Abstract
Current developmental neurotoxicity (DNT) testing in animals faces major limitations, such as high cost and time demands as well as uncertainties in their methodology, evaluation and regulation. Therefore, the use of human-based 3D in vitro systems in combination with high-content image analysis (HCA) might contribute to DNT testing with lower costs, increased throughput and enhanced predictivity for human hazard identification. Human neural progenitor cells (hNPCs) grown as 3D neurospheres mimic basic processes of brain development including hNPC migration and differentiation and are therefore useful for DNT hazard identification. HCA of migrated neurospheres creates new challenges for automated evaluations because it encompasses variable cell densities, inconsistent z-layers and heterogeneous cell populations. We tackle those challenges with our Omnisphero software, which assesses multiple endpoints of the ‘Neurosphere Assay.’ For neuronal identification, Omnisphero reaches a true positive rate (TPR) of 83.8 % and a false discovery rate (FDR) of 11.4 %, thus being comparable to the interindividual difference among two researchers (TPR = 94.3, FDR = 11.0 %) and largely improving the results obtained by an existing HCA approach, whose TPR does not exceed 50 % at a FDR above 50 %. The high FDR of existing methods results in incorrect measurements of neuronal morphological features accompanied by an overestimation of compound effects. Omnisphero additionally includes novel algorithms to assess ‘neurosphere-specific’ endpoints like radial migration and neuronal density distribution within the migration area. Furthermore, a user-assisted parameter optimization procedure makes Omnisphero accessible to non-expert end users.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodevelopmental toxicants represent a threat for society as a small loss in IQ points may not necessarily be recognized by individuals, but poses personal as well as monetary burdens on whole populations (Bellanger et al. 2013; Weiss 1988). To protect developing brains from unnecessary damage, testing for developmental neurotoxicity (DNT) of compounds is desired (Bal-Price et al. 2015; Goldman and Koduru 2000). Current DNT toxicity testing in animals faces major limitations, such as high cost and time demands, extensive animal usage, as well as uncertainties in their methodology, evaluation and regulation (Tsuji and Crofton 2012). As a result, till 2012 only, around 110 DNT guideline studies have been conducted (Tsuji and Crofton 2012). Due to this lack of information, there is still an ongoing debate on the contribution of environmental chemicals to human neurodevelopmental diseases such as attention deficit hyperactivity disorders, autism and many others (Grandjean and Landrigan 2014; Kalkbrenner et al. 2014; McDonald and Paul 2010). For studying risks of compounds as contributing etiological factors in such diseases, more information on compounds’ DNT hazard and potency needs to be acquired. In line with the proposed paradigm shift in toxicology away from apical endpoint testing in animal models toward mode-of-action-based hazard assessment in in vitro systems and in non-mammal in vivo models, several alternative methods for detecting DNT have been developed (Bal-Price et al. 2012; Fritsche et al. 2015).
For evaluating DNT-related endpoints in such systems reproducibly and in a higher throughput, novel technologies, like high-content image analysis (HCA), are indispensable (NRC 2007). HCA approaches are currently mainly designed for two-dimensional (2D) single cell type cultures (Harrill et al. 2010, 2011a; Ryan et al. 2016; Wilson et al. 2014) with only a few exceptions for more complex heterogeneous 2D (Anderl et al. 2009; Harrill et al. 2011b) and none for organoid cultures. Due to the self-organized nature of cell–cell contacts between multiple cell types, the latter is closer to the in vivo situation than homogeneous 2D cultures (Alépée et al. 2014). Therefore, we have developed the ‘Neurosphere Assay’ as a promising module of an envisioned DNT testing strategy (Baumann et al. 2015) (Fig. 1a, b, e). The assay is based on primary human neural progenitor cell (hNPC) clusters, which grow in 3D and mimic several neurodevelopmental endpoints including migration and differentiation into neurons, astrocytes and oligodendrocytes in vitro (Baumann et al. 2014; Moors et al. 2009). Exposure of these neurosphere cultures toward test compounds allows to assess disturbances of these processes (Barenys et al. 2016; Baumann et al. 2015; Gassmann et al. 2010; Moors et al. 2007, 2009). Quantification of hNPC migration and neuronal differentiation including assessment of neuronal morphology by HCA requires advanced scanning and data analysis procedures, because the neurospheres’ 3D structure results in assay-specific challenges. First, neurospheres contain a 3D neurosphere core, which, if not corrected for, leads to unfocused images. We omitted the 3D neurosphere core from analysis, since its evaluation would require a 3D reconstruction, thus significantly increasing the image acquisition time. Second, the density of cells within the migration area of a neurosphere varies from areas close to the neurosphere core to the periphery of the migration area. This is an analytical issue as most HCA studies rely on adjusting cell densities in the samples to facilitate object discrimination (Harrill et al. 2010). Third, neurospheres consist of a heterogeneous cell population of neurons and glia cells. The composition of this heterogeneous cell population is strongly dependent on the differentiation time, with increased percentage of neurons and oligodendrocytes and decreased number of nestin-/glial fibrillary acidic protein (GFAP)-positive cells over time (Fig. 1m–r and Online Resource, S.1.4). Within this study, hNPC were differentiated for 5 days obtaining on average 18 ± 3 % of neurons, 8 ± 2 % oligodendrocytes and 75 ± 6 % of nestin-/GFAP-positive cells [mean ± standard error of the mean; Fig. 1n and (Barenys et al. 2016)]. Finally, it is desirable to measure neurosphere-specific endpoints like radial glia and neuronal migration by HCA, as these endpoints cannot be measured in a conventional monolayer culture. By plating a 3D cell aggregate, we obtain a guided radial NPC migration originating from the neurosphere core. Migrated cells differentiate over time into neurons, astrocytes and oligodendrocytes forming a multiple cell layer with neurons migrating on top of the glia cells (Alépée et al. 2014; Baumann et al. 2016). This allows on the one hand to measure the average migration distance of all cells, which is dictated by the glia cell migration. On the other hand, this enables to specifically measure neuronal migration by assessing the neuronal density distribution, which describes distributions of neurons within the migration area formed by the glia cells.
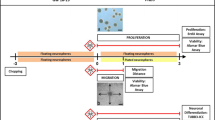
Omnisphero workflow and cell composition over time. Neurospheres (a) were plated on PDL-/laminin-coated multiwell plates (b) and incubated with either acrylamide, MeHgCl or EGF over 5 days. c Afterward cells were fixed and immunocytochemically stained and whole wells (196 images per well of a 96 well plate) were imaged [nuclei channel (green), neuron channel (magenta)] using the ArrayScan VTI (Thermo Fisher). The in-built NPBA analyzes images for neuron quantification (j) and neuronal morphology (k). Omnisphero combines all 196 image tiles (d) to one large image montage (e). The complete image montage is binarized, and binary nuclei images are used for nucleus quantification using a watershed algorithm (f). Connected components in the overlap of the binarized nucleus channel and the binarized neuron channel are used for identifying neuron candidates (g). The binary neuron image is further processed with a skeletonization algorithm (h). i Shows the final Neuron Tracer algorithm which uses the skeletons from (h) to verify the neuronal positions determined by the overlap neuron identification algorithm (g) and to assess neuronal morphology (k). Beside these classical endpoints, it is also possible to assess radial migration distance and neuronal density distributions utilizing spatial information of the image montage (l). To characterize the cell type composition of the formed migration area in the system, we performed time course experiments quantifying the raw numbers (m) and percentages (n) of neurons, oligodendrocytes and nestin-/GFAP-positive cells for 5 days. o Representative image of a 5 days differentiated neurosphere stained for Hoechst (blue), βIII-tubulin (red), O4 (green), nestin (yellow) and magnification of single cells highlighted in the yellow box. To further characterize the nestin-positive cell population, we performed triple staining of Hoechst (blue), nestin (red) and GFAP (green) of 5 days differentiated neurospheres (p Hoechst, nestin and GFAP, q Hoechst and GFAP, r Hoechst and nestin and representative magnifications highlighted in yellow boxes), revealing that most cells are double positive for GFAP and nestin (color figure online)
To tackle these challenges, we implemented the new software Omnisphero which identifies the ‘classical’ endpoints neuron quantification and neuronal morphology but also novel endpoints like radial migration and neuronal density distributions within the Neurosphere Assay. Furthermore, we assessed Omnisphero’s accuracy and sensitivity with respect to an extensive manual ground truth annotation and manual migration distance measurements. These results were further compared to the Neuronal Profiling BioApplication version 4.1 (NPBA) module of the vHCS-Scan (build 6585) software. Omnisphero is accessible to non-expert end users through a graphical user interface (GUI) and an automated parameter optimization. While our main target is to facilitate automated HCA DNT screenings, our image analysis approaches may also be applied in other 3D in vitro systems such as in the migration assay of tumor spheroids (Vinic et al. 2013).
Methods
Cell culture
hNPCs (Lonza, Verviers, Belgium) from gestational week 16 to 20 were grown as neurospheres in proliferation medium. Differentiation was initiated via growth factor withdrawal in differentiation medium and plating on poly-d-lysine (PDL)-/laminin-coated 96-well plates (Online Resource S.1.1–1.2).
Time course experiments
For a detailed description of time course experiments for system characterization included in Fig. 1m–r, we refer to Online Resource, S.1.2–1.4, S2.
Chemical exposure, cytotoxicity assay and immunocytochemical stainings
Neurospheres were incubated with increasing concentrations of either EGF, acrylamide or MeHgCl under differentiating conditions for 5 days. Cell viability was assessed, cells were fixed, and immunostaining was performed (Online Resource, S.1.2–1.3). For each compound, four individual experiments with three technical replicates per concentration were conducted.
Image preprocessing
Preprocessing
Microscopic images of complete microtiter well plates were generated for the nuclei channel (Hoechst 33258) and the neuron channel (βIII-tubulin) utilizing the ArrayScan VTI (Thermo Fisher Scientific). For details, see Online Resource, S.2. Images were recorded in tiles, which were subsequently stitched to obtain a whole neurosphere image which displays a complete well of a 96-well plate (Fig. 1e).
Identification of nuclei
Coordinates of nuclei were identified as centroid coordinates in the whole neurosphere images utilizing the Spot Detector BioApplication version 4 to later enable a direct comparison between the coordinates of cell nuclei of neurons identified by Omnisphero and the NPBA. We further implemented an independent nucleus identification algorithm in Omnisphero for users not working with the NPBA (Online Resource, S.3.1 and Fig. 1f).
Neurosphere core removal
The core of the neurosphere is occupied by densely clustered progenitor cells which are not relevant for morphological analysis. Thus, the neurosphere core is masked out from the image using a thresholding approach (Online Resource, S.3.2) and omitted from further analysis.
Skeletonization
For the neuron channel, the morphological skeleton S N of every connected component N in the neuron binary channel is computed using a refined version of the approach from Wang et al. (2013) based on the method proposed by Bai et al. (2007) (Fig. 1h and Online Resource, S.3.3).
Determining standard analysis endpoints
Automated quantification of neurons
We define a nucleus as a neuron nucleus if there is a closed connected component in the overlay of the binarized neuron and nucleus image and if the nucleus is uniquely assigned to one skeleton S N. If the closed connected component exceeds a predefined size threshold (Fig. 1g), the nucleus is considered as a neuron nucleus candidate. Those candidates are investigated further by the Neuron Tracer algorithm which utilizes the skeletonization results (Fig. 1i). A neuron nucleus candidate associated with a skeleton S N is rejected if length or roundness of the skeleton falls below a user-defined threshold. Furthermore, areas with low signal-to-noise ratio in the neuron channel are disregarded. Neuron nuclei candidates located on the remaining skeletons S N are counted as neuron nuclei. Whenever no neuron nucleus candidate is associated with a skeleton, the nearest neighbor nucleus at the endpoints of the skeleton can be assigned if it is located within a certain distance and angle to an endpoint. The parameters involved in these steps are determined using an automated parameter optimization (see “Automated parameter optimization” section).
Neuronal morphology
Neuronal morphology is characterized on the single cell level through four features normalized per neuron, namely the number, total length and average length of neurites adjacent to one neuron nucleus and the number of branching points within these neurites. In order to measure only morphological properties of the neurites, the cell soma has to be excluded. The soma of hNPCs can be considered equivalent to the area of the neuron nuclei. Therefore, all vertices covered by a nucleus N are not considered part of the respective skeleton S N, resulting in a new set of subskeletons representing the neurites (Online Resource, S.4).
Validation
As validation measures, we determined the numbers of true positives (TPs) and false positives (FPs) as identified by an automated algorithm compared to a manual ground truth annotation utilizing a manual counting tool in Omnisphero. Furthermore, we determined the true positive rate (TPR) as the ratio of TPs to the total number of manually annotated neurons as well as the false discovery rate (FDR) as the proportion of FPs to all automatically identified neurons (Online Resource, S.6.1).
Determining extended analysis endpoints
Migration area and migration distance
The migration area is spanned between the boundary of the neurosphere core and the furthest migrated cells. These boundaries are obtained by subdividing the image into 64 wedges centered at the centroid coordinate of the neurosphere core (see Neurosphere core removal) and determining for each wedge one coordinate located on the boundary of the neurosphere core and one at the rim of the furthest migrated cells. The average distance between those coordinates for all 64 wedges delivers the average migration distance (Online Resource, S.5.1 and Fig. 1l).
Neuronal density distribution
The neuronal density distribution is assessed by subdividing the migration area into ten rings with equal width. Within each ring, the ratio of neurons to cell nuclei is calculated and normalized to the average ratio within the entire migration area, resulting in a distance-dependent density function (Online Resource, S.5.2 and Fig. 1l).
Automated parameter optimization
The different analysis steps of our approach involve three parameter categories: (1) general image preprocessing parameters for thresholding the neuron and nucleus channel as well as thresholds for identifying and eliminating low signal-to-noise areas in the neuron channel, (2) parameters for the minimum overlap between connected components, and (3) parameters regarding neurite morphology like neurite length, the minimum distance and tolerance angle for searching neuron nuclei starting at skeleton endpoints. To avoid the obvious difficulty to adjust many parameters manually, we use a small annotated reference data set to optimize these parameters (Online Resource, S.7.1–7.2).
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 6 (La Jolla, California). Dose–response curves for viability, neuronal quantification, neuronal morphology, migration distance and neuronal density distributions were obtained from four independent experiments with three technical replicates per exposure. The raw values for viability (CTB: fluorescence signal) of each technical replicate within one plate were corrected by subtraction of a background control (containing only medium and CTB reagent), averaged and normalized to the mean of the solvent controls (differentiation medium for acrylamide and EGF or 0.015 % DMSO in differentiation medium for MeHgCl). Data were further analyzed as percentage of control and are presented as percentage of control ± SEM (standard error of the mean). Neuronal quantification, morphology and migration distance raw values (percentage of neurons as number neurons/number of nuclei, neurite length in µm, number of branching points and migration distance in µm) were averaged for all technical replicates within one plate. The resulting mean values were averaged among the four individual experiments. Data are presented as mean ± SEM. For dose–response curves, EC50 values were determined by using a sigmoidal dose–response (variable slope) fit with values of solvent controls as upper constraint and zero as lower constraint. For the neuronal density distributions, the average slopes of the four independent experiments were determined using a nonlinear regression (straight line). Results are shown as mean ± SEM. Significant differences within one method were analyzed using the analysis of variance (ANOVA) with a post hoc Sidak test (p < 0.05). Intermethodological differences were analyzed using a multiple t test assuming non-consistent standard deviations and the Holm–Sidak method for determining the statistical significance (α < 0.05). For box plot diagrams, the Tukey representation was chosen. Differences between two groups were calculated using unpaired t test and F-test for comparison of variances. Bland–Altman diagrams were plotted as average against differences of automated methods and manual evaluation using raw values of each single well of all experiments. Raw values were transformed to logarithmic scale. Bias and 95 % confidence intervals were retransformed to normal scale resulting in percentage values.
Results
Methodological comparison: neuronal differentiation
The Omnisphero Neuron Tracer algorithm exhibits a significantly higher TPR, lower FDR and a lower variance for those values among the full concentration range of the three model substances compared to the NPBA (Fig. 2a–f, j, k).
Methodological comparison of neuronal quantification. a–c TPR of the NPBA, the Omnisphero Neuron Tracer algorithm and manual evaluation for acrylamide, EGF and MeHgCl. d–f Corresponding FDR for all methods. j Box plot representation of combined TPR data of (a–c) and k respective box plot representation of FDR of (d–f). g–i Dose–response curves for neuronal differentiation and viability by means of Alamar Blue assay and respective EC50 values. l Bland–Altman diagram for comparison of manual evaluation versus automated evaluation. Data are plotted as average against differences of log-transformed raw values. Bias is shown as continuous line and confidence intervals as dotted lines and is given as percentage values. Results are obtained from four independent experiments with three technical replicates per concentration and are presented as mean ± SEM. Significant differences among one method are indicated as * and intermethodological significant differences as #. Significant differences of the mean in the box plot representation are indicated as ** and difference in the variance as ## (color figure online)
Comparison between automated and manual evaluation of neuron cell body coordinates revealed an average TPR of 83.8 % for our Omnisphero Neuron Tracer algorithm compared to 41.9 % for the existing approach implemented in NPBA. The average FDR of our Neuron Tracer algorithm is 11.4 % and thus much lower than the 52.1 % FDR of NPBA. Average TPR and variation of both automated methods are represented as box plot diagrams in Fig. 2j and corresponding data for FDR in Fig. 2k, respectively. Statistical analyses revealed a significant difference between the mean values of the automated methods for both TPR and FDR (Fig. 2j, k). The Neuron Tracer algorithm shows a significantly lower variance compared to the NPBA, revealing a much higher robustness of this method. The highest variance is found for the FDR of the NPBA especially for high concentrations indicated by the non-symmetric box plot in Fig. 2k.
Comparison of concentration–response curves for percentage of neurons (number of neurons counted at day five normalized to the number of cell nuclei in the total migration area) and calculated EC50 values obtained by automated methods and manual evaluation revealed no statistically significant intermethodological differences (Fig. 2g–i). EC50 values are consistently higher for NPBA compared to manual and Neuron Tracer evaluation. Significant effects of tested substances on neuronal differentiation observed by automated and manual evaluations correspond to each other (acrylamide: 0.35 mM for all methods; EGF: 0.5 ng/ml for all methods; MeHgCl: 0.11 µM for all methods). Bland–Altman diagrams revealed a high accordance between manual and Neuron Tracer evaluation (Fig. 2l). The NPBA shows a proportional error overestimating values for high and underestimating values for low concentrations resulting in a wide 95 % confidence interval (from 57.3 to −53.1 %).
Methodological comparison: neurite outgrowth
In order to compare different methods, total neurite length, average neurite length, number of branching points and number of neurites were determined using the same skeletonization-based approach (see Skeletonization) for coordinates obtained by automated methods and by manual evaluation. Differences within the results can therefore be attributed to differences in identified neuron coordinates of the different methods. The manual evaluation was taken as the gold standard to study intermethodological comparisons using the Bland–Altman representation. The Omnisphero Neuron Tracer showed the lowest bias and the lowest range of the 95 % confidence interval among automated methods indicating the highest accordance toward manual evaluation (Fig. 3a–d). The NPBA tends to overestimate the morphological features which becomes most obvious for the number of branching points (Fig. 3d, i) for which values determined using the coordinates identified by NPBA are between 25 and 30 % higher compared to the manual evaluation. The GUI of Omnisphero revealed the source of this overestimation as a false identification of neuron nuclei located next to neurites by the NPBA (Fig. 3e, f). Calculation of the bias which is always positive for NPBA reveals a systematic error of the method which persists for average neurite length and total neurite length (Fig. 3a, b, g, h), but not for the number of neurites (Fig. 3c). For a complete set of all concentration–response curves, we refer to Online Resource, Fig. S.7.
Neuronal morphology. Bland–Altman diagrams of combined data of all three model substances for total neurite length (a), average neurite length (b), number of neurites (c) and number of branching points (d) for comparison of the two automated evaluations versus manual evaluation. e Representative images of FPs identified by NPBA visualized with the GUI of Omnisphero are indicated as red dots. f Representative skeleton images with artificial branching points originating from FP’s identified by NPBA located next to a neurite. Examples of dose–response curves for total neurite length (g), average neurite length (h) and number of branching points (i). Results are obtained from four independent experiments with three technical replicates per concentration and are presented as mean ± SEM. Significant differences among one method are indicated as *. Bland–Altman diagrams are plotted as average against differences of log-transformed raw values. Bias is shown as continuous lines and confidence intervals as dotted lines and is given as percentage values (color figure online)
Extended endpoints: migration distance and neuronal density distribution
Both manual evaluation and Omnisphero reveal an increase in migration distance upon EGF treatment and a concentration-dependent decrease in migration distance induced by acrylamide and MeHgCl with no significant difference between methods (Fig. 4a–c).
Migration distance and neuronal density distributions. a–c Migration distance obtained by manual and automated measurements of Omnisphero for the three model substances. d–f Average slope of each neuronal density function obtained by manual evaluation, Omnisphero and NPBA for all three model substances and all concentrations. g Neuronal density graphs of untreated samples and h, i EGF-treated samples (0.5 ng/ml and 5 ng/ml EGF). g, i Equal distributions are indicated as homogeneous green-colored migration areas and h altered density distributions as color-coded migration areas. Results are obtained from four independent experiments with three technical replicates per condition and are shown as mean ± SEM. Significant differences among one method are indicated as * (color figure online)
The average slope of neuronal density distribution graphs showed no significant difference between manual evaluation, Omnisphero and NPBA (Fig. 4d–f). Untreated neurospheres showed a close to ideal equal distribution of neurons for all distances (Fig. 4g). Treatment with acrylamide and MeHgCl did not influence this neuronal density distribution at any concentrations indicated by a stable slope (Fig. 4d, f). Low concentrations of EGF (0.5–2.5 ng/ml, Fig. 4h), however, significantly altered the neuronal density distribution. Under these concentrations, the density of neurons was higher in the vicinity of the neurosphere core and decreased with increasing distance. This effect disappeared at higher concentrations (Fig. 4i).
Discussion
There is a substantial gap in both knowledge and data for compounds’ DNT hazard and potency (Grandjean and Landrigan 2014; Tsuji and Crofton 2012). To close this gap, screening tools are needed that reliably predict DNT hazard in a higher throughput than the rat in vivo guideline studies (Bal-Price et al. 2015). Among the multiple test systems useful for studying a variety of DNT endpoints across a number of species, human-based cell models, such as stem/progenitor cell cultures, are currently suggested as most predictive for human hazard (Fritsche et al. 2015; Jennings 2015). This is due to the instance that they are the correct species, can be grown in 3D (Alépée et al. 2014), consist of multiple cell types (Moors et al. 2009) and thus allow evaluation of a large variety of endpoints (Fritsche et al. 2015). To study multiple neurodevelopmentally relevant endpoints within the Neurosphere Assay in medium throughput, we developed the Omnisphero software to facilitate substance screening for DNT hazard assessment of compounds.
In this article, we mainly address the two questions: (1) how robust is the Omnisphero approach for delivering reliable assessments of DNT screenings involving a large number of substances and (2) how do the results acquired with the novel methods compare to the existing approaches of manual counting and the NPBA approach.
Omnisphero detects neurons with a TPR of 83.8 % and a FDR of 11.4 % and thus exceeds the NPBA TPR of 41.9 % with a FDR of 52.1 %. Remarkably, the performance of Omnisphero remains constant or even improves compared to NPBA over the full range of concentrations for all substances. The FDR of NPBA increases significantly with increasing concentrations (Fig. 2d–f). The overall higher variability of NPBA shown in the box plot diagram in Fig. 2j, k will narrow down the size of effects detectable with this approach. Besides this quantitative analysis of TPR and FDR, the GUI of Omnisphero also facilitates to qualitatively assess the reliability of an automated method by displaying the neuron coordinates. In particular, it is possible to specifically display the coordinates of all unidentified or falsely identified neurons. We utilized this feature to identify potential subpopulations of cells not detectable or falsely detected by a specific algorithm in order to spot systematic errors of this algorithm. In fact, we identified a systematic error for NPBA which tends to assign glial nuclei as neuronal when passed by a neurite (see Fig. 3e). This can be explained by the algorithm used by NPBA, which identifies neurons by measuring relative overlap between the neuron fluorescence channel and a ring placed around the identified nucleus. Neurites crossing a non-neuron nucleus will produce a corresponding overlap, so that areas with a high neuron density are particularly prone to produce false positive neurons. In contrast, the approach in Omnisphero is based on a combination of overlap criteria and a skeletonization, which prevents this particularly frequent source of FP neurons by the NPBA. Overall, our results strongly indicate that the quantification of neuronal features by Omnisphero is highly robust and far exceeds the robustness of existing approaches. To validate the algorithms of Omnisphero for substance screening, we treated neurospheres over 5 days with three model substances with well-known specific effects on DNT endpoints (differentiation and migration) starting at non-cytotoxic concentrations: acrylamide (Park et al. 2010), EGF (Ayuso-Sacido et al. 2010) and MeHgCl (Baumann et al. 2015; Moors et al. 2009). All substances are expected to decrease neuronal differentiation in a concentration-dependent manner, yet by different mechanisms: inducing NPC apoptosis as well as general neurotoxicity in the case of acrylamide (Erkekoglu and Baydar 2014; Park et al. 2010) and inhibiting neuronal differentiation by EGF (Ayuso-Sacido et al. 2010) and MeHgCl (Baumann et al. 2015). Concentration–response curves for all three substances generated by manual evaluation, which is considered the ‘gold standard,’ were compared to the automated methods. All methods delivered comparable curve shapes with significant effects starting at 0.35 mM for acrylamide, 0.5 ng/ml for EGF and 0.11 µM for MeHgCl (Fig. 2g–i). Calculated EC50 values revealed no statistically significant difference between methods, but were always higher for NPBA (Fig. 2g–i). In order to figure out the reason for this overestimation, we created Bland–Altman diagrams (Fig. 2l), demonstrating a proportional error of NPBA, leading to an overestimation of high concentrations and an underestimation for low concentrations. The overestimation of the percentages of neurons for high concentrations in the concentration–response curves is attributed to the high FDR of the NPBA (Fig. 2k). The underestimation for low concentrations is more likely attributed to the low TPR of the NPBA. Since high concentrations decrease the number of neurons to below 1 % of the control value, even high FDR alterations will not significantly alter the curve fitting for determining the EC50 value. In contrast, the underestimation for low concentrations can play a more crucial role, especially when concentration–response curves display a slight slope. In this case, the underestimation for low concentrations could result in missing of low concentration effects leading to significantly higher EC50 values.
Besides neuronal quantification, we evaluated neuronal morphology on the single cell level after 5 days of differentiation in the same samples: total neurite length, average neurite length, number of branching points and number of neurites (Fig. 3). MeHgCl reduced neurite outgrowth in rat, but not in human embryonic stem cell-derived neuronal cultures at non-cytotoxic concentrations (Harrill et al. 2011a). In line with these results, we observed that MeHgCl affected neuronal morphology of human NPC-derived neurons only for the highest concentration of MeHgCl tested (0.33 µM, Fig. 3i), which exceeds the concentration reducing neuronal differentiation (0.11 µM) and decreased cell viability (Fig. 2i). So far nothing is known for EGF’s ability to interfere with neuronal morphology. Since EGF inhibits neuronal differentiation (Ayuso-Sacido et al. 2010), we expected an EGF-induced delayed neuronal maturation possibly resulting in reduction in neurite outgrowth and branching. Indeed, we observed a reduction in total and average neurite length, number of branching points and a significant decrease in neurite number upon EGF exposure (Online Resource Fig.S. 7a–d) at concentrations not reducing viability (Fig. 2h). The effects of acrylamide on neuron morphology of developing neurons are, to the best of our knowledge, not known. Acrylamide’s mode of actions is associated with three mechanisms suspected to induce neurite degeneration in fully differentiated neurons: inhibition of kinesin-based fast axonal transport, alteration of neurotransmitter levels and direct inhibition of neurotransmission (Erkekoglu and Baydar 2014). Since 5 days differentiated neurons from hNPCs are relatively immature, acrylamide effects on neurite outgrowth are expected to be limited. This is in line with the automated and manual results: Only the NPBA detects effects in total neurite length (Fig. 3g) for the highest acrylamide concentration applied, while the number of neurons was significantly reduced at a subcytotoxic concentration of 0.35 mM (Fig. 2g). However, one limitation of assessing neurite outgrowth is the dynamic range of values (Harrill et al. 2011a), which for developing neurons in the neurosphere system (50–60 µm) is much lower compared to, for example, primary cortical cultures (129.8 ± 12.6 µm). Therefore, small compound effects on this endpoint cannot be distinguished from in vitro model variability. Comparison of the evaluation performances for endpoints concerning neuronal morphology revealed similar curve shapes for manual and automated methods (Online Resource, S.7). While curves of manual evaluation and of the Omnisphero algorithm correspond very precisely, the curve of NPBA indicates an overestimation of total neurite length (Fig. 3a, g), of average neurite length (Fig. 3b, h) and, most notably, of the number of branching points (Fig. 3d, i). In order to identify a potential systematic error, we again generated Bland–Altman diagrams (Fig. 3a–d), revealing a systematic error for NPBA as well as a higher variability compared to the Neuron Tracer. The NPBA algorithm overestimates the number of branching points by up to 39.2 % (Fig. 3d). Utilizing the GUI of Omnisphero, we were able to identify falsely assigned neuron nuclei by the NPBA located next to a neurite as the source of the artificial branching points (Fig. 3e, f). This overestimation effect tends to result in an artificially high sensitivity of NPBA for adverse outcomes. This is demonstrated by NPBA detecting significant effects of EGF on average neurite length (Fig. 3h) and of acrylamide on total neurite length (Fig. 3g), which are not confirmed by either manual evaluation or the Omnisphero algorithms. These significant effects on neuronal morphology originate from the higher measures of the control values (Fig. 3g, h). Additionally, the higher overall variability of NPBA leads to the loss of significant results for the total number of branching points in the case of MeHgCl (Fig. 3i).
All model substances tested interfere with cell migration in vitro and/or in vivo. MeHgCl decreases migration in vitro and in vivo, acrylamide disturbs migration in vivo (Kakita et al. 2002; Ogawa et al. 2011), and EGF induces cellular migration in vitro and in vivo (Ayuso-Sacido et al. 2010; Puehringer et al. 2013). Both manual and automatic evaluations of radial migration detected either concentration-dependent increases (EGF) or decreases (acrylamide, MeHgCl) for radial migration with no detectable intermethodological differences, further indicated by a close to ideal overlap of plotted curves of Omnisphero and manual evaluation (Fig. 4a–c).
The last endpoint evaluated was the neuronal density distribution, which provides the unique opportunity of measuring specific neuronal migration in vitro and is, to the best of our knowledge, the first time assessed in a 3D in vitro system. Untreated neurospheres display a close to ideal equal distribution of neurons migrating on the glia scaffold (Baumann et al. 2016) (Fig. 4g). MeHgCl did not alter neuronal migration by any applied concentration (Fig. 4f). Because MeHgCl interferes with SH-groups of proteins (Bernhoft 2012), our data indicate that glial cell migration might be more sensitive to MeHgCl exposure (Fig. 4c) than neuronal migration itself. However, so far the precise molecular mechanisms behind neuronal migration disturbances in MeHgCl-exposed humans are not clarified (Kakita et al. 2002), but based on our data there is room to speculate that disturbances of neuronal migration due to migrational defects of the glial scaffold might be involved. Acrylamide is also suspected to alter migration resulting in a distorted distribution of neurons (Ogawa et al. 2011). Comparable to MeHgCl, acrylamide decreased the overall migration distance without specific effects on neuron positioning (Fig. 4a, d) pointing toward a non-neuron-specific effect on migration. In contrast to the other substances, EGF is reported to specifically induce neuronal migration in vivo and at 20 ng/ml in vitro (Ayuso-Sacido et al. 2010; Puehringer et al. 2013). In the Neurosphere Assay, increased neuronal migration will manifest in a higher neuronal density toward the periphery of the migration area than close to the neurosphere core, while inhibition of neuronal migration will accumulate neurons in areas close to the neurosphere core. Very low concentrations of EGF (0.5–2.5 ng/ml) caused the latter with neuronal gathering in the central migration areas (Fig. 4e, h). Increasing EGF concentrations up to 20 ng/ml restored equal distribution of neurons across the migration area (Fig. 4e, i). Because all EGF concentrations enhanced the total radial migration distance (Fig. 4b), the restoration of equal neuronal distribution at higher EGF concentrations is actually only possible by increased neuronal migration. Thus, our HCA allows evaluation of migration of specific cell types in this mixed culture 3D system.
Investigation of different training set sizes in the automated parameter optimization (Online resource S.7.2) of Omnisphero revealed that a manual evaluation time of roughly 1 h delivers comparable precise results for parameter settings than those obtained by experts (manual parameter set: TPR = 82.4; FDR = 12.5 %; quality index (QI, defined in Online Resource, S.7.1) = 70.6 % and automatically optimized with a reduced training set: TPR = 81.0; FDR = 14.5 %; QI = 67.2 %). It is important to note that parameters for one condition, in our case 5 days differentiated hNPCs, remain constant and can therefore be used for multiple experiments. In order to exclude a bias introduced by the manual annotation of one researcher, we compared the manual annotation used in this study to an additional researcher (Online Resource, S.6.2). In order to assess potential bias of a manual ground truth annotation, we performed two independent ground truth annotations by two different experts on one and the same data set. Cohen’s kappa value, as an established measure of interrater agreement (Cohen 1968; Stang et al. 2006), yields an agreement of κ = 0.90, which qualifies for perfect agreement (Landis and Koch 1977). This is also reflected by a high TPR of 94.3 % and a low FDR of 11.0 % by the second researcher compared to the first, exceeding the TPR and FDR values obtained by Omnisphero (κ = 0.81) and NPBA (κ = 0.38).
In summary, our novel approach implemented in the Omnisphero software facilitates a highly robust HCA-based evaluation of primary human neurospheres contributing to DNT in vitro testing. Omnisphero assesses effects of three model substances on the Neurosphere Assay and achieves accuracy and precision that is comparable to manual evaluation. In contrast to existing software, Omnisphero is also capable of analyzing spatial distribution of cells within the entire well including endpoints like radial migration and neuronal density distributions. Through the automated parameter optimization, Omnisphero is highly automated and thus suitable for users without data analysis or programming experience in studies involving large numbers of experiments. Furthermore, the migration endpoints can be transferred to other 3D cell systems such as tumor spheroids, where radial migration is a measure for invasiveness (Vinic et al. 2013).
References
Alépée N, Bahinski A, Daneshian M, Wever BD, Fritsche E (2014) State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology. Altex 31:441–477. doi:10.14573/altex1406111
Anderl JL, Redpath S, Ball AJ (2009) A neuronal and astrocyte co-culture assay for high content analysis of neurotoxicity. J Vis Exp JoVE. doi:10.3791/1173
Ayuso-Sacido A, Moliterno JA, Kratovac S et al (2010) Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells. J Neurooncol 97:323–337. doi:10.1007/s11060-009-0035-x
Bai X, Latecki LJ, Liu WY (2007) Skeleton pruning by contour partitioning with discrete curve evolution. IEEE Trans Pattern Anal Mach Intell 29:449–462. doi:10.1109/TPAMI.2007.59
Bal-Price AK, Coecke S, Costa L et al (2012) Advancing the science of developmental neurotoxicity (DNT): testing for better safety evaluation. Altex 29:202–215. doi:10.14573/altex.2012.2.202
Bal-Price A, Crofton KM, Leist M et al (2015) International STakeholder NETwork (ISTNET): creating a developmental neurotoxicity (DNT) testing road map for regulatory purposes. Arch Toxicol 89:269–287. doi:10.1007/s00204-015-1464-2
Barenys M, Gassmann K, Baksmeier C et al (2016) Epigallocatechin gallate (EGCG) inhibits adhesion and migration of neural progenitor cells in vitro. Arch Toxicol. doi:10.1007/s00204-016-1709-8
Baumann J, Barenys M, Gassmann K, Fritsche E (2014) Comparative human and rat “neurosphere assay” for developmental neurotoxicity testing. Curr Protoc Toxicol 59:12.21.1–12.21.24. doi:10.1002/0471140856.tx1221s59
Baumann J, Gassmann K, Masjosthusmann S et al (2015) Comparative human and rat neurospheres reveal species differences in chemical effects on neurodevelopmental key events. Arch Toxicol. doi:10.1007/s00204-015-1568-8
Baumann J, Dach K, Barenys M et al (2016) Application of the neurosphere assay for DNT hazard assessment: challenges and limitations. Methods Pharmacol Toxicol. doi:10.1007/7653
Bellanger M, Pichery C, Aerts D et al (2013) Economic benefits of methylmercury exposure control in Europe: monetary value of neurotoxicity prevention. Environ Health 12:3. doi:10.1186/1476-069X-12-3
Bernhoft RA (2012) Mercury toxicity and treatment: a review of the literature. J Environ Public Health 2012:1–10. doi:10.1155/2012/460508
Cohen J (1968) Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull 70(4):213–220
Erkekoglu P, Baydar T (2014) Acrylamide neurotoxicity. Nutr Neurosci 17:49–57. doi:10.1179/1476830513Y.0000000065
Fritsche E, Alm H, Baumann J et al (2015) Literature review on in vitro and alternative developmental neurotoxicity (DNT) testing methods. EFSA Support Publ 778:1–186. doi:10.2903/sp.efsa.2015.EN-778
Gassmann K, Abel J, Bothe H et al (2010) Species-specific differential AhR expression protects human neural progenitor cells against developmental neurotoxicity of PAHs. Environ Health Perspect 118:1571–1577. doi:10.1289/ehp.0901545
Goldman LR, Koduru S (2000) Chemicals in the environment and developmental toxicity to children: a public health and policy perspective. Environ Health Perspect 108(Suppl):443–448. doi:10.2307/3454535
Grandjean P, Landrigan PJ (2014) Neurobehavioural effects of developmental toxicity. Lancet Neurol 13:330–338. doi:10.1016/S1474-4422(13)70278-3
Harrill JA, Freudenrich TM, Machacek DW, Stice SL, Mundy WR (2010) Quantitative assessment of neurite outgrowth in human embryonic stem cell-derived hN2 cells using automated high-content image analysis. Neurotoxicology 31:277–290. doi:10.1016/j.neuro.2010.02.003
Harrill JA, Freudenrich TM, Robinette BL, Mundy WR (2011a) Comparative sensitivity of human and rat neural cultures to chemical-induced inhibition of neurite outgrowth. Toxicol Appl Pharmacol 256:268–280. doi:10.1016/j.taap.2011.02.013
Harrill JA, Robinette BL, Mundy WR (2011b) Use of high content image analysis to detect chemical-induced changes in synaptogenesis in vitro. Toxicol In Vitro 25:368–387. doi:10.1016/j.tiv.2010.10.011
Jennings P (2015) The future of in vitro toxicology. Toxicol In Vitro 29:1217–1221. doi:10.1016/j.tiv.2014.08.011
Kakita A, Inenaga C, Sakamoto M, Takahashi H (2002) Neuronal migration disturbance and consequent cytoarchitecture in the cerebral cortex following transplacental administration of methylmercury. Acta Neuropathol 104:409–417. doi:10.1007/s00401-002-0571-3
Kalkbrenner AE, Schmidt RJ, Penlesky AC (2014) Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Amy Curr Probl Pediatr Adolesc Health Care 44:277–318. doi:10.1016/j.cppeds.2014.06.001.Environmental
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
McDonald ME, Paul JF (2010) Timing of increased autistic disorder cumulative incidence. Environ Sci Technol 44:2112–2118. doi:10.1021/es902057k
Moors M, Cline JE, Abel J, Fritsche E (2007) ERK-dependent and -independent pathways trigger human neural progenitor cell migration. Toxicol Appl Pharmacol 221:57–67. doi:10.1016/j.taap.2007.02.018
Moors M, Rockel TD, Abel J et al (2009) Human neurospheres as three-dimensional cellular systems for developmental neurotoxicity testing. Environ Health Perspect 117:1131–1138. doi:10.1289/ehp.0800207
NRC (2007) Toxicity testing in the 21st century: a vision and a strategy. The National Academies Press, London, pp 1–216
Ogawa B, Ohishi T, Wang L et al (2011) Disruptive neuronal development by acrylamide in the hippocampal dentate hilus after developmental exposure in rats. Arch Toxicol 85:987–994. doi:10.1007/s00204-010-0622-9
Park HR, Kim M-S, Kim SJ et al (2010) Acrylamide induces cell death in neural progenitor cells and impairs hippocampal neurogenesis. Toxicol Lett 193:86–93. doi:10.1016/j.toxlet.2009.12.015
Puehringer D, Orel N, Lüningschrör P et al (2013) EGF transactivation of Trk receptors regulates the migration of newborn cortical neurons. Nat Neurosci 16:407–415. doi:10.1038/nn.3333
Ryan KR, Sirenko O, Parham F et al (2016) Neurite outgrowth in human induced pluripotent stem cell-derived neurons as a high-throughput screen for developmental neurotoxicity or neurotoxicity. Neurotoxicology. doi:10.1016/j.neuro.2016.02.003
Stang A, Pohlabeln H, Muller KM, Jahn I, Giersiepen K, Jockel KH (2006) Diagnostic agreement in the histopathological evaluation of lung cancer tissue in a population-based case-control study. Lung Cancer 52(1):29–36. doi:10.1016/j.lungcan.2005.11.012
Tsuji R, Crofton KM (2012) Developmental neurotoxicity guideline study: issues with methodology, evaluation and regulation. Congenit Anom 52:122–128. doi:10.1111/j.1741-4520.2012.00374.x
Vinic M, Box C, Zimmermann M, Eccles SA (2013) Target identification and validation in drug discovery target identification and validation. Drug Discov 986:187–214. doi:10.1007/978-1-62703-311-4
Wang C, Gui C-P, Liu H-K, Zhang D, Mosig A (2013) An image skeletonization-based tool for pollen tube morphology analysis and phenotyping. J Integr Plant Biol 55:131–141. doi:10.1111/j.1744-7909.2012.01184.x
Weiss B (1988) Neurobehavioral toxicity as a basis for risk assessment. Trends Pharmacol Sci 9:59–62. doi:10.1016/0165-6147(88)90118-6
Wilson MS, Graham JR, Ball AJ (2014) Multiparametric high content analysis for assessment of neurotoxicity in differentiated neuronal cell lines and human embryonic stem cell-derived neurons. Neurotoxicology 42:33–48. doi:10.1016/j.neuro.2014.03.013
Funding
This project was funded by the DFG through the research training group 1033 and by the German Ministry for Education and Research (16V0899).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Martin R. Schmuck and Thomas Temme: shared first authorship. Axel Mosig and Ellen Fritsche: shared senior authorship.
Open source software (under the GPLv3 license) is available at https://www.omnisphero.com.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmuck, M.R., Temme, T., Dach, K. et al. Omnisphero: a high-content image analysis (HCA) approach for phenotypic developmental neurotoxicity (DNT) screenings of organoid neurosphere cultures in vitro. Arch Toxicol 91, 2017–2028 (2017). https://doi.org/10.1007/s00204-016-1852-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1852-2