Abstract
There is an ongoing debate whether the intake of soy-derived isoflavones (sISO) mediates beneficial or adverse effects with regard to breast cancer risk. Therefore, we investigated whether nutritional exposure to a sISO-enriched diet from conception until adulthood impacts on 17β-estradiol (E2)-induced carcinogenesis in the rat mammary gland (MG). August-Copenhagen-Irish (ACI) rats were exposed to dietary sISO from conception until postnatal day 285. Silastic tubes containing E2 were used to induce MG tumorigenesis. Body weight, food intake, and tumor growth were recorded weekly. At necropsy, the number, position, size, and weight of each tumor were determined. Plasma samples underwent sISO analysis, and the morphology of MG was analyzed. Tumor incidence and multiplicity were reduced by 20 and 56 %, respectively, in the sISO-exposed rats compared to the control rats. Time-to-tumor onset was shortened from 25 to 20 weeks, and larger tumors developed in the sISO-exposed rats. The histological phenotype of the MG tumors was independent of the sISO diet received, and it included both comedo and cribriform phenotypes. Morphological analyses of the whole-mounted MGs also showed no diet-dependent differences. Lifelong exposure to sISO reduced the overall incidence of MG carcinomas in ACI rats, although the time-to-tumor was significantly shortened.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of isolated soy-derived isoflavones (sISO) or soy extracts poses both benefits and risks. A meta-analysis of epidemiological studies recently found an inverse relationship between soy consumption and the incidence of breast cancer in both pre- and postmenopausal women in Asian countries, yet not in women from Western countries (Chen et al. 2014). Furthermore, soy consumption has been associated with a lower recurrence rate of breast cancer in Chinese women (Shu et al. 2009). Interestingly, the average daily sISO intake ranges from 20 to 50 mg in Asian societies versus <1 mg in Western societies (Arai et al. 2000; Nagata 2010; Magee and Rowland 2012). Recent studies have demonstrated that the possible benefit of dietary isolated soy-derived isoflavones (sISO) regarding breast cancer risk depends on the period in life when exposure occurs. For example, in the Shanghai Women’s Health Study, the risk of premenopausal breast cancer in women who consumed a large amount of soy-containing foods consistently during both adolescence and adulthood was significantly reduced, yet a similar effect was not observed for the risk of postmenopausal breast cancer (Lee et al. 2009). In another population-based case–control study, girls that were exposed to soy prepubertally had a reduced incidence of breast cancer later in life (Shu et al. 2001). Our own studies have shown that the responsiveness toward estrogenic stimuli of estrogen target organs in rats is modified by chronic exposure to sISO, although with varying effects. For example, while attenuated proliferative responses were observed in the mammary gland (MG) epithelium in response to subcutaneous 17β-estradiol (E2) treatment (Molzberger et al. 2012, 2013; Blei et al. 2015), an increase in uterine wet weights was found to be induced via activation of estrogen receptor (ER) α (Möller et al. 2010).
Female August-Copenhagen-Irish (ACI) rats rarely develop mammary tumors spontaneously. However, permanently elevated endogenous E2 levels can induce mammary tumors in this model (Shull et al. 1997; Turan et al. 2004). Therefore, hormonal carcinogenesis of the MG in intact ACI rats represents a highly relevant model for human breast cancer. Moreover, estrogen-induced cancers in this model exhibit aneuploidy, similar to invasive ductal breast carcinomas in women.
The aim of the present study was to investigate whether nutritional exposure to a sISO-enriched diet from conception until adulthood impacts on E2-induced carcinogenesis in the female rat MG and whether attenuation of a proliferative response to E2 treatment can be extrapolated to E2-dependent carcinogenesis. In addition, the relevant mechanisms were investigated.
Materials and methods
Soy isoflavone-enriched diets and E2-releasing tubes
Commercially available soy bean extract Novasoy®650 (ADM, Decatur, IL, USA) containing 67.7 % (w/w) sISO (Blei et al. 2015) was purchased and stored at −30 °C. For the sISO-enriched diet [sISO aglycone equivalents: ~500 parts-per-million (ppm)], 1.05 g of Novasoy®650 extract was added to 1.0 kg of R/MH phytoestrogen-free, closed-formula, natural ingredient diet (Ssniff, Soest, Germany). As a control, a R/MH phytoestrogen-free diet was used (Ssniff). The sISO content of each produced lot was examined for compliance.
Silicone tubes (Trelleborg Sealing Solutions, Stuttgart, Germany) 10 mm in length (inner diameter 1.9 mm; wall thickness 0.6 mm) were filled with 4 mg E2 (Sigma-Aldrich, Steinheim, Germany) dissolved in absolute ethanol (Cohen and Milligan 1993). Following vaporization of the alcohol, the tubes were closed by Elastosil® RTV1 silastic glue (Wacker, Munich, Germany) and were stored at room temperature. Empty silastic tubes served as controls.
Animal subjects and experimental techniques
All animal handling and experimental techniques have been approved by the Animal Care and Use Committee (Registration No.: 24-9168.11-1/2011-7) according to the Animal Care and Use Committee guidelines regulated by German federal law for animal welfare and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Animals were housed under controlled conditions (12-h light/12-h dark cycle, 20 ± 1 °C, 50–80 % humidity) and had ad libitum access to food (diet) and water. Breeding ACI/SegHsd rats (~40 weeks old; ~200 g; Harlan, Eystrup, Germany) were randomly allocated to receive either the control or sISO-enriched diet prior to mating. Pregnant dams were maintained on their established dietary regimen throughout gestation and the lactation period. Female offspring were maintained under the same established dietary regimen as their mothers. Six offspring animals were killed each on PND 21 (prepuberty), PND 50 ± 1 (puberty), PND 81 ± 2 (adolescence), and PND 97 ± 2 (young adulthood). The greatest number of terminal end buds (TEB) was observed at PND 50 (Fig. 1a), with 35.0 ± 7.3 TEB for the dietary sISO group and 44.5 ± 10.6 TEB per MG for the control group. Consequently, PND 50 was designated as the time point at which to start the E2 treatment. Briefly, female ACI offspring were randomly divided into two different treatment groups (n = 10/group) and were maintained on the diet received since conception. One group was implanted with two 4 mg E2-containing silastic tubes on PND 45 and PND 175, while the second group was implanted with empty silastic tubes at the same time points. Prior to implantation, the rats were lightly anesthetized by O2/CO2 (1:1) inhalation. The implants were inserted between the scapulae through a skin incision which was subsequently closed with a wound clip.
Morphology of whole-mounted MGs without indications of palpable tumors. a An age-dependent assessment of the number of TEB in female ACI rats administered sISO-free or sISO-rich diets. The number of TEB was significantly reduced in the latter compared with the former (two-way ANOVA/Tukey; p < 0.002). Significantly more TEB were also observed on PND 50 compared to the other time points (two-way ANOVA/Tukey; p < 0.05). b Representative images of carmine alum-stained PND 285 MG whole mounts at 1.6× and 4×. Elongation (c) and area (d) of the epithelial tree in animals from both diet groups with or without implanted of E2-releasing silastic tubes on PND 285. The values for each assessed animal are shown with the arithmetic mean (white square), median (minus), and SD (whisker) values. A two-way ANOVA/Tukey analysis revealed no statistical differences between the groups. The horizontal lines represent the median values, and the vertical lines represent the SDs
Palpation to detect tumors and assessments of body weight and food intake were performed weekly for the duration of the study (36 weeks). The dates for the appearance and location of each tumor were recorded. Rats were euthanized when the mammary tumors reached more than 2 cm2 in size, when the animals exhibited extreme body weight loss (or other signs of poor health status), or at the end of experimental period on PND 285. Specific growth rates (SGRs) were estimated from tumor volume measurements during exponential growth using the formula: SGR = ln (V2/V1)/(t2 − t1) (Mehrara et al. 2007).
Blood and tissue collection
Animals were subjected to light anesthesia by O2/CO2 (1:1) inhalation prior to killing by CO2 inhalation. After killing and the recording of the final body weights, blood samples were collected from the portal vein and transferred to EDTA-containing collection tubes (Sarstedt, Nümbrecht, Germany) and stored on ice. In addition, silastic tubes were recollected for measurement of the unreleased E2.
During necropsy, all of the rats were examined for the presence and location of mammary tumors. Mammary tumors were quickly removed and their size and wet weights were recorded. Portions of each tumor were fixed in 4 % formaldehyde for paraffin embedding or were immediately frozen in liquid nitrogen for molecular analysis. Unless there was a tumor, the #4 abdominal right MG was prepared as a carmine alum-stained whole mount (n = 5–10) (de Assis et al. 2010), while the #4 abdominal left MG was embedded in paraffin. The #5 abdominal left MG was directly frozen and stored in liquid nitrogen. Liver, uteri, and ovaries were also collected, weighed, and stored in liquid nitrogen. To assess epithelial growth, the distance from the lymph node to the end of the epithelial tree was determined with light microscopy (de Assis et al. 2010). The area of the epithelial tree was determined with Image J 1.45s analysis software (National Institutes of Health, USA).
Quantification of sISO and their metabolites
Levels of sISO in the diets and plasma samples were measured by liquid chromatography-diode array detection (Molzberger et al. 2013) and ultra-high-performance liquid chromatography-tandem mass spectrometry (Soukup et al. 2014), respectively. For the latter, 100 µl of rat plasma and 5 µl of internal standard solution containing 5 µM 13C3-daidzein and 5 µM 13C3-genistein-7-glucuronide in DMSO were added to each sample. Briefly, the samples were diluted with 400 µl of water, and extraction and analysis were performed (Soukup et al. 2014). The analytes, daidzein-4′-glucuronide-7-sulfate and genistein-4′-glucuronide-7-sulfate (Toronto Research Chemicals, North York, Canada), were also implemented in the UHPLC-MS/MS method. The measuring conditions used in the scheduled multiple reaction monitoring (sMRM) for these analytes are provided in Online Resource 1. The limit of quantification (LOQ) and limit of detection (LOD) for all the analytes in rat plasma are summarized in Online Resource 2.
Quantification of E2 and its metabolites
Amounts of E2 in the silastic implants were quantified by high-performance liquid chromatography with an ultraviolet detector (Online Resources 3 and 4). Analyses of E2 and its metabolites in plasma samples were performed with a mass spectrometer (Varian 300-MS; Bruker Daltonics, Bremen, Germany) interfaced with a Varian 450-GC system. Plasma levels were quantified using the ratios of the peak areas of the analytes and their respective deuterated derivatives (Online Resource 5).
Histological characterization
Sections (3 μm) of paraffin-embedded MG tumors were stained with hematoxylin and eosin and were examined by two pathologists (MCB and MHM) blinded to the treatments. Tumors were classified essentially according to Russo et al. (1990).
Analysis of Ki67 transcript levels
Total RNA was extracted from MGs (Chomczynski and Sacchi 1987) using the PeqGOLD TriFast™ protocol (PeqLab, Erlangen, Germany). Following extraction, RNA samples were treated with Deoxyribonuclease 1 (Roche Diagnostic GmbH, Mannheim, Germany) and then were incubated with MMLV-Reverse Transcriptase (Promega, Madison, WI, USA) and oligo (dT)18 primers for first-strand cDNA synthesis. The resulting cDNAs were amplified with Platinum® TaqDNA polymerase (Invitrogen, Karlsruhe, Germany) using a CXF96 instrument (Bio-Rad Laboratories GmbH, Munich, Germany), SybrGreen® I (Sigma-Aldrich, Taufkirchen, Germany), and primers for Rps18 (fwd: CGTGAAGGATGGGAAGTATAGC; rev: TATTAACAGCAAAGGCCCAAAG) and Ki67 (fwd: AACCAGGACTTTGTGCTCTGTAA; rev: CTCTTTTGGCTTCCATTTCTTC). Transcript levels were quantitated for each animal in duplicate using independently produced cDNA. Relative gene expression rates were calculated using the ΔCT method (Pfaffl 2001).
Ki67 protein expression in MGs without palpable tumors
Immunohistological staining of Ki67 in MGs without palpable tumors was performed as previously described (Helle et al. 2014), except that the dissected MGs were fixed in 4 % formaldehyde overnight at 4 °C.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). For normally distributed data, differences between the means were assessed either using one-way, two-way ANOVA followed by Tukey post hoc test or by Welch’s t test (Origin Pro 9.1 software, Northampton, MA, USA). Kaplan–Meier curves were generated (Origin Pro 9.1) and were used to compare tumor onset between the sISO-rich and control diet groups using a log-rank (Mantel–Cox) test. Welch’s t test with subsequent p value adjustment after the Holm multiple comparison test was applied to detect statistically significant differences in the Ki67 mRNA profiles. SGRs were analyzed with nonparametric Kruskal–Wallis ANOVA. p values ≤0.05 were considered significant.
Results
Body and organ weights
There were no significant differences in body weight, food uptake, or uterine and ovarian wet weights among the rats that received a sISO-free or sISO-rich diet and were implanted with control or E2-releasing tubes. However, E2 treatment significantly increased the liver wet weights by approximately 10 % in both the control (p = 0.037) and sISO-rich (p = 0.018) diet groups (Table 1). Only one animal was lost due to spontaneous and severe deterioration of health.
E2 content in the implanted devices and plasma samples
To induce the formation of MG tumors, E2-releasing silastic tubes were implanted. The silastic tubes contained an average of 3.98 ± 0.71 mg E2 prior to implantation (n = 14), and 3.11 ± 1.06 mg E2 (n = 12) and 3.50 ± 0.51 mg E2 (n = 16), following retrieval from the sISO-free- and sISO-fed animals, respectively. In the plasma samples collected from the rats implanted with the control tubes, the E2 concentrations were under the LOD or LOQ for 7/10 samples tested. The remaining three samples had E2 levels slightly above the LOQ (33 fmol/ml). Rats fed the sISO-rich diet had average plasma concentration of 67 ± 27 fmol/ml. When implanted with the E2-releasing tubes, the average plasma concentration of E2 increased to 242 ± 108 fmol/ml (sISO-free diet) and 253 ± 180 fmol/ml (sISO-rich diet; Online Resource 6).
MG morphology
MG morphology endpoints and growth of the MG ductal tree have been found to be suitable predictors for the effects of dietary manipulations early in life on mammary cancer risk later in life (de Assis et al. 2010). The MGs of the rats in both dietary groups exhibited ductal differentiation with ‘virgin’ morphology on PND 285 when they were implanted with E2-free tubes. The MG phenotype included elongated, branched ductal structures that infilled the margin of the fat pad without transformation of the ductal epithelial cells into lobulo-alveolar arrangements (Fig. 1b). When the animals were implanted with the E2-releasing silicone tubes, the appearance of the MG changed to a ‘pregnancy/lactating’ morphology with highly differentiated lobulo-alveolar structures (Fig. 1b), while the growth and area of the MG ductal tree remained largely unchanged (Fig. 1c, d).
Tumor burden
Mammary tumors only formed in the rats that were implanted with E2-releasing silicone tubes. In the animals that received a sISO-rich diet throughout their lifetime, the first tumor was palpable after 20 weeks. In comparison, the first palpable tumor in the rats administered the sISO-free control diet developed after 25 weeks. Most of the tumors exhibited classical morphological features of mammary ductal carcinomas, with comedo (in 59 % of tumors), cribriform (46 %), alveolar (36 %), squamous (28 %), solid (15 %), and papillary (8 %) phenotypes observed. Necrotic areas were observed as well (21 %). Most tumors appeared to be partially encapsulated even though they were microscopically invasive. The dietary background of the experimental groups did not affect the spectrum of histological patterns observed for the induced tumors (data not shown).
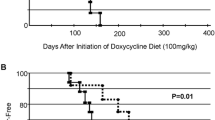
At necropsy, there were no benign tumors or macroscopically visible lesions in the regional lymph nodes or lungs suggestive of metastases. A 50 % incidence of palpable tumors was observed after 26 weeks compared to 31 weeks in the rats receiving the sISO-rich diet versus the sISO-free diet (Fig. 2). The difference in the time-to-tumor appearance was highly significant (p = 0.00002). The final cumulative mammary tumor incidences at the end of 36 weeks were 60 and 80 % for the sISO-rich and sISO-free diet groups, respectively (Table 2). Although the final incidence of palpable tumors was thus only slightly reduced in the animals receiving the sISO-rich diet, tumor multiplicity was reduced by 56 % in this group compared to the controls (p = 0.018; Table 2). However, the tumors that grew in the sISO-rich diet group reached a larger size than the tumors that grew in the sISO-free diet group (Table 2). In contrast, estimates of the tumor SGRs from the first appearance of tumor until killing revealed no significant differences between the two groups (Table 2).
Kaplan–Meier tumor-free survival curves for ACI rats that received a sISO diet with E2-free (black circle) versus E2-releasing tubes (black triangle) and a sISO-free diet with control (white circle) versus E2-releasing tubes (white triangle). Tumor development was significantly faster for the sISO-rich fed rats than in the sISO-free fed rats that were implanted with E2-releasing tubes [log-rank (Mantel–Cox) test; p = 0.00002]
Proliferation in MGs without palpable tumors
To assess the proliferative response in the MG, levels of Ki67 mRNA and protein were determined in MGs without indications for palpable tumors. Animals of both dietary groups that were implanted with E2-free tubes had low Ki67 mRNA levels and Ki67 labeling index values (Fig. 3). For rats that were not exposed to sISO and were implanted with E2-releasing silicone tubes, the Ki67 mRNA levels increased by ~75 % compared to their respective controls. However, E2-treated animals that had received a sISO-rich diet for their lifetime and were implanted with E2-releasing silicone tubes exhibited a 264 % increase in Ki67 mRNA levels compared to their control counterparts (p = 0.003; Fig. 3a). A corresponding increase in epithelial cell Ki67 labeling index values was also observed for the two E2-treated groups (p = 0.040 and p = 0.030, respectively). Furthermore, there was no synergistic effect from a lifelong sISO-rich diet and chronic E2 treatment observed based on the Ki67 proliferation index values (Fig. 3b, c).
Proliferation detected for the MGs of the four experimental ACI rat groups. a Levels of Ki67 mRNA were determined by qPCR and were normalized to Rps18 for the rats that received a sISO-rich diet or sISO-free diet and were implanted with empty or E2-releasing tubes. Welch’s t test with subsequent p value adjustment after the Holm multiple comparison test revealed a significant increase in Ki67 mRNA levels in the MGs of the rats that received a sISO-rich diet and were implanted with E2-releasing silastic tubes (p = 0.003) compared to rats that were implanted with control tubes. Levels of Ki67 mRNA also significantly differed between the MGs of the rats that received a sISO-free diet versus the sISO-rich diet (p = 0.017) and between the MGs of the rats that received the sISO-rich diet versus the sISO-free diet, and each were implanted with E2-releasing tubes (p = 0.025). The horizontal lines represent the median values, and the vertical lines represent the SDs. b Representative images of MG tissue sections from the four experimental groups that were stained for Ki67 (and counterstained with hematoxylin). The magnifications used for each panel and the inset panel at the top left of each are 40× and 200×, respectively. Scale bar 100 µm. c Epithelial Ki67 labeling index values for the MGs of each treatment group. Statistical analysis (two-way ANOVA/Tukey) revealed an E2 treatment-dependent effect and not a diet-dependent effect (p = 0.003). The values for each assessed animal are shown with the arithmetic mean (white square), median (minus), and SD (whisker) values
Dietary sISO content, exposure, and plasma levels
The sISO content of the enriched diet was 476 ppm total sISO aglycone equivalents consisting of 236 ppm of genistein aglycone equivalents, 195 ppm of daidzein aglycone equivalents, and 45 ppm of glycitein aglycone equivalents. Moreover, the sISO administered were mainly glucosides (Blei et al. 2015; Online Resource 7). Age-dependent sISO exposure was calculated using dietary sISO content, body weight, and food intake. The average exposure in the offspring was 54 mg/kg/day directly after weaning, and this leveled out to 30 mg/kg/day toward the end of the experiment (data not shown).
In the plasma samples collected at necropsy, daidzein-7-glucuronide, genistein-7-glucuronide, and equol-7-glucuronide were the main metabolites detected (Online Resource 8). Animals that received the sISO diet and were implanted with E2-releasing devices or E2-free devices had total genistein and daidzein aglycone equivalent concentrations of 1936.2 ± 883.6 and 2357.7 ± 347.9 nM, respectively, compared with 8.7 ± 6.9 and 9.0 ± 5.6 nM, respectively, for the sISO-free control rats.
Discussion
The aim of this study was to investigate whether continuous exposure to a diet containing nutritionally relevant levels of sISO from conception until adulthood has an effect on estrogen-dependent carcinogenesis in the female rat MG. Chronic dietary exposure to sISO reduced the incidence and multiplicity of MG cancers in E2-treated ACI rats, while tumor latency was shortened and tumor growth was enhanced once the tumors were established (Fig. 2). These data suggest that dietary sISO intake over known sensitive periods of MG development among them late gestational, neonatal, and pubertal stages of development protected against E2-induced breast cancer development, but not against tumor growth. This is consistent with the findings that female SD rats developed significantly fewer DMBA-induced mammary tumors compared with controls when they were exposed to a diet containing the isoflavone genistein (250 mg/kg) from the time of conception until PND 21 (Fritz et al. 1998). The protective effect of genistein has also been observed in animals that were only prenatally exposed to isoflavone genistein (Lamartiniere et al. 2002). However, no difference in the incidence or multiplicity of tumors was observed between rats fed a standard diet or a diet enriched with genistein (~200 mg/kg) starting 1 week before DMBA administration. Moreover, for animals that received a diet containing 200 mg/kg isoflavone daidzein or 16 % soy protein isolate, tumor incidence and multiplicity were reduced (Constantinou et al. 2001). Nevertheless, irrespective of the differences between these studies in terms of dosages and compound matrices (aglycone vs. soy extract), it is obvious that both timing and duration are critical factors in determining the protective effects of sISO exposure against mammary carcinogenesis in rats.
A similar acceleration in tumor growth (as observed in our rat study) was recently described for MTB-IGFIR transgenic mice that overexpress type I insulin-like growth factor receptor in a doxycycline-inducible manner that were continuously fed either a casein-based diet or a soy-based diet containing 332 ppm total sISO aglycone equivalents throughout their embryonic and postnatal development (Watson et al. 2015). Following the induction of the IGFIR transgene by a parallel doxycycline treatment starting on PND 45, the average tumor onset for the mice receiving the soy diet was 10 weeks earlier than that of the mice that received a control casein diet. However, the potential for sISO to trigger tumor growth remains controversial. Concerns regarding the estrogenic effects of sISO exposure are based on data indicating that genistein stimulates the proliferation of ER-positive breast tumor cells in vitro (Seo et al. 2006) and in several types of rodent models in vivo. For example, in ovariectomized athymic mice, dietary levels of 150 and 300 ppm of genistein resulted in a dose-dependent increase in the growth of xenografted ER-positive MCF-7 cells (Allred et al. 2001). This finding was reproduced in ovariectomized SD rats that were chemically induced to form tumors by 1-methyl-1-nitrosourea (Allred et al. 2004). Consistent with the notion that estrogenic stimulation is a key factor mediating this effect, dietary genistein did not stimulate the growth of ER-negative MDA-MB-231 cells that were implanted into nude mice (Santell et al. 2000). However, long-term administration of a diet containing 500 ppm genistein significantly decreased in rats the incidence of spontaneous MG fibroadenoma, yet increased the incidence of spontaneous mammary adenoma and adenocarcinoma, compared with female rats that received a control diet (NTP report 2008). Collectively, these data and the current findings suggest that high dietary levels of genistein may stimulate the growth of initiated or preexisting mammary tumors in animals, although any stimulatory effect on cancer cell growth is probably limited to ER-positive tumor cells, either via direct activation of the ER on cancer cells or through ER-mediated paracrine factors induced in the stroma. TEB are considered the most fragile structures in the developing MG. Due to their high number, their undifferentiated status, and their high mitotic activity during puberty, TEB represent a critical target for chemical and radiation carcinogens, as well as dietary factors, and therefore play a key role in tumor initiation. In one arm of the animal experiment of the present study, animals that received continuous exposure to dietary sISO from conception exhibited fewer TEB on PND 50 compared to the control group. A similar observation was made for female 50-day-old SD rats that were postnatally treated with subcutaneous injections of genistein (Lamartiniere et al. 1995). We hypothesize that a reduction in the number of TEB represents an important contribution to the overall mechanism by which sISO exert its protective effects on breast cancer development. It has also been shown that prepubertal exposure to sISO, independent of the administration route, accelerates developmental processes in the MG, thereby leading to greater differentiation of the MG at puberty, and thus a reduced number of less differentiated, proliferative TEB (Murrill et al. 1996; Lamartiniere et al. 1998, 2002). While this may account for the observed reduction in tumor incidence in the DMBA-treated rat model, it involves DNA damage at the time of carcinogen injection (~PND 50) and the subsequent mutagenic events depend on the rate of cell proliferation at the time of injection. However, this model is fundamentally different from carcinogenesis in E2-treated ACI rats. In the ACI rat model, the continuous estradiol-driven proliferation and genotoxic effects of E2 metabolites (Li et al. 2004; Turan et al. 2004) rather than a single carcinogenic challenge at a sensitive stage probably lead to the observed carcinogenic events. The higher degree of MG differentiation induced by peripubertal sISO exposure is often discussed as a potential chemopreventive mechanism of sISO (Messina and Hilakivi-Clarke 2009). In the present study, there were no differences in the degree of MG differentiation in whole mounts compared to control rats fed a sISO-free diet. On the other hand, the observation that Ki67 transcripts, but not the Ki67 labeling index values, were increased in the MGs without palpable tumors indicates that accelerated MG epithelial growth did occur, consistent with the increased growth rates observed for the MG tumors in the sISO-rich group.
A consistent finding in the literature is that concentrations of genistein <10 µM appear to stimulate cancer cell growth, while concentrations >10 µM inhibit cancer cell growth in vitro (Taylor et al. 2009), similar to the known biphasic effect of E2. To explain the tumor growth-promoting activities of sISO, the receptor expression profile of the tumor tissue, as well as the plasma concentrations of sISO, is of importance. In the present study, the plasma concentration of total genistein and daidzein aglycone equivalent was ~2 µM. These plasma levels are comparable to those reported in humans with high sISO intake (Adlercreutz et al. 1993; Arai et al. 2000). Furthermore, the main phase II metabolites detected were 7-glucuronides and all animals were equol producers. Similar findings have been reported for female Wistar rats (Blei et al. 2015). However, humans mainly produce sulfoglucuronides as their major phase II metabolite (Hosoda et al. 2011; Soukup et al. 2014) and only 30–50 % produce equol (Lampe et al. 1998; Setchell et al. 2003). Therefore, it remains to be investigated whether sISO metabolite profiles play a role in the development and/or promotion of breast cancer. Taken together, these results and those of the present study support the hypothesis that the potential benefit of dietary sISO regarding breast cancer risk depends on the period in life when exposure occurs.
Conclusions
The sISO diet protected the female ACI rat MG from initiation of E2-induced tumorigenesis, most likely via the effects of sISO on the TEB, resulting in fewer TEB around puberty. As a result, significantly fewer tumors developed. However, once tumors or tumor precursors were established, plasma concentrations of daidzein plus genistein of ~2 µM promoted tumor progression and growth, potentially due to MG proliferation. Thus, lifelong dietary exposure to a sISO-rich diet started in utero reduced the overall burden of E2-induced mammary carcinomas compared to the animals that were maintained on a sISO-free control diet. However, the onset of palpable tumors was significantly shorter in sISO-exposed rats, thereby leading to larger tumors by the end of the study, yet the tumor SGR was not influenced. Thus, chronic exposure to dietary sISO mediated both MG tumor preventive and growth-promoting properties in E2-treated female ACI rats, and these results should be further investigated.
References
Adlercreutz H, Markkanen H, Watanabe S (1993) Plasma concentrations of phyto-oestrogens in Japanese men. Lancet 342:1209–1210
Allred CD, Allred KF, Ju YH et al (2001) Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res 61:5045–5050
Allred CD, Allred KF, Ju YH et al (2004) Dietary genistein results in larger MNU-induced, estrogen-dependent mammary tumors following ovariectomy of Sprague-Dawley rats. Carcinogenesis 25:211–218. doi:10.1093/carcin/bgg198
Arai Y, Uehara M, Sato Y et al (2000) Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol 10:127–135. doi:10.2188/jea.10.127
Blei T, Soukup ST, Schmalbach K et al (2015) Dose-dependent effects of isoflavone exposure during early lifetime on the rat mammary gland: studies on estrogen sensitivity, isoflavone metabolism, and DNA methylation. Mol Nutr Food Res 59:270–283. doi:10.1002/mnfr.201400480
Chen M, Rao Y, Zheng Y et al (2014) Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PLoS ONE 9:e89288. doi:10.1371/journal.pone.0089288
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159
Cohen PE, Milligan SR (1993) Silastic implants for delivery of oestradiol to mice. J Reprod Fertil 99:219–223. doi:10.1530/jrf.0.0990219
Constantinou AI, Lantvit D, Hawthorne M et al (2001) Chemopreventive effects of soy protein and purified soy isoflavones on DMBA-induced mammary tumors in female Sprague-Dawley rats. Nutr Cancer 41:75–81. doi:10.1080/01635581.2001.9680615
de Assis S, Warri A, Cruz MI, Hilakivi-Clarke L (2010) Changes in mammary gland morphology and breast cancer risk in rats. J Vis Exp. doi:10.3791/2260
Fritz WA, Coward L, Wang J, Lamartiniere CA (1998) Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis 19:2151–2158
Helle J, Kräker K, Bader MI et al (2014) Assessment of the proliferative capacity of the flavanones 8-prenylnaringenin, 6-(1.1-dimethylallyl)naringenin and naringenin in MCF-7 cells and the rat mammary gland. Mol Cell Endocrinol 392:125–135. doi:10.1016/j.mce.2014.05.014
Hosoda K, Furuta T, Ishii K (2011) Metabolism and disposition of isoflavone conjugated metabolites in humans after ingestion of kinako. Drug Metab Dispos 39:1762–1767. doi:10.1124/dmd.111.038281
Lamartiniere CA, Moore JB, Brown NM et al (1995) Genistein suppresses mammary cancer in rats. Carcinogenesis 16:2833–2840. doi:10.1093/carcin/16.11.2833
Lamartiniere CA, Zhang JX, Cotroneo MS (1998) Genistein studies in rats: potential for breast cancer prevention and reproductive and developmental toxicity. Am J Clin Nutr 68:1400S–1405S
Lamartiniere CA, Cotroneo MS, Fritz WA et al (2002) Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr 132:552S–558S
Lampe JW, Karr SC, Hutchins AM, Slavin JL (1998) Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med 217:335–339
Lee SA, Shu XO, Li H et al (2009) Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women’s Health Study. Am J Clin Nutr 89:1920–1926
Li K-M, Todorovic R, Devanesan P et al (2004) Metabolism and DNA binding studies of 4-hydroxyestradiol and estradiol-3,4-quinone in vitro and in female ACI rat mammary gland in vivo. Carcinogenesis 25:289–297. doi:10.1093/carcin/bgg191
Magee PJ, Rowland I (2012) Soy products in the management of breast cancer. Curr Opin Clin Nutr Metab Care 15:586–591. doi:10.1097/MCO.0b013e328359156f
Mehrara E, Forssell-Aronsson E, Ahlman H, Bernhardt P (2007) Specific growth rate versus doubling time for quantitative characterization of tumor growth rate. Cancer Res 67:3970–3975. doi:10.1158/0008-5472.CAN-06-3822
Messina M, Hilakivi-Clarke L (2009) Early intake appears to be the key to the proposed protective effects of soy intake against breast cancer. Nutr Cancer 61:792–798. doi:10.1080/01635580903285015
Möller FJ, Diel P, Zierau O et al (2010) Long-term dietary isoflavone exposure enhances estrogen sensitivity of rat uterine responsiveness mediated through estrogen receptor alpha. Toxicol Lett 196:142–153
Molzberger AF, Vollmer G, Hertrampf T et al (2012) In utero and postnatal exposure to isoflavones results in a reduced responsivity of the mammary gland towards estradiol. Mol Nutr Food Res 56:399–409. doi:10.1002/mnfr.201100371
Molzberger AF, Soukup ST, Kulling SE, Diel P (2013) Proliferative and estrogenic sensitivity of the mammary gland are modulated by isoflavones during distinct periods of adolescence. Arch Toxicol 87:1129–1140. doi:10.1007/s00204-012-1009-x
Murrill WB, Brown NM, Zhang J-X et al (1996) Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation. Carcinogenesis 17:1451–1457. doi:10.1093/carcin/17.7.1451
Nagata C (2010) Factors to consider in the association between soy isoflavone intake and breast cancer risk. J Epidemiol 20:83–89. doi:10.2188/jea.JE20090181
National Toxicology Program (2008) Toxicology and carcinogenesis studies of genistein (CAS No. 446-72-0) in Sprague-Dawley rats (feed study). Natl Toxicol Program Tech Rep Ser (545):1–240
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ (1990) Comparative study of human and rat mammary tumorigenesis. Lab Investig J Tech Methods Pathol 62:244–278
Santell RC, Kieu N, Helferich WG (2000) Genistein inhibits growth of estrogen-independent human breast cancer cells in culture but not in athymic mice. J Nutr 130:1665–1669
Seo H-S, DeNardo DG, Jacquot Y et al (2006) Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res Treat 99:121–134. doi:10.1007/s10549-006-9191-2
Setchell KD, Brown NM, Desai PB et al (2003) Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr 133:1027–1035
Shu XO, Jin F, Dai Q et al (2001) Soyfood intake during adolescence and subsequent risk of breast cancer among chinese women. Cancer Epidemiol Biomark Prev 10:483–488
Shu XO, Zheng Y, Cai H et al (2009) Soy food intake and breast cancer survival. JAMA 302:2437–2443
Shull JD, Spady TJ, Snyder MC et al (1997) Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis 18:1595–1601. doi:10.1093/carcin/18.8.1595
Soukup ST, Al-Maharik N, Botting N, Kulling SE (2014) Quantification of soy isoflavones and their conjugative metabolites in plasma and urine: an automated and validated UHPLC-MS/MS method for use in large-scale studies. Anal Bioanal Chem. doi:10.1007/s00216-014-8034-y
Taylor CK, Levy RM, Elliott JC, Burnett BP (2009) The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr Rev 67:398–415. doi:10.1111/j.1753-4887.2009.00213.x
Turan VK, Sanchez RI, Li JJ et al (2004) The effects of steroidal estrogens in ACI rat mammary carcinogenesis: 17β-estradiol, 2-hydroxyestradiol, 4-hydroxyestradiol, 16α-hydroxyestradiol, and 4-hydroxyestrone. J Endocrinol 183:91–99. doi:10.1677/joe.1.05802
Watson KL, Stalker L, Jones RA, Moorehead RA (2015) High levels of dietary soy decrease mammary tumor latency and increase incidence in MTB-IGFIR transgenic mice. BMC Cancer. doi:10.1186/s12885-015-1037-z
Acknowledgments
The authors are grateful to Dr. Carolin Kleider for help in determining E2 levels in the implants and for performing the UHPLC-MS/MS analysis of estrogens in plasma, to Antje Beyer for support with the animal experiments and technical assistance, and to Dr. Katja Schmalbach for assistance with the biostatistical analysis. This work is part of the joint research project, IsoCross, entitled, ‘Isoflavones: Cross-species comparison of metabolism, estrogen sensitivity, epigenetics and carcinogenesis’ that is funded by the German Research Foundation (Grants: VO 410/12-1, KU 1079/10–1, LE 1329/10–1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Human and animal participants
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Möller, F.J., Pemp, D., Soukup, S.T. et al. Soy isoflavone exposure through all life stages accelerates 17β-estradiol-induced mammary tumor onset and growth, yet reduces tumor burden, in ACI rats. Arch Toxicol 90, 1907–1916 (2016). https://doi.org/10.1007/s00204-016-1674-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1674-2