Abstract
Phenyl valerate is used for detecting and measuring neuropathy target esterase (NTE) and has been used for discriminating esterases as potential target in hen model of organophosphorus delayed neuropathy. In previous studies we observed that phenyl valerate esterase (PVase) activity of an enzymatic fraction in chicken brain might be due to a butyrylcholinesterase protein (BuChE), and it was suggested that this enzymatic fraction could be related to the potentiation/promotion phenomenon of the organophosphate-induced delayed neuropathy (OPIDN). In this work, PVase activity of purified human butyrylcholinesterase (hBuChE) is demonstrated and confirms the novel observation that a relationship of BuChE with PVase activities is also relevant for humans, as is, therefore the potential role in toxicity for humans. The KM and catalytic constant (kcat) were estimated as 0.52/0.72 µM and 45,900/49,200 min−1 respectively. Furthermore, this work studies the inhibition by preincubation of PVase and cholinesterase activities of hBuChE with irreversible inhibitors (mipafox, iso-OMPA or PMSF), showing that these inhibitors interact similarly in both activities with similar second-order inhibition constants. Acethylthiocholine and phenyl valerate partly inhibit PVase and cholinesterase activities, respectively. All these observations suggest that both activities occur in the same active center. The interaction with a reversible inhibitor (ethopropazine) showed that the cholinesterase activity was more sensitive than the PVase activity, showing that the sensitivity for this reversible inhibitor is affected by the nature of the substrate. The present work definitively establishes the capacity of BuChE to hydrolyze the carboxylester phenyl valerate using a purified enzyme (hBuChE). Therefore, BuChE should be considered in the research of organophosphorus targets of toxicity related with PVase proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to organophosphorus (OP) esters can cause several toxic effects, including acute cholinergic clinical episodes, intermediate syndrome, organophosphate-induced delayed neuropathy (OPIDN) and chronic neurological effects. Other toxic effects with unknown molecular targets have been described (COT 1999; Jamal et al. 2002).
Several observations support the view that OPs have additional non-acetylcholinesterase (AChE) targets (Lockridge and Schopfer 2006; Terry 2012), which emerge especially given the limited correlation between certain behavioral responses and the magnitude and regional selectivity of AChE inhibition in the brain (McDaniel and Moser 2004). The wide variety of long-term neuropsychiatric symptoms that have been associated with OP exposures suggests that other non-AChE targets might be implicated (Terry 2012). Linking these sets of OP pesticide-binding proteins to their toxicological relevance is needed to understand low-dose, long-term OP neurotoxicity and OP detoxification pathways (Lockridge and Schopfer 2006; Costa 2006). However, the effects of low doses for long-term exposure remain unclear (Sogorb and Vilanova 2010).
Phenyl valerate (PV) was the substrate used in the identification and characterization of neuropathy target esterase (NTE; Johnson 1975; Chemnitius et al. 1983; Carrington and Abou-Donia 1984; Vilanova et al. 1990; Glynn et al. 1994, 1998) and other serine hydrolases of neural tissue and brain (Céspedes et al. 1997; Escudero et al. 1997; Barril et al. 1999; Estévez et al. 2004, 2010, 2011).
In soluble brain fraction of chicken, three enzymatic components with phenyl valerate esterase (PVase) activity have been discriminated using irreversible inhibitors as follows: mipafox (OPIDN-inducer), paraoxon (non OPIDN-inducer) and phenylmethylsulfonyl fluoride (PMSF) (Mangas et al. 2011, 2012): Eα, Eβ, and Eγ. PMSF is not an OP but is an irreversible NTE inhibitor that protects OPIDN development when dosed before a neuropathic dose of a neuropathic OP, but PMSF enhances neuropathy severity when dosed after a low non-neuropathic dose of a neuropathy inducer (Pope et al. 1990; Lotti et al. 1991). Mangas and coworkers (Mangas et al. 2012) suggested that the inhibitory kinetic properties of Eα PVase activity with organophosphates are compatible with being a target of the potentiation/promotion phenomenon of PMSF on OPIDN and for understanding the effects of low-dose exposures to OPs. Benabent et al. (2014a, b) showed that PV (the substrate of PVase activity) partially inhibited cholinesterase activity in soluble brain fractions of chicken, and vice versa, acetylthiocholine (AtCh) shows some inhibition on PVase activity components. This scenario suggested that PVase components may contain enzymes that hydrolyze acetylcholine. By a proteomic analysis, we demonstrate that a fraction enriched with the Eα component of PVase activity contains butyrylcholinesterase (BuChE; Mangas et al. 2016).
To clarify if the relationship of BuChE with PVase activity is also relevant for humans, here we test and demonstrate that purified human butyrylcholinesterase (hBuChE) is able to hydrolyze PV. The relationship between the catalytic center of PVase activity and its AtCh hydrolyzing activity (ChE) is evaluated by assessing the interactions between substrates and inhibitors.
Materials and methods
Chemicals
Sodium dodecyl sulfate (SDS; purity 99%) was obtained from Panreac Química S.L.U. (Barcelona, Spain). Ellmant’s reagent, 5,5′-dithio-bis-2-nitrobenzoate (DTNB, purity 99%) acetylthiocholine iodide (purity ≥ 98) phenylmethylsulfonyl fluoride (PMSF), paraoxon, tetraisopropylpyrophosphoramide (iso-OMPA) and 10-(2-diethylaminopropyl) phenothiazine (ethopropazine) were purchased from Sigma (Madrid, Spain). N, N′-di-isopropylphosphorodiamidefluoridate (mipafox, purity > 98%) and phenyl valerate were attained from Lark Enterprise (Webster, MA, USA). All the other reagents were obtained from Merck SL (Madrid, Spain) and were of analytical grade.
Solutions
“Phosphate buffer”, which is mentioned throughout contained 0.1 M phosphate, pH 7.4, 1 mM EDTA. A stock solution of inhibitors: 10 mM mipafox was prepared in 10 mM Tris–citrate buffer (pH 6.0); 50 mM PMSF in dimethylsulfoxide; 10 mM paraoxon in dried acetone; 10 mM iso-OMPA in dried acetone; 10 mM ethopropazine in water. All the inhibitors were diluted to the appropriate concentration in water immediately before the kinetic assays.
A stock solution of substrate PV (168 mM) was prepared in dried N, N-diethylformamide, and was diluted in water at the concentrations indicated in each assay immediately before the enzymatic assays. AtCh was prepared in water before use at the concentrations indicated in each assay immediately before the enzymatic assays.
To stop the enzymatic reaction and color development, the following solutions were prepared. SDS-AAP solution (for PVase activity): a 2% SDS (sodium dodecyl sulfate) that contained 1.23 mM aminoantypirine in phosphate buffer. SDS-DTNB solution (for ChE activity): a 2% SDS solution that contained 6 mM DTNB in phosphate buffer.
Enzyme
Purified human BuChE isolated from human plasma was a gift from Dr David Lenz and Dr Douglas Cerasoli [USAMRICD (US Army Medical Research Institute of Chemical Defense), Aberdeen Proving Ground, MD, USA]. All the enzyme concentrations refer to the concentration of catalytic sites, i.e., monomers.
Measure of esterase activities
PVase activity and ChE activity were both inhibited and measured by a similar strategy and procedure to enable the comparison of the response to inhibitors and to evaluate the interaction between the substrates under comparable conditions. Enzyme preparation was pre-incubated with the inhibitor for the desired time (needed for irreversible inhibitor), the substrate was added for a fixed time of 10 min and the reaction was stopped with a mixture that contained SDS plus the color reagent (SDS-AAP or SDS-DTNB solutions for PVase or ChE, respectively).
An automated Work Station (Beckman Biomek 2000) was employed for pipetting and incubating process with inhibitors and substrates.
PVase activity
PVase activity was measured according to Mangas et al. (2011) by following a procedure based on the colorimetric method for the NTE assay developed by Johnson (1977), and using an automated Work Station (Beckman Biomek 2000) for the full procedure as follows: samples of 200-μl volume of the enzyme preparation of 2 nM human butyrylcholinesterase (buffer in blanks for spontaneous hydrolysis) were incubated with 200 μl of PV at the concentration indicated in each experiment. The mixture was incubated for 10 min at 37 °C for the enzyme reaction with the substrate. The reaction was stopped by adding 200 μl of SDS-AAP solution and after mixing. Next 100 μl of 1.21 mM potassium ferricyanide was added and left for 5 min for color development. A 300-μl volume from each microtube was transferred to a 96-well microplate. Absorbance was read at 510 nm in a microplate reader (Beckman Coluter AD 340). Blanks for the spontaneous hydrolysis (samples without hBuChE) were included and the same procedure was applied.
ChE activity
Assays were carried out according to Benabent et al. (2014b). Samples of a 200-μl volume of the enzyme preparation of 4 nM hBuChE (buffer in the blanks) were incubated with 200 µL of AtCh in ultrapure water at the concentration indicated in each experiment for 10 min at 37 °C to measure enzymatic activity. The enzymatic reaction was stopped by adding 200 μl of SDS-DTNB solution. Then 200 μL of phosphate buffer (diluted enzyme preparation in the blanks) was added. The final assay volume was 800 μL. After mixing and waiting for at least 5 min, a 300-μL volume from each microtube was transferred to a 96-well microplate to read absorbance at 410 nm.
Interactions with inhibitors
For the irreversible inhibitors (mipafox, iso-OMPA, PMSF), the inhibitor was preincubated for 30 min with the enzyme preparation before adding the substrate to measure residual activity. With the reversible inhibitor (ethopropazine) the inhibitor and substrate were added simultaneously. For the substrates interaction, both substrates PV and AtCh were added simultaneously. Then after running the enzyme substrate reaction for 10 min, PVase or ChE activity was measured. The specific conditions are shown for each experiment in the Results or in the legends of figures.
Mathematical models
Irreversible inhibitors
Exponential decay models were fitted to the fixed-time inhibition data of PVase and ACth activity of one, two sensitive enzymatic components, or more, with or without a resistant fraction, using the version 8 of Sigma Plot software. The general model equation for inhibition is as follows:
where k1, k2, k3,…, kn are the second-order inhibition constants; t is the inhibition time (30 min in fixed the inhibition time experiments); E10, E20,…, En0 and R are the proportion (amplitude) of enzymatic components E1, E2, En,… and R, respectively. For the purpose of obtaining a coherent solution in the interactive computing estimation, some restrictions were applied: (1) all the parameters (rate constants and amplitudes) should have positive values (>0); (2) the following complementary restriction was also applied: E10 + E20 + E30 +⋯+En 0 + R = 100%.
The best fitting model (according to the F test) is shown in the “Results” section.
The I50 (30 min) values in the mipafox and iso-OMPA fixed-time inhibition experiments were obtained by applying the following equation:
where ki is the second-order rate of inhibition.
If the spontaneous hydrolysis of PMSF was considered, then the general model was as follows (Estévez et al. 2012):
where kh is the chemical hydrolysis constant of the PMSF and t is the inhibition time (30 min in the fixed inhibition time experiments). The I 50 (30 min) values in the PMSF fixed-time inhibition experiments were obtained by applying the following equation:
where ki is the second-order rate of inhibition and kh is the rate constant of chemical hydrolysis.
Reversible inhibitors
For the reversible inhibitors (ethopropazine), the following model equations were applied:
When considering a sensitive enzymatic component, the mathematical model was as follows (Copeland 2000, 2005)
where I is the inhibitor concentration and I 50 is the concentration that inhibits 50% of total activity.
When considering a sensitive enzymatic component plus other one resistant, the mathematical models were as follows.
When considering two sensitive enzymatic components and another one resistant, the mathematical models were as follows:
where E 1 and E 2 are the proportions of activities of the sensitive enzymatic components and R is the resistant component, I is the inhibitor concentration, I150 and I250 are the concentrations that inhibit 50% of the activity of the sensitive components.
Determination of variability
Each point in the graphs represents the mean of three replicates (SD < 5%). Different experiments were done on distinct days with various preparations of enzyme, substrates and inhibitors. The “Results” section shows the kinetic parameters obtained in two independent experiments, while the figures show one of these experiments.
Results
Hydrolysis of PV by h-BuChE
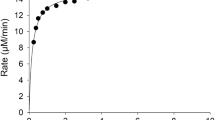
Incubation of PV at concentrations within the 0.001–3.000 mM range with 2 nM hBuChE (1 nM in reaction volume) yielded a behavior of a rectangular hyperbola (plot with black circles in Fig. 1) and activity up to ~40 µM min− 1 (up to 3 mM PV). The estimated V max, K M, and catalytic constant (k cat) are shown in Table 1.
Inhibition of PVase activity by AtCh. The procedure is described in “Materials and methods”. PV concentrations (substrate): 0.00126, 0.0252, 0.0525, 0.105, 0.294, 1.008 and 3.003 mM in 420 µl of the enzyme–substrate reaction volume. Inhibitor concentrations (AtCh): 0 (black circles), 0.5 (white circles) and 1 mM (black triangles) in 420 µl of the enzyme–substrate reaction volume. The level lines obtained from 3D fit for 0, 0.5 and 1 mM of AtCh
Hydrolysis of AtCh by hBuChE
Incubation of AtCh at concentrations within the 0.005–1 mM range with 4 nM hBuChE (2 nM in the reaction volume) showed activity up to ~53 µM·min−1. Saturation was not reached under the experimental conditions (plot with black circles in Fig. 2), so it was not possible to estimate the V max and K M.
Inhibition of ChE activity by PV. The procedure is described in the Materials and Methods. AtCh concentrations (substrate): 0, 0.05, 0.1, 0.3, 0.5, 0.8, and 1 mM in 420 µl of the enzyme–substrate reaction volume. PV concentrations: 0 (black circles), 0.5 (white circles) and 1 mM (black triangles) in 420 µl of the enzyme–substrate reaction volume
Inhibition of the PVase activity with AtCh
PVase activity was inhibited by 0.5 and 1 mM of AtCh (inhibition by 1 mM of AtCh was 7% in the presence of 0.5 mM PV).
Figure 1 shows the 3D fit to the data (Estévez et al. 2004) with the mathematical equation deduced from the model of two substrates that compete for a same active site according to the Michaelis–Menten kinetic reaction (Cornish-Bowden 2004). This mathematical equation was as follows:
where V max is the maximun rate, S1 is PV concentration, S2 the AtCh concentration, K M1 is the Michaelis–Menten constant for PVase activity and K M2 for ChE activity. The V max estimated in two independent experiments was 48.0/53.8 µM min−1. K M1 was 0.59/0.88 mM and K M2 was 1.62/1.95 mM.
Inhibition of ChE activity with PV
ChE activity was inhibited by 0.5 and 1 mM of PV (~19% inhibition for 0.05 mM AtCh and in the presence of 1 mM PV, and ~15% for 1 mM AtCh in the presence of 1 mM PV). As Fig. 2 illustrates, inhibition of ChE activity took place at all PV concentrations. The rate values did not reach a plateau, so it was not possible to estimate the V max and Km for the AtCh and PV hydrolisis reactions.
PVase and ChE activities inhibition by iso-OMPA, mipafox and PMSF
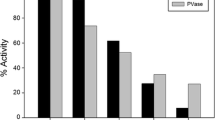
hBuChE was pre-incubated at different concentrations with iso-OMPA (up to 1818 µM), mipafox (up to 50 µM) or PMSF (up to 50 µM) for 30 min at 37 °C. Then substrate (2.7 mM PV or 1 mM AtCh in the reaction volume) was added to measure residual PVase or ChE activity (Fig. 3 for PVase activity and Fig. 4 for ChE activity). Exponential decay models were fitted to the fixed time inhibition data of PVase or ChE activity.
Fixed time inhibition curve of PVase activity with irreversible inhibitors. A 100-µl volume of 0.2 nM hBuChE was incubated with a 10-µl volume of a iso-OMPA, b mipafox or c PMSF for 30 min at 37 °C, then the mixture was incubated with 100-µl volume of 5.4 mM PV for 10 min at 37 °C and residual PVase activity was measured. The curves show the model that best fitted the data provided in the “Results” section. Each point represents the mean of three replicates (SD < 5%). The insets in panels a and b show the inhibition within a low concentration range
Fixed time inhibition curve of ChE activity with irreversible inhibitors. A 100-µl volume of 0.4 nM hBuChE was incubated with a 10-µl volume of a iso-OMPA, b mipafox or c PMSF for 30 min at 37 °C, then the mixture was incubated with 100-µl volume of 2 mM AtCh for 10 min at 37 °C, and residual ChE activity was measured. The curves show the model that best fitted to the data provided in the “Results” section. Each point represents the mean of three replicates (SD < 5%). The inset in panel a shows the inhibition within the 0–50 nM range
The best fitting model (according to the ‘F’ test) for iso-OMPA was E = E 0·e−k·30·I for both activities, which consisted of a sensitive component; for mipafox it was E = E10·e−k1·30·I + E20·e−k2·30·I for both activities, which consisted of two sensitive components; for PMSF it was \(E = E_{0}\cdot {e^{\left( {\frac{{ki}}{{kh}}} \right) \cdot I \cdot {e^{ - kh \cdot 30}}}}\) (Estévez et al. 2012) for both activities, which consisted of a sensitive component.
The kinetic parameters are shown in Table 2.
Inhibition by ethopropazine of ChE and PVase activities
hBuChE was incubated with ethopropazine and 1 mM AtCh (Fig. 5a) or 2.7 mM PV (Fig. 5b). The mathematical models used to fit the inhibition data were those shown in the “Materials and methods” section. The best fitting model (according to the ‘F’ test) for ChE activity was as follows:
which consisted of a sensitive enzymatic component.
Inhibition of PVase and ChE activities with ethopropazine. A 100-µl volume of 0.4 nM hBuChE was incubated with ethopropazine and 100-µl volume of a 5.4 mM PV or b 1 mM AtCh for 10 min at 37 °C, and residual activities were measured. The curves show the model that best fitted the data provided in the “Results” section. Each point represents the mean of three replicates (SD < 5%).The insets in panels a and b show the inhibition within the low concentration range
The best fitting model (according to the ‘F’ test) for PVase activity was as follows:
which consisted of a sensitive component plus a resistant one of around 3.5% of total activity.
The estimated I50s are shown in Table 2.
Discussion
The novelty of this work is that it has proven that human BuChE hydrolyzes PV. This work confirms the relevance to humans of previous published works done in a chicken brain fraction of Eα PVase activity (Benabent et al. 2014a, b; Mangas et al. 2016). PV is a substrate which has been used to detect and describe proteins considered potential targets of OP toxicity. It was also observed that AtCh inhibited PVase activity and PV inhibited ChE activity. Different irreversible inhibitors were used. The results of the inhibition experiments showed that these inhibitors interact similarly with both activities, which suggests that both are related with the same active catalytic center. However, ChE activity is more sensitive than PVase activity to reversible inhibitor ethopropazine.
Hydrolysis of PV by hBuChE
Mangas and coworkers (Mangas et al. 2011, 2012) kinetically distinguished an enzymatic component of PVase activity in chicken brain soluble fraction called Eα. This component interacted with AtCh (Benabent et al. 2014a, b) and was sensitive to mipafox (I50 for 30 min, 0.004 µM) and to paraoxon (I50 for 30 min, 0.009–0.011 µM), iso-OMPA and ethopropazine, but was resistant to PMSF and BW284C51. A subsequent molecular identification study showed that BuChE was the only candidate responsible for PVase activity in component Eα (Mangas et al. 2014, 2016).
The results of this work confirm that the human enzyme (hBuChE) also displays PVase activity. No activation or inhibition by the substrate was observed under the assay conditions.
Comparison with other reported substrates of hBuChE
Other substrates have been reported to be hydrolyzed by BuChE. The K M estimated for PV (0.52/0.72 mM) was similar to the KM obtained for the reaction with other substrates like o-nitrophenylacetate, α-naphthylacetate, propionylthiocholine, o-nitrophenylbutyrate, acetylcholine, AtCh and butyrylthiocholine at a high substrate concentration, but higher than the KM of butyrylthiocholine and AtCh at low substrate concentration (Table 1). The estimated kcat was similar to the kcat estimated in the literature for the reaction with butyrylthiocholine, with AtCh, phenylacetate, o-nitrophenylbutyrate and the reaction with acetylcholine (Table 1).
Inhibition of PVase and ChE activities with AtCh and PV
PVase activity was inhibited by the presence of AtCh in the medium and ChE activity was inhibited with PV, which suggests that both substrates interact at the same active site. According to the Michaelis–Menten kinetic reaction (Cornish-Bowden 2004), the KM estimated for AtCh in the PVase reaction was higher than the highest AtCh concentration used in the ChE assay (Fig. 2). However both reactions, or only one of them, could result in non-Michaelis–Menten kinetic reaction because it was not possible to estimate KM in the ChE assay since the rate values for ChE assay did not reach a plateau (Fig. 2). Therefore, kinetic behavior could be more complex than the model applied to fit the data in the PVase assay. It is known that hBuChE shows substrate activation at high AtCh concentrations (Masson et al. 1993), but no activation was found under the experimental conditions in the PVase assay.
Inhibition of PVase and ChE activities by irreversible inhibitors (iso-OMPA, mipafox and PMSF)
An enzymatic component was estimated in the inhibition with iso-OMPA and PMSF in the assays of PVase and ChE activities. However, two sensitive components were estimated in the inhibition assays with mipafox in both activities. The second-order rate constants and I50 estimated for each irreversible inhibitor were similar in both activities, which indicates that these irreversible inhibitors could alter PVase and ChE activities similarly and their interaction with hBuChE is independent of the nature of the substrate.
For iso-OMPA, both activities showed similar I50 (30 min) to the human cortex and plasma BuChE with butyrylthiocholine as a substrate (6.7 and 1 µM, respectively; Atack et al. 1989).
PMSF seemed to respond differently with BuChE of distinct species. Kraut and coworkers (2000) reported that the second-order inhibition constant of PMSF in mouse BuChE was 10 × 10−3 µM−1 min−1 at 25 °C, without considering the hydrolysis constant of PMSF. This second-order inhibition constant is comparable to the constants deduced at 37 °C in this work. Mangas and coworkers (2016) identified BuChE in the enriched fraction of PVase activity Eα in chicken brain, which was resistant to PMSF. However, hBuChE is sensitive to PMSF. Mouse BuChE and AChE were inactivated by PMSF, but Torpedo californica AChE was found to be resistant to PMSF inhibition (Kraut et al. 2000). Human BuChE could interact with PMSF differently from chicken brain BuChE, which occurs with AChE of different species. Nevertheless, the published results of inhibition with PMSF should be considered cautiously because PMSF is quickly hydrolyzed and the reported results could be differently affected by this possible artifact according to data published by other authors who did not take this effect into account.
With mipafox, according to F test the best mathematical model to fit the inhibition data showed two enzymatic entities with similar I50 in both activities. This could be interpreted as a more complex molecular mechanism than the molecular mechanism proposed in the irreversible inhibition reaction. The most sensitive component observed in both activities is compatible with a reaction in which mipafox is binding to hBuChE to slow down the activity, but to not totally inactivate it. The least sensitive component is the proportion of the activity that could be irreversibly inactivated by mipafox. The second-order inhibition rate constant of the most sensitive component is comparable to the constant estimated by Kropp and Richardson (2007), with butyrylthiocholine used as substrate in horse serum (1.28 µM−1 min−1). Chemnitius and coworkers estimated inhibition second-order rate constants with the same substrate in porcine left ventricular heart muscle (8.5 × 10−3 µM−1 min−1; Chemnitius et al. 1997) and human left ventricular heart muscle (5.3 × 10−3 µM−1 min−1; Chemnitius et al. 1999). These constants are comparable to the constants for the least sensitive component estimated herein (Table 2). It is also comparable to the I50 estimated by Petroianu and coworkers in human blood plasma with AtCh (0.824 µM) and butyrylthiocholine (0.35 µM) as substrates (Petroianu et al. 2004) for 10 min at 25 °C.
Inhibition by ethopropazine (reversible inhibitor) of the ChE and PVase activities
An enzymatic component was estimated in the inhibition with ethopropazine of ChE activity. However, two enzymatic components were estimated in PVase activity, one sensitive and the other resistant, which only represented 3% of total activity. Different sensitivity can be expected because reversible inhibition depends on the nature of the substrate and the interaction between the substrate and inhibitor.
Relationship with previous observations of the PVase activity of BuChE
In the soluble fraction of chicken brain, three main enzymatic components of PVase activity (Eα, Eβ, and Eγ) were discriminated by inhibitory kinetic approaches using an inducer (mipafox), a noninducer (paraoxon) and an enhancer (PMSF) of neuropathy. PVase component Eα was inhibited with AtCh, iso-OMPA and ethopropazine but not with BW284C51 (Benabent et al. 2014a, b). A detailed proteomic analysis of a fraction enriched in Eα activity demonstrated that the only protein responsible for all PVase activity in component Eα was probably BuChE (Mangas et al. 2016). Eα is spontaneously reactivated after inhibition with paraoxon. Due to the high sensitivity of Eα esterases with paraoxon and/or mipafox, it has been suggested that it might either play a role in toxicity in the low-level long-term exposure of organophosphate compounds or have a protective effect in relation to the spontaneous reactivation of some OPs, such as paraoxon, and other di-ethyl/dimethyl phosphates, which may be considered a biodegradation reaction (Mangas et al. 2014). PMSF can interact with Eα at a concentration that does not inhibit, but which strongly modifies its sensitivity to other esterase inhibitors. These results were interpreted as the covalent irreversible interaction of PMSF at sites other than the substrate catalytic center because PMSF was quickly removed through chemical hydrolysis. It was suggested that this kind of interaction should be considered to interpret the OPIDN potentiation/promotion phenomenon of PMSF (Mangas et al. 2012). These esterases have been suggested to be possible secondary targets of OPs, whose inhibition may be related to some neurotoxicological effects of OPs with unidentified targets (Mangas et al. 2011). Therefore, BuChE should be considered among the candidates of possible targets of OP related to the mechanism of the potentiation/promotion phenomenon of OPIDN, and also in the understanding of the toxic neurological effect of low-dose chronic exposure to OPs.
Final remarks
The results of this work show that human BuChE possesses PVase activity, which supports the findings obtained in soluble chicken brain. Relevance for humans is confirmed and, therefore, the potential role in toxicity hypothesized for PVase proteins may be extended to human cholinesterase. The capacity of BuChE to hydrolyze the carboxylester PV has been definitively established and confirmed using a purified enzyme (hBuChE). Therefore, BuChE should be considered in research into OP targets of toxicity related with PVase proteins, which would be especially interesting for understanding the mechanism of the potentiation/promotion phenomenon of OPIDN, as well as the toxic neurological effect of low-dose long-term exposure to OPs.
Abbreviations
- AChE:
-
Acetylcholinesterase
- AtCh:
-
Acetylthiocholine
- BuChE:
-
Butyrylcholinesterase protein
- ChE:
-
AtCh hydrolyzing activity
- DTNB:
-
5,5′-Dithio-bis-2-nitrobenzoate
- hBuChE:
-
Human butyrylcholinesterase
- kcat :
-
Catalytic constant
- NTE:
-
Neuropathy target esterase
- OP:
-
Organophosphorus
- OPIDN:
-
Organophosphate-induced delayed neuropathy
- PMSF:
-
Phenylmethylsulfonyl fluoride
- PV:
-
Phenyl valerate
- PVase:
-
Phenyl valerate esterase
- SDS:
-
Sodium dodecyl sulfate
References
Atack JR, Yu QS, Soncrant TT, Brossi A, Rapoport SI (1989) Comparative inhibitory effects of various physostigmine analogs against acetyl- and butyrylcholinesterases. J Pharmacol Exp Ther 249(1):194–202
Barril J, Estévez J, Escudero MA, Céspedes MV, Ñíguez N, Sogorb MA, Monroy A, Vilanova E (1999) Peripheral nerve soluble esterases are spontaneously reactivated after inhibition by paraoxon: implications for a new definition of neuropathy target esterase. Chem Biol Interact 119–120:541–550
Benabent M, Vilanova E, Mangas I, Sogorb MA, Estévez J (2014a) Interaction between substrates suggests a relationship between organophosphorus-sensitive phenyl valerate- and acetylcholine-hydrolyzing activities in chicken brain. Toxicol Lett 230:132–138
Benabent M, Vilanova E, Sogorb MA, Estévez J (2014b) Cholinesterase assay by an efficient fixed time endpoint method. MethodsX. 1:258–263
Carrington CD, Abou-Donia MB (1984) The correlation between the recovery rate of neurotoxic esterase activity and sensitivity to organophosphorus-induced delayed neurotoxicity. Toxicol Appl Pharmacol 75(2):350–357
Céspedes MV, Escudero MA, Barril J, Sogorb MA, Vicedo JL, Vilanova E (1997) Discrimination of carboxylesterases ofchicken neural tissue by inhibition with a neuropathic, nonneuropathicorganophosphorus compounds and neuropathy promoter. Chem Biol Interact 106(3):191–200
Chemnitius JM, Haselmeye, r K.H., Zech R (1983) Neurotoxic esterase. Identification of two isoenzymes in hen brain. Arch Toxicol 53(3):235–244
Chemnitius JM, Haselmeyer KH, Gonska BD, Kreuzer H, Zech R (1997) Mipafox differential inhibition assay for heart muscle cholinesterases: substrate specificity and inhibition of three isoenzymes by physostigmine and quinidine. Gen Pharmacol 28(4):567–575
Chemnitius JM, Sadowski R, Winkel H, Zech R (1999) Organophosphate inhibition of human heart muscle cholinesterase isoenzymes. Chem Biol Interact 119–120:183–192
Copeland AR (2000) Enzymes: a practical introduction to structure, mechanism, and data analysis. 2nd edn. Wiley
Copeland AR (2005) Evaluation of enzyme inhibitors in drug discovery. Wiley
Cornish-Bowden A (2004) In principles of enzyme kinetics, 3rd edn. In: Cornish-Bowden A (ed) Portland Press, pp 36–39
Costa LG (2006) Current issues in organophosphate toxicology. Clin Chim Acta 366(1–2):1–13
COT (1999) Organophosphates. A report of the committee on toxicology of chemicals in food: consumer products and the Environment UK Department of Health, London
Escudero MA, Céspedes MV, Vilanova E (1997) Chromatographic discrimination of soluble neuropathy target esterase isoenzymes and related phenyl valerate esterases from chicken brain, spinal cord, and sciatic nerve. J Neurochem 68(5):2170–2176
Estévez J, García-Pérez AG, Barril J, Pellín M, Vilanova E (2004) The inhibition of the high sensitive peripheral nerve soluble esterases by mipafox. A new mathematical processing for the kinetics of inhibition of esterases by organophosphorus compounds. Toxicol Lett 151(1):171–181
Estévez J, Barril J, Vilanova E (2010) Inhibition with spontaneous reactivation and the “ongoing inhibition” effect of esterases by biotinylated organophosphorus compounds: S9B as a model. Chem Biol Interact 187(1–3):397–402
Estévez J, García-Pérez A, Barril J, Vilanova E (2011) Inhibition with spontaneous reactivation of carboxyl esterases by organophosphorus compounds: paraoxon as a model. Chem Res Toxicol 24(1):135–143
Estévez J, Barril J, Vilanova E (2012) Kinetics of inhibition of soluble peripheral nerve esterases by PMSF: a non-stable compound that potentiates the organophosphorus-induced delayed neurotoxicity. Arch Toxicol 86:767–777
Glynn P, Read DJ, Guo R, Wylie S, Johnson MK (1994) Synthesisand characterization of a biotinylated organophosphorus ester fordetection and affinity purification of a brain serine esterase: neuropathytarget esterase. Biochem J 301(Pt 2):551–556
Glynn P, Holton JL, Nolan CC, Read DJ, Brown L, Hubbard A, Cavanagh JB (1998) Neuropathy target esterase: immunolocalizationto neuronal cell bodies and axons. Neurosci 83(1):295–302
Hou S, Xue L, Yang W, Fang L, Zheng F, Zhan CG (2013) Substrate selectivity of high-activity mutants of human butyrylcholinesterase. Org Biomol Chem 11(43):7477–7485
Jamal GA, Hansen S, Julu, P.O.O. (2002) Low level exposures to organophosphorus esters may cause neurotoxicity. Toxicology 181–182:23–33
Johnson MK (1975) Structure-activity relationships for substrates and inhibitors of hen brain neurotoxic esterase. Biochem Pharmacol 24(7):797–805
Johnson MK (1977) Improved assay of neurotoxic esterase for screening organophosphates for delayed neurotoxicity potential. Arch Toxicol 37(2):113–115
Kaplan D, Ordentlich A, Barak D, Ariel N, Kronman C, Velan B, Shafferman A (2001) Does “butyrylization” of acetylcholinesterase through substitution of the six divergent aromatic amino acids in the active center gorge generate an enzyme mimic of butyrylcholinesterase? BioChemistry 40(25):7433–7445
Kraut D, Goff H, Pai RK, Hosea NA, Silman I, Sussman JL, Taylor P, Voet JG (2000) Inactivation studies of acetylcholinesterase with phenylmethylsulfonyl fluoride. Mol Pharmacol 57(6):1243–1248
Kropp TJ, Richardson RJ (2007) Mechanism of aging of mipafox-inhibited butyrylcholinesterase. Chem Res Toxicol 20(3):504–510
Lockridge O, La Du BN (1978) Comparison of atypical and usual human serum cholinesterase. Purification, number of active sites, substrate affinity, and turnover number. J Biol Chem 253(2):361–366
Lockridge O, Schopfer LM (2006) Biomarkers of exposure. In: Gupta RC (ed) Toxicology of organophosphate and carbamate compounds. Academic Press, San Diego, pp 703–715
Lotti M, Caroldi S, Capodicasa E, Moretto A (1991) Promotion of organophosphate-induced delayed polyneuropathy by phenylmethanesulfonyl fluoride. Toxicol Appl Pharmacol 108:234–241
Mangas I, Vilanova E, Estévez J (2011) Kinetics of the inhibitory interaction of organophosphorus neuropathy inducers and non-inducers in soluble esterases in the avian nervous system. Toxicol App Pharmacol 256:360–368
Mangas I, Vilanova E, Estévez J (2012) Phenylmethylsulfonyl Fluoride a Potentiator of Neuropathy, alter the interaction of organophosphorus compounds with soluble brain esterases. Chem Res Toxicol 25:2393–2401
Mangas I, Vilanova E, Benabent M, Estevez J (2014) Separating esterase targets of organophosphorus compounds in the brain by preparative chromatography. Toxicol Lett 225:167–176
Mangas I, Radić Z, Tailor P, Ghassemian M, Candela H, Vilanova E, Estévez J (2016) Butyrylcholinesterase identification in a pheny lvalerate esterase enriched fraction sensitive to low mipafox concentrations in chicken brain. Arch Toxicol. doi:10.1007/s00204-016-1670-6
Masson P, Lockridge O (2010) Butyrylcholinesterase for protection from organophosphorus poisons: catalytic complexities and hysteretic behavior. Arch Biochem Biophys 494(2):107–120
Masson P, Adkins S, Gouet P, Lockridge O (1993) Recombinant human butyrylcholinesterase G390V, the fluoride-2 variant, expressed in Chinese hamster ovary cells, is a low affinity variant. J Biol Chem 268(19):14329–14341
McDaniel KL, Moser VC (2004) Differential profiles of cholinesterase inhibition and neurobehavioral effects in rats exposed to fenamiphos or profenofos. Neurotoxicol Teratol 26(3):407–415
Petroianu G, Kühn F, Arafat K, Zuleger K, Missler A (2004) In vitro protection of plasma cholinesterases by metoclopramide from inhibition by mipafox. J Appl Toxicol 24(2):143–146
Pope CN, Padilla S (1990) Potentiation of organophosphorus-induced delayed neurotoxicity by phenylmethylsulfonyl fluoride. J Toxicol Environ Health 31:261–273
Reiner E, Simeon-Rudolf V, Skrinjaric-Spoljar M (1995) Catalytic properties and distribution profiles of paraoxonase and cholinesterase phenotypes in human sera. Toxicol Lett 82–83:447–452
Sogorb MA, Vilanova E (2010). Detoxication of anticholinesterase pesticides. In: Satoh T, Gupta RG (eds) Anticholinesterase pesticides: metabolism, neurotoxicity and epidemiology. Willey, pp 121–133
Sun H, El Yazal J, Lockridge O, Schopfer LM, Brimijoin S, Pang YP (2001) Predicted Michaelis-Menten complexes of cocaine-butyrylcholinesterase. Engineering effective butyrylcholinesterase mutants for cocaine detoxication. J Biol Chem 276(12):9330–9336
Terry AV (2012) Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Ther 134(3):355–365
Vilanova E, Barril J, Carrera V, Pellin MC (1990) Soluble and particulate forms of the organophosphorus neuropathy target esterase in hen sciatic nerve. J Neurochem 55(4):1258–1265
Weingand-Ziade A, Ribes F, Renault F, Masson P (2001) Pressure- and heat-induced inactivation of butyrylcholinesterase: evidence for multiple intermediates and the remnant inactivation process. Biochem J 356(Pt 2):487–493
Acknowledgements
We are most grateful to Dr David Lenz and Dr Douglas Cerasoli [USAMRICD (US Army Medical Research Institute of Chemical Defense), Aberdeen Proving Ground, MD, USA] for the supply of hBuChE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Funding sources statement
Institutional funds.
Rights and permissions
About this article
Cite this article
Mangas, I., Vilanova, E. & Estévez, J. Phenyl valerate esterase activity of human butyrylcholinesterase. Arch Toxicol 91, 3295–3305 (2017). https://doi.org/10.1007/s00204-017-1946-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-017-1946-5