here, we investigated whether perinatal exposure to low doses of a GBH alters the female reproductive performance, and/or induced second-generation effects related to congenital anomalies or growth alterations. Pregnant rats (F0) received a GBH through food, in a dose of 2 mg (GBH-LD: GBH-low dose group) or 200 mg (GBH-HD: GBH-high dose group) of glyphosate/kg bw/day from gestational day (GD) 9 until weaning. Body weight gain and vaginal canal-opening of F1 females were recorded. Sexually mature F1 females were mated to evaluate their reproductive performance by assessing the pregnancy rate, and on GD19, the number of corpora lutea, the implantation sites (IS) and resorption sites. To analyze second-generation effects on F2 offspring, we analyzed the fetal morphology on GD19, and assessed the fetal length and weight, and the placental weight. GBH exposure neither altered the body weight gain of F1 females, nor vaginal opening onset. Although all GBH-exposed F1 rats became pregnant, a lower number of IS was detected. F2 offspring from both GBH groups showed delayed growth, evidenced by lower fetal weight and length, associated with a higher incidence of small for gestational age fetuses. In addition, higher placental weight and placental index were found in F2 offspring from GBH-HD dams. Surprisingly, structural congenital anomalies (conjoined fetuses and abnormally developed limbs) were detected in the F2 offspring from GBH-HD group. In conclusion, perinatal exposure to low doses of a GBH impaired female reproductive performance and induced fetal growth retardation and structural congenital anomalies in F2 offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infertility remains a highly prevalent global condition and it is estimated to affect over 10% of women worldwide (WHO 2017). Moreover, no specific cause is identified in one-third of the couples experiencing infertility (Macklon 2017). Growing evidence suggests that some lifestyle factors and increasing environmental exposure to endocrine disrupting chemicals (EDCs) might contribute to trends in the occurrence of reproductive health problems (Den Hond et al. 2015; Ziv-Gal and Flaws 2016). Epidemiological studies have shown a close correlation between environmental and/or occupational exposure to pesticides and adverse reproductive disorders in men and women (Chevrier et al. 2013; Mostafalou and Abdollahi 2017). Some deleterious effects on reproductive health outcomes included: decreased fertility, miscarriages, stillbirth, low birth weight or birth defects (Chiu et al. 2015; García et al. 2017; Rappazzo et al. 2016; Razi et al. 2016).
Glyphosate (N-[phosphonomethyl]-glycine) is a systemic herbicide used for weed control both in domestic gardening and in extensive crops (Bromilow and Chamberlain 2000; Perego et al. 2017). In 1996, genetic engineering revolution applied to agriculture cultures introduced genetically modified herbicide-tolerant crops and glyphosate-based herbicides (GBHs). Since that time, culture areas destined to genetically modified crops have been extended mostly in South American countries like Argentina, Brazil, Bolivia, Paraguay and Uruguay (Fischer et al. 2014), and GBHs have become the most globally used herbicides (Benbrook 2016). Additionally, in the last decade, farmers increased glyphosate application rates and spray more often to combat glyphosate-resistant weeds (Benbrook 2016). Lately, it has been found that GBHs can also be used as desiccants in the growing season to accelerate harvest operations, especially in small grain crops (Cuhra et al. 2016). Given the extensive and increased usage of GBHs, glyphosate and its primary metabolite aminomethylphosphonic acid (AMPA) have been detected in the air (Chang et al. 2011), soil (Avila-Vazquez et al. 2017; Primost et al. 2017), respirable dust (Mendez et al. 2017), water (Battaglin et al. 2014; Ronco et al. 2016), as well as in food that can be consumed by humans or livestock (Bai and Ogbourne 2016). In addition, a trend towards increasing glyphosate concentrations was detected in urine samples from farm and non-farm individuals in Europe, probably reflecting more sensitive analytical techniques, more frequent use in agricultural practice and/or higher residues in food items (Krüger et al. 2014a; Niemann et al. 2015). This evidence shows that there is a risk of environmental exposition to GBHs, stressing concerns about their potential effects on human health.
Some research works on GBHs revealed different effects indicative of endocrine disruption. Mesnage et al. (2017) reported that glyphosate activates the estrogen receptor alpha via a ligand-independent mechanism in in vitro assays. On the other hand, using an in vivo model, we found that GBH can modulate the expression of estrogen-sensitive genes in the rat uterus (Varayoud et al. 2017). Experimental studies performed in rodents have associated early exposure to GBH during development with male and female reproductive toxicity. Male offspring exposed during pregnancy and lactation exhibited a decrease in sperm production and signs of degenerating spermatids (Dallegrave et al. 2007). Using a similar experimental approach, Romano et al. (2012) found that GBH-exposed male offspring showed an early onset of puberty, and behavioral and histological changes related to reproductive parameters. Recently, we showed that the administration of GBH by subcutaneous injections during the first week of life affected: (1) the uterine morphology and differentiation in neonatal and prepubertal rats (Guerrero Schimpf et al. 2017), and (2) the reproductive performance and endocrine-dependent mechanisms involved in the decidualization process (Ingaramo et al. 2017, 2016).
The goal of the present study was to investigate the effect of perinatal (gestational and lactational) exposure of F1 female rats to low doses of a GBH administered through food on their reproductive performance and the feto-placental parameters of their progeny. Here, we presented a GBH exposure model through diet using a laboratory pellet chow-based paste. We determined the real doses of glyphosate achieved and the serum levels of glyphosate and AMPA in F0 dams to validate our model. In F1 female rats, we assessed the onset of puberty recording the vaginal canal-opening. The reproductive performance was evaluated by the determination of: (1) pregnancy rate, (2) the number of corpora lutea (CLs), implantation (IS), and resorption (RS) sites on gestational day (GD) 19. We also analyzed feto-placental parameters of F2 offspring to evaluate possible second-generation effects, related to congenital anomalies or growth alterations.
Materials and methods
Chemicals
The glyphosate formulation used in this study was MAGNUM SUPER II marketed in Argentina by Grupo Agros S.R.L. It is a liquid water-soluble formulation containing 66.2% of glyphosate potassium salt (equivalent to 54% w/v of glyphosate acid), as its active ingredient, coadjuvants and inert ingredients. This GBH was chosen based on the fact that it is one of the herbicides most commonly used in our country, and that it is representative of formulations with high content of glyphosate indicated against weeds difficult to eradicate.
Animals
The procedures used in this study were approved by the Institutional Ethics Committee of the Facultad de Bioquímica y Ciencias Biológicas (Universidad Nacional del Litoral, Santa Fe, Argentina) and were performed in accordance with the principles and procedures outlined in the Guide for the Care and Use of Laboratory Animals issued by the United States National Academy of Sciences.
We used inbred Wistar-derived strain rats that were bred in the Department of Human Physiology (Universidad Nacional del Litoral), and housed in a controlled environment (22 ± 2 °C; lights on from 06:00 to 20:00 h) in stainless steel cages with wood bedding.
Experimental design
Nulliparous female rats at the proestrus stage were caged overnight with males of proven fertility. Every morning, vaginal smears were performed to check for the presence of spermatozoa (Montes and Luque 1988). The first day on which a sperm-positive smear was detected was considered gestational day 1 (GD1). Pregnant females (F0) were housed singly and randomly assigned to one of the following oral treatment groups: control group (n = 7) provided with a laboratory pellet chow-based paste, GBH-low dose group (GBH-LD; n = 7) provided with paste supplemented with GBH in a dose of 2 mg of glyphosate/kg bw/day, and GBH-high dose group (GBH-HD; n = 7) provided with paste supplemented with GBH in a dose of 200 mg of glyphosate/kg bw/day. The doses of glyphosate were selected based on the no-observed adverse effect level (NOAEL) of 1000 mg/kg bw/day for maternal toxicity established in rat (Williams et al. 2000). The dose of 2 mg/kg bw/day is in the order of magnitude of the reference dose (RfD) of 1 mg/kg bw/day recently reassigned for glyphosate by the Environmental Protection Agency (EPA 2017) based on developmental toxicity studies. Moreover, this dose is representative of the glyphosate residues found in soybean grains (Arregui et al. 2004; Test Biotech 2013), and is in the order of magnitude of the environmental levels detected in our country (Peruzzo et al. 2008; Primost et al. 2017). The laboratory chow-based paste was prepared by blending optimized quantities of pellet chow (Nutrición Animal, Santa Fe, Argentina) and water; for GBH treatment groups a glyphosate commercial formulation was added to the water according to the above described doses. The mixture was covered and stood overnight, after that it was homogenized to form a paste and chow balls were prepared to each treatment. The pellet-based paste for control and GBH groups was prepared freshly, i.e., the same day the food was replaced. We checked for glyphosate integrity in the dietary matrix by measuring its concentration during three consecutive days by Ultra performance liquid chromatography–tandem mass spectrometer (UHPLC–MS/MS) according to the protocols described in Oulkar et al. (2017). A preliminary study was carried out to ensure that the addition of GBH does not alter food consumption, and to estimate the average amount of chow-based paste daily consumed by F0 dams during pregnancy and lactation. These data allowed us to calculate the amount of the active ingredient (glyphosate) to add to the pellet chow. Tap water was supplied ad libitum in glass bottles with rubber stoppers surrounded by a steel ring. F0 pregnant females received the oral treatment from GD9 until the end of weaning (on lactational day (LD) 21). In our colony, embryo implantation occurs in the evening of GD5 (Milesi et al. 2015). Therefore, we began the oral treatment of F0 dams on GD9 to avoid potential implantation failures prompted by GBH. To determine the dose of glyphosate administered, individual maternal weight and food intake were recorded three times per week throughout the treatment period. With these data, we determined the relative body weight gain and loss of F0 dams during gestation and lactation periods, respectively. In addition, we collected trunk blood of F0 mothers at two different moments: (1) at the end of gestation, GD22, and (2) at weaning, LD21. We determined the serum levels of glyphosate and its metabolite AMPA according with the method described in “Glyphosate and AMPA determination in F0 dams’ serum” from Materials and Methods.
After delivery (postnatal day (PND) 0), F1 pups were weighed and sexed according to the anogenital distance, and litters of eight pups (preferably four males and four females) were left with F0 lactating mothers. The following parameters were analyzed: length of gestation, birth weight of male and female pups, litter size and maternal care. At weaning (PND21), female offspring were housed in groups of four rats according to the treatment group (control or GBH-exposed) with free access to pellet laboratory chow and tap water. Male offspring were used in other experiments. Body weight of F1 females were recorded between birth and PND90 (PND 1, 7, 14, 21, 60 and 90). We determined the onset of puberty by checking the vaginal canal-opening on PND30. On PND90, F1 females were caged with males of proven fertility to evaluate their reproductive performance by determining the pregnancy rates and the number of CLs, IS and RS on GD19 (see “Evaluation of reproductive performance in F1 females” from Materials and Methods). At this stage, we also determined feto-placental parameters in their progeny (F2) (see “Feto-placental parameters in F2 offspring” from Materials and Methods). A schematic representation of the experimental design is shown in Fig. 1.
Glyphosate and AMPA determination in F0 dams’ serum
Glyphosate and its metabolite AMPA concentrations were measured in serum of F0 dams on GD22 (at the end of the gestation period) and LD21 (at the end of the lactation period) by UHPLC–MS/MS. The analytical procedure consisted of FMOC derivatization based on the method proposed by Bernal et al. (2010) with modifications. Sample extracts were then analyzed using a previously optimized and validated UHPLC–MS/MS methodology as described by Sasal et al. (2015). Glyphosate (97.0%), AMPA (98.0%), glyphosate-FMOC (91.5%), AMPA-FMOC (98.0%) and isotopically labeled standards (ILS) [1,2-13C2 15N] glyphosate (98%) and [13C2 15N] AMPA were from Dr. Ehrenstorfer (Augsburg, Germany). All other supplies used were of the best commercially available analytical grade.
500 µL of rat serum (blank, blank spiked with standards, or experimental serum samples) were transferred to an Eppendorf tube, and 250 µL of acetonitrile (MeCN) was added. The mixture was vortexed for 1 min, placed in an ultrasound device for 10 min, and centrifuged at 15,000 rpm for 10 min at room temperature to precipitate the proteins. 500 µL of the supernatant were collected and transferred to a different Eppendorf tube and the same precipitation step was repeated once again. The supernatant (500 µL) was then spiked with internal standard (40 µL of ILS 1 mg L−1) and was derivatized with the addition of 84 µL borate buffer (40 mM pH 9), 84 µL FMOC-Cl (6 g L−1), and acetonitrile. After a 2 h reaction at room temperature, the derivatization was quenched by acidification to pH 3 with formic acid. After the derivatization reaction was completed, the extracts were cleaned-up by liquid–liquid partition with dichloromethane (500 µL extract/500 µL DCM). Finally, 10 µL of this solution was injected into the UHPLC–MS/MS system.
Validation was carried out following the Document SANTE/11945 (SANTE 2015), by determining recovery, selectivity, limits of quantification and detection, linearity, precision and accuracy. The recovery of glyphosate and AMPA was determined in 6 replicates at 3 concentrations levels (1, 10 and 100 µg L−1), comparing the peak areas of glyphosate and AMPA from standard samples with those from: (1) extracted blank serum samples from control rats, spiked with the same amounts of the compounds and then treated as described above (Recovery assay); (2) and to check the possible matrix effect on the ESI ionization, extracted blank serum samples from control rats that were treated as described above and then spiked with the same amounts of glyphosate and AMPA (matrix-matched serum). The average percentage recoveries were between 80–100% with relative standard deviation (RSD) lower than 20% for all residues.
To check the selectivity of the method, extracts from blank and spiked serum samples were assayed. The limit of detection (LOD) and quantification (LOQ) were determined by injecting a number of extracts from blank serum samples (n = 6) and measuring the magnitude of the background response. The LOD was experimentally estimated as three times the signal-to-noise ratio (S/N) and LOQ as the lowest concentration that could be measured with an intra-assay precision CV% and relative bias less than 20%. The LOD and LOQ for glyphosate and AMPA were 1 and 2 µg L−1, respectively. Precision was expressed as the % RSD and accuracy was calculated through the relative error (% RE).
Evaluation of reproductive performance in F1 females
F1 control (n = 25) and GBH-exposed (GBH-LD, n = 20; GBH-HD, n = 20) female rats at the proestrus stage were bred to untreated fertile males. The presence of spermatozoa in vaginal smears was registered as an index of pregnancy. The pregnancy rate was calculated as the number of pregnant females/number of females housed with a male × 100. Pregnant females with sperm-positive smears were housed separately and submitted to a fertility test on GD19. The ovaries from the pregnant rats were dissected, and the number of profusely irrigated CLs was counted by direct visualization using a stereomicroscope (Leica Corp., Buffalo, NY, USA). The two-horned uteri were removed and visually inspected to identify the number of RS and IS. The RS were defined as endometrial sites with an appended amorphous mass without a fetus. The number of IS was defined as the result of the total number of placentas with fetuses plus the total number of RS. With these data, we calculated the rate of pre-implantation loss as follows: [number of CLs − number of IS/number of CLs] × 100 (Perobelli et al. 2012).
Feto-placental parameters in F2 offspring
To determine second-generation effects on fetal development, F2 fetuses were removed from uteri on GD19. Fetuses and their respective placentas were examined for their morphology and weighed; with these data we calculated the placental index as follows: (placental weight/fetal body weight). Fetal length from the top of the head to the bottom of the buttocks (crown–rump length) was also measured. F2 fetuses were classified according to their weight on GD19 as small (SGA) (< 10th percentile), appropriate (AGA) (10–90th percentile) or large (LGA) (> 90th percentile) for gestational age, using a frequency distribution curve (MacDonald et al. 2005). This curve was constructed with weight values of F2 fetuses from control group of our colony (data not shown), which followed the classic bell-shaped Gaussian distribution.
Statistical analysis
Results are expressed as the mean ± SEM. The data from the number of IS, CLs, and feto-placental parameters were analyzed using one-way ANOVA followed by Dunnett’s test for the multiple comparisons (after Bartlett’s test for the homogeneity of the variance). The analysis of the number of RS was conducted using a generalized linear model with a negative binomial response with the glm.nb function from R statistical software. Occurrence of unilateral pregnancy was assessed using Fisher’s exact test. Incidence of SGA or LGA F2 fetuses and the relative risk associated with these categories were analyzed by Chi square test. Occurrence of congenital anomalies in F2 fetuses was also analyzed by Chi square test. All statistical analyses were performed using R statistical software (The R Foundation for Statistical Computing version 3.4.1). Differences were considered significant at p < 0.05.
Results
Glyphosate concentration in the dietary matrix
To check glyphosate integrity in the dietary matrix, we measured its concentration in the pellet-based paste of control and GBH groups during three consecutive days that correspond to the period of food replacement. As it is shown in Table 1, for both GBH diets glyphosate concentrations were within the expected values and did not change over time, suggesting that glyphosate integrity was preserved. As expected, no detectable levels of glyphosate were found in control diet.
Data about F0 dams and glyphosate and AMPA serum levels
GBH treatment through food did not produce signs of embryotoxicity, abnormal maternal or nursing behaviors as it was expected according to the doses evaluated in this study (below the NOAEL dose). In fact, the length of gestation was unaltered, all F0 pregnant dams successfully delivered their pups, the numbers of F1 live-born pups per litter were similar between groups, and the litter sex ratio showed no alterations and was within the normal range (50% females and 50% males). Also, no difference was found in weight at birth in GBH-exposed F1 males or females compared to the control group. In addition, no gross malformations were observed in F1 pups at birth. Moreover, no changes were detected in the F0 dams´ body weight gain during pregnancy. However, the relative body weight loss from LD1 until weaning was higher in F0 dams treated with the low dose of GBH (GBH-LD group). These results are shown in Table 2.
Food intake during pregnancy and lactation was not affected in F0 dams exposed to the herbicide. Moreover, no changes were detected in the average body weight of F0 dams. The real average doses of glyphosate, calculated based on the dams’ average body weight and food consumption during pregnancy and lactation are shown in Table 3. Although the real glyphosate doses effectively reached in both GBH groups resulted a bit higher than the theoretical doses (3.69 ± 0.07 instead of 2 mg/kg bw/day in GBH-LD group, and 352.2 ± 4.78 instead of 200 mg/kg bw/day in GBH-HD group), they were in the same order of magnitude. Moreover, as originally setting out, a 100-fold difference was maintained between the two real glyphosate doses.
The mean serum concentrations of glyphosate in F0 dams at the end of the lactation period (LD21) were: 0.039 ± 0.006 mg/L for GBH-LD group, and 3.8 ± 1.2 mg/L for GBH-HD group. Again, it is worth noting that a 100-fold difference was observed when comparing glyphosate concentrations of F0 dams from the two treated groups. As for AMPA serum concentrations, the main metabolite of glyphosate, no detectable levels were found neither in GBH-LD nor in GBH-HD treated dams. Finally, no glyphosate or AMPA levels were detected in control animals. Similar results were obtained on GD22 (data not shown). All these data are shown in Table 3.
Effect of perinatal GBH treatment on body weight and vaginal opening in F1 females
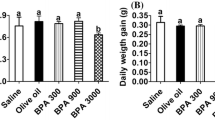
To determine whether perinatal exposure to GBH altered the growth or the onset of puberty of F1 female rats, we recorded their weight from birth until adulthood (PND90) and the day at which the vaginal canal-opening occurred, respectively. No differences were observed in the body weight at any of the studied points (Fig. 2a). Similarly, no changes were detected in the onset of vaginal canal-opening as a consequence of GBH exposure (Fig. 2b).
Effect of perinatal GBH treatment on weight and onset of vaginal canal-opening in F1 female rats. a Body weight curves of control (empty circle) and perinatally exposed F1 female rats to GBH-LD (empty diamond) or GBH-HD (empty square) through diet from birth until adulthood. b Onset of vaginal canal-opening expressed as postnatal day (PND) for each experimental group. All data are presented as mean ± SEM (Control, n = 25; GBH-LD, n = 20; GBH-HD, n = 20)
Reproductive performance of perinatally GBH-exposed F1 females on GD19
Perinatal exposure of F1 female rats to GBH-LD and GBH-HD doses did not affect the pregnancy rates (Fig. 3a). The fertility test performed on GD19 revealed neither changes in the number of CLs (CLs/rat: 11–13) (Fig. 3b) nor in the number of resorption sites (Fig. 3c). Interestingly, both GBH-exposed groups showed a lower number of implantation sites (Fig. 3e). When we analyzed the percentage of pre-implantation loss (i.e., number of oocytes not fertilized or embryo loss before implantation), a significant increase was detected in both GBH-exposed groups (Fig. 3d).
Evaluation of reproductive performance in control and GBH perinatal exposed F1 rats recorded on gestational day 19 (GD19). a The pregnancy rates were calculated by the average of females that were pregnant with a fertile male. b The number of CLs and d pre-implantation loss rate are expressed as the mean ± SEM for each experimental group (Control, n = 25; GBH-LD, n = 20; GBH-HD, n = 20). c The numbers of resorption sites in each individual pregnant rat were plotted, and the horizontal lines are the mean for each group (n ≥ 20 per group) with its corresponding SEM. Asterisks indicate statistical significance compared to the control (*, p < 0.05). e Photographs of representative uteri collected on GD19 from a control and a GBH-LD or GBH-HD perinatally exposed F1 female rat. Note the lower number of implantation sites in the uterus of a GBH-LD and GBH-HD F1 rat compared to a control rat. Each black arrow indicates an implantation site and the gray arrow indicates a resorption site
A peculiar effect was found in some of the F1 females from GBH-LD and GBH-HD groups, which presented unilateral pregnancies. In fact, in 2 out of 20 rats from GBH-LD group and in 3 out of 20 rats from GBH-HD group, embryo implantation occurred only in one uterine horn, even though the number of CLs in the ovary adjacent to the no pregnant uterine horn was normal. However, these findings did not show to be statistically significant (Fig. 3e-GBH-HD).
Feto-placental parameters of F2 offspring
As oral exposure to a GBH during perinatal period affected F1 female reproductive performance, we investigated whether it could also have an influence on the development of their progeny, by analyzing feto-placental parameters. We detected that F2 fetuses (fetuses/litters: Control, n = 213/20; GBH2, n = 152/15; GBH200, n = 117/13) from F1 females exposed to both doses of GBH showed a decrease in length and body weight (Table 4). From the weight frequency distribution curve, we found that 57.5 and 48.2% of the F2 fetuses from GBH-LD and GBH-HD groups, respectively, were SGA fetuses, i.e., with a weight < 10th percentile. In the control group, only 23.7% of the fetuses were SGA. The relative risk of being SGA was 2.43 [95% CI (1.66, 3.55); p < 0.0001] for GBH-LD F2 fetuses and 2.04 [95% CI (1.33, 3.12); p = 0.0012] for GBH-HD F2 fetuses. A representative image of these alterations is shown in Fig. 4. In addition to low weight and length, F2 fetuses from F1 females perinatally exposed to GBH-HD exhibited higher placental weight (Table 4) and placental index (Fig. 5a). Differences in size of F2 fetuses and their corresponding placentas from control and GBH-HD group can be appreciated in Fig. 5b.
Fetal morphology of F2 offspring
On GD19, each fetus was subjected to a careful evaluation to detect structural anomalies. Surprisingly, fetal malformations were detected in some of the F2 fetuses from mothers perinatally exposed to the high dose of GBH (200 mg/kg bw/day). Fetal abnormalities were observed in 3/117 fetuses, each one from different F1 mothers (i.e., 3/13 litters affected). A statistically significant correlation was found between perinatally GBH-HD exposure and fetal anomalies (p < 0.05). These anomalies included conjoined fetuses and abnormally developed limbs (Fig. 6). In the last case, we observed fetuses which lacked one of their extremities or exhibited a longitudinal reduction of the tail (Fig. 6b, c). No structural anomalies were observed in F2 fetuses from GBH-LD or control mothers.
Fetal morphology of F2 offspring from GBH-HD exposed F1 female rats. In the photographs are shown structural congenital anomalies, such as, a conjoined fetuses and abnormally developed limbs, in b a fetus lacking one of its anterior extremities (indicated by the black arrow) and in c a fetus lacking one of its posterior extremities and displaying a longitudinal reduction of the tail (indicated by black arrows). 3 structural anomalies were detected in 117 GBH-HD F2 fetuses analyzed (3/117) in (3/13) litters (*, p < 0.05)
Discussion
In the present study, we investigated the effects of in utero and lactational exposure to low doses of a GBH, administered orally to F0 mothers, on the development and reproductive outcome of F1 female rats. We also evaluated feto-placental parameters of F2 offspring to assess possible second-generation effects.
Here, we presented an oral exposure model in which GBH was administered through food by means of the incorporation into a laboratory pellet chow-based paste. This exposure model proved to be successful since F0 dams consumed a similar amount of food regardless of the treatment received. In a study performed by Beuret et al. (2005), GBH was administered during the gestational period through drinking water, and a reduction in water and food consumption was reported. The authors suggested that glyphosate could have affected palatability of water (Beuret et al. 2005). Another point to highlight about our model, it was that the real glyphosate doses achieved were in the same order of magnitude as the theoretical doses. Moreover, a 100-fold difference was maintained between the two real glyphosate doses. With regards to the serum concentrations of glyphosate in GBH-LD and GBH-HD F0 dams, similar values were obtained on GD22 and on LD21, indicating that glyphosate concentration remained apparently constant along the treatment. As for AMPA serum concentrations, no detectable levels were found neither in GBH-LD nor in GBH-HD treated dams. According to Anadón et al. (2009), glyphosate is poorly metabolized to AMPA after oral administration in Wistar rats, and this metabolite could be the result of intestinal microbial action. The metabolite AMPA has raised great concern for being more persistent than glyphosate in the environment (Battaglin et al. 2005). Similar to glyphosate, genotoxic effects were reported for AMPA in in vitro and in vivo studies using Comet assay and micronucleus test, respectively (Mañas et al. 2009; Roustan et al. 2014). Furthermore, some studies found that AMPA is even more toxic than its parent compound in human umbilical, embryonic and placental cells and also in CHO-K1 cells (Benachour and Séralini 2009; Roustan et al. 2014). Based on these findings and considering that no AMPA was detected in the serum of both GBH-treated groups, we suggest that this metabolite would not have great influence on the adverse effects detected in our work.
Our results showed that a GBH administered orally during pregnancy and lactation in a dose of 2 or 200 mg of glyphosate/kg bw/day did not induce maternal toxicity, i.e., mortality, or changes in gestational body weight gain and length. Lactational body weight loss was unaffected in the GBH-HD group, but it was decreased in the GBH-LD exposed dams. Moreover, no adverse effects were detected in the F1 offspring variables, such as birth weight, litter size and sex ratio in both GBH groups. These results are in accordance with those reported by Dallegrave et al. (2007), who found no maternal or offspring toxicity after oral treatment with a GBH in a dose of 50, 150 or 450 mg of glyphosate/kg bw/day during pregnancy and lactation. Similar findings were observed by Gallegos et al. (2016), who reported that exposure to a GBH during the same sensitive period in a dose of 100 or 200 mg of glyphosate /kg bw/day did not affect maternal weight gain during pregnancy, gestational length, litter size or birth weight of pups.
It has been shown that exposure to EDCs during fetal development can promote functional deficits and altered programming, leading to increased disease risk later in life and even across successive generations, the so-called fetal origin of adult diseases (Skogen and Øverland 2012). As for GBHs, it has been recently debated over the possibility about signs of endocrine activity (Vandenberg et al. 2017). Studies in cell culture showed that glyphosate promotes cell proliferation and induces endocrine-mediated effects on end points relevant to toxicity (Mesnage et al. 2017; Thongprakaisang et al. 2013). Furthermore, in vivo studies revealed male and female reproductive development impairment (Guerrero Schimpf et al. 2017; Romano et al. 2012).
In our experiment, perinatal exposure of F1 females to GBH affected neither the body weight gain from birth until adulthood, nor the day of vaginal canal-opening, indicating no effect on the beginning of puberty. By contrast, in the study of Dallegrave et al. (2007), a delay in the vaginal canal-opening was reported for all the GBH studied doses. Regarding to reproductive outcome, all F1 females from control and GBH-treated groups became successfully pregnant. The number of CLs recorded in the ovaries showed no differences between the experimental groups, suggesting that neither the ovulation rate nor the CLs “activation” were altered as a consequence of perinatal exposure to GBH. Similarly, no differences were detected in the number of resorption sites between the control and GBH-treated rats, ruling out a post-implantation failure. However, we found a lower number of implantation sites in the females exposed to both doses of GBH, in association with an increase of the rate of pre-implantation embryo loss. The fact that F1 pregnant dams exposed in utero and during lactation to GBH exhibited pre-implantation embryo loss without difference in the number of corpora lutea, might suggest that uterine organogenesis might have been affected during the embryonic development, and then at adulthood might have failed to reach the receptive state which is critical for implantation. Nevertheless, as successful implantation not only requires an intricate program of uterine preparation but also the development of the embryo to the blastocyst stage (Review in Varayoud et al. 2014), we cannot rule out that other problems such as reduction of fertilization rate of the oocytes, defects in tubal transport and/or embryo development are also implicated in GBH-induced implantation failures. A strange feature of this experiment was the incidence of unilateral pregnancies in rats perinatally exposed to both doses of GBH. Taking into account that the number of CLs in the ovary adjacent to the non-pregnant uterine horn was normal, the origin of this abnormal implantation pattern is unknown and deserves further investigation. Even though this feature occurred at low frequency, it is worthy to mention that it has not been recorded in the historical controls from our colony or in previous studies of our lab with other EDCs (Altamirano et al. 2015; Kass et al. 2012). Moreover, to the best of our knowledge, such abnormal implantation pattern has not been previously reported in others works leading to study the effects of EDCs exposure on female fertility. Recently, using a different experimental approach, we demonstrated that neonatal exposure to a GBH, administered subcutaneously during the first postnatal week in a dose of 2 mg of glyphosate/kg bw/day, altered uterine development in prepubertal rats (Guerrero Schimpf et al. 2017) and induced post-implantation embryo loss at adulthood (Ingaramo et al. 2016). Indeed, adult female rats neonatally treated with GBH, showed a normal rate of pregnancy, but an increased number of resorption sites, in association with an impaired decidualization response (Ingaramo et al. 2016, 2017). Taken together, our findings provide novel evidence about different adverse reproductive outcomes caused by GBH depending on the administration route (oral vs. subcutaneous injection), and/or the timing of GBH exposure (in utero and lactational exposure vs. neonatal exposure). It has been suggested that the timing, nature, and severity of endocrine system impacts will vary depending on the levels and timing of GBH exposures, the tissues exposed, the age and health status, and other biotic or abiotic stressors impacting the developmental stage and/or physiology of the exposed organism (Myers et al. 2016). Finally, our present and previous results contribute to increased evidence about the potential activity as endocrine disrupters of GBHs, and indicate that regardless of window and route of exposure, the herbicide prompted long-term adverse effects on female reproductive capability.
The original but also worrying finding of our work was the occurrence of second-generation adverse effects caused by GBH exposure. F2 fetuses whose mothers had been exposed in utero and during lactation to GBH-LD and GBH-HD exhibited a decrease in the body weight and length. Similar effects on F2 fetal weight were found by gestational dexamethasone (glucocorticoid) overexposure in a three generation animal model (Drake et al. 2011). However, to our knowledge, no information is available regarding how pesticides applied indirectly to F1 generation animals could affect the fetal parameters studied here in their progeny. Considering low-weight fetuses, a higher incidence of SGA fetuses (weight below the 10th percentile) was detected in both GBH groups, evidencing delayed growth. It has been reported that fetal growth restriction might be caused by inherent fetal abnormalities, such as chromosomal or other congenital anomalies, maternal disease or placental insufficiency (Barron and Thomson 1983; Kramer 1987). Furthermore, SGA has been shown to predispose for some growth and development disorders, as well as chronic diseases later in life (Saenger et al. 2007). A number of long-term risks for SGA children have been identified, including higher systolic blood pressure (Barker 1995), glucose intolerance, hyperinsulinism, type 2 diabetes (Barker et al. 1993; Hales et al. 1991), precocious pubarche (Verkauskiene et al. 2013), and ovarian hyperandrogenism (Ibáñez et al. 1998). Finally, several epidemiological studies have been associated low birth weight and length with environmental or occupational maternal exposure to pesticides (Burdorf et al. 2011; Quintana et al. 2017; Sathyanarayana et al. 2010; Whyatt et al. 2004).
In addition to low weight and length, F2 fetuses from F1 dams perinatally exposed to GBH-HD exhibited higher placental weight and placental index (placental to fetal weight ratio). Placenta is responsible for maternal transfer of nutrients to the fetus to propitiate fetal growth and development (Fowden et al. 2008). The placental index decreases across gestation and reflects the balance between fetal and placental growth; as the placenta matures, the fetal weight increases (Macdonald et al. 2014). Placenta has a high functional reserve capacity and a remarkable potential to increase its growth as a required adaptation. It has been reported that placental hypertrophy is an adaptation mechanism to preserve fetal well-being in adverse environments (Quintana et al. 2017). As in our experiment higher placental index was associated to an increase in placental weight as well as to a decrease in fetal weight, we proposed that placental hypertrophy might be induced as a compensatory reaction to fetal growth restriction. Similar to our finding, compensatory placental hypertrophy has been reported in rats exposed to drugs and chemicals like indomethacin (Wellstead et al. 1989) and tributyltin (Adeeko et al. 2003). Humans studies have pointed out that placental weight and placental index are predictive factors for maternal disease and obstetric outcome, perinatal morbidity and mortality, and impaired fetal growth and development (Burkhardt et al. 2006; Macdonald et al. 2014). A retrospective study of 18,386 pregnancies found a high placental index among pregnancies characterized by poor outcomes, such as hypertensive disorders and small for gestational age infants (Londero et al. 2013). In addition, other studies have demonstrated an association between higher placental weight and placental index with pesticide exposure in pregnant women living in rural areas (Acosta-Maldonado et al. 2009; Quintana et al. 2017).
A surprising finding of our work was the occurrence of structural congenital anomalies in the F2 offspring whose mothers had been exposed to the high dose of GBH. Such fetal malformations included conjoined fetuses and abnormally developed limbs. Although fetal abnormalities in F2 occurred in a relatively small number of animals, a statistically significant correlation was found with GBH exposure. Also, it is worthy of consideration that we have never detected structural congenital anomalies in our own animal facility. For these reasons, we consider our results are of great relevance and that developmental disorders we found were prompted by GBH and were not the result of spontaneous changes. Alterations in F2 generation suggest a mechanism of transgenerational induction of congenital anomalies by GBH. Transgenerational inheritance of disease involves the germ line transmission of altered chromosomal or epigenetic information in the absence of direct exposure (Skinner et al. 2013). It has been shown that most environmental toxicants have the capacity to alter the epigenome, rather than to modify the DNA sequence (Skinner et al. 2011). If epigenetic modifications occur in a somatic cell, these may promote disease in the exposed individual; but they will not be transmitted to the next generation. Otherwise, if the toxicant modifies the epigenome of the germ line permanently, then the disease promoted can become transgenerationally transmitted to subsequent progeny (Skinner et al. 2011). Different EDCs have been shown to induce disease across generations following ancestral exposure during fetal gonadal sex determination, and this was associated with an alteration in the epigenetic programming of the germ line. Some EDCs include dioxin (Bruner-Tran and Osteen 2011; Manikkam et al. 2012a), the fungicide vinclozolin (Anway et al. 2006; Nilsson et al. 2008), the pesticide methoxichlor (Anway et al. 2005), and a pesticide mixture (permethrin and diethyltoluamide) (Manikkam et al. 2012b), and the transgenerational effects consist of reduced fertility, decreased spermatogenic capacity, increased incidence of premature birth, among others. As for glyphosate herbicides, studies in zebrafish and the amphibian Xenopus laevis showed that developmental exposures induce craniofacial and brain malformations, revealing the teratogenic potential of these compounds (Paganelli et al. 2010; Roy et al. 2016). Birth defects were also detected in young pigs fed with glyphosate-contaminated soybean (Krüger et al. 2014b). In the present work, structural anomalies in GBH group were not detected in the F1 generations at birth (no teratogenic effects were observed), but were found in the F2 offspring indicating transgenerational induction of congenital defects. To the extent of our knowledge, this is the first study showing second-generation adverse effects as a consequence of GBH exposure.
In recent years, GBHs has received significant attention by the general population and regulatory agencies around the world. Human health concerns about these herbicides have been raised principally because of: (1) extensive and increased used of GBHs over the past decade, together with their new use as pre-harvest desiccants that may increase the risk of dietary exposure; (2) detection of glyphosate and its metabolites in foods; and (3) studies from laboratory and domesticated animals as well as human populations, suggesting that current exposure levels to GBHs could induce adverse health outcomes (Myers et al. 2016). So far, few epidemiological and biomonitoring studies have deal with the impact of GBHs on human disease. A longitudinal study from Thailand found that pregnant women who work in agriculture or live in families that work in agriculture have higher exposure to the GBHs (Kongtip et al. 2017). In this work, the serum concentrations of glyphosate in pregnant women at childbirth and in the umbilical cord were in the range of 0.2–189.1 ng/ml and 0.2–94.9 ng/ml, respectively, which are comparable with the glyphosate’s serum levels we detected in the GBH-LD group (i.e., 39 ng/ml). More recently, a birth cohort study was performed by Parvez et al. (2018) who determined exposure frequency to GBHs, potential exposure pathways, and associations with fetal growth and pregnancy length. They reported that > 90% of pregnant women had detectable glyphosate urine levels (range 0.5–7.20 ng/mL), and that the higher levels were found in women who lived in rural areas, and in those who consumed > 24 oz. of caffeinated beverages per day. They also found a significant correlation between high glyphosate urine levels and shortened gestational lengths. Although epidemiological data are limited, recent studies using direct estimation of exposure (by direct measurement of glyphosate in urine or blood) allow increasing evidence that at current levels of occupational and environmental exposures there might be a risk for human reproductive and developmental health. The results from our experimental animal model support previous epidemiological evidence, showing detrimental effects on reproductive health at environmentally relevant doses of glyphosate.
Another matter of concern focused on whether the adverse effects caused by GBHs are prompted by the active principle (glyphosate), the co-formulants, or both acting in a synergic way. Several studies comparing GBHs, co-formulants and glyphosate effects have revealed that GBHs are more toxic than the active ingredient using cell line assays, suggesting that co-formulants would not be innocuous compounds (Defarge et al. 2016; Mesnage et al. 2013; Richard et al. 2005). On the contrary, it has been recently reported that glyphosate induced the proliferation of estrogen-dependent MCF-7 human breast cancer cells and activated ERα in vitro, while the commercial GBH formulation or their adjuvants alone did not show estrogenic activity (Mesnage et al. 2017). Taking into account the controversy about this issue, under the condition of our experiment, we cannot ascribe the reproductive and developmental adverse effects we found to glyphosate, the co-formulants or both acting together. Another point of consideration is the fact that the composition of commercial GBH formulations vary between brands and countries (co-formulants and glyphosate salt type and concentration are variable), and therefore is it difficult to compare their effects. For that reason, in light of our results we could not ensure that the adverse effects we detected will be prompted by other GBHs’ formulations. Overall, further research is needed to fully understand glyphosate and co-formulants contribution to GBH effects.
Conclusion
Our results demonstrated that perinatal exposure to low doses of a GBH alters the female reproductive outcome and induces second-generation adverse effects in rats. The most salient results include: (1) impaired reproductive capability characterized by an increase in the rate of pre-implantation embryo loss in F1 females, and (2) fetal growth retardation and structural congenital anomalies in their progeny (F2 generation). Our present and previous findings suggest that GBHs might act as endocrine disrupters, and highlight the importance of assessing different administration routes, doses and window/lengths of exposure to GBHs, since the effects on fertility can be different.
References
Acosta-Maldonado B, Sánchez-Ramírez B, Reza-López S, Levario-Carrillo M (2009) Effects of exposure to pesticides during pregnancy on placental maturity and weight of newborns: a cross-sectional pilot study in women from the Chihuahua State, Mexico. Hum Exp Toxicol 28:451–459
Adeeko A et al (2003) Effects of in utero tributyltin chloride exposure in the rat on pregnancy outcome. Toxicol Sci 74:407–415
Altamirano GA, Muñoz-de-Toro M, Luque EH, Gómez AL, Delconte MB, Kass L (2015) Milk lipid composition is modified by perinatal exposure to bisphenol A. Mol Cell Endocrinol 411:258–267
Anadón A et al (2009) Toxicokinetics of glyphosate and its metabolite aminomethyl phosphonic acid in rats. Toxicol Lett 190:91–95
Anway MD, Cupp A, Uzumcu M, Skinner MK (2005) Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308:1466–1469
Anway MD, Leathers C, Skinner MK (2006) Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology 147:5515–5523
Arregui MC, Lenardon A, Sanchez D, Maitre MI, Scotta R, Enrique S (2004) Monitoring glyphosate residues in transgenic glyphosate-resistant soybean. Pest Manag Sci 60:163–166
Avila-Vazquez M, Maturano E, Etchegoyen A, Difilippo F, Maclean B (2017) Association between cancer and environmental exposure to glyphosate. Int J Clin Med 8:73–85
Bai SH, Ogbourne SM (2016) Glyphosate: environmental contamination, toxicity and potential risks to human health via food contamination. Environ Sci Pollut Res 23:18988–19001
Barker DJ (1995) Fetal origins of coronary heart disease. BMJ 311:171–174
Barker DJ, Hales CN, Fall C, Osmond C, Phipps K, Clark P (1993) Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36:62–67
Barron S, Thomson A (1983) Obstetrical epidemiology. Academic Press, London
Battaglin W, Kolpin D, Scribner E, Kuivila K, Sandstrom M (2005) Glyphosate, other herbicides, and transformation products in Midwestern streams. J Am Water Resour Assoc 41:323–332
Battaglin W, Meyer M, Kuivila K, Dietze J (2014) Glyphosate and its degradation product AMPA occur frequently and widely in US soils, surface water, groundwater, and precipitation. J Am Water Resour Assoc 50:275–290
Benachour N, Séralini GE (2009) Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem Res Toxicol 22:97–105
Benbrook CM (2016) Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28:3
Bernal J, Bernal JL, Martin MT, Nozal MJ, Anadón A, Martínez-Larrañaga MR, Martínez MA (2010) Development and validation of a liquid chromatography–fluorescence–mass spectrometry method to measure glyphosate and aminomethylphosphonic acid in rat plasma. J Chromatogr B 878:3290–3296
Beuret CJ, Zirulnik F, Giménez MS (2005) Effect of the herbicide glyphosate on liver lipoperoxidation in pregnant rats and their fetuses. Reprod Toxicol 19:501–504
Bromilow RH, Chamberlain K (2000) The herbicide glyphosate and related molecules: physicochemical and structural factors determining their mobility in phloem. Pest Manag Sci 56:368–373
Bruner-Tran KL, Osteen KG (2011) Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol 31:344–350
Burdorf A, Brand T, Jaddoe V, Hofman A, Mackenbach J, Steegers E (2011) The effects of work-related maternal risk factors on time to pregnancy, preterm birth and birth weight: the Generation R study. Occup Environ Med 68:197
Burkhardt T, Schäffer L, Schneider C, Zimmermann R, Kurmanavicius J (2006) Reference values for the weight of freshly delivered term placentas and for placental weight–birth weight ratios. Eur J Obstet Gynecol Reprod Biol 128:248–252
Chang FC, Simcik MF, Capel PD (2011) Occurrence and fate of the herbicide glyphosate and its degradate aminomethylphosphonic acid in the atmosphere. Environ Toxicol Chem 30:548–555
Chevrier C, Warembourg C, Gaudreau E, Monfort C, Le Blanc A, Guldner L, Cordier S (2013) Organochlorine pesticides, polychlorinated biphenyls, seafood consumption, and time-to-pregnancy. Epidemiology 24:251–260
Chiu Y et al (2015) Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum Reprod 30:1342–1351
Cuhra M, Bøhn T, Cuhra P (2016) Glyphosate: too much of a good thing? Front Environ Sci 4:28
Dallegrave E, Mantese FD, Oliveira RT, Andrade AJ, Dalsenter PR, Langeloh A (2007) Pre-and postnatal toxicity of the commercial glyphosate formulation in Wistar rats. Arch Toxicol 81:665–673
Defarge N, Takács E, Lozano VL, Mesnage R, Spiroux de Vendômois J, Séralini GE, Székács A (2016) Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int J Environ Res Public Health 13:264. https://doi.org/10.3390/ijerph13030264
Den Hond E et al (2015) Human exposure to endocrine disrupting chemicals and fertility: a case–control study in male subfertility patients. Environ Int 84:154–160
Drake AJ, Liu L, Kerrigan D, Meehan RR, Seckl JR (2011) Multigenerational programming in the glucocorticoid programmed rat is associated with generation-specific and parent of origin effects. Epigenetics 6:1334–1343
EPA (2017) Glyphosate. Dietary exposure analysis in support of registration review. https://www.regulations.gov/document?D=EPA-HQ-OPP-2009-0361-0071 Accessed 12 Apr 2018
Fischer R, Byerlee D, Edmeades G (2014) Crop yields and global food security: will yield increase continue to feed the world? Australian Centre for International Agricultural Research, Canberra
Fowden A, Forhead A, Coan P, Burton G (2008) The placenta and intrauterine programming. J Neuroendocrinol 20:439–450
Gallegos CE, Bartos M, Bras C, Gumilar F, Antonelli MC, Minetti A (2016) Exposure to a glyphosate-based herbicide during pregnancy and lactation induces neurobehavioral alterations in rat offspring. Neurotoxicology 53:20–28
García J, Ventura MI, Requena M, Hernández AF, Parrón T, Alarcón R (2017) Association of reproductive disorders and male congenital anomalies with environmental exposure to endocrine active pesticides. Reprod Toxicol 71:95–100
Guerrero Schimpf M, Milesi MM, Ingaramo PI, Luque EH, Varayoud J (2017) Neonatal exposure to a glyphosate based herbicide alters the development of the rat uterus. Toxicology 376:2–14
Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter P (1991) Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303:1019–1022
Ibáñez L, Potau N, Francois I, de Zegher F (1998) Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab 83:3558–3562
Ingaramo PI, Varayoud J, Milesi MM, Guerrero Schimpf M, Muñoz-de-Toro M, Luque EH (2016) Effects of neonatal exposure to a glyphosate-based herbicide on female rat reproduction. Reproduction 152:403–415
Ingaramo PI, Varayoud J, Milesi MM, Guerrero Schimpf M, Alarcón R, Muñoz-de-Toro M, Luque EH (2017) Neonatal exposure to a glyphosate-based herbicide alters uterine decidualization in rats. Reprod Toxicol 73:87–95
Kass L, Altamirano GA, Bosquiazzo VL, Luque EH, Muñoz-de-Toro M (2012) Perinatal exposure to xenoestrogens impairs mammary gland differentiation and modifies milk composition in Wistar rats. Reprod Toxicol 33:390–400
Kongtip P et al (2017) Glyphosate and paraquat in maternal and fetal serums in thai women. J Agromed 22:282–289
Kramer MS (1987) Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ 65:663
Krüger M, Schledorn P, Schrödl W, Hoppe H-W, Lutz W, Shehata AA (2014a) Detection of glyphosate residues in animals and humans. J Environ Anal Toxicol 4:210. https://doi.org/10.4172/2161-0525.1000210
Krüger M, Schrödl W, Pedersen I, Shehata AA (2014b) Detection of glyphosate in malformed piglets. J Environ Anal Toxicol 4:230. https://doi.org/10.4172/2161-0525.1000230
Londero AP, Bertozzi S, Visentin S, Fruscalzo A, Driul L, Marchesoni D (2013) High placental index and poor pregnancy outcomes: a retrospective study of 18,386 pregnancies. Gynecol Endocrinol 29:666–669
MacDonald MG, Seshia MM, Mullett MD (2005) Avery’s neonatology pathophysiology and management of the newborn. Lippincott Williams and Wilkins, Philadelphia
Macdonald E, Natale R, Regnault T, Koval J, Campbell M (2014) Obstetric conditions and the placental weight ratio. Placenta 35:582–586
Macklon N (2017) Recurrent implantation failure is a pathology with a specific transcriptomic signature. Fertil Steril 108:9–14
Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK (2012a) Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One 7(9):e46249
Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK (2012b) Pesticide and insect repellent mixture (permethrin and DEET) induces epigenetic transgenerational inheritance of disease and sperm epimutations. Reprod Toxicol 34:708–719
Mañas F, Peralta L, Raviolo J, Ovando HG, Weyers A, Ugnia L, Cid MG, Larripa I, Gorla N (2009) Genotoxicity of AMPA, the environmental metabolite of glyphosate, assessed by the Comet assay and cytogenetic tests. Ecotoxicol Environ Safety 72(3):834–837
Mendez MJ, Aimar SB, Aparicio VC, Haberkon NBR, Buschiazzo DE, De Gerónimo E, Costa JL (2017) Glyphosate and aminomethylphosphonic acid (AMPA) contents in the respirable dust emitted by an agricultural soil of the central semiarid region of Argentina. Aeol Res 29:23–29
Mesnage R, Bernay B, Séralini GE (2013) Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 313:122–128
Mesnage R et al (2017) Evaluation of estrogen receptor alpha activation by glyphosate-based herbicide constituents. Food Chem Toxicol 108:30–42
Milesi MM, Alarcón R, Ramos JG, Muñoz-de-Toro M, Luque EH, Varayoud J (2015) Neonatal exposure to low doses of endosulfan induces implantation failure and disrupts uterine functional differentiation at the pre-implantation period in rats. Mol Cell Endocrinol 401:248–259
Montes G, Luque E (1988) Effects of ovarian steroids on vaginal smears in the rat. Acta Anat 133:192–199
Mostafalou S, Abdollahi M (2017) Pesticides: an update of human exposure and toxicity. Arch Toxicol 91:549–599
Myers JP et al (2016) Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. Environ Health 15:19. https://doi.org/10.1186/s12940-016-0117-0
Niemann L, Sieke C, Pfeil R, Solecki R (2015) A critical review of glyphosate findings in human urine samples and comparison with the exposure of operators and consumers. Journal für Verbraucherschutz Lebensmittelsicherheit 10:3–12
Nilsson EE, Anway MD, Stanfield J, Skinner MK (2008) Transgenerational epigenetic effects of the endocrine disruptor vinclozolin on pregnancies and female adult onset disease. Reproduction 135:713–721
Oulkar DP, Hingmire S, Goon A, Jadhav M, Ugare B, Thekkumpurath AS, Banerjee K (2017) Optimization and validation of a residue analysis method for glyphosate, glufosinate, and their metabolites in plant matrixes by liquid chromatography with tandem mass spectrometry. J Assoc Off Anal Chem Int 100:631–639
Paganelli A, Gnazzo V, Acosta H, López SL, Carrasco AE (2010) Glyphosate-based herbicides produce teratogenic effects on vertebrates by impairing retinoic acid signaling. Chem Res Toxicol 23:1586–1595
Parvez S et al (2018) Glyphosate exposure in pregnancy and shortened gestational length: a prospective Indiana birth cohort study. Environ Health 17:23. https://doi.org/10.1186/s12940-018-0367-0
Perego MC, Caloni F, Cortinovis C, Schutz LF, Albonico M, Tsuzukibashi D, Spicer LJ (2017) Influence of a roundup formulation on glyphosate effects on steroidogenesis and proliferation of bovine granulosa cells in vitro. Chemosphere 188:274–279
Perobelli JE, Alves TR, de Toledo FC, Fernandez CDB, Anselmo-Franci JA, Klinefelter GR, Kempinas WDG (2012) Impairment on sperm quality and fertility of adult rats after antiandrogen exposure during prepuberty. Reprod Toxicol 33:308–315
Peruzzo PJ, Porta AA, Ronco AE (2008) Levels of glyphosate in surface waters, sediments and soils associated with direct sowing soybean cultivation in north pampasic region of Argentina. Environ Pollut 156:61–66
Primost JE, Marino DJ, Aparicio VC, Costa JL, Carriquiriborde P (2017) Glyphosate and AMPA,“pseudo-persistent” pollutants under real-world agricultural management practices in the Mesopotamic Pampas agroecosystem, Argentina. Environ Pollut 229:771–779
Quintana MM, Vera B, Magnarelli G, Guiñazú N, Rovedatti MG (2017) Neonatal, placental, and umbilical cord blood parameters in pregnant women residing in areas with intensive pesticide application. Environ Sci Pollut Res 24:20736–20746
Rappazzo KM, Warren JL, Meyer RE, Herring AH, Sanders AP, Brownstein NC, Luben TJ (2016) Maternal residential exposure to agricultural pesticides and birth defects in a 2003 to 2005 North Carolina birth cohort. Birth Defects Res Part A Clin Mol Teratol 106:240–249
Razi S, Rezaeian M, Dehkordi FG, Manshoori A, Goujani R, Vazirinejad R (2016) Exposure to pistachio pesticides and stillbirth: a case–control study. Epidemiol Health 38:e2016016. https://doi.org/10.4178/epih.e2016016
Richard S, Moslemi S, Sipahutar H, Benachour N, Seralini GE (2005) Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ Health Perspect 113:716–720
Romano MA et al (2012) Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Arch Toxicol 86:663–673
Ronco A, Marino D, Abelando M, Almada P, Apartin C (2016) Water quality of the main tributaries of the Paraná Basin: glyphosate and AMPA in surface water and bottom sediments. Environ Monit Assess 188:1–13
Roustan A, Aye M, De Meo M, Di Giorgio C (2014) Genotoxicity of mixtures of glyphosate and atrazine and their environmental transformation products before and after photoactivation. Chemosphere 108:93–100
Roy NM, Carneiro B, Ochs J (2016) Glyphosate induces neurotoxicity in zebrafish. Environ Toxicol Pharmacol 42:45–54
Saenger P, Czernichow P, Hughes I, Reiter EO (2007) Small for gestational age: short stature and beyond. Endocr Rev 28:219–251
SANTE (2015) Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed. European commission, SANTE/11945/2015. http://www.eurl-pesticides.eu Accessed 22 Dec 2017
Sasal MC et al (2015) Glyphosate loss by runoff and its relationship with phosphorus fertilization. J Agric Food Chem 63:4444–4448
Sathyanarayana S, Basso O, Karr CJ, Lozano P, Alavanja M, Sandler DP, Hoppin JA (2010) Maternal pesticide use and birth weight in the agricultural health study. J Agromed 15:127–136
Skinner MK, Manikkam M, Guerrero-Bosagna C (2011) Epigenetic transgenerational actions of endocrine disruptors. Reprod Toxicol 31:337–343
Skinner MK, Haque CG-BM., Nilsson E, Bhandari R, McCarrey JR (2013) Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS One 8(7):e66318
Skogen JC, Øverland S (2012) The fetal origins of adult disease: a narrative review of the epidemiological literature. J R Soc Med Short Rep 3:1–7
Test Biotech (2013) High levels of residues from spraying with glyphosate found in soybeans in Argentina. http://www.testbiotech.org/en/node/926 Accessed 12 Apr 2018
Thongprakaisang S, Thiantanawat A, Rangkadilok N, Suriyo T, Satayavivad J (2013) Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem Toxicol 59:129–136
Vandenberg LN et al (2017) Is it time to reassess current safety standards for glyphosate-based herbicides? J Epidemiol Community Health 71:613–618
Varayoud J, Ramos JG, Muñoz-de-Toro M, Luque EH (2014) Long-lasting effects of neonatal bisphenol A exposure on the implantation process. Vitam Horm 94:253–275
Varayoud J, Durando M, Ramos JG, Milesi MM, Ingaramo PI, Muñoz-de-Toro M, Luque EH (2017) Effects of a glyphosate-based herbicide on the uterus of adult ovariectomized rats. Environ Toxicol 32:1191–1201
Verkauskiene R, Petraitiene I, Albertsson WK (2013) Puberty in children born small for gestational age. Horm Res Paediatr 80:69–77
Wellstead JR, Bruce NW, Rahima A (1989) Effects of indomethacin on spacing of conceptuses within the uterine horn and on fetal and placental growth in the rat. Anat Rec 225:101–105
WHO (2017) Sexual and reproductive health: infertility is a global public health issue. http://www.who.int/reproductivehealth/topics/infertility/perspective/en/ Accessed 22 Dec 2017
Whyatt RM et al (2004) Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect 112:1125–1132
Williams GM, Kroes R, Munro IC (2000) Safety evaluation and risk assessment of the herbicide roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol 31:117–165
Ziv-Gal A, Flaws JA (2016) Evidence for bisphenol A-induced female infertility: a review (2007–2016). Fertil Steril 106:827–856
Acknowledgements
We thank Juan Grant and Juan C. Villarreal for technical assistance and animal care. We are grateful to Department of Mathematics and Laboratorio de Investigación y Servicios en Bioestadística (LISEB), in particular to Professor Stella Vaira, from Facultad de Bioquímica y Ciencias Biológicas (UNL) for the help with the statistical analyses. This work was supported by grants from the UNL (CAI+D 2016 PIC 50420150100085LI), the Argentine National Agency of Scientific and Technological Promotion (ANPCyT; PICT 2014 N° 2125, PICT 2014 N° 1522, PICT 2014 N° 1628) and CONICET. MMM, JV, and EHL are Career Investigators of the CONICET. VL is a fellow of Bunge and Born Foundation (Argentina); GP is an undergraduate student of Facultad de Bioquímica y Ciencias Biológicas (UNL); MRR is Professor of Facultad de Ingeniería Química (UNL); LDD is a fellow of the CONICET.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The manuscript does not contain clinical studies or patient data.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of the animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Milesi, M.M., Lorenz, V., Pacini, G. et al. Perinatal exposure to a glyphosate-based herbicide impairs female reproductive outcomes and induces second-generation adverse effects in Wistar rats. Arch Toxicol 92, 2629–2643 (2018). https://doi.org/10.1007/s00204-018-2236-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-018-2236-6