Abstract
MicroRNAs (miRNAs) have been shown to be critical mediators of many cellular and developmental processes and have been implicated in different human diseases. Since the observation of extracellular miRNAs present in various biofluids, much attention and excitement have been garnered toward understanding the functional roles of these circulating extracellular miRNAs and establishing their potential use as noninvasive diagnostic biomarkers. Here, we will review the current state of miRNA biomarkers for many human diseases, including their emerging use in toxicological applications, and discuss the current challenges in the field, with an emphasis on technical issues that often hinder discovery-based miRNA biomarker studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
MicroRNAs: discovery, synthesis, and function
Noncoding RNAs (ncRNAs) are a diverse subset of RNA molecules that unlike coding messenger RNAs (mRNAs) are not translated into a protein product, but function intrinsically as critical structural molecules or regulators of various cellular processes. Aside from the historically well-studied transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs), other classes of ncRNAs have come into the spotlight, such as long noncoding RNAs (lncRNAs) and microRNAs (miRNAs), which have garnered extreme interest in the past few years. MiRNAs are a class of short noncoding RNAs that have been shown to modulate proteome by affecting the stability of specific mRNA, or through inhibiting protein translation (Fabian et al. 2010). These short regulators were first identified by Victor Ambrose’s group in Caenorhabditis elegans, and it was thought that this type of regulatory RNA was present only in non-vertebrates (Lee et al. 1993). However, with the advent of genome sequencing, over 25,000 individual miRNAs have now been identified in more than 200 species including viruses, plants, and humans (Kozomara and Griffiths-Jones 2014). In addition, some of the miRNA sequences are highly conserved across species; this strong evolutionary conservation suggests its critical involvement in regulating key biological processes. The miRNA targeting mechanisms appear to differ between plants and animals, which may suggest miRNAs evolving twice from an ancestral RNA-mediated gene-silencing process in early eukaryotes (Axtell et al. 2011).
The biogenesis of miRNA has been reviewed in recent years by others (for example, Ha and Kim 2014). Like protein-coding transcripts, miRNAs are transcribed as part of a larger primary miRNA (pri-miRNA). Through a series of enzyme complex (including the ribonuclease, Drosha, and the RNA-binding protein, DGCR8), the 5′ cap and 3′ poly A tail are removed to generate a shorter (~70 bp) precursor miRNA (pre-miRNA). The pre-miRNA is then transported into cytoplasm and processed by the ribonuclease, Dicer, into a 19- to 24-bp mature miRNA duplex. The miRNA then incorporates into the RNA-induced silencing complex (RISC) and interacts with target mRNA through imperfect base-paring usually at its 3′ untranslated regions (UTRs). This miRNA–mRNA interaction promotes degradation of the transcript, or inhibits protein translation by blocking the progression or binding of ribosome (Pillai 2005). MiRNAs have been implicated to function in a wide category of normal cellular processes including differentiation, proliferation, cell death, signal transduction, stress response, and metabolism (Huang et al. 2011). With its diverse function, it is not surprising that miRNAs have also been observed to be involved in many diseases, including cardiovascular, neurological, and metabolic diseases, as well as various forms of cancers (Erson and Petty 2008).
Extracellular miRNAs
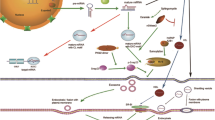
Recently, miRNAs have been detected in the extracellular environment, circulating in various biological fluids including sera, plasma, urine, tears, saliva, seminal fluid, cerebrospinal fluid (CSF), extracellular fluid (ECF), and others. While the exact role for these extracellular miRNAs remains to be precisely determined, there has been speculation that at least a fraction of the extracellular miRNAs may function as mediators of paracrine or endocrine signaling between cells. RNAs are often degraded at extracellular environment due to the high ribonucleases activity. Nonetheless, the presence of many miRNA species in these biofluids suggests that they are able to escape degradation through some protective mechanisms. MiRNA has been observed to be complexed with lipoproteins and RNA-binding proteins including HDL (Vickers et al. 2011; Wagner et al. 2013), AGO2 (Arroyo et al. 2011; Turchinovich et al. 2011), and NPM1 (Wang et al. 2010b). These RNA–protein complexes maybe the results of cell death and autophagy and do not necessarily represent actively exported miRNAs of physiological significance. Besides complexed with proteins, miRNA has also been found in lipid vesicles including microvesicles, exosomes, and apoptotic bodies. Among them, the exosome has drawn a significant interest in recent years.
Work by Valadi et al. 2007 showed that miRNAs were present in exosomes, a class of secreted vesicles between 30 and 100 nm in size, originating from multi-vesicle bodies (MVBs), and released through fusion with the cellular membrane (Denzer et al. 2000; Valadi et al. 2007). The uptake of exosomes has been shown to have a significant biological impact in the recipient cells. For example, it has been shown that macrophages can transfer miR-223 via exosomes into breast cancer cell lines and enhance their invasiveness by targeting MEF2C (myocyte enhancer factor 2C) and CTNNB1 (β-catenin) (Yang et al. 2011). It has also been demonstrated that rat primary mesenchymal stromal cells can transfer miR-133b via exosomes to primary neurons and astrocytes, promoting neurite outgrowth (Xin et al. 2012). While these findings suggest that miRNAs can be transferred between cells via exosomes, the mechanisms that dictate their selective packaging into the exosomes, delivery to target cells, and subsequent activation remain elusive. Kosaka et al. have identified a ceramide-dependent pathway for sorting miRNA into exosomes in human cell lines. They showed that disrupting the biosynthesis of the sphingolipid ceramide, through perturbation of the critical enzyme neutral sphingomyelinase 2 (nSMase2), affected the levels of miRNAs present in exosomes (Kosaka et al. 2010). In addition, mutations in KRAS affect the miRNA content in exosome (Cha et al. 2015), which may affect the tumor microenvironment and influence tumor cell growth and migration (Demory Beckler et al. 2013). The work with KRAS in particular shows how exosomal miRNAs may have a significant clinical impact on disease progression and diagnosis. Sequence motifs present in miRNAs are also play a role in export into exosomes. For example, in primary T lymphoblasts, sumoylated hnRNPPA2B1 has been shown to recognize a G/A-rich motif present in the 3′ end of miRNAs and can facilitate their exported into exosomes (Villarroya-Beltri et al. 2013). In B cell lines, miRNAs with non-template additions of terminal uridines (Us) were more frequently observed in exosomes, whereas miRNAs with non-template additions of adenines (As) were enriched in cells (Koppers-Lalic et al. 2014). These findings suggested that (1) a role for exosomes in miRNA trafficking through cell membrane, (2) exosomal miRNAs maybe functional, and (3) specific cellular mechanisms are responsible for miRNA packaging and export. Much more work needs to be done to elucidate the detailed mechanism involved in this process.
Role for extracellular miRNAs as potential biomarkers for disease
While the observation of circulating nucleic acids in plasma and serum has been sustained over the last 65 years (Ayala et al. 1951; Mandel and Metais 1948), the recent observation of circulating miRNAs present in different biofluids has spurred the interest of using these extracellular miRNAs as biomarkers for different diseases. Many of the current disease biomarkers are proteins that are circulating in body fluids. Examples include the prostate-specific antigen (PSA, KLK3) used to screen for prostate cancer and troponin (TNNT2) to diagnose myocardial infarction. Unfortunately, the discovery of new protein-based biomarkers has many technical challenges, such as low protein abundancy in samples and difficulties in developing high affinity capture reagent. MiRNAs offer many advantages over their protein counterparts in many of these regards. Here, we will provide some updates on the current status and challenges of identifying circulating miRNAs as biomarkers.
Cancer
As extracellular miRNAs were first observed in cancer cell lines (Chen et al. 2008; Mitchell et al. 2008), and because of the recent excitement in the approach of ‘liquid biopsy’ to detect and monitor cancer, considerable efforts have gone into identifying circulating miRNAs involved in or associated with various forms of cancer, and how they might possibly influence the tumor microenvironment. This section is not considered to be exhaustive list of all the studies looking at circulating miRNAs involved in cancer, as many excellent reviews have been written on this topic (see our recent meta-analysis and review—He et al. 2015).
Lung cancer
Lung cancer is the most common cause of cancer-related death worldwide, with non-small cell lung cancer (NSCLC) accounts for the majority of cases. Signatures of specific miRNAs present in tumor tissues have already been shown to be useful in predicting survival and relapse in NSCLC (Yu et al. 2008). Chen et al. made one of the first observational reports of circulating miRNAs and found miR-25 and miR-223 to be elevated in serum samples from NSCLC patients versus healthy controls. In one study, miRNA-1, miR-30d, miR-499, and miR-486 were highlighted as serum-based predictive markers (Hu et al. 2010). Shen et al. identified a six miRNA signature (see Table 1) showing similar concentration changes between plasma and corresponding tumor tissues in NSCLC patients and were able to discriminate NSCLC patients from healthy controls. Another group showed that serum levels of miR-486-5p were predictive of survival in NSCLC (Petriella et al. 2015). Conversely, elevated serum levels of miR-21 have been shown to be associated with poor prognosis in NSCLC patients in several studies (Gao et al. 2010; Liu et al. 2012b; Wang et al. 2011) and a marker of metastasis to other organs (Liu et al. 2012b). Interestingly, elevated levels of miR-21 have also been observed in the sputum of NSCLC patients (Xie et al. 2010). Additionally, when looking at circulating miRNA biomarkers, several groups have found that larger panels of circulating miRNAs (between 10 and 34) increase the sensitivity of NSCLC detection and can predict early-stage NSCLC (see Table 1; Bianchi et al. 2011; Boeri et al. 2011; Wozniak et al. 2015).
Breast cancer
While there has been lots of effort looking at genetic risk factors for developing breast cancer, there is lack of reliable noninvasive diagnostic markers. Work has been done to identify circulating miRNA that may have diagnostic potential for breast cancer. Examining serum of breast cancer patients versus controls, Mar-Aguilar et al. identified a seven miRNA signature that were increased in the serum of cancer patients (Table 1). Further work has shown the combination of miR-451 and miR-145 concentration changes in plasma was able to discriminate cancer patients from healthy controls (Ng et al. 2013). The concentrations of miR-505-5p and miR-96-5p were also identified as being significantly increased in the serum of early-stage breast cancer patients versus controls, and miR-505-5p levels decreased in early-stage breast cancer patients that had undergone treatment (Matamala et al. 2015). The levels of several miRNAs (miR-148b, miR-376c, miR-409-3p, and miR-801) have been observed to be increased in plasma of breast cancer patients compared to healthy controls (Cuk et al. 2013).
A major emphasis of cancer treatment and intervention has been directed at the process of metastasis. Various studies have looked at the changes of miRNAs in patients with metastatic breast cancer (van Schooneveld et al. 2012; see Table 2). Several groups have observed the increase in circulating miR-10b concentration in metastatic breast cancer patients (Chen et al. 2013; Zhao et al. 2012), and silencing of miR-10b has been shown to inhibit metastasis in a mouse mammary tumor model (Ma et al. 2010). Studies suggesting that the potential of using specific miRNA as both biomarker and therapeutic target and using circulating miRNAs as prognostic marker for patients with metastatic breast cancer has also been reported. Madhavan et al. identified a panel of circulating miRNAs (Table 2) associated with overall survival of patients with metastatic breast cancer. Several members of this panel, like the miR-200 family, are enriched in exosomes (Meng et al. 2016) and involved in promoting metastasis and tumor progression (Feng et al. 2014).
Colorectal cancer
Colorectal cancer (CRC) is the third most deadly form of cancer worldwide. The potential of using the changes of circulating miRNA as diagnostic marker for CRC has been examined. The concentration of miR-21 has been consistently shown to be increased in both plasma and serum of CRC patients among various studies (Krichevsky and Gabriely 2009), as well as enriched in exosomes isolated from serum of CRC patients (Ogata-Kawata et al. 2014). A recent meta-analysis has shown that miR-21 can discriminate between CRC patients and healthy controls and may have prognostic utility (Shan et al. 2015). In addition, study found elevated levels of miR-135b, miR-95, miR-222, miR-17-3p and miR-92 in the plasma of CRC patients (Ng et al. 2009). Further work by Huang et al. confirmed elevated levels of miR-92 along with miR-29 in a different cohort of CRC patients. Interestingly, elevated miR-29 and miR-141 levels can be used to identify patients with metastatic CRC. The level of circulating miR-141 can also be used as a prognostic marker, as elevated miR-141 is associated with poor outcome in metastatic CRC patients (Cheng et al. 2011; Wang and Gu 2012). Some circulating miRNAs have been shown to be able to predict treatment response. For example, Hansen et al. 2015) have shown that miR-126 can be used to evaluate response to treatment in metastatic CRC patients undergoing chemotherapy (Table 2).
Pancreatic cancer
Currently, pancreatic cancer is the fourth most deadly cancer worldwide, with very limited diagnostic tools available to catch early occurrences. Resultantly, the five-year survival rate for the most common type of pancreatic cancer, pancreatic ductal adenocarcinoma (PDAC), is only about 5 %, so identifying diagnostic and prognostic biomarkers for PDAC is urgently needed. Four miRNAs (miR-21, miR-155, miR-210, and miR-196a) previously shown to be implicated in PDAC have been shown to be increased in the plasma of PDAC patients versus healthy controls (Wang et al. 2009). Interestingly, miR-210 is hypoxia-inducible, which may reflect the environment of tumor growth in PDAC patients (Ho et al. 2010). Liu et al. identified a seven miRNA panel (see Table 2) that was both significantly elevated in serum from PDAC patients, and able to discriminate between PDAC patients and chronic pancreatitis (CP) patients (Liu et al. 2012a). The level of miR-21 in serum can also have similar diagnostic utility when combined with other miRNAs, such as miR-34a (Alemar et al. 2016), or miR-483-3p (Abue et al. 2015). In the latter case, miR-483-3p was able to discriminate patients with PDAC from patients with intraductal papillary mucinous neoplasm (IPMN), a less severe form of pancreatic tumor that can give rise to PDAC. In order to benchmark the use of circulating miRNAs as diagnostic or prognostic biomarkers in pancreatic cancers, comparative studies with current biomarkers must be done. The most commonly used biomarker for pancreatic cancer is carbohydrate antigen 19-9 (CA 19-9), which unfortunately suffers from low specificity (Goonetilleke and Siriwardena 2007). Morimura et al. have shown the elevated miR-18a levels in plasma from preoperative PDAC patients and recurrent PDAC patients after surgery, whereas CA 19-9 level did not change. Work by Liu et al. showed that two miRNAs, miR-16 and miR-196a, were elevated in the plasma of PDAC patients and when combined with CA 19-9 provided the most effective diagnosis of PDAC. The level of circulating miR-196 in plasma has also been shown to predict survival rates in advanced-stage PDAC patients, where elevated miR-196a correlates with a lower survival rate (Kong et al. 2011b). Looking at whole blood samples from PDAC patients, Schultz et al. identified two miRNA signatures (see Table 2) that outperformed CA 19-9 in discriminating PDAC patients from healthy controls, but performed best when used in combination with CA 19-9. Examining plasma of PDAC patients, Chen et al. observed elevated levels of miR-182 in PDAC patients that was able to discriminate PDAC patients from healthy controls. The elevated level of miR-182 was also associated with shortened overall survival and disease-free survival (Chen et al. 2014b). The pancreas secretes local biofluids, such as pancreatic juice and bile. While these biofluids are more invasively obtained than serum or plasma, they offer an opportunity to examine the local tumor microenvironment more closely. Wang et al. found the concentrations of miR-205, miR-210, miR-492, and miR-1247 in pancreatic juice increased in PDAC patients and were able to discriminate between PDAC and non-PDAC controls alone, or with increased sensitivity when combined with CA 19-9 (Wang et al. 2014). Cote et al. examined several miRNAs known to be involved in PDAC and found five (miR-10b, miR-155, miR-106b, miR-30c, and miR-212) that were elevated in bile of PDAC patients versus healthy controls. Moreover, performance of this miRNA panel in discriminating PDAC patients versus control patients was moderately enhanced when using bile versus plasma, suggesting that miRNA in bile may offer more precise diagnostic performance in detecting PDAC.
Diabetes
Diabetes mellitus (DM) and its associated ailments pose a significant impact on human health. Reliable diagnostic and prognostic biomarkers are needed for DM and their related conditions, such as cardiovascular diseases, kidney failure, and pathologies related to DM induced disruption of macro- and microvascular functions. Several miRNAs play critical roles in β-cell development and function and have been implicated in diabetes pathogenesis. Some DM-associated miRNAs are involved in insulin resistance in target tissues such as adipose, muscle, and liver cells (Chen et al. 2014a; Guay and Regazzi 2013). Analyzing the changes of circulating miRNAs in patients with DM provides the possibility of using miRNA to identify prediabetic individuals, assess β-cell function, and monitor the development of DM-related conditions.
T1DM
Looking at serum miRNA levels between newly diagnosed T1DM patients versus healthy controls, Nielsen et al. found that the concentration of a subset of miRNAs (Table 3) that are involved β-cell function and development was increased in sera from T1DM patients. One miRNA, miR-25, was negatively associated with residual β-cell function and positively associated with glycemic control suggesting potential clinical utilities in T1DM. Similarly when examining miRNA levels in plasma and urine, Osipova et al. found elevated levels of miR-21 and miR-210 in both plasma and urine in T1DM patients compared to healthy controls. The miR-21 also showed modest discriminatory power in distinguishing T1DM patients from controls. Additionally, in peripheral blood mononuclear cells (PBMCs), the miR-21 levels are also altered in T1DM patients (Salas-Pérez et al. 2013). The kidneys are drastically affected by microvascular perturbations caused by T1DM, which often result in progressive kidney failure, or diabetic nephropathy (DN). If left untreated, DN can rapidly progress to end-stage renal disease (ESRD) and eventually complete kidney failure. Work by our group has profiled miRNAs present in urine in T1DM patients undergoing various stages of DN and found that several miRNAs (see Table 3) were dysregulated in T1DM patients with DN, compared to matched controls of T1DM patients without DN (Argyropoulos et al. 2013). Microalbuminuria is one of the earliest indications of onset of DN and is a useful time point to asses DN status. When looking at T1DM patients that were albuminuric versus non-albuminuric, we identified gender-dependent miRNA signatures that could identify whether an individual with T1DM would develop microalbuminuria (Argyropoulos et al. 2015). Interestingly, many of the targets of these miRNAs identified in urine are associated with biological pathways perturbed in DN and other renal and kidney diseases, such as VEGF, EGF, FGF, and TGF-β/BMP signaling. Similarly, Pezzolesi et al. tested 5 miRNAs regulated by TGF-β/BMP in plasma and found let-7c-5p and miR-29a-3p were associated with protection against rapid ESRD progression and let-7b-5p and miR-21-5p were associated with rapid ESRD progression. Notably, let-7b-5p and miR-21-5p have been previously shown to be involved in renal cell function in DN, suggesting that these miRNAs might be targets for therapeutic intervention for the development of DN.
Another complication that can arise due to microvascular disruption in T1DM is diabetic retinopathy (DR), where damage to the vasculature surrounding the retina can cause vision problems potentially leading to blindness. Utilizing serum samples from a previous clinical trial DR, Zampetaki et al. identified two miRNAs (miR-27b and miR-320a) that were highly associated with the incidence and progression of NPDR (non-proliferative diabetic retinopathy). Furthermore, they performed proteomic experiments on cultured endothelial cells and identified the antiangiogenic factor, thrombospondin-1, as common target of miR-27b and miR-320a. In NPDR patients, successive hemorrhaging of the vasculature surrounding the retina can cause the transition from NPDR to proliferative diabetic retinopathy (PDR), where the risk of blindness and related ocular complications increases significantly. Looking at the transition from NPDR to PDR, Qing et al. identified a three miRNA signature (miR-21, miR-181c, and miR-1179) that could differentiate PDR from both NPDR and controls. While miR-21 has been shown to be directly involved in angiogenesis by activating VEGF expression through the PTEN/AKT pathway (Liu et al. 2011), the roles of miR-181c and miR-1179 in T1DM or DR are not clear.
T2DM
One of the first studies to assess the circulating miRNAs profile changes associated with T2DM identified 13 miRNAs (Table 4) that showed concentration changes (Zampetaki et al. 2010). Of these, five of the most significantly changed miRNA: miR-15a, miR-126, miR-223, miR-320, and miR-28-3p were able to distinguish T2DM patients from healthy controls. Furthermore, using cell culture and mouse models they found that hypoglycemia triggered a decreases in miR-126 levels in endothelial cells, where it has been previously shown to play a critical role in maintaining vascular integrity and angiogenesis (Fish et al. 2008). Interestingly, when looking at serum of prediabetic patients that will eventually develop T2DM, miR-126 levels are also decreased but went back to healthy baseline levels when patients undergo treatment (diet control and insulin treatment) suggesting possible diagnostic application for miR-126 (Liu et al. 2014). Similarly, Kong et al. identified a signature of seven diabetes-related miRNAs (Table 4) that were elevated in T2DM compared to prediabetics and individuals with normal glucose tolerance. While this signature was able to identify individuals with T2DM from individuals that were prediabetic or had normal glucose tolerance, it failed to separate prediabetic from normal glucose tolerance. This suggests the circulating miRNAs panel identified might not be suitable for predicting which individuals may be susceptible to develop T2DM. Of these miRNAs, miR-146 has been shown to play a critical role in regulating oxidative stress caused by iron metabolism, which has been implicated in T2DM-related pathologies (Balasubramanyam et al. 2011; Kozakowska et al. 2012). Additionally, miR-146 levels are elevated in the plasma of newly diagnosed T2DM individuals versus healthy controls (Rong et al. 2013). Ortega et al. identified a signature of 10 miRNAs (Table 4) that showed concentration changes in plasma between T2DM patients and those with normal glucose tolerance. When some T2DM patients underwent treatment with metformin (a glucose regulator), they saw a shift of concentration in plasma for several of these miRNAs (miR-140, miR-222, and miR-195) toward the levels of individuals with normal glucose tolerance, suggesting their potential use in disease management. Notably, this group had previously showed that many of these miRNAs (Table 4) gave similar levels of concentration changes in a separate cohort of morbidly obese patients and showed that after surgical weight loss miR-140 levels returned close to that of healthy controls (Ortega et al. 2013). This is significant as obesity is a strong preexisting risk factor for the development of T2DM. In the same study, they also identified circulating miRNAs that are specifically associated with obesity (miR-15a, miR-423-5p and miR-520c-3p; Ortega et al. 2013, 2014). In a similar study, Pescador et al. identified several miRNAs showing concentration differences among obese, non-obese diabetic, and obese diabetic individuals (Table 4). Together, the levels of miR-15b, miR-138, and miR-376a in serum were found to have moderate discriminatory power in distinguishing obese patients from all other cohorts, while miR-503 and miR-138 were able to distinguish non-obese diabetic from obese diabetic individuals. The results of these studies suggest that obesity and obesity-related T2DM may have their own unique circulating miRNA signatures.
Like with T1DM, complications related to microvascular network dysfunction can arise from T2DM. Comparing to healthy controls, one study identified a five miRNA panel (miR-571, miR-661, miR-770-5p, miR-892b, and miR-1303) in serum that were elevated in T2DM patients with multiple microvascular complications including diabetic retinopathy, diabetic neuropathy, diabetic nephropathy, and diabetic foot (Wang et al. 2016). As discussed previously, many of the circulating miRNAs relevant to pathophysiological conditions and/or disease states are associated with extracellular vesicles (exosomes and microvesicles) and microparticles (HDL and LDL). Because microparticles (MPs) are observed to be increased in circulation in T2DM patients (Leroyer et al. 2008; Tushuizen et al. 2007), Jansen et al. isolated circulating MPs from T2DM patients and healthy controls and characterized the profiles of nine miRNAs (Table 4) previously implicated in T2DM and vascular function. They found significant reduction of miR-26a and miR-126 in MP isolated from T2DM patients versus healthy controls and found that patients with lower levels of miR-26a and miR-126 were at higher risk of coronary artery disease. This is likely significant, because miR-126 is one of the mostly widely reported miRNAs to be altered in T2DM in various studies. In addition to MPs, extracellular vesicles have been investigated as a potential source for miRNAs that may be useful biomarkers in T2DM. Delić et al. identified 16 miRNAs (Table 4) present in urinary exosomes that were differentially regulated in microalbuminuric T2DM patients with DN. Of these, miR-320c has been shown to regulate TGF-b signaling and involved in renal cell tubular injury (Nassirpour et al. 2014); therefore, miR-320c could be a potential biomarker candidate associated with renal pathophysiology for T2DM patients.
When looking at the landscape of studies examining circulating miRNA profiles in both T1DM and T2DM, there has been consistency in reported biomarker candidates (including miR-210 and miR-21, in T1DM; and miR-126 and miR-146 in T2DM); however, additional studies have to be done to confirm the consistency of the miRNA signatures and look more closely on factors that may affect the levels of these miRNA before they can be considered as clinical relevant biomarkers.
Cardiovascular diseases
Cardiovascular disease is a leading cause of death in developed and developing countries that includes a collection of diseases related to vascular dysfunction, such as coronary artery disease (CAD), and diseases related to heart dysfunction, such as heart failure (HF) and myocardial infarction (MI). Much like diabetes, cardiovascular diseases have their own set of risk factors that can influence development and progression of cardiovascular diseases and their associated complications. Indeed, many miRNAs (coined the ‘myomiRs’) have been implicated with direct role in cardiovascular development, function, and disease (see Maegdefessel 2014 and Romaine et al. 2015 for recent reviews), so there is potential to identify circulating miRNAs that may be as potential biomarkers for cardiovascular diseases. We summarized the findings relate to HF and MI, as these are the two most prevalent cardiac diseases globally.
Heart failure
One of the first groups was Corsten et al. to identify circulating miRNAs associated with cardiovascular disease. They investigated plasma levels of several myomiRs in patients suffering from various states of cardiac stress and damage, including HF and MI. They found in HF patients, miR-499 levels were elevated, and in MI patients, miR-208b and miR-499 were elevated when compared to healthy controls. In MI patients, the miR-208b and mir-499 levels in plasma correlated with troponin T, a biomarker that has been used as an indicator of cardiac damage (Corsten et al. 2010). Another study identified a signature of four miRNAs (miR-22, miR-92b, miR-320a, and miR-423-5p) that showed a significant increase in the serum of HF patients versus healthy controls and was able to discriminate between the two cohorts effectively (Goren et al. 2012). Furthermore, HF patients with elevated miRNA signature correlated well with other clinical prognostic parameters indicative of HF, such as B-type natriuretic peptide (BNP) levels and dilation of the left ventricle and atrium. Ellis et al. identified a plasma miRNA signature (Table 5) that had moderate discriminatory capability to identify HF patients from chronic obstructive pulmonary disease (COPD) patients and healthy controls. Combining the miRNA signatures and BNP into a single diagnostic panel achieved the best specificity and sensitivity, suggesting the possibility of using a combined miRNAs and traditional biomarkers in clinic for HF diagnosis (Ellis et al. 2013). Cardiac resynchronization therapy (CRT) is an effective measure used to treat patients suffering from HF; however, there are no biomarkers to predict response to CRT treatment. One study looking at response of HF patients to CRT identified miR-30d, as being elevated in HF patients that responded well to CRT (and inversely correlated with levels of troponin T) compared to non-responder and controls (Melman et al. 2015). They also showed miR-30d protects cardiomyocytes against apoptosis by inhibiting the tumor necrosis factor signaling pathway, and the miRNA is enriched in extracellular vesicles derived from cardiomyocytes released into the environment after stress-induced damage. This finding suggests that miR-30d is a promising biomarker candidate to evaluate HF patient response to CRT. More recently, Akat et al. systematically studied miRNAs in circulation, and those expressed in the myocardium in HF patients, and found that the concentration of many myocardial myomiRs (Table 5) was elevated in circulation among HF patients and scaled comparably to troponin T levels and severity of the HF (advanced versus stable) (Akat et al. 2014). Furthermore, circulating miRNAs returned to near-normal levels after treatment with a left ventricular assist device (LVAD), suggesting some prognostic value of these miRNAs.
Myocardial infarction
The circulating roles of muscle- and cardiac-enriched miRNAs have also been investigated in MI patients. Elevated levels of miR-1, mir-133a, mir-208a, and mir-499 have been observed in the plasma of MI patients, as early as 4 h post-MI (Wang et al. 2010a). Of these, miR-208a served as the best candidate; they found it was undetectable in non-MI patients and rapidly detected in MI patients. Further work identified myomiR signature in MI patients that provided prognostic value after treatment with coronary reperfusion and showed similar results with an induced MI mouse model (Table 5; D’Alessandra et al. 2010). Because of the similar clinical presentations of MI and HF, and comparable troponin T and myomiR concentration changes, it would be useful in clinic having biomarker to distinguish the two conditions. Olivieri et al. further investigated the myomiRs and found that miR-499-5p had the ability to discriminate MI from HF and control patients based on relative concentration changes—about 80-fold increase in MI patients versus about 20-fold increase in HF patients when compared to healthy controls. This concentration difference-based miRNA biomarker showed a better performance than high-sensitivity troponin T in differentiating MI from HF patients (Olivieri et al. 2013). Interestingly, elevated plasma miR-499-5p has been associated with poor prognosis (increased mortality rate) within a 30-day window post-MI (Gidlöf et al. 2013).
Not all studies agree with the diagnostic and prognostic values of the circulating myomiR-based biomarkers. One of the larger studies, found that elevated myomiR levels in plasma of MI patients performed poorly as diagnostic or prognostic biomarkers when compared to (or combined with) troponin T (Devaux et al. 2012). In addition to the conventional myomiRs discussed here, additional circulating miRNAs have been described as potential biomarkers for MI. Long et al. examined several miRNAs that are involved in cardiac hypertrophy in the plasma of MI patients and found a signature of miR-30a, miR-195, and let-7b provided good discriminatory function (Long et al. 2012). Zhong et al. showed that plasma miR-19a, a miRNA with no prior association with cardiac function, performed superior than the miR-1, troponin T, and BNP in detecting MI (Zhong et al. 2014). Huang et al. identified a two miRNAs signature, miR-125b and miR-320b, that provided good discriminatory function to identify MI patients. These miRNAs regulate many genes and pathways associated with cardiovascular disease, such as TGF-B, apoptosis, and cytokine signaling (Huang et al. 2014).
While there seems to be a consensus that many of the myomiRs and other miRNAs are elevated in circulation during HF and MI, their source remains controversial. Recent work has suggested that these miRNAs are released into the extracellular environment from damaged myocardium after injury (De Rosa et al. 2011; Gidlöf et al. 2013); however, the possibility of selective extracellular release through lipid vesicles such as exosomes has been supported with data in human cell culture and mouse models (Hergenreider et al. 2012; Jansen et al. 2013) and cannot be ruled out.
Toxicology and drug-induced organ damage
Various miRNAs have reproducible and consistent organ-specific expression patterns that may be useful readouts of organ’s health state during different diseases and exposure to toxins or xenobiotics. As discussed previously, the cardiac-enriched myomiRs have been a rich source of circulating miRNA to monitor the outcome and progression of HF and MI, but other organ-specific miRNAs have also shown promise in monitoring organ status. Our own work has shown that plasma measurements of the liver-enriched miRNAs, miR-122 and miR-192 can be used to accurately monitor drug-induced liver injury—DILI in an acetaminophen overdose mouse model, with higher sensitivity than the most commonly use biomarker, alanine aminotransferase (ALT) (Wang et al. 2009). Additionally, elevated serum concentration of miR-122 and miR-192 has been observed in acetaminophen-overdosed patients (Starkey Lewis et al. 2011), and patients with hepatitis-induced liver diseases (Zhang et al. 2010) and cholestatic liver injury (Shifeng et al. 2013). We have also demonstrated that many other transcripts (both endogenous and exogenous) are perturbed during DILI reflecting not only damage to the liver but also to other organ systems including the kidneys. Another group also reported incidences of kidney damage during acetaminophen overdose in patients and identified a plasma miRNA signature specific to either liver (including miR-122) or kidney damage (Vliegenthart et al. 2015) (Table 6). Using urine miRNA profiles, several groups have identified miRNA signatures associated with cisplatin-induced kidney injury in rats (Kanki et al. 2014; Pavkovic et al. 2014, 2015), with recent work by Kanki et al. identifying a urine miRNA signature (miR-21, miR-200, miR-423; Table 6) that is predicted to target genes associated with kidney development and function (Pavkovic et al. 2016). Some preliminary work has been done to identify circulating miRNAs associated with drug-induced myocardial injury or cardiotoxicity. The myomiR, miR-208, has been identified by several groups as a plasma biomarker for isoproterenol-induced myocardial injury (Ji et al. 2009; Nishimura et al. 2015). MiR-579 and miR-1254 were identified by Zhao et al. as having elevated plasma concentrations in bevacizumab-induced cardiotoxicity in CRC patients and were able to discriminate between CRC patients with bevacizumab-induced cardiotoxicity versus control patients (Zhao et al. 2014). MiRNAs have been shown to be affected by toxin exposure (reviewed recently by Vliegenthart et al. 2015), and recent attention has focused on those in circulation. Several studies (in animal models and in humans) have looked at the effect of smoking on miRNAs in circulation. In mice, exposure to cigarette smoke results in the concentration changes of several different miRNAs (see Table 7), many of which are involved in the inflammatory response and have tumor-suppressor functions (Yuchuan et al. 2014). Elevated serum concentrations of miR-206 and miR-133b were observed when rats were exposed to nicotine-derived nitrosamine ketone (NNK), a major component of cigarette smoke (Wu et al. 2013). In humans, exposure to cigarette smoke leads to alterations in the concentration of many plasma miRNAs (Takahashi et al. 2013), including a decrease in platelet-derived microvesicles and miRNAs (Badrnya et al. 2014; Table 7). Environmental exposure to pollutants, such as particulate matter (PM) and harmful chemicals, has been shown to have a serious long-term health effects. Looking at exposure to metal-rich PM, Motta et al. noticed that plasma concentrations of four miRNAs: miR-29a, miR-146a, miR-421 and let-7 g, which are involved in the inflammatory response, were increased when compared to controls. Another study also looking at metal-rich PM exposure observed perturbations in plasma microvesicle-associated miRNAs that are involved in apoptosis, inflammation, and cardiovascular disease (Table 7) in patients exposed to PM (Bollati et al. 2015). Assessing exposure to the industrial chemical, perfluorooctanoic acid (PFOA), one group noticed increased expression of miR-26b and miR-199a-3p (an oncomiR) in individuals exposed to high levels of PFOA versus healthy controls (Wang et al. 2012). Deng et al. examined the plasma miRNAs profile of individuals exposed to polycyclic aromatic hydrocarbons (PAHs) and identified a six miRNA signature (Table 7) altered in individuals exposed to PHA versus non-exposed controls. They further observed that many of these affected miRNAs are predicted to target many genes involved in ROS metabolism, DNA damage and repair, and genome stability.
Progresses, challenges, and future work
The field-circulating miRNA made significant advances since it was first reported in 2008 by Lawrie et al. and Mitchell et al. and proposed their use as biomarkers for different disease conditions. As briefly touched in earlier sections of the review, we have a much better understanding on the possible function of circulating miRNA, factors that may affect the spectrum of miRNA, how miRNA gaining its stability in extracellular environment, the list of miRNAs that are preferentially packaged in exosome, the method to purify exosome and HDL/LDL miRNA particles, the cellular proteins and processes that may affect the export of miRNAs into exosome, and how to profile miRNA in different body fluids. However, there are still many questions and technical issues need to be resolved.
Even though with great enthusiasm on the idea of using circulating miRNA as disease biomarkers, there is no miRNA candidates that has successfully went into clinical development. One of the biggest reasons is the lack of consistency on findings. This is largely caused by the different sample preparation methods and different measurement platforms. The low concentration of RNA in sample, high sequence conservation among miRNA family members, and short sequence length of miRNA all add to the technical challenges for circulating miRNA measurement. Protein biomarkers have been used extensively due to standards and guidelines implemented by governing bodies (such as the FDA) to ensure that the appropriate biomarker is validated and meet the requirements for clinical utility for a given application. Currently, there are no standards or guidelines setup for the development of miRNAs as clinical biomarkers; however, the NIH recently established the Extracellular RNA Communication Consortium (ERCC) to create dialog within the community of researchers studying circulating miRNAs, with the goal of establishing guidelines and standards when performing circulating miRNA studies.
Technical issues on miRNA measurement
While miRNAs have obvious advantages over protein-based biomarkers, such as higher sensitivity and easier to develop assay with high specificity, there still are various points during a circulating miRNA study where various factors can influence the spectrum of miRNAs isolated, which may cause the miRNA candidates identified are not reflecting the true disease state. These artifacts can be introduced during sample preparation, RNA isolation, miRNA measurement method, and data normalization and analysis. During sample preparation (depending on the biofluid being collected), several steps can be taken, such as limiting contamination from other elements (such as RBCs when preparing plasma, serum or CSF), to ensure the consistency of samples in a study. Recent reports have shown that various factors during RNA isolation, such as the isolation method being used and day-to-day batch effects, can influence the miRNA spectra that are isolated from biofluids (El-Khoury et al. 2016; Yuan et al. 2016). Though the majority of studies discussed here do not take exosome-derived miRNAs into accord, the interest in miRNAs isolated from exosomes has come under considerable interest recently and will be very important for future studies. Unfortunately, the most common technique for exosome isolation, ultracentrifugation, has many variables (angle of the rotor, centrifugal force, type of tubes, etc.) that can affect the quality of the exosome preparation (Théry et al. 2006). Commercial precipitation-based methods are easier to use and do not require expensive equipment, but often co-precipitate significant amount of other microvesicles and proteins (Witwer et al. 2013).
Depending on the study being performed, there are several techniques that can be used to quantify miRNA species in a given sample. However, due to their small size, sequence variation at the 3′ ends (isomiRs), and sequence conservation among family members, precise measurement of miRNA concentration in the sample can be complicated. Common methods used in miRNA studies include qRT-PCR, microarrays, and next-generation sequencing (NGS) techniques. Newer platforms, such as Nanostring nCounter, have also been used. qRT-pCR has been considered the ‘gold standard’ of measuring individual miRNAs as it is sensitive, quantifiable, cheap and require low amounts of starting material. Two primary commercial qRT-pCR assays are used in most of the studies presented here, TaqMan (ThermoFischer) and LNA (Exiqon). However, when compared to other methods qRT-PCR can be low throughput and cannot detect novel miRNAs, and technical issues like primer design limit its scale and use. Microarrays have an advantage over qRT-PCR, as they can assay many miRNAs simultaneously; however, they require larger amounts of starting material, not entirely quantifiable, and cannot identify novel miRNAs, and probe design can be cumbersome. NGS-based miRNA measurement (miRNA-seq) allows for the identification of novel miRNAs and comprehensive repertoire of isomiR and avoids the sequence-dependent primer and probe issues of qRT-PCR and microarrays. However, the sequencing of miRNAs from biofluids can be challenging, as inconsistencies exist between sequencing library construction kits, and sequence bias introduced during library construction and computational support is needed to analyze the data (Yuan et al. 2016). One of the goals of the ERCC is to develop standardized library preparation and sequencing protocols for researchers to use, which will eliminate many of the current issues associated with miRNA-seq.
While there is much promise to using miRNAs as biomarkers for human diseases, overcoming many of these technical issues will be critical to properly isolate, measure, and analyze circulating miRNAs in biofluids. Building and learning from these challenges, and implementing core standards and guidelines will ensure successful migration of miRNA biomarker from the bench to the bedside.
References
Abue M, Yokoyama M, Shibuya R, Tamai K, Yamaguchi K, Sato I, Tanaka N, Hamada S, Shimosegawa T, Sugamura K et al (2015) Circulating miR-483-3p and miR-21 is highly expressed in plasma of pancreatic cancer. Int J Oncol 46:539–547
Akat KM, Moore-McGriff D, Morozov P, Brown M, Gogakos T, Correa Da Rosa J, Mihailovic A, Sauer M, Ji R, Ramarathnam A et al (2014) Comparative RNA-sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers. Proc Natl Acad Sci USA 111:11151–11156
Alemar B, Izetti P, Gregório C, Macedo GS, Castro MAA, Osvaldt AB, Matte U, Ashton-Prolla P (2016) miRNA-21 and miRNA-34a are potential minimally invasive biomarkers for the diagnosis of pancreatic ductal adenocarcinoma. Pancreas 45:84–92
Argyropoulos C, Wang K, McClarty S, Huang D, Bernardo J, Ellis D, Orchard T, Galas D, Johnson J (2013) Urinary microRNA profiling in the nephropathy of type 1 diabetes. PLoS ONE 8:e54662
Argyropoulos C, Wang K, Bernardo J, Ellis D, Orchard T, Galas D, Johnson JP (2015) Urinary microRNA profiling predicts the development of microalbuminuria in patients with type 1 diabetes. J Clin Med 4:1498–1517
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL et al (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA 108:5003–5008
Axtell MJ, Westholm JO, Lai EC (2011) Vive la différence: biogenesis and evolution of microRNAs in plants and animals. Genome Biol 12:221
Ayala W, Moore LV, Hess EL (1951) The purple color reaction given by diphenylamine reagent. I. With normal and rheumatic fever sera. J Clin Invest 30:781–785
Badrnya S, Baumgartner R, Assinger A (2014) Smoking alters circulating plasma microvesicle pattern and microRNA signatures. Thromb Haemost 112(1):128–136
Balasubramanyam M, Aravind S, Gokulakrishnan K, Prabu P, Sathishkumar C, Ranjani H, Mohan V (2011) Impaired miR-146a expression links subclinical inflammation and insulin resistance in Type 2 diabetes. Mol Cell Biochem 351:197–205
Bianchi F, Nicassio F, Marzi M, Belloni E, Dall’Olio V, Bernard L, Pelosi G, Maisonneuve P, Veronesi G, Di Fiore PP (2011) A serum circulating miRNA diagnostic test to identify asymptomatic high-risk individuals with early stage lung cancer: serum miRNAs to diagnose asymptomatic NSCLC. EMBO Mol Med 3:495–503
Boeri M, Verri C, Conte D, Roz L, Modena P, Facchinetti F, Calabrò E, Croce CM, Pastorino U, Sozzi G (2011) MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci USA 108:3713–3718
Bollati V, Angelici L, Rizzo G, Pergoli L, Rota F, Hoxha M, Nordio F, Bonzini M, Tarantini L, Cantone L et al (2015) Microvesicle-associated microRNA expression is altered upon particulate matter exposure in healthy workers and in A549 cells. J Appl Toxicol JAT 35:59–67
Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, DemoryBeckler M, Weaver AM, Vickers K, Prasad N, Levy S et al (2015) KRAS-dependent sorting of miRNA to exosomes. eLife 4:e07197
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X et al (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18:997–1006
Chen W, Cai F, Zhang B, Barekati Z, Zhong XY (2013) The level of circulating miRNA-10b and miRNA-373 in detecting lymph node metastasis of breast cancer: potential biomarkers. Tumour Biol J Int Soc Oncodev Biol Med 34:455–462
Chen H, Lan H-Y, Roukos DH, Cho WC (2014a) Application of microRNAs in diabetes mellitus. J Endocrinol 222:R1–R10
Chen Q, Yang L, Xiao Y, Zhu J, Li Z (2014b) Circulating microRNA-182 in plasma and its potential diagnostic and prognostic value for pancreatic cancer. Med Oncol Northwood Lond Engl 31:225
Cheng H, Zhang L, Cogdell DE, Zheng H, Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR, Zhang W (2011) Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS ONE 6:e17745
Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, Wagner DR, Staessen JA, Heymans S, Schroen B (2010) Circulating microRNA-208b and microRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet 3:499–506
Cote GA, Gore AJ, McElyea SD, Heathers LE, Xu H, Sherman S, Korc M (2014) A pilot study to develop a diagnostic test for pancreatic ductal adenocarcinoma based on differential expression of select miRNA in plasma and bile. Am J Gastroenterol 109:1942–1952
Cuk K, Zucknick M, Heil J, Madhavan D, Schott S, Turchinovich A, Arlt D, Rath M, Sohn C, Benner A et al (2013) Circulating microRNAs in plasma as early detection markers for breast cancer. Int J Cancer 132:1602–1612
D’Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, Rubino M, Carena MC, Spazzafumo L, De Simone M et al (2010) Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J 31:2765–2773
De Rosa S, Fichtlscherer S, Lehmann R, Assmus B, Dimmeler S, Zeiher AM (2011) Transcoronary concentration gradients of circulating microRNAs. Circulation 124:1936–1944
Delić D, Eisele C, Schmid R, Baum P, Wiech F, Gerl M, Zimdahl H, Pullen SS, Urquhart R (2016) Urinary Exosomal miRNA Signature in Type II Diabetic Nephropathy Patients. PLoS ONE 11:e0150154
Demory Beckler M, Higginbotham JN, Franklin JL, Ham A-J, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ (2013) Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteom MCP 12:343–355
Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ (2000) Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 113(Pt 19):3365–3374
Devaux Y, Vausort M, Goretti E, Nazarov PV, Azuaje F, Gilson G, Corsten MF, Schroen B, Lair M-L, Heymans S et al (2012) Use of circulating microRNAs to diagnose acute myocardial infarction. Clin Chem 58:559–567
El-Khoury V, Pierson S, Kaoma T, Bernardin F, Berchem G (2016) Assessing cellular and circulating miRNA recovery: the impact of the RNA isolation method and the quantity of input material. Sci Rep 6:19529
Ellis KL, Cameron VA, Troughton RW, Frampton CM, Ellmers LJ, Richards AM (2013) Circulating microRNAs as candidate markers to distinguish heart failure in breathless patients. Eur J Heart Fail 15:1138–1147
Erson AE, Petty EM (2008) MicroRNAs in development and disease. Clin Genet 74:296–306
Fabian MR, Sonenberg N, Filipowicz W (2010) Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 79:351–379
Feng X, Wang Z, Fillmore R, Xi Y (2014) MiR-200, a new star miRNA in human cancer. Cancer Lett 344:166–173
Fish JE, Santoro MM, Morton SU, Yu S, Yeh R-F, Wythe JD, Ivey KN, Bruneau BG, Stainier DYR, Srivastava D (2008) miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15:272–284
Gao W, Yu Y, Cao H, Shen H, Li X, Pan S, Shu Y (2010) Deregulated expression of miR-21, miR-143 and miR-181a in non small cell lung cancer is related to clinicopathologic characteristics or patient prognosis. Biomed Pharmacother Bioméd Pharmacothérapie 64:399–408
Gidlöf O, Smith JG, Miyazu K, Gilje P, Spencer A, Blomquist S, Erlinge D (2013) Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc Disord 13:12
Goonetilleke KS, Siriwardena AK (2007) Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 33:266–270
Goren Y, Kushnir M, Zafrir B, Tabak S, Lewis BS, Amir O (2012) Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail 14:147–154
Guay C, Regazzi R (2013) Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol 9:513–521
Ha M, Kim VN (2014) Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15:509–524
Hansen TF, Carlsen AL, Heegaard NHH, Sørensen FB, Jakobsen A (2015) Changes in circulating microRNA-126 during treatment with chemotherapy and bevacizumab predicts treatment response in patients with metastatic colorectal cancer. Br J Cancer 112:624–629
He Y, Lin J, Kong D, Huang M, Xu C, Kim T-K, Etheridge A, Luo Y, Ding Y, Wang K (2015) Current state of circulating microRNAs as cancer biomarkers. Clin Chem 61:1138–1155
Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M et al (2012) Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14:249–256
Ho AS, Huang X, Cao H, Christman-Skieller C, Bennewith K, Le Q-T, Koong AC (2010) Circulating miR-210 as a novel hypoxia marker in pancreatic cancer. Transl Oncol 3:109–113
Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu Y, Chen Y, Xu L, Zen K, Zhang C et al (2010) Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol 28:1721–1726
Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X (2010) Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer 127:118–126
Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ (2011) Biological functions of microRNAs: a review. J Physiol Biochem 67:129–139
Huang S, Chen M, Li L, He MA, Hu D, Zhang X, Li J, Tanguay RM, Feng J, Cheng L et al (2014) Circulating microRNAs and the occurrence of acute myocardial infarction in Chinese populations. Circ Cardiovasc Genet 7:189–198
Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, Wenzel D, Vosen S, Franklin BS, Fleischmann BK et al (2013) Endothelial microparticle-mediated transfer of microRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation 128:2026–2038
Jansen F, Wang H, Przybilla D, Franklin BS, Dolf A, Pfeifer P, Schmitz T, Flender A, Endl E, Nickenig G, Werner N (2016) Vascular endothelial microparticles-incorporated microRNAs are altered in patients with diabetes mellitus. Cardiovasc Diabetol 15:49. doi:10.1186/s12933-016-0367-8
Ji X, Takahashi R, Hiura Y, Hirokawa G, Fukushima Y, Iwai N (2009) Plasma miR-208 as a biomarker of myocardial injury. Clin Chem 55:1944–1949
Kanki M, Moriguchi A, Sasaki D, Mitori H, Yamada A, Unami A, Miyamae Y (2014) Identification of urinary miRNA biomarkers for detecting cisplatin-induced proximal tubular injury in rats. Toxicology 324:158–168
Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, Dong Q, Pang Z, Guan Q, Gao L et al (2011a) Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol 48:61–69
Kong X, Du Y, Wang G, Gao J, Gong Y, Li L, Zhang Z, Zhu J, Jing Q, Qin Y et al (2011b) Detection of differentially expressed microRNAs in serum of pancreatic ductal adenocarcinoma patients: miR-196a could be a potential marker for poor prognosis. Dig Dis Sci 56:602–609
Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MAJ, Sadek P, Sie D, Zini N, Middeldorp JM, Ylstra B, de Menezes RX et al (2014) Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep 8:1649–1658
Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285:17442–17452
Kozakowska M, Ciesla M, Stefanska A, Skrzypek K, Was H, Jazwa A, Grochot-Przeczek A, Kotlinowski J, Szymula A, Bartelik A et al (2012) Heme oxygenase-1 inhibits myoblast differentiation by targeting myomirs. Antioxid Redox Signal 16:113–127
Kozomara A, Griffiths-Jones S (2014) miRBase: annotating high confidence microRNAs using deep sequencing data. Nucl Acids Res 42:D68–D73
Krichevsky AM, Gabriely G (2009) miR-21: a small multi-faceted RNA. J Cell Mol Med 13:39–53
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854
Leroyer AS, Tedgui A, Boulanger CM (2008) Microparticles and type 2 diabetes. Diabetes Metab 34(Suppl 1):S27–32
Liu L-Z, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung H-F, Lai L, Jiang B-H (2011) MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS ONE 6:e19139
Liu R, Chen X, Du Y, Yao W, Shen L, Wang C, Hu Z, Zhuang R, Ning G, Zhang C et al (2012a) Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin Chem 58:610–618
Liu X-G, Zhu W-Y, Huang Y-Y, Ma L-N, Zhou S-Q, Wang Y-K, Zeng F, Zhou J-H, Zhang Y-K (2012b) High expression of serum miR-21 and tumor miR-200c associated with poor prognosis in patients with lung cancer. Med Oncol Northwood Lond Engl 29:618–626
Liu Y, Gao G, Yang C, Zhou K, Shen B, Liang H, Jiang X (2014) The role of circulating microRNA-126 (miR-126): a novel biomarker for screening prediabetes and newly diagnosed type 2 diabetes mellitus. Int J Mol Sci 15:10567–10577
Long G, Wang F, Duan Q, Yang S, Chen F, Gong W, Yang X, Wang Y, Chen C, Wang DW (2012) Circulating miR-30a, miR-195 and let-7b associated with acute myocardial infarction. PLoS ONE 7:e50926
Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA (2010) Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol 28:341–347
Madhavan D, Peng C, Wallwiener M, Zucknick M, Nees J, Schott S, Rudolph A, Riethdorf S, Trumpp A, Pantel K et al (2016) Circulating miRNAs with prognostic value in metastatic breast cancer and for early detection of metastasis. Carcinogenesis 37:461
Maegdefessel L (2014) The emerging role of microRNAs in cardiovascular disease. J Intern Med 276(6):633–644
Mandel P, Metais P (1948) Comptes rendus séances société. Biol Ses Fil 142:241–243
Mar-Aguilar F, Mendoza-Ramírez JA, Malagón-Santiago I, Espino-Silva PK, Santuario-Facio SK, Ruiz-Flores P, Rodríguez-Padilla C, Reséndez-Pérez D (2013) Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis Markers 34:163–169
Matamala N, Vargas MT, González-Cámpora R, Miñambres R, Arias JI, Menéndez P, Andrés-León E, Gómez-López G, Yanowsky K, Calvete-Candenas J et al (2015) Tumor microRNA expression profiling identifies circulating microRNAs for early breast cancer detection. Clin Chem 61:1098–1106
Melman YF, Shah R, Danielson K, Xiao J, Simonson B, Barth A, Chakir K, Lewis GD, Lavender Z, Truong QA et al (2015) Circulating microRNA-30d is associated with response to cardiac resynchronization therapy in heart failure and regulates cardiomyocyte Apoptosis A translational pilot study. Circulation 131:2202–2216
Meng X, Müller V, Milde-Langosch K, Trillsch F, Pantel K, Schwarzenbach H (2016) Diagnostic and prognostic relevance of circulating exosomal miR-373, miR-200a, miR-200b and miR-200c in patients with epithelial ovarian cancer. Oncotarget 7:16923
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A et al (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105:10513–10518
Morimura R, Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Nagata H, Konishi H, Shiozaki A, Ikoma H, Okamoto K et al (2011) Novel diagnostic value of circulating miR-18a in plasma of patients with pancreatic cancer. Br J Cancer 105:1733–1740
Motta V, Angelici L, Nordio F, Bollati V, Fossati S, Frascati F, Tinaglia V, Bertazzi PA, Battaglia C, Baccarelli AA (2013) Integrative analysis of miRNA and inflammatory gene expression after acute particulate matter exposure. Toxicol Sci Off J Soc Toxicol 132:307–316
Nassirpour R, Mathur S, Gosink MM, Li Y, Shoieb AM, Wood J, O’Neil SP, Homer BL, Whiteley LO (2014) Identification of tubular injury microRNA biomarkers in urine: comparison of next-generation sequencing and qPCR-based profiling platforms. BMC Genom 15:485
Nielsen LB, Wang C, Sørensen K, Bang-Berthelsen CH, Hansen L, Andersen M-LM, Hougaard P, Juul A, Zhang C-Y et al (2012) Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. J Diabetes Res 2012:e896362
Ng EKO, Chong WWS, Jin H, Lam EKY, Shin VY, Yu J, Poon TCW, Ng SSM, Sung JJY (2009) Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut 58:1375–1381
Ng EKO, Li R, Shin VY, Jin HC, Leung CPH, Ma ESK, Pang R, Chua D, Chu K-M, Law WL et al (2013) Circulating microRNAs as specific biomarkers for breast cancer detection. PLoS ONE 8:e53141
Nishimura Y, Kondo C, Morikawa Y, Tonomura Y, Torii M, Yamate J, Uehara T (2015) Plasma miR-208 as a useful biomarker for drug-induced cardiotoxicity in rats. J Appl Toxicol JAT 35:173–180
Ogata-Kawata H, Izumiya M, Kurioka D, Honma Y, Yamada Y, Furuta K, Gunji T, Ohta H, Okamoto H, Sonoda H et al (2014) Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS ONE 9:e92921
Olivieri F, Antonicelli R, Lorenzi M, D’Alessandra Y, Lazzarini R, Santini G, Spazzafumo L, Lisa R, La Sala L, Galeazzi R et al (2013) Diagnostic potential of circulating miR-499-5p in elderly patients with acute non ST-elevation myocardial infarction. Int J Cardiol 167:531–536
Ortega FJ, Mercader JM, Catalán V, Moreno-Navarrete JM, Pueyo N, Sabater M, Gómez-Ambrosi J, Anglada R, Fernández-Formoso JA, Ricart W et al (2013) Targeting the circulating microRNA signature of obesity. Clin Chem 59:781–792
Ortega FJ, Mercader JM, Moreno-Navarrete JM, Rovira O, Guerra E, Esteve E, Xifra G, Martínez C, Ricart W, Rieusset J et al (2014) Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabet Care 37:1375–1383
Osipova J, Fischer D-C, Dangwal S, Volkmann I, Widera C, Schwarz K, Lorenzen JM, Schreiver C, Jacoby U, Heimhalt M et al (2014) Diabetes-associated microRNAs in pediatric patients with type 1 diabetes mellitus: a cross-sectional cohort study. J Clin Endocrinol Metab 99:E1661–1665
Pavkovic M, Riefke B, Ellinger-Ziegelbauer H (2014) Urinary microRNA profiling for identification of biomarkers after cisplatin-induced kidney injury. Toxicology 324:147–157
Pavkovic M, Riefke B, Frisk A-L, Gröticke I, Ellinger-Ziegelbauer H (2015) Glomerulonephritis-induced changes in urinary and kidney microRNA profiles in rats. Toxicol Sci Off J Soc Toxicol 145:348–359
Pavkovic M, Robinson-Cohen C, Chua AS, Nicoara O, Cárdenas-González M, Bijol V, Ramachandran K, Hampson L, Pirmohamed M, Antoine DJ et al (2016) Detection of drug-induced acute kidney injury in humans using urinary KIM-1, miR-21, -200c, and -423. Toxicol Sci Off J Soc Toxicol 152:205–213
Pescador N, Pérez-Barba M, Ibarra JM, Corbatón A, Martínez-Larrad MT, Serrano-Ríos M (2013) Serum circulating microRNA profiling for identification of potential type 2 diabetes and obesity biomarkers. PLoS ONE 8:e77251
Petriella D, De Summa S, Lacalamita R, Galetta D, Catino A, Logroscino AF, Palumbo O, Carella M, Zito FA, Simone G et al (2015) miRNA profiling in serum and tissue samples to assess noninvasive biomarkers for NSCLC clinical outcome. Tumour Biol J Int Soc Oncodev Biol, Med
Pezzolesi MG, Satake E, McDonnell KP, Major M, Smiles AM, Krolewski AS (2015) Circulating TGF-β1-regulated miRNAs and the risk of rapid progression to ESRD in type 1 diabetes. Diabetes 64:3285–3293
Pillai RS (2005) MicroRNA function: Multiple mechanisms for a tiny RNA? RNA 11:1753–1761
Qing S, Yuan S, Yun C, Hui H, Mao P, Wen F, Ding Y, Liu Q (2014) Serum MiRNA biomarkers serve as a fingerprint for proliferative diabetic retinopathy. Cell Physiol Biochem 34(5):1733–1740
Romaine SP, Tomaszewski M, Condorelli G, Samani NJ (2015) MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart 101(12):921–928
Rong Y, Bao W, Shan Z, Liu J, Yu X, Xia S, Gao H, Wang X, Yao P, Hu FB et al (2013) Increased microRNA-146a levels in plasma of patients with newly diagnosed type 2 diabetes mellitus. PLoS ONE 8:e73272
Salas-Pérez F, Codner E, Valencia E, Pizarro C, Carrasco E, Pérez-Bravo F (2013) MicroRNAs miR-21a and miR-93 are down regulated in peripheral blood mononuclear cells (PBMCs) from patients with type 1 diabetes. Immunobiology 218:733–737
Schultz NA, Dehlendorff C, Jensen BV et al (2014) MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA 311:392–404
Shan L, Ji Q, Cheng G, Xia J, Liu D, Wu C, Zhu B, Ding Y (2015) Diagnostic value of circulating miR-21 for colorectal cancer: a meta-analysis. Cancer Biomark Sect Dis Markers 15:47–56
Shen J, Todd NW, Zhang H, Yu L, Lingxiao X, Mei Y, Guarnera M, Liao J, Chou A, Lu CL et al (2011) Plasma microRNAs as potential biomarkers for non-small-cell lung cancer. Lab Investig J Tech Methods Pathol 91:579–587
Shifeng H, Danni W, Pu C, Ping Y, Ju C, Liping Z (2013) Circulating liver-specific miR-122 as a novel potential biomarker for diagnosis of cholestatic liver injury. PLoS ONE 8:73133
Starkey Lewis PJ, Dear J, Platt V, Simpson KJ, Craig DGN, Antoine DJ, French NS, Dhaun N, Webb DJ, Costello EM et al (2011) Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatol Baltim Md 54:1767–1776
Takahashi K, Yokota S-I, Tatsumi N, Fukami T, Yokoi T, Nakajima M (2013) Cigarette smoking substantially alters plasma microRNA profiles in healthy subjects. Toxicol Appl Pharmacol 272:154–160
Théry C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In: Board Juan Bonifacino Al (ed) Curr Protoc Cell Biol Chapter 3, Unit 3.22
Turchinovich A, Weiz L, Langheinz A, Burwinkel B (2011) Characterization of extracellular circulating microRNA. Nucl Acids Res 39:7223–7233
Tushuizen ME, Nieuwland R, Rustemeijer C, Hensgens BE, Sturk A, Heine RJ, Diamant M (2007) Elevated endothelial microparticles following consecutive meals are associated with vascular endothelial dysfunction in type 2 diabetes. Diabet Care 30:728–730
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659
van Schooneveld E, Wouters MC, Van der Auwera I, Peeters DJ, Wildiers H, Van Dam PA, Vergote I, Vermeulen PB, Dirix LY, Van Laere SJ (2012) Expression profiling of cancerous and normal breast tissues identifies microRNAs that are differentially expressed in serum from patients with (metastatic) breast cancer and healthy volunteers. Breast Cancer Res BCR 14:R34
Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT (2011) MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13:423–433
Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sánchez-Madrid F (2013) Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 4:2980
Vliegenthart ADB, Shaffer JM, Clarke JI, Peeters LEJ, Caporali A, Bateman DN, Wood DM, Dargan PI, Craig DG, Moore JK et al (2015) Comprehensive microRNA profiling in acetaminophen toxicity identifies novel circulating biomarkers for human liver and kidney injury. Sci Rep 5:15501
Wagner J, Riwanto M, Besler C, Knau A, Fichtlscherer S, Röxe T, Zeiher AM, Landmesser U, Dimmeler S (2013) Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol 33:1392–1400
Wang L-G, Gu J (2012) Serum microRNA-29a is a promising novel marker for early detection of colorectal liver metastasis. Cancer Epidemiol 36:e61–67
Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, Frazier ML, Killary AM, Sen S (2009) MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res Phila Pa 2:807–813
Wang G-K, Zhu J-Q, Zhang J-T, Li Q, Li Y, He J, Qin Y-W, Jing Q (2010a) Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 31:659–666
Wang K, Zhang S, Weber J, Baxter D, Galas DJ (2010b) Export of microRNAs and microRNA-protective protein by mammalian cells. Nucl Acids Res 38:7248–7259
Wang Z-X, Bian H-B, Wang J-R, Cheng Z-X, Wang K-M, De W (2011) Prognostic significance of serum miRNA-21 expression in human non-small cell lung cancer. J Surg Oncol 104:847–851
Wang J, Zhang Y, Zhang W, Jin Y, Dai J (2012) Association of perfluorooctanoic acid with HDL cholesterol and circulating miR-26b and miR-199-3p in workers of a fluorochemical plant and nearby residents. Environ Sci Technol 46:9274–9281
Wang J, Raimondo M, Guha S, Chen J, Diao L, Dong X, Wallace MB, Killary AM, Frazier ML, Woodward TA et al (2014) Circulating microRNAs in pancreatic juice as candidate biomarkers of pancreatic cancer. J Cancer 5:696–705
Wang C, Wan S, Yang T, Niu D, Zhang A, Yang C, Cai J, Wu J, Song J, Zhang C-Y, et al. (2016) Increased serum microRNAs are closely associated with the presence of microvascular complications in type 2 diabetes mellitus. Sci Rep 6:20032. doi:10.1038/srep20032
Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, et al. (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2. doi:10.3402/jev.v2i0.20360
Wozniak MB, Scelo G, Muller DC, Mukeria A, Zaridze D, Brennan P (2015) Circulating microRNAs as non-invasive biomarkers for early detection of non-small-cell lung cancer. PLoS ONE 10:e0125026
Wu J, Yang T, Li X, Yang Q, Liu R, Huang J, Li Y, Yang C, Jiang Y (2013) Alteration of serum miR-206 and miR-133b is associated with lung carcinogenesis induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Toxicol Appl Pharmacol 267:238–246
Xie Y, Todd NW, Liu Z, Zhan M, Fang H, Peng H, Alattar M, Deepak J, Stass SA, Jiang F (2010) Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer Amst Neth 67:170–176
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells Dayt Ohio 30:1556–1564
Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang J-D, Song E (2011) Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer 10:117
Yu S-L, Chen H-Y, Chang G-C, Chen C-Y, Chen H-W, Singh S, Cheng C-L, Yu C-J, Lee Y-C, Chen H-S et al (2008) MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell 13:48–57
Yuan T, Huang X, Woodcock M, Du M, Dittmar R, Wang Y, Tsai S, Kohli M, Boardman L, Patel T et al (2016) Plasma extracellular RNA profiles in healthy and cancer patients. Sci Rep 6:19413
Yuchuan H, Ya D, Jie Z, Jingqiu C, Yanrong L, Dongliang L, Changguo W, Kuoyan M, Guangneng L, Fang X et al (2014) Circulating miRNAs might be promising biomarkers to reflect the dynamic pathological changes in smoking-related interstitial fibrosis. Toxicol Ind Health 30:182–191
Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E et al (2010) Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 107:810–817
Zampetaki A, Willeit P, Burr S, Yin X, Langley SR, Kiechl S, Klein R, Rossing P, Chaturvedi N, Mayr M (2016) Angiogenic microRNAs linked to incidence and progression of diabetic retinopathy in type 1 diabetes. Diabetes 65:216–227
Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, Fei M, Sun S (2010) Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem 56:1830–1838
Zhao F-L, Hu G-D, Wang X-F, Zhang X-H, Zhang Y-K, Yu Z-S (2012) Serum overexpression of microRNA-10b in patients with bone metastatic primary breast cancer. J Int Med Res 40(3):859–866
Zhao Z, He J, Zhang J, Liu M, Yang S, Li N, Li X (2014) Dysregulated miR1254 and miR579 for cardiotoxicity in patients treated with bevacizumab in colorectal cancer. Tumour Biol J Int Soc Oncodev Biol Med 35:5227–5235
Zhong J, He Y, Chen W, Shui X, Chen C, Lei W (2014) Circulating microRNA-19a as a potential novel biomarker for diagnosis of acute myocardial infarction. Int J Mol Sci 15:20355–20364
Acknowledgments
This work was supported by research contracts from DOD W911NF-10-2-0111 and DTRA HDTRA1-13-C-0055.
Author contributions
Vikas Ghai and Kai Wang wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ghai, V., Wang, K. Recent progress toward the use of circulating microRNAs as clinical biomarkers. Arch Toxicol 90, 2959–2978 (2016). https://doi.org/10.1007/s00204-016-1828-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1828-2