Abstract
Glyphosate has been the most widely used herbicide during the past three decades. The US Environmental Protection Agency (EPA) classifies glyphosate as ‘practically non-toxic and not an irritant’ under the acute toxicity classification system. This classification is based primarily on toxicity data and due to its unique mode of action via a biochemical pathway that only exists in a small number of organisms that utilise the shikimic acid pathway to produce amino acids, most of which are green plants. This classification is supported by the majority of scientific literature on the toxic effects of glyphosate. However, in 2005, the Food and Agriculture Organisation (FAO) reported that glyphosate and its major metabolite, aminomethylphosphonic acid (AMPA), are of potential toxicological concern, mainly as a result of accumulation of residues in the food chain. The FAO further states that the dietary risk of glyphosate and AMPA is unlikely if the maximum daily intake of 1 mg kg−1 body weight (bw) is not exceeded. Research has now established that glyphosate can persist in the environment, and therefore, assessments of the health risks associated with glyphosate are more complicated than suggested by acute toxicity data that relate primarily to accidental high-rate exposure. We have used recent literature to assess the possible risks associated with the presence of glyphosate residues in food and the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glyphosate [N-(phosphonomethyl) glycine] is the most widely used herbicide in the world (Duke and Powles 2008) with an estimated global demand of half a million tonnes per annum (Székács and Darvas 2012) and $5.5 billion in sales in 2011 (Krebs 2011). Dr. Henri Martin synthesised glyphosate in 1950 (Parrot et al. 1995); however, it was not commercialised as a herbicide until 1974 (Duke and Powles 2008). The popularity of glyphosate revolves around its efficiency in killing weeds at low cost but is also due to its perceived low toxicity, rapid absorption by plants, and slow evolution of glyphosate resistance in weeds (Duke and Powles 2008).
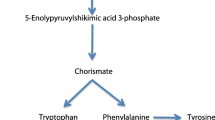
Glyphosate is categorised as a non-selective, systemic, post-emergence herbicide (Duke and Powles 2008), which acts as an inhibitor of the enzyme, 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), in the shikimate pathway (Duke and Powles 2008). The shikimate pathway produces aromatic amino acids used for synthesis of proteins and plays an important role in the production of secondary metabolites such as lignin (Haslam 2014). It is currently understood that inhibition of EPSPS deregulates the shikimate pathway, resulting in uncontrolled carbon flow, mostly going into shikimate (Duke et al. 2003a). This depletes pools of compounds needed for carbon fixation, causing a general disruption of the organisms metabolism (Siehl 1997; Duke et al. 2003a; Duke and Powles 2008).
Glyphosate is categorised by the EPA as a ‘least toxic’ (category IV) substrate for animals (Williams et al. 2000). This is based primarily on toxicity data and also due to the unique mode of action of glyphosate being confined to a small range of organisms, primarily green plants. It is also due to the perception that glyphosate is rapidly mineralised from the environment (Mamy et al. 2005). In reality, the half-lives of glyphosate and its major metabolite, AMPA can be lengthy, ranging between 0.8–151 and 10–98 days, respectively (Table 1). Most likely, the relatively large range in persistence of glyphosate and AMPA resulted from varying soil properties and environmental conditions. For example, glyphosate and AMPA showed half-lives of up to 151 and 98 days, respectively, in one study based on clay soil in Sweden (Bergström et al. 2011) and 10 and 10 days, respectively, in a different study based on loamy soil in China (Zhang et al. 2015). Prolonged half-life and slow degradation may increase the risk of long-term environmental contamination (Al-Rajab and Schiavon 2010), particularly with frequent and repeated applications that are typical in agricultural settings. Therefore, this review summarises eco-toxicological effects of glyphosate on non-targeted species and possible human health risks posed by glyphosate contamination of food.
Fate pathways of glyphosate
Once applied, glyphosate may undergo mineralisation, immobilisation or leaching, but it does not undergo volatilisation to a significant degree because it has an extremely low vapour pressure (Mamy et al. 2005; Al-Rajab and Schiavon 2010). Glyphosate mineralisation is considered the primary degradation mechanism, resulting in the production of AMPA, methylphosphonic acid, glycine and sarcosine (Fig. 1) (Mamy et al. 2005; Kwiatkowska et al. 2014). AMPA is the main metabolite of glyphosate and is further mineralised to methylamine and phosphate, with decomposition finally producing CO2 and NH3 (Borggaard and Gimsing 2008; Al-Rajab and Schiavon 2010). Bergström et al. (2011) propose that the degradation pathway to produce sarcosine might also be important.
Mineralisation
Glyphosate and AMPA mineralisation are affected by soil biochemical properties and can occur within a very short period of time in certain situations (Mamy et al. 2005). Soil properties that accelerate glyphosate mineralisation include high soil pH and phosphate content or low soil Cu and Fe content, primarily driven by increased microbial mineralisation (Morillo et al. 2000; Mamy et al. 2005; Ghafoor et al. 2011; Zhang et al. 2015). Microbial activities are highly dependent on soil labile organic carbon (C) availability (Bai et al. 2012a; Bai et al. 2014). However, increased labile organic C may not always stimulate microbial activity and mineralisation because glyphosate sorption is often increased by organic matter in soil (Mamy et al. 2005). Increased glyphosate adsorption to organic C is likely to be beneficial to the environment because it delays leaching and allows gradual release and degradation in soil. However, repeated glyphosate application may eventually lead to saturation of the organic C system. Ultimately, the combination of soil biochemical properties, microbial diversity and microbial activity drives glyphosate degradation through mineralisation.
Immobilisation and leaching
Glyphosate exhibits a high adsorption coefficient and is quickly immobilised following application in most natural situations (Bergström et al. 2011; Syan et al. 2014). Soil organic matter, clay and minerals are influential factors in glyphosate immobilisation. For example, adsorption to soil, of up to 20 % of the initial glyphosate quantity, can occur within 3 h of application, although no further immobilisation was observed in the following 3 days (Shushkova et al. 2009). High adsorption depends on low pH and phosphate concentration, high organic matter and clay content, and high Al and Fe concentrations in the soil (Gimsing et al. 2004; Laitinen et al. 2009; Shushkova et al. 2009; Syan et al. 2014). Conversely, soils with low organic matter, high phosphate, low Al and Fe and high pH are prone to glyphosate and AMPA losses, mainly due to decreased adsorption capacity and increased leaching (Laitinen et al. 2009; Shushkova et al. 2009).
Glyphosate leaching and resultant water contamination are a growing concern as both glyphosate and AMPA residues have been frequently reported in water sources (Fig. S1). For example, glyphosate and AMPA were detected in 40 and 55 %, respectively, of 3700 soil, water and sediment samples collected from 38 sites in the USA between 2001 and 2010 (Battaglin et al. 2014). All sample concentrations were under 700 μg L−1, which is below accepted maximum contamination levels (MCLs) (Battaglin et al. 2014) (Fig. S1). AMPA is also a degradation product of the artificial sweetener, acesulfame, and it is conceivable that this sweetener is a source of water AMPA. However, glyphosate and AMPA concentrations in water samples were strongly correlated, suggesting that AMPA was more likely to have originated from glyphosate rather than acesulfame (Van Stempvoort et al. 2014).
Ecological risks of glyphosate and AMPA residues in the environment
Both glyphosate and AMPA can be relatively persistent in the environment, which may result in a wide range of ecological risks. However, it is not easy to predict the significance and extent of those risks when there is a lack of toxicity, health and safety data on repeated and long-term exposures to glyphosate and AMPA. Thus, we have reviewed the potential presence of glyphosate and AMPA residues in soil, water and non-target crops, such as those that may be consumed by humans, and we discuss the potential risks for health in that context.
Glyphosate in soil
Despite glyphosate and AMPA being regarded as non-toxic to soil micro-organisms (Busse et al. 2001; Araújo et al. 2003), studies that have investigated microbial composition and soil microbial diversity do not always support this perspective. For example, earthworms are a critical bioindicator of soil health, and earthworm biomass was reduced following glyphosate application to soils (Johnson-Maynard and Lugo-Perez 2006; García-Pérez et al. 2014). In a coffee plantation subjected to repeated glyphosate application for 22 years, soil earthworm biomass was significantly lower than those plantations with no application in the past 7 years (García-Pérez et al. 2014). However, other studies report no direct effects of glyphosate on earthworms (Pereira et al. 2009; Zhou et al. 2012; Fusilero et al. 2013). For example, one study showed that earthworms may survive after glyphosate application but glyphosate may affect cocoon hatching leading to decreased earthworm populations in soil (Correia and Moreira 2010; Pelosi et al. 2014). In another study, sub-lethal glyphosate application in a glasshouse did not affect the survival of earthworms but resulted in changed soil chemistry, which may have other implications for water quality and other soil dwellers (Santadino et al. 2014). Interestingly, it has been reported that although AMPA may not increase earthworm mortality, juveniles produced in contaminated soil may have reduced body mass affecting their function in the system (Domínguez et al. 2016).
The majority of studies that assess earthworm responses to glyphosate have been undertaken under laboratory conditions and responses may not necessarily be observed under field conditions. In general, the eco-toxicity of herbicides is difficult to assess under field conditions, as results will be confounded by numerous factors including levels of organic matter, nutrient application, soil type, soil cover and weather conditions (Yu and Zhou 2005; Fusilero et al. 2013). Despite this, field studies must be undertaken such that the long-term effects of glyphosate on soil ecology can be thoroughly assessed. Furthermore, there is a need to create assessment criteria that allow laboratory data to be directly comparable to field data (Casabé et al. 2007; Pelosi et al. 2014).
There are also conflicting reports on the impact of soil microbial diversity and biomass following glyphosate application (Kremer and Means 2009; Bai et al. 2012b; Zabaloy et al. 2012; Druille et al. 2013; Arango et al. 2014). It seems clear that the effects of glyphosate on soil microbial biomass are dose-dependent, but recent evidence suggests that the effects may also be transitional (Nguyen et al. 2016). However, it should be noted that microbial biomass and activity are not indicative of microbial composition and it remains unclear as to the extent that soil microbial communities will respond to different management practises including repeated glyphosate applications (Nguyen et al. 2016).
Some studies report that the observed shifts in soil community compositions due to glyphosate application altered soil nutrient availability and nutrient balance (Kremer and Means 2009), which may influence plant performance (Wolmarans and Swart 2014) and, ultimately, ecosystem productivity. However, many conflicting reports exist as to whether using glyphosate or glyphosate resistant species can result in nutrient imbalance (reviewed in Wolmarans and Swart 2014). Glyphosate binds strongly to nutrients in the soil, which could significantly reduce nutrient availability in soil (Duke et al. 2012). However, the concentrations of glyphosate added to soil even at the highest recommended doses are up to 500 times lower than concentrations of different soil nutrients. Therefore, even if all of the applied glyphosate is bound by the soil nutrients, the decrease in soil nutrient concentrations will be negligible and any impact on nutrient imbalance is unlikely to affect crop yield (Duke et al. 2012).
Glyphosate in water
Detection of both glyphosate and AMPA residues in water sources is becoming frequent. Although runoff is one source of water contamination, some formulations are approved for the control of aquatic weeds (Annett et al. 2014) and therefore, direct application can also result in contamination. In some cases, the reported concentrations are cause for concern. For example, glyphosate concentrations of over 400 μg L−1 are potentially toxic to some aquatic species including amphibians and fish (King and Wagner 2010; Annette et al. 2014; Braz-Mota et al. 2015). The presence of glyphosate in marine ecosystems has also been reported and its persistence in seawater is now an area of active research (Mercurio et al. 2015). However, several studies suggest that the levels of glyphosate residues in water are not capable of causing toxicity in aquatic species (Levine et al. 2015; Struger et al. 2008; Solomon and Thompson 2003). Since the toxicity of glyphosate is both dose and species dependent (Annett et al. 2014), there appears to be a need for additional research to assess the potential impacts of glyphosate in aquatic systems.
In terms of the risks posed to humans, the majority of the reported residue concentrations are below MCLs, and therefore, the acute toxicity risks posed to humans are minimal. The MCL for glyphosate before posing a risk to human health is considered to be 700 μg L−1 in the USA (EPA, 2015) and 1000 μg L−1 in Australia (Australian Drinking Water Guidelines 6 2011). In Europe, the acceptable concentration in drinking water is less than 0.1 μg L−1 and the tolerable risk is reported to be 77 μg L−1 (Horth and Blackmore 2009). Water treatment to reduce glyphosate concentrations is costly but, according to European guidelines, is necessary to reduce the risk of glyphosate residues in human drinking water. These treatments do not impact on glyphosate levels in the source water and, therefore, the long-term effects of glyphosate on aquatic species remain a potential concern.
Glyphosate is not approved to be used in water to control weeds, and the majority of glyphosate exposures would be caused by runoff or accidental glyphosate spills. Hence, applying proper management practises may minimise eco-toxicity risks of glyphosate for aquatic species including reduced application frequencies and using vegetation buffers (Saunders and Pezeshki 2015).
Glyphosate in non-target plant species
Both glyphosate and AMPA residues are found in non-target plant species (e.g. crops) following glyphosate application to weeds, even after the recommended withholding period in harvested crops (Table S1). Furthermore, both glyphosate and AMPA have also been detected in crop plants and native forest foliage following application of glyphosate to adjacent crops. The concentration of glyphosate and AMPA residues in different crop species and samples varies significantly (Table 2). For example, glyphosate and AMPA residues were observed in 25 and 8.3 % of analysed cannabis samples, respectively (Lanaro et al. 2015). Similarly, concentrations ranged from 1000 to 0.3 mg kg−1 in tree foliage sampled within 3 days of glyphosate application (Table 2). Such unusually high glyphosate residues (e.g. 1000 mg kg−1) in tree foliage can be explained by direct absorption into tree leaves following drift contamination from aerial herbicide application (Newton et al. 1994).
In addition to the health risks potentially caused by food contamination, glyphosate contamination can have phytotoxic effects. Phytotoxicity can influence plant performance through reduced absorption of essential nutrients (Mateos-Naranjo and Perez-Martin 2013), nutrient imbalance, yield reduction and compromised food quality (Bott et al. 2008; Zobiole et al. 2010). Plant biomass has been reduced up to 50 % in some non-target plant species following glyphosate contamination (Alister et al. 2005; Mateos-Naranjo and Perez-Martin 2013). Therefore, the potential negative impacts of glyphosate contamination on non-target plant performance and productivity, especially reduced crop yield and quality, should not be dismissed (Alister et al. 2005; Reddy et al. 2008; Zobiole et al. 2010). However, other studies show no negative effects of glyphosate on plant yield (Bohm et al. 2014; Duke 2015) and given the complicated influences of soil type, soil nutrient availability and environmental conditions, yield reduction and nutrient deficiency may not be directly caused by glyphosate (Duke et al. 2012).
Toxicological effects of glyphosate and AMPA
Glyphosate and AMPA are considered low risk to mammals, primarily because of low skin and gastrointestinal absorption (Williams et al. 2000; Greim et al. 2015). Both glyphosate and AMPA are excreted in urine, with half-lives between 3 and 15 h without any changes in their structure (Anadon et al. 2009). For these reasons, combined with a battery of acute toxicity data, glyphosate and AMPA are classified in the least toxic category (category IV; practically non-toxic and not an irritant) by the EPA (Williams et al. 2000). However, given recent data regarding glyphosate contamination in the environment, acute toxicity may not be as important as chronic, sub-chronic and reproductive toxicity, which occur at lower concentrations. Drawing upon several case studies, it has been concluded that there is no robust evidence of cytotoxicity, genotoxicity, DNA damage, carcinogenicity or reproductive toxicity from glyphosate and AMPA (Williams et al. 2000; Greim et al. 2015; EFSA 2015). However, much of the data referenced by those authors is relatively old and/or from unpublished data, and in this review, we summarise additional literature, which builds upon previous conclusions.
Acute poisoning
Worst case exposure causing acute poisoning in adult humans has been reported to be 125 and 5 μg kg−1 day−1 for glyphosate and AMPA, respectively (Williams et al. 2000). Fatalities caused by exposures of that order have occurred in 3.2 % of cases, with a median time to death of 20 h, mainly due to cardiorespiratory toxicity (Roberts et al. 2010). Rat oral and dermal LD50 are reported to be much higher at >5000 mg kg−1 bw, although there is also a lower LD50 value reported (>2000 mg kg−1 bw) (Greim et al. 2015). Importantly, the reported values may differ as a result of using different formulations of glyphosate. Most commercial formulations of glyphosate contain surfactants to facilitate penetration of the active ingredient and increase efficacy. As a result, recent research tends to focus on the toxicity of the formulation rather than the active ingredient (Currie et al. 2015). For example, neat glyphosate had the least toxicity (~2 g L−1) in vitro, whereas Roundup® 400 and 450 had the highest toxicity (~0.001 g L−1) (Gasnier et al. 2009). In another study, however, it was found that glyphosate itself, rather than the surfactants in the formulation, affected mechanisms of morphogenesis in vertebrate embryos (Paganelli et al. 2010). It is therefore important that both commercial formulations and neat glyphosate are used to estimate acute toxicity parameters.
Chronic and sub-chronic toxicity
The no-observed-adverse-effect-level (NOAEL) for chronic toxicity is recommended to be 560 mg kg−1 bw day−1 (Greim et al. 2015) (Table 3). Williams et al. (2000) concluded that rodents can even tolerate a daily glyphosate uptake of 20,000 mg kg−1 bw day−1. However, as summarised by Cox (1995), daily consumption of glyphosate between 60 and 2500 mg kg−1 for 90 days in rats and mice resulted in liver damage, increased bile acids, chronic kidney inflammation, decreased body weight and increased potassium and phosphorous in the blood. In recent studies, there are reports of even lower rates causing irreversible damage to mammals (Table 4). For example, rats that were exposed to glyphosate at rates between 5 and 490 mg kg−1 every 48 h for 75 days had irreversible damage to hepatocytes (Benedetti et al. 2004). In a separate study, mild liver damage was reported in rats following sub-chronic exposure of glyphosate (56 and 560 mg kg−1) for between 35 and 90 days (Çağlar and Kolankaya 2008). Some studies show that even one exposure of glyphosate at very low concentrations is sufficient to change cell functions and cause cytotoxicity (Table 4). For example, sub-agricultural doses of both glyphosate and Roundup®400 caused disruption of the human endocrine system at 0.5 ppm, inhibition of transcriptional activities of oestrogen receptors at 2 ppm, and cytotoxicity at 10 ppm in vitro (Gasnier et al. 2009).
Genotoxicity
Genotoxicity caused by glyphosate has regularly been questioned and often rejected (Williams et al. 2000; Greim et al. 2015). The main reason for this conclusion was that the majority of previous studies reporting DNA damage used unreasonably high doses of glyphosate. However, other studies that used sub-agricultural doses of both glyphosate and Roundup® have observed DNA damage to human cells (Gasnier et al. 2009; Prasad et al. 2009; Koller et al. 2012). DNA damage was reported when buccal epithelial cells were exposed to glyphosate and Roundup® at concentrations between 10 and 20 mg L−1 or between 225- and 1350-fold lower than recommended agricultural rates (Koller et al. 2012) and, in a separate study, following application at 5 ppm to human liver HepG2 cells (Gasnier et al. 2009). Exposure of caiman embryos to Roundup® at different sub-lethal rates also resulted in DNA damage (Poletta et al. 2009). Even considering this data, Kier and Kirkland (2013) concluded that glyphosate was not genotoxic, suggesting that the observed DNA damage was due to cytotoxicity rather than genotoxicity. Irrespective of the cause being direct or indirect, recent evidence indicates that DNA damage may occur at relatively low concentrations of glyphosate.
Reproductive toxicity
The potential for glyphosate to cause reproductive toxicity has been reported to be ‘very slim’ (Williams et al. 2000), with a NOAEL between 300 mg kg−1 bw day−1 (Greim et al 2015) and 50 mg kg−1 bw day−1 (Lu 1995). Other studies suggest that glyphosate exposure even at NOAEL concentrations may cause adverse effects on the reproductive function of offspring (Dallegrave et al. 2007; Romano et al. 2012). Rats exposed to Roundup® at rates between 50 mg kg−1 (recommended NOAEL by Lu (1995)) and 450 mg kg−1 for 21 days during pregnancy showed no adverse effects but, interestingly, male offspring had damage to their reproductive organs (Dallegrave et al. 2007). Similarly, treatment of female Wistar rats (50 mg kg−1) caused reproductive toxicity in male offspring (Romano et al. 2012 with changes observed to male offspring behaviour and reproductive parameters; the result of hyper-secretion of androgens and increased gonadal activity (Romano et al. 2012). Given these findings, it seems that additional research is needed to improve our understanding of the effects of glyphosate and Roundup® on mammalian reproduction (Dallegrave et al. 2007).
Carcinogenicity
Whether glyphosate is carcinogenic or not, it is heavily debated in the literature. Some authors argue that given glyphosate genotoxicity has been rejected, carcinogenicity caused by mutations is not possible (Williams et al. 2000; Kier and Kirkland 2013). Furthermore, there are studies that indicate glyphosate is not carcinogenic when exposure is within acceptable NOAEL (Table 3). For example, carcinogenicity was not observed when rats drank water containing glyphosate at rates within NOAEL for 2 years (Chruscielska et al. 2000). Although the reliability of this study has been questioned, in general, data rejecting glyphosate induced carcinogenicity has been evaluated as reliable and Greim et al. (2015) concluded that there was no statistically significant relationship between carcinogenicity and glyphosate exposure. However, those authors acknowledged that further research was needed before the carcinogenic potential of glyphosate can be completely excluded. In contrast, there is a body of research that reports potential carcinogenic cases in mouse skin, breast, kidney, intestine, liver and thyroid tissues (Cox 1995; George et al. 2010). Furthermore, the possibility of glyphosate causing tumour promotion in skin cells and proliferation in breast cells has been reported in vivo mouse and in vitro human models, respectively (George et al. 2010; Thongprakaisang et al. 2013). In one of these studies, hormone induced breast cancer was stimulated at glyphosate concentrations as low as 10−12 M (Thongprakaisang et al. 2013), 600-fold lower than the acceptable European glyphosate residue concentration in drinking water (Fig. S1). More recently, the International Agency for Research on Cancer (IARC) classified glyphosate as ‘probably carcinogenic to humans’ (group 2A) (Guyton et al. 2015). To arrive at this conclusion, the IARC Working Group considered previous findings from the US EPA, recent published scientific literature and publically available government reports. In contrast, the European Food Safety Authority (EFSA) (2015) reported that glyphosate is unlikely to be carcinogenic. EFSA believes that IARC has not considered all of the relevant literature and is open to further clarify their assessment to address all concerns raised by other parties (http://www.efsa.europa.eu/en/press/news/160113).
Possible risks of glyphosate to human health via food contamination
Glyphosate and AMPA residues in food consumed by humans are of potential toxicological concern if the residues are above acceptable daily intake levels (FAO 2005). Traces of glyphosate and AMPA have been observed in both plant and animal material suggesting that residues do exist in different food sources (Reddy et al. 2004; Druart et al. 2011; Bernal et al. 2012) (Table 2). Maximum residue limits (MRL) of glyphosate have been reviewed by the European Food Safety Authority in 2015 and generally range from 0.025 to 2 mg kg−1 in different food sources (EFSA 2015). However, a MRL up to 30 mg kg−1 was proposed for some cereals including rye, wheat, oat and barley (EFSA 2015). Surprisingly, no MRL has been established for fish tissue consumed by humans (McQueen et al. 2012), most likely due to the fact that glyphosate is not applied directly to water, it is not lipophilic (Glyphosate–Renewal Assessment Report 2013) and also there are no legal testing requirements for bioconcentration of glyphosate in fish. However, one study was reported for different aquatic species achieving a maximum bioconcentration factor (BCF) of 10 (Glyphosate–Renewal Assessment Report 2013), which is below the BCF trigger value of 1000 provided in Annex VI of the Stockholm Convention on Persistent Organic Pollutants (http://chm.pops.int/Default.aspx?tabid=2806). Based on this data, bioaccumulation of glyphosate was assessed as unlikely (Glyphosate–Renewal Assessment Report 2013). Unfortunately, the details of this study were not provided, including the species studied and therefore, it is difficult to interpret the applicability of this assessment. Considering these MRLs, the theoretical maximum daily intake (TMDI) for glyphosate of 23.8 μg kg−1 day−1 for adults as suggested by Williams et al. (2000) and the glyphosate residues reported in various studies (Table 2), the likelihood of a human experiencing toxic side effects following long-term consumption of contaminated food ranges from possible to improbable. For example, food consumed by approximately 40 expecting mothers was assessed for glyphosate residues and despite the fact that glyphosate residues were detected in 75 % of the food items studied, the total concentration was less than 0.4 % of acceptable daily intake (McQueen et al. 2012). Williams et al. (2000) argued that, in reality, less than 50 % of harvested crop samples will contain high residue levels, and these levels would be further decreased by food processing. In support of this, food composite residue assessments have shown lower glyphosate residues than expected accepted daily intakes (Gimou et al. 2008; McQueen et al. 2012). Therefore, glyphosate residues in food would decrease below the predicted TMDI, thus posing no risk for humans (Williams et al. 2000). However, more recent studies demonstrate that food or feed produced from genetically modified glyphosate resistant (GR) crops contain significantly higher residue concentrations compared with non-GR crops, likely because of different application practises between crops (Bøhn et al. 2014; Swanson et al. 2014). These changes in application practises also increase the chance of drift and contamination of other crops and therefore, become a substantial source of concern as GR-crops become more widely used.
It is important to note that MRLs are typically determined based on the sensitivity of relevant analytical methods rather than on toxicology or eco-toxicity data. Glyphosate falls into this category with Limit of Quantitation (LoQ) values between 0.01 and 0.05 mg kg−1 for validated analytical methods, which vary in different tissues (Glyphosate–Renewal Assessment Report 2013). Thus, available MRLs may not necessarily suggest a safe level of a pesticide residue in or on food or feed.
Harvested crops and/or food composites may contain glyphosate and AMPA residues at levels that are unlikely to result in exposure to the currently accepted TMDI through direct consumption. However, glyphosate and AMPA residues are clearly present in food that can be consumed by humans or livestock (Table 2), and chronic exposure to glyphosate or AMPA through consumption of contaminated products may be a potential risk to human health. Recent research even suggests that there has been an increase in glyphosate in human urine samples (an indication of dietary exposure). This could be explained by the improved sensitivity of analytical techniques, but is also potentially a result of increased glyphosate usage (Niemann et al. 2015). Those authors suggested that the reported concentrations are not sufficient to be of concern to human health (Niemann et al. 2015). However, it is important to note that the literature on the risks of low concentration chronic exposure to glyphosate is minimal; Mesnage et al. (2015) suggest that low glyphosate concentrations may result in risks to human health and call for further studies to be undertaken before conclusions regarding the safety of glyphosate are made. It would therefore seem prudent to modify glyphosate application practises such that residues in the food are minimised, and as a matter of priority to undertake additional testing to better understand the risks.
Conclusion
Glyphosate is the most widely used herbicide in the world and its demand continues to grow. Although, the majority of glyphosate is mineralised following application, the half-lives of glyphosate and its metabolites are long under certain conditions, and glyphosate and AMPA residues can persist in soil, water and plants in some circumstances. In fact, recent research suggests that contamination of soil, water and some food occurs at concentrations that may pose ecological risks. However, the majority of literature concludes that the levels of contamination do not pose a risk to most organisms and are unlikely to cause an environmental risk, if recommended application rates are followed and repeat applications are avoided.
In 2015, the EFSA reported that glyphosate and its major metabolite, AMPA, may be present in food consumed by humans. Whilst it is unlikely that human exposure will reach TMDI levels through consumption of contaminated crops or other food, this review showed not only that it is possible, but also that chronic glyphosate exposure at low concentrations can potentially result in risks to human health. However, this review also revealed a striking dearth of glyphosate and AMPA food residues analysis in the peer-reviewed literature, including a complete absence of data for any species of fish. More recently, and despite conflicting reports in the literature, the carcinogenic classification of glyphosate was changed to ‘probably carcinogenic to humans’ by the IARC, a classification based on limited evidence of carcinogenicity on human studies but sufficient evidence from animal research (Guyton et al. 2015). However, this classification was rejected by European Food Safety authorise (EFSA 2015). Taken together, completion of additional studies that analyse glyphosate and AMPA residues in food and that explore the potential of chronic glyphosate toxicity seems prudent.
Glyphosate is a valuable and important weed management tool for agricultural professionals and hobby gardeners alike. However, in the light of recent research, there is a need to identify the most sensitive environmental and toxicological scenarios to inform future best practice management for glyphosate use such that it can remain effective, whilst ensuring minimal environmental contamination and no impact on human health.
References
Accinelli C, Screpanti C, Vicari A, Catizone P (2004) Influence of insecticidal toxins from Bacillus thuringiensis subsp. kurstaki on the degradation of glyphosate and glufosinate-ammonium in soil samples. Agr Ecosys Environ 103:497–507
Al-Rajab AJ, Schiavon M (2010) Degradation of 14C-glyphosate and aminomethylphosphonic acid (AMPA) in three agricultural soils. J Environ Sci 22:1374–1380
Alister C, Kogan M, Pino I (2005) Differential phytotoxicity of glyphosate in maize seedlings following applications to roots or shoot. Weed Res 45:27–32
Anadon A, Martinez-Larranaga M, Martínez M, Castellano V, Martínez M, Martin M, Nozal M, Bernal J (2009) Toxicokinetics of glyphosate and its metabolite aminomethyl phosphonic acid in rats. Toxicol letters 190:91–95
Annett R, Habibi HR, Hontela A (2014) Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J Appl Toxicol 34:458–479
Araújo AS, Monteiro RT, Abarkeli RB (2003) Effect of glyphosate on the microbial activity of two Brazilian soils. Chemosphere 52:799–804
Arango L, Buddrus-Schiemann K, Opelt K, Lueders T, Haesler F, Schmid M, Ernst D, Hartmann A (2014) Effects of glyphosate on the bacterial community associated with roots of transgenic Roundup Ready® soybean. Eur J Soil Biol 63:41–48
Arregui MC, Lenardón A, Sanchez D, Maitre MI, Scotta R, Enrique S (2004) Monitoring glyphosate residues in transgenic glyphosate-resistant soybean. Pest Manag Sci 60:163–166
Sciences AL (1997) HR-001: 24-month oral chronic toxicity and oncogenicity study in rats. The Institute of Environmental Toxicology, Tokyo
Australian Drinking Water Guidelines 6, 2011. http://www.clearwater.asn.au/user-data/.../Aust_drinking_water_guidelines, Downloded 23-Feb-15.
Bai SH, Blumfield T, Xu Z, Chen C, Wild C (2012a) Soil organic matter dynamics and nitrogen availability in response to site preparation and management during revegetation in tropical Central Queensland, Australia. J Soils Sediments 12:386–395
Bai SH, Blumfield T, Xu Z, Chen C, Wild C (2012b) Effects of pre-planting site management on soil organic matter and microbial community functional diversity in subtropical Australia. Appl Soil Ecol 62:31–36
Bai SH, Blumfield T, Xu Z, Chen C, Wild C (2014) Soil carbon and nitrogen dynamics in the first year following herbicide and scalping in a revegetation trial in south-east Queensland, Australia. Environ Sci Pollut Res 21:5167–5176
Battaglin W, Meyer M, Kuivila K, Dietze J (2014) Glyphosate and its degradation product AMPA occur frequently and widely in US soils, surface water, groundwater, and precipitation. J Am Water Resour Assoc 50:275–290
Benedetti ASL, Vituri CDL, Trentin AG, Domingues MAC, Alvarez-Silva M (2004) The effects of sub-chronic exposure of Wistar rats to the herbicide Glyphosate-Biocarb®. Toxicol Letters 153:227–232
Bergström L, Börjesson E, Stenström J (2011) Laboratory and lysimeter studies of glyphosate and aminomethylphosphonic acid in a sand and a clay soil. J Environ Qual 40:98–108
Bernal J, Martin MT, Soto ME, Nozal MJ, Marotti I, Dinelli G, Bernal JL (2012) Development and application of a LC-MS method to evaluate the glyphosate and aminomethylphosphonic acid dissipation in maize plants after foliar treatment. J Agr Food Chem 60:4017–4025
Borggaard OK, Gimsing AL (2008) Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag Sci 64:441–456
Bohm B, Mariza G, Rombaldi CV, Genovese MI, Castilhos D, Rodrigues Alves BJ, Rumjanek NG (2014) Glyphosate effects on yield, nitrogen fixation, and seed quality in glyphosate-resistant soybean. Crop Sci 54:1737–1743
Bøhn T, Cuhra M, Traavik T, Sanden M, Fagan J, Primicerio R (2014) Compositional differences in soybeans on the market: glyphosate accumulates in Roundup Ready GM soybeans. Food Chem 153:207–215
Bott S, Tesfamariam T, Candan H, Cakmak I, Römheld V, Neumann G (2008) Glyphosate-induced impairment of plant growth and micronutrient status in glyphosate-resistant soybean (Glycine max L.). Plant Soil 312:185–194
Braz-Mota S, Sadauskas-Henrique H, Duarte RM, Val AL, Almeida-Val VM (2015) Roundup® exposure promotes gills and liver impairments, DNA damage and inhibition of brain cholinergic activity in the Amazon teleost fish Colossoma macropomum. Chemosphere 135:53–60
Çağlar S, Kolankaya D (2008) The effect of sub-acute and sub-chronic exposure of rats to the glyphosate-based herbicide Roundup. Environ Toxicol Pharmacol 25:57–62
Casabé N, Piola L, Fuchs J, Oneto ML, Pamparato L, Basack S, Giménez R, Massaro R, Papa JC, Kesten E (2007) Ecotoxicological assessment of the effects of glyphosate and chlorpyrifos in an Argentine soya field J Soils Sediments 7:232–239
Chan P, Mahler J (1992) NTP technical report on the toxicity studies of glyphosate (CAS No. 1071-83-6) administered in dosed feed to F344/N rats and B6C3F1 mice. Toxicity report 16:1–D3
Cheminova (1993) Glyphosate: 104 week dietary carcinogenicity study in mice. Inveresk Research International, Ltd., Tranent
Chruscielska K, Brzezinski J, Kita K, Kalhorn D, Kita I, Graff stein B, Korzeniowski P (2000) Glyphosate—evaluation of chronic activity and possible far-reaching eff ects. Part 1. Studies on chronic toxicity. Pestycydy 3-4:11–20
Correia FV, Moreira JC (2010) Effects of glyphosate and 2, 4-D on earthworms (Eisenia foetida) in laboratory tests. Bull Environ Contam Toxicol 85:264–268
Cox C (1995) Glyphosate, part 1: toxicology. Journal of Pesticide Reform 108:1–13
Cuervo JL, Fuentes CL (2014) Mineralization and sorption of 14C-glyphosate in samples from three soil types collected in El Espinal, Colombia. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales 38:287–297
Currie Z, Prosser RS, Rodriguez-Gil JL, Mahon K, Poirier D, Solomon KR (2015) Toxicity of Cupside 480SL® Sprasy mixture formulation of glyphosate to aquatic organisms. Environ Toxicol Chem 34:1178–1184
Dallegrave E, Mantese FD, Oliveira RT, Andrade AJ, Dalsenter PR, Langeloh A (2007) Pre-and postnatal toxicity of the commercial glyphosate formulation in Wistar rats. Arch Toxicol 81:665–673
Domínguez A, Brown GG, Sautter KD, de Oliveira CM, de Vasconcelos EC, Niva CC, Bartz ML, Bedano JC (2016) Toxicity of AMPA to the earthworm Eisenia andrei Bouché, 1972 in tropical artificial soil. Scientific Reports 6:19731
Druart C, Millet M, Scheifler R, Delhomme O, De Vaufleury A (2011) Glyphosate and glufosinate-based herbicides: fate in soil, transfer to, and effects on land snails. J. Soils Sediments 11:1373–1384
Druille M, Cabello MN, Omacini M, Golluscio RA (2013) Glyphosate reduces spore viability and root colonization of arbuscular mycorrhizal fungi. Appl Soil Ecol 64:99–103
Duke SO (2015) Perspectives on transgenic, herbicide-resistant crops in the United States almost 20 years after introduction. Pest Manag Sci 71:652–657
Duke SO, Lydon J, Koskinen WC, Moorman TB, Chaney RL, Hammerschmidt R (2012) Glyphosate effects on plant mineral nutrition, crop rhizosphere microbiota, and plant disease in glyphosate-resistant crops. J Agr Food Chem 60:10375–10397
Duke SO, Powles SB (2008) Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 64:319–325
Duke SO, Baerson SR, Rimando AM (2003a) Glyphosate. Encyclopedia of agrochemicals. J.R. Plimmer, D.W. Gammon, N.N. Ragsdale, eds., Wiley, New York.
Duke SO, Rimando AM, Pace PF, Reddy KN, Smeda RJ (2003b) Isoflavone, glyphosate, and aminomethylphosphonic acid in seeds of glyphosate-treated, glyphosate-resistant soybean. J Agr Food Chem 51:340–344
Environmental Protection Agency (2015) Basic information about glyphosate in drinking water. http://water.epa.gov/drink/contaminants/basicinformation/glyphosate.cfm#four. Viewed 23-Feb-15.
EFSA (European Food Safety Authority) (2015) Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate 1. EFSA J 13(11):4302
Ehling S, Reddy TM (2015) Analysis of glyphosate and aminomethylphosphonic acid in nutritional ingredients and milk by derivatization with Fluorenylmethyloxycarbonyl chloride and liquid chromatography−mass spectrometry. J Agr Food Chem 63:10562–10568
Estes FL (1979) 90-Day Subacute Rat Toxicity Study. Unpublished report, International Research and Development Corporation, Mattawan, Michigan.
Schwebda F (1996) Combined chronic toxicity and carcinogenicity study with glyphosate technical in Wistar rats. Rallis India, Ltd., Bangalore
Schwebda F (2001) Carcinogenicity study with glyphosate technical in Swiss albino mice. Rallis India, Ltd., Bangalore
FAO (2005) Glyphosate (158), Pesticide residues in food. FAO plant production and protection paper 183. Joint FAO/WHO Meetings on Pesticide Residues, Food and Agriculture Organisation, Rome, Italy, pp. 122–144. http://www.fao.org/docrep/009/a0209e/a0209e0d.htm#bm13. Accessed 16-Mar-15.
Fusilero MA, Mangubat J, Ragas RE, Baguinon N, Taya H, Rasco E (2013) Weed management systems and other factors affecting the earthworm population in a banana plantation. Eur J Soil Biol 56:89–94
García-Pérez JA, Alarcón-Gutiérrez E, Perroni Y, Barois I (2014) Earthworm communities and soil properties in shaded coffee plantations with and without application of glyphosate. Appl Soil Ecol 83:230–237
Gasnier C, Dumont C, Benachour N, Clair E, Chagnon M-C, Séralini G-E (2009) Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicol 262:184–191
George J, Prasad S, Mahmood Z, Shukla Y (2010) Studies on glyphosate-induced carcinogenicity in mouse skin: a proteomic approach. J Proteom 73:951–964
Ghafoor A, Jarvis N, Thierfelder T, Stenström J (2011) Measurements and modeling of pesticide persistence in soil at the catchment scale. Sci Total Environ 409:1900–1908
Gimou M-M, Charrondiere U, Leblanc J-C, Pouillot R (2008) Dietary exposure to pesticide residues in Yaoundé: the Cameroonian total diet study. Food Additi Contam 25:458–471
Gimsing AL, Borggaard OK, Bang M (2004) Influence of soil composition on adsorption of glyphosate and phosphate by contrasting Danish surface soils. Eur J Soil Sci 55:183–191
Glyphosate–Renewal Assessment Report (2013) Glyphosate_RAR_01_Volume_1_2013–12-18_san.pdf
Greim H, Saltmiras D, Mostert V, Strupp C (2015) Evaluation of carcinogenic potential of the herbicide glyphosate, drawing on tumor incidence data from fourteen chronic/carcinogenicity rodent studies. Crit Rev Toxicol 45:185–208
Grunewald K, Schmidt W, Unger C, Hanschmann G (2001) Behavior of glyphosate and aminomethylphosphonic acid in soils and water of reservoir Radeburg II catchment (Saxony/Germany). J Plant Nutr Soil Sci 164:65–70
Guyton KZ, Loomis D, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K (2015) Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. The Lancet Oncology 16:490–491
Harris CA, Gaston CP (2004) Effects of refining predicted chronic dietary intakes of pesticide residues: a case study using glyphosate. Food Addit Contam 21:857–864
Haslam E (2014) The shikimate pathway: biosynthesis of natural products series. Elsevier, Amsterdam
Holson JF (1991) A developmental toxicology study of AMPA in rats. Unpublished report, WIL Research Laboratories, Inc., Ashland, OH.
Horth H, Blackmore K (2009) Survey of glyphosate and AMPA in groundwaters and surface waters in Europe. Report by WRc plc, Swindon, Wiltshire, United Kingdom No: UC8073 2
Jensen PK, Wujcik CE, McGuire MK, McGuire MA (2016) Validation of reliable and selective methods for direct determination of glyphosate and aminomethylphosphonic acid in milk and urine using LC-MS/MS. Journal of Environmental Science and Health, Part B 51:254–259
Johnson-Maynard J, Lugo-Perez J (2006) Earthworm populations, microbial biomass and coffee production in different experimental agroforestry management systems in Costa Rica. Carib J Sci 42:397–409
Kier LD, Kirkland DJ (2013) Review of genotoxicity studies of glyphosate and glyphosate-based formulations. Crit Rev Toxicol 43:283–315
King JJ, Wagner RS (2010) Toxic effects of the herbicide Roundup® Regular on Pacific Northwestern amphibians. Northwest Nat 91:318–324
Knezevich AL (1983) A chronic feeding study of glyphosate (Roundup technical) in mice. Unpublished report, Bio/Dynamics, Inc., East Millstone, NJ.
Koller V, Fürhacker M, Nersesyan A, Mišík M, Eisenbauer M, Knasmueller S (2012) Cytotoxic and DNA-damaging properties of glyphosate and Roundup in human-derived buccal epithelial cells. Arch Toxicol 86:805–813
Krebs C (2011) Farmers look to broader strategies to battle weeds. AG J. March 11
Kremer RJ, Means NE (2009) Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. Eur J Agron 31(3):153–161
Kwiatkowska M, Huras B, Bukowska B (2014) The effect of metabolites and impurities of glyphosate on human erythrocytes (invitro). Pestic Biochem Phys 109:34–43
Laitinen P, Rämö S, Nikunen U, Jauhiainen L, Siimes K, Turtola E (2009) Glyphosate and phosphorus leaching and residues in boreal sandy soil. Plant Soil 323:267–283
Lanaro R, Costa JL, Cazenave SO, Zanolli-Filho LA, Tavares MF, Chasin AA (2015) Determination of herbicides paraquat, glyphosate, and aminomethylphosphonic acid in marijuana samples by capillary electrophoresis. J Forensic Sci 60(Suppl 1):S241–S247
Lankas GR (1981) A lifetime feeding study of glyphosate (Roundup technical) in rats. Unpublished report, Bio/Dynamics, Inc., East Millstone, NJ.
Levine SL, von Mérey G, Minderhout T, Manson P, Sutton P (2015) Aminomethylphosphonic acid has low chronic toxicity to Daphnia magna and Pimephales promelas. Environ Toxicol Chem. doi:10.1002/etc.2940
Lu FC (1995) A review of the acceptable daily intakes of pesticides assessed by WHO. Regul. Toxicol Pharm 21:352–364
Ma J, Bu Y, Li X (2015) Immunological and histopathological responses of the kidney of common carp (Cyprinus carpio L.) sublethally exposed to glyphosate. Environ Toxicol Pharm 39:1–8
Mamy L, Barriuso E, Gabrielle B (2005) Environmental fate of herbicides trifluralin, metazachlor, metamitron and sulcotrione compared with that of glyphosate, a substitute broad spectrum herbicide for different glyphosate-resistant crops. Pest Manag Sci 61:905–916
Mateos-Naranjo E, Perez-Martin A (2013) Effects of sub-lethal glyphosate concentrations on growth and photosynthetic performance of non-target species Bolboschoenus maritimus. Chemosphere 93:2631–2638
Mercurio P, Flores F, Mueller JF, Carter S, Negri AP (2015) Glyphosate persistence in seawater. Mar Pollut Bull 85:385–390
Mesnage R, Defarge N, Spiroux de Vendômois J, Séralini GE (2015) Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem Toxicol 84:133–153
Monsanto (1981) A lifetime feeding study of glyphosate (roundup ® technical) in rats. Bio/dynamics Inc., East Millstone
Monsanto (1983) A chronic feeding study of glyphosate (roundup ® technical) in mice. Bio/dynamics, Inc., East Millstone
Monsanto (1990) Chronic study of glyphosate administered in feed to albino rats. Monsanto Agricultural Company, St. Louis
Morillo E, Undabeytia T, Maqueda C, Ramos A (2000) Glyphosate adsorption on soils of different characteristics: influence of copper addition. Chemosphere 40:103–107
McQueen H, Callan AC, Hinwood AL (2012) Estimating maternal and prenatal exposure to glyphosate in the community setting. Intern J Hyg Environ Health 215:570–576
Newton M, Horner LM, Cowell JE, White DE, Cole EC (1994) Dissipation of glyphosate and aminomethylphosphonic acid in North American forests. J Agr Food Chem 42:1795–1802
Niemann L, Sieke C, Pfeil R, Solecki R (2015) A critical review of glyphosate findings in human urine samples and comparison with the exposure of operators and consumers. J Verbr Lebensm 10:3–12
Nufarm (2009) Glyphosate technical: dietary carcinogenicity study in the mouse. Harlan Laboratories Ltd, Derbyshire, UK
Nguyen DB, Rose MT, Rose TJ, Morris SG (2016) Van Zwieten L. Impact of glyphosate on soil microbial biomass and respiration: a meta-analysis Soil Biology and Biochemistry 92:50–57
Paganelli A, Gnazzo V, Acosta H, López SL, Carrasco AE (2010) Glyphosate-based herbicides produce teratogenic effects on vertebrates by impairing retinoic acid signaling. Chem Res Toxicol 23:1586–1595
Parrot F, Bedry R, Favarel-Garrigues J-C (1995) Glyphosate herbicide poisoning: use of a routine aminoacid analyzer appears to be a rapid method for determining glyphosate and its metabolite in biological fluids. Clin Toxicol 33:695–698
Pelosi C, Barot S, Capowiez Y, Hedde M, Vandenbulcke F (2014) Pesticides and earthworms. A review. Agronr Sustain Develop 34:199–228
Pereira JL, Antunes SC, Castro BB, Marques CR, Gonçalves AM, Gonçalves F, Pereira R (2009) Toxicity evaluation of three pesticides on non-target aquatic and soil organisms: commercial formulation versus active ingredient. Ecotoxicology 18:455–463
Poletta G, Larriera A, Kleinsorge E, Mudry M (2009) Genotoxicity of the herbicide formulation Roundup®(glyphosate) in broad-snouted caiman (Caiman latirostris) evidenced by the Comet assay and the Micronucleus test. Mut. Res./gen. Tox En 672:95–102
Prasad S, Srivastava S, Singh M, Shukla Y (2009) Clastogenic effects of glyphosate in bone marrow cells of Swiss albino mice. J Toxicol 2009:308985
Reddy KN, Rimando AM, Duke SO (2004) Aminomethylphosphonic acid, a metabolite of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. J Agr Food Chem 52:5139–5143
Reddy KN, Rimando AM, Duke SO, Nandula VK (2008) Aminomethylphosphonic acid accumulation in plant species treated with glyphosate. J Agr Food Chem 56:2125–2130
Reyna MS (1990) Two generation reproduction feeding study with glyphosate in sprague–dawley rats. Unpublished report, Monsanto Environmental Health Laboratory, St. Louis, MO.
Reyna MS, Ruecker FA (1985) Twelve month study of glyphosate administered by gelatin capsule to beagle dogs. Unpublished report, Mosanto Environmental Health Laboratory, St. Louis, MO.
Roberts DM, Buckley NA, Mohamed F, Eddleston M, Goldstein DA, Mehrsheikh A, Bleeke MS, Dawson AH (2010) A prospective observational study of the clinical toxicology of glyphosate-containing herbicides in adults with acute self-poisoning. Clin Toxicol 48:129–136
Romano MA, Romano RM, Santos LD, Wisniewski P, Campos DA, de Souza PB, Viau P, Bernardi MM, Nunes MT, de Oliveira CA (2012) Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Arch Toxicol 86:663–673
Rubio F, Guo E, Kamp L (2014) Survey of glyphosate residues in honey, corn and soy products. J Environ Anal Toxicol 4:249. doi:10.4172/2161-0525.1000249
Schroeder RE (1981) A three-generation reproduction study with glyphosate in rats. Unpublished report, Bio/Dynamics, Inc., East Millstone, NJ.
Busse MD, Ratcliff AW, Shestak CJ, Powers RF (2001) Glyphosate toxicity and the effects of long-term vegetation control on soil microbial communities. Soil Biol Biochem 33:1777–1789
Santadino M, Coviella C, Momo F (2014) Glyphosate sublethal effects on the population dynamics of the earthworm Eisenia fetida (Savigny, 1826). Water Air Soil Pollut 225:1–8
Siehl DL (1997) Inhibitors of EPSP synthase, glutamine synthase and histidine synthesis, in Herbicide Activity: Toxicology, Biochemistry and Molecular Biology, ed. By Roe R.M/, Burton, J.D. and Kuhr, R.J., IOS Press, Amsterdam, The Netherlands, pp. 37–67.
Shehata AA, Schrödl W, Aldin AA, Hafez HM, Krüger M (2013) The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Current Microbiol 66:350–358
Shushkova T, Vasilieva G, Ermakova I, Leontievsky A (2009) Sorption and microbial degradation of glyphosate in soil suspensions. Appl Biochem Microbiol 45:599–603
Solomon K, Thompson D (2003) Ecological risk assessment for aquatic organisms from over-water uses of glyphosate. Journal of Toxicology and Environmental Health Part B: Crit Rev 6:289–324
Saunders LE, Pezeshki R (2015) Glyphosate in runoff waters and in the root-zone: A review toxics 3:462–480
Stout LD, Johnson CW (1987) 90-day study of glyphosate administered in feed to sprague–sawley rats. Unpublished report, Monsanto Environmental Health Laboratory, St. Louis, MO.
Stout LD, Ruecker FA (1990) Chronic study of glyphosate administered in feed to albino rats. Unpublished report, Monsanto Environmental Health Laboratory, St. Louis, MO.
Struger J, Thompson D, Staznik B, Martin P, McDaniel T, Marvin C (2008) Occurrence of glyphosate in surface waters of southern Ontario. Bull Environ Contam Toxicol 80:378–384
Swanson NL, Leu A, Abrahamson J, Wallet B (2014) Genetically engineered crops, glyphosate and the deterioration of health in the United States of America. J. Organic Systems 9:6–37
Syan HS, Prasher SO, Pageau D, Singh J (2014) Dissipation and persistence of major herbicides applied in transgenic and non-transgenic canola production in Quebec. Eur J Soil Biol 63:21–27
Syngenta (2001) Glyphosate acid: two year dietary toxicity and oncogenicity study in rats. Central Toxicology Laboratory, Alderley Park Macclesfi eld, Cheshire, UK: Syngenta.
Székács A, Darvas B (2012) Forty years with glyphosate. Herbicides–properties, synthesis and control of weeds Ed Hasaneen, MNAE-G, InTech, Croatia.
Tasker EJ (1980a) Teratology study in rats. Unpublished report, International Research and Development Corporation, Mattawan, MI.
Tasker EJ (1980b) Teratology study in rabbits. Unpublished report, International Research and Development Corporation, Mattawan, MI.
Thongprakaisang S, Thiantanawat A, Rangkadilok N, Suriyo T, Satayavivad J (2013) Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem Toxicol 59:129–136
Tierney WJ (1979) A three month feeding study of glyphosate (Roundup technical) in mice. Unpublished report, Bio/Dynamics, Inc., East Millstone, NJ.
Tompkins EC (1991) 90-day oral (capsule) toxicity study in dogs with AMPA. Unpublished report, WIL Research Laboratories, Inc., Ashland, OH.
Van Stempvoort D, Roy J, Brown S, Bickerton G (2014) Residues of the herbicide glyphosate in riparian groundwater in urban catchments. Chemosphere 95:455–463
WHO (World Health Organisation), 1994. Glyphosate. Environmental Health Criteria 159. The Internal Programme on Chemical Safety (IPCS), WHO, Geneva. Cited in: Buffin, D., Jewell, T. (2001). Health and Environmental Impact of Glyphosate: The Implications of Increased use of Glyphosate in Association with Genetically Modified Crops. In: Riley, P., Taylor, M., Diamand, E., Barron, H., (Eds.). UK, pp. 1–40.
Williams GM, Kroes R, Munro IC (2000) Safety evaluation and risk assessment of the herbicide roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol 31:117–165
Wolmarans K, Swart WJ (2014) Influence of glyphosate, other herbicides and genetically modified herbicide-resistant crops on soil microbiota: a review. South Afr J Plant Soil 31:177–186
Yang C, Shen S, Wang M, Li J (2013) Mild salinization stimulated glyphosate degradation and microbial activities in a riparian soil from Chongming Island, China.
Yu Y, Zhou QX (2005) Adsorption characteristics of pesticides methamidophos and glyphosate by two soils. Chemosphere 58:811–816
Zabaloy MC, Gómez E, Garland JL, Gómez MA (2012) Assessment of microbial community function and structure in soil microcosms exposed to glyphosate. Appl Soil Ecol 61:333–339
Zhang C, Hu X, Luo J, Wu Z, Wang L, Li B, Wang Y, Sun G (2015) Degradation dynamics of glyphosate in different types of citrus orchard soils in China. Molecules 20:1161–1175
Zhou CF, Wang YJ, Yu YC, Sun RJ, Zhu XD, Zhang HL, Zhou DM (2012) Does glyphosate impact on Cu uptake by, and toxicity to, the earthworm Eisenia fetida? Ecotoxicology 21:2297–2305
Zobiole LH, Oliveira RS Jr, Visentainer JV, Kremer RJ, Bellaloui N, Yamada T (2010) Glyphosate affects seed composition in glyphosate-resistant soybean. J Agr Food Chem 58:4517–4522
Acknowledgments
The authors thank Helen Wallace and Stephen Trueman for their input to this article. This research was funded by EcoBiotics. EcoBiotics played no role in the preparation of this review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bai, S.H., Ogbourne, S.M. Glyphosate: environmental contamination, toxicity and potential risks to human health via food contamination. Environ Sci Pollut Res 23, 18988–19001 (2016). https://doi.org/10.1007/s11356-016-7425-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7425-3