Abstract
Soy and its primary isoflavonic component, genistein, have been demonstrated to act via a novel mechanism for breast cancer chemoprevention, i.e. programming. Programming is defined as developmental modifications at the molecular level that result in permanent and irreversible modifications that determine how cells and tissues respond later in life, even in the absence of the initial effector. Depending on the chemical effector and the changes in the biochemical blue-print the adult host may be rendered more or less susceptible for biochemical insult. Exposure of prepubertal rats to physiological concentrations of genistein via the diet protects against chemically-induced mammary cancer. Genistein during the prepubertal period increases mammary protein expression of p-AKT, annexin A2, EGF-receptor, gelsolin and GTP-cyclohydrolase-1, while decreasing expression of cleaved-caspase-3 and protein disulfide-isomerase A3, actions consistent with increased cell proliferation and differentiation, cell turnover, and tissue remodeling. In mature rat mammary glands, cleaved-caspases-3 and 9, cleaved-poly(ADP-ribose) polymerase, fetuin B, β-casein and Ki-67 were increased, while tyrosine hydrolase, annexin A2, EGF-receptor, phosphoglycerate kinase-1, steroid receptor co-activators 1–3, and vascular endothelial growth factor-receptor-2 were down-regulated, actions consistent with increased apoptosis and reduced potential for carcinogenesis. Recent epidemiology reports confirm the laboratory findings on carcinogenesis, demonstrating that adolescent girls ingesting soy containing genistein are at reduced risk for breast cancer. Toxicology studies in animals and epidemiology with genistein and soy demonstrate little or no toxicity. We recommend clinical studies in adolescent girls to determine if soy and genistein can suppress mammary cancer development by programming for cell/tissue differentiation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Soybeans (Glycine max) have been cultivated in China for over 13,000 years. The soybean is the basis for soy milk, tofu, tempeh, and soy protein. While it has been used in other regions of Asia as a food source, it was not until the early twentieth century that the soybean was used for more than animal feed in the West. Soy contains numerous phytochemicals, including the diphenolic isoflavones, genistein and daidzein, of which genistein (4,7,4′-trihydroxyisoflavone) is the predominant and bioactive isoflavone of soy diet. In soy foods, the isoflavones are in the form of glycosides. After ingestion, the isoflavones undergo enzymatic hydrolysis to release the bioactive aglycones, genistein and daidzein (Fig. 2.1).
Soy has been touted as a health supplement with little or no toxicity. It is a good source of protein, low in fat and calories, cholesterol-free, and provides bone-healthy minerals like calcium, potassium, and magnesium. Regularly eating soy appears to reduce the risk of diabetes, especially in people who are overweight. The carbohydrates in soy are complex, hence they break down slowly in the body, limiting their impact on blood sugar [1]. People with diabetes are at increased risk of heart and kidney diseases, and soy is beneficial against these diseases. It can lower levels of low density lipoproteins (LDL), a benefit for heart health. Anderson et al. [2] reported that the consumption of soy protein rather than animal protein significantly decreased serum concentrations of total cholesterol, LDL cholesterol, and triglycerides. Soy lowers the amount of proteinuria, which is a common complication of diabetes. For postmenopausal women concerned about osteoporosis, soy can be a valuable dietary addition.
In women, phytoestrogens have been found to have weak estrogen-like activity. Isoflavones appear to work by binding and stimulating estrogen-receptor sites on cells and blocking out the natural estrogens. They can be helpful in improving symptoms of estrogen depletion such as postmenopausal syndrome. Due to the phytoestrogen content of soy, many women decide to include it in their diet as they enter menopause. During the menopause, the body’s natural production of estrogen declines and symptoms may ensue. As phytoestrogens act as a weak estrogen, they help relieve symptoms by providing a source of weak estrogen stimulation. Soy isoflavones, especially genistein, have attracted a great deal of research and studies suggest that women consuming a soy-rich diet have a lower risk of breast cancer.
2.2 Genistein In Vitro Studies
In cell culture, genistein has been reported to have growth promoting properties at nanomolar concentrations and inhibitory effects at micromolar concentrations [3, 4]. It has been demonstrated that genistein binds to the estrogen receptors-α and -β, with a higher affinity for the latter [5]. Genistein has been reported to be an anti-oxidant and to inhibit protein tyrosine kinases [6, 7], topoisomerase [8], cell proliferation and angiogenesis [9]. In ovariectomized female SCID mice (immunocompromised) implanted with human MC-7 breast cancer cells, genistein was reported to stimulate cell growth [10]. However, in mice with intact ovaries, genistein had no effect on the growth of human tumor cell growth [11]. Many of the studies resulting in toxicity are either carried out in in vitro systems using supraphysiological concentrations and/or animals administered genistein in a non-physiological manner (injections), the latter not taking into account bioavailability.
2.3 Genistein In Vivo Studies
Epidemiological studies show that Asian women consuming a diet high in soy products have a lower lifetime incidence of breast cancer [12, 13]. Yet, Asians who immigrate to the United States and adopt a Western diet lose this protection. Using rodents [14], researchers have reported soy-containing diets protecting against chemically-induced mammary cancer in animal models [15–17]. Subsequently, researchers investigated the potential of early exposure to injections of genistein in female rats to protect against chemically-induced mammary cancer using the chemical carcinogen, 7, 12-dimethylbenz(a)anthracene (DMBA). The reason for trying genistein was threefold: (1) it was previously reported that estrogen administered during the neonatal period protected against mammary tumor development [18, 19], (2) the structural similarity of genistein to estrogen, and (3) the epidemiology reports that Asian women consuming a traditional diet high in soy have a lower incidence of breast cancer but when they migrate to the U.S. the second, but not the first, generation lose this protection [13]. Fortuitously, genistein injected neonatally (days 2, 4 and 6 postpartum) to Sprague-Dawley rats did suppress DMBA-induced mammary cancer [20, 21]. This was followed by demonstrating that injections of genistein during the prepubertal period (postnatal days 16, 18 and 20) also suppressed DMBA-induced mammary cancer in rats [22, 23]. Subsequently, Hilakivi-Clarke et al. confirmed that prepubertal injections of genistein suppressed DMBA-induced mammary cancer in rats [24].
2.4 Dietary Genistein
Switching to a more physiological means of genistein administration, three groups of female rats (n = 30/treatment group) were fed 0, 25, and 250 mg genistein/kg AIN-76A diet starting two weeks before breeding and continuing through conception and parturition, until being discontinued at the time of weaning (21 days postpartum [25]. From day 21 postpartum, all female offspring from the three treatment groups were fed AIN-76A diet only, which is free of phytoestrogens. At day 50 postpartum, DMBA (80 mg/kg BW) was administered by gavage to all female offspring to induce mammary tumors. These specific dietary concentrations of genistein were chosen because, in a rodent model, they yielded serum concentrations of total genistein (aglycone and glycoside) similar to serum concentrations of total genistein found in men eating a diet high in soy [26, 27].
Control animals (no genistein in the diet) developed 8.8 tumors per rat, whereas dietary genistein suppressed DMBA-induced mammary tumor development in a dose-dependent manner (Fig. 2.2). Rats exposed to 25 and 250 mg genistein/kg AIN-76A diets had 7.1 and 4.4 mammary tumors per rat, respectively. We concluded that the chemoprevention of perinatal dietary genistein [25] was similar to our previous reports of neonatal and prepubertal genistein injections rendering a protective effect against DMBA-induced mammary cancer [20–23]. Demonstrating that lifetime protection against mammary cancer could be achieved by perinatal exposure to genistein in the diet suggests a differentiation/programming effect on the mammary target tissue [22, 25].
Ontogeny of palpable mammary tumors in female Sprague-Dawley CD rats exposed perinatally to genistein in the diet from conception until 21 days postpartum. After weaning, the offspring were fed AIN-76A diet only. On day 50 postpartum, all animals were treated with 80 mg DMBA/kg body weight [25]. Reprinted with permission from Fritz WA, Coward L, Wang J, Lamartiniere, CA (1998) Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis 19:2151–2158 (Oxford University Press)
2.5 Prenatal Genistein Treatment
To determine whether the prenatal period was the sensitive period for genistein to program against chemically-induced mammary cancer, we provided one group of pregnant females with 250 mg genistein/kg diet and the other group (controls) with no genistein in the diet during pregnancy. At parturition, both groups of dams and their offspring were fed the base diet, AIN-76A, containing no genistein. At day 50 postpartum, both groups of female offspring were gavaged with DMBA. Both groups developed the same number of tumors/rat, demonstrating that the prenatal period did not influence DMBA-induced mammary cancer chemoprevention [28], and strongly suggesting that the early postnatal period (neonatal/prepubertal) is responsible for the chemoprevention that we observed in our perinatal dietary genistein study [25].
2.6 Bioavailability
Our prenatal genistein findings proved to be contrary to that of Hilakivi-Clarke et al. [29] who reported that injecting pregnant rats with genistein resulted in the adult female offspring having increased susceptibility for DMBA-induced mammary cancer. We speculated that this contradiction was due to different routes of administration and bioavailability between the two studies. Measuring blood genistein concentrations from 21 day fetal-, 7 day neonatal-, and 21 day prepubertal rats exposed perinatally to 250 mg genistein/kg AIN-76A diet, we determined the circulating genistein concentrations to be 43, 726, and 1810 nmol/L, respectively [25, 30]. The 21 day fetal blood concentration [29] was 2.4 % that of the 21 day old prepubertal blood concentration [25]. This demonstrated that dietary genistein had good bioavailability during postnatal life, but poor bioavailability prenatally. It is noteworthy to point out that prepubertal rats start eating solid feed out of the jars at 14–16 days postpartum accounting, in part, for the high genistein concentration at day 21 postpartum. Furthermore, we determined that approximately 46 % of circulating total genistein was free genistein 24 h after injection of rats with genistein [31]. This is in contrast to less than 2 % being aglycone (free) genistein from dietary administration [25]. Thus, the bioavailability of injected genistein is substantially greater than that of oral genistein (23-fold), and this supraphysiological concentration can account for increased DMBA-induced mammary tumors in the Hilakivi-Clarke et al. report [29]. An awareness of timing of exposure, route of administration, metabolism, bioavailability, and biological action explains why prenatal injections of genistein to rats resulted in increased mammary tumorigenesis, while prenatal dietary genistein does not change susceptibility to DMBA-induced mammary cancer in the offspring [25, 28, 30, 31].
2.7 “Reading the Blueprint”
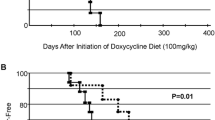
Because most breast cancers have been demonstrated to be estrogen dependent, we were concerned that genistein, an isoflavone phytoestrogen, may contribute to mammary cancer development. More specifically, women who have been diagnosed with breast cancer inquire whether soy products, including genistein, will protect from, or cause a recurrence of their cancer. To address this concern in the laboratory, rats were fed AIN-76A diet ± 250 mg genistein/kg diet at three time periods, and all females were treated with DMBA at day 50 to induce mammary cancer. As seen in Fig. 2.3, rats exposed to the control diet, AIN-76A only, from birth until the end of the experiment (Zero/DMBA/Zero) had the highest average number of tumors (8.9 tumors/rat) [28]. Rats exposed to genistein from days 1 to 21 postpartum only (Gen/DMBA/Zero) developed 4.3 tumors, which confirmed the earlier work of Fritz et al. [25]. Furthermore, rats exposed to genistein from days 1–21 and 100–180 (Gen/DMBA/Gen) developed the fewest number of tumors (2.8 tumors/rat). The later genistein feeding was initiated 50 days after the DMBA treatment, the time of onset of palpable mammary tumors. The results showed that genistein fed to adult rats previously exposed prepubertally to genistein provided these animals with additional protection against mammary cancer (Table 2.1). Prepubertal genistein exposure appears to permanently affect the mammary gland in a way that determines how that individual later responds to the same or similar chemical stimuli, i.e. the “blue-print” for gene and protein expression is set. In this case, genistein acquired via the diet during the prepubertal period programmed future (adult) genistein response against mammary cancer susceptibility [25, 28].
Ontogeny of palpable mammary tumors in female Sprague-Dawley CD rats exposed prepubertally and/or as adults to genistein in the diet. Group 1 was fed control AIN-76A diet starting after parturition and continued throughout the study (Zero/DMBA/Zero). Group 2 was fed AIN-76A diet containing 250 mg genistein/kg diet, starting after parturition through day 21 only and then AIN-76A onward (Gen/DMBA/Zero). Group 3 was fed genistein-containing diet after parturition through day 21, AIN-76A only through day 100 postpartum, and genistein-containing diet (Gen/DMBA/Gen) from day 100 onward. All animals received 80 mg DMBA/kg body weight at day 50. Each group consisted of 25 rats. Reprinted with permission from Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel, R-M, Elgavish A (2002) Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr 132:552S-558S (American Society for Nutritional Sciences)
2.8 Mammary Gland Development
Via the elegant studies of Jose and Irma Russo, we know that developmental alterations to the mammary gland can determine cancer susceptibility. The development of the mammary gland in rats starts in utero. From birth through the first week postpartum in the rat, the mammary gland is composed of a single primary or main lactiferous duct that branches into 3–5 secondary ducts [32, 33]. During the second week, further sprouting of ducts occurs up to the sixth generation. This sprouting of ducts causes an increase in density of terminal end buds in the growing periphery of the mammary gland (Fig. 2.4). Some of the terminal end buds differentiate in response to each estrous cycle, giving rise to alveolar buds which can be found in type I lobules. Type I lobules can mature to type II lobules. These lobules respond to hormones of pregnancy by differentiating further into type III lobules, which form the functional units of the lactating gland [34, 35]. The differentiation of terminal end buds to lobules appears to be a basic and protective mechanism against chemical carcinogenesis. Terminal end buds and terminal ducts are less differentiated structures that are more susceptible to chemical carcinogenesis than the more differentiated alveolar buds and lobules [32]. This is due to the increased mitotic activity of terminal end bud and terminal duct cells at day 21 as opposed to cells in alveolar buds and lobules in mature animals [34]. In the human, the development of the mammary gland is similarly initiated in utero [36]. Further development and differentiation requiring active cell proliferation takes place almost simultaneously with the formation of lobules type 1–4.
Terminal ductal structures in rat mammary glands. (A) Whole mounts of fourth abdominal mammary glands from female Sprague Dawley CD rats. Note nipple at upper corner and lymph nodes at bottom right. (B) The upper structure is a terminal end bud, the lower structure is a terminal duct, (C) Lobule I, (D) Lobule II. Reprinted with permission from Brown NM, Manzolillo PA, Zhang J-X, Wang J, Lamartiniere CA (1998) Prenatal TCDD and predisposition to mammary cancer in the rat. Carcinogenesis 19(9):1623-1629 (Oxford University Press)
Evidence of age-related breast cancer susceptibility is evident from reports in girls exposed to cancer causing agents early in life. In female patients who were exposed to radiation via fluoroscopy an average of 102 times over a period of several years, the greatest risk of breast cancer occurred among women who were first treated between the ages of 15 and 19 years, with no excess risk being associated with women who were over 30 years old at the time of first exposure [37]. This increased breast cancer risk did not appear until 15 years after the initial exposure and was present at the end of 40 years of observation. Also, after World War II bombing of Hiroshima and Nagasaki [38], young girls who were 10–19 years old when exposed to the ionizing radiation of the atomic bomb were followed and found to have a higher lifetime risk of breast cancer after the age of 35. These reports suggest that the early period of a women’s life is critical for predisposition to, or for protection against, breast cancer.
2.9 Cellular Mechanism of Action
Analysis of mammary gland morphology in mature rats exposed to genistein early in life revealed that its cellular mechanism of action is enhancement of mammary gland differentiation [21, 22, 25, 28, 30, 39, 40]. We studied this in 50 day old rats since this is the time that the carcinogen, DMBA, is administered in the rat model. We found reduced number of terminal end buds and increased number of lobules in adult animals exposed neonatally or prepubertally to genistein (before administering the DMBA). Mammary terminal end buds are terminal ductal structures found primarily in young animals (and humans) and contain many undifferentiated epithelial cells [32, 34]. They are characterized by having a high mitotic index, hence they are most susceptible to chemical carcinogens [33]. While terminal end buds are the structures most susceptible to chemical carcinogenesis in the mammary glands, lobules are the most differentiated and least susceptible to chemical carcinogens. Importantly, a similar cellular mechanism of action involving increased mammary terminal ductal differentiation also occurs in the rat mammary and human breast via hormones of pregnancy [34–36].
Further evidence that genistein enhances differentiation was obtained by measuring β-casein in mammary glands. β-Casein is a milk protein and biomarker of mature mammary glands and differentiated cells [34]. Using western blot analysis, we found that prepubertal genistein treatment increased beta-casein expression in mammary glands of prepubertal and adult rats [28]. In the adult rats beta-casein was measured 30 days after genistein treatment, so even in the absence of circulating genistein, the rat mammary gland still produced β-casein, indicating permanent, non-reversible differentiation.
The potential of the soy components, daidzein and equol, have also been investigated for potential to alter mammary gland development and for mammary cancer prevention. Daidzein is the second most plentiful of the isoflavones, and equol is an intestinal bacterial metabolite of daidzein. Perinatal exposure of female rats via 250 and 1000 mg daidzein/kg AIN-76A diet did not alter mammary gland development at day 50 or the ontogeny of chemically-induced mammary tumors in rats treated with DMBA on day 50 [41]. Brown et al. investigated the potential of 250 mg equol/kg diet during the neonatal (0–21 days) or prepubertal (21–35 days) period to alter mammary gland development and predisposition for mammary cancer. By day 50, early equol exposure resulted in a decrease in immature terminal end structures and an increase in mature lobules [42]. Despite these morphological changes to the mammary gland, neonatal and prepubertal exposures to equol had no long-term chemoprevention against mammary tumors induced by DMBA. The fact that equol enhanced mammary gland differentiation, but did not render chemoprevention, suggest that there is more than gland differentiation as the mechanism for genistein chemoprevention.
2.10 Genistein: Cell Proliferation and Apoptosis
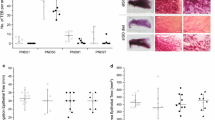
Cell turnover is a key process involved in mammary gland development and the pathogenesis of tumor formation. Hence, we investigated if early prepubertal exposure to genistein treatment impacted normal cell proliferation and apoptosis in the mammary gland. At postnatal day 21, rats prepubertally exposed to dietary genistein exhibited a 53 % increase in cell proliferation and a 45 %, but not statistically significant decrease in apoptosis in mammary terminal ductal structures (Fig. 2.5A & B) [43]. Using the ratio of cell proliferation to apoptosis to estimate cell turnover in mammary terminal ductal structures, genistein exposure increased the ratio compared with controls by 2.6-fold (Fig. 2.5C). The latter is suggestive of active remodeling in the mammary gland from the presence of genistein.
Cell Proliferation, apoptosis and cell turn-over in mammary gland terminal end bud epithelial cells of 21 and 50 day old rats exposed prepubertally via lactating dams fed genistein in the diet from days 2–21 postpartum. The pictures in sections (A & D) illustrate cell proliferation via immunohistochemical staining for antigen Ki-67, and sections (B & E) are for apoptosis via ApopTag in situ labelling kit. Sections (C & F) reflect cell turn over, i.e. ratio of cell proliferation to apoptosis. Reprinted with permission from Wang J, Jenkins S and Lamartiniere CA (2014) Cell proliferation and apoptosis in rat mammary glands following combinational exposure to bisphenol A and genistein. BMC Cancer 14:379 doi:10.1186/1471-2407-14-379 (BioMed Central)
Because Sprague Dawley rats are most susceptible to chemically-induced mammary cancer at day 50, we also investigated cell proliferation and apoptosis at that age. This time point is 30 days after the last dietary genistein treatment and a time point that, due to metabolism and disposition, animals are free of circulating genistein. While cell proliferation was not changed in mammary glands of 50 day old rats exposed prepubertally to genistein (Fig. 2.5D), cell apoptosis was increased over two-fold (Fig. 2.5E) [43]. Cell turnover was calculated to be decreased by 55 % (Fig. 2.5F). Summarily, genistein administered to rats during the prepubertal period stimulates mammary cell proliferation at day 21, but not at day 50, while apoptosis is increased at the latter age. The increase in cell proliferation during early postnatal mammary gland development correlates with differentiation of the mammary terminal ductal structures (from day 21 to day 50), and is to be associated with less chemically-induced cancer in the adult animals [21, 22, 25, 28, 30]. Of added importance is that increased rate of apoptosis at time of carcinogen exposure probably contributes to chemoprevention by killing DNA-damaged cells.
2.11 Molecular Mechanism of Genistein Action
Day 21 mammary glands. One reason cancer researchers have investigated genistein as a chemoprevention agent are the reports that it inhibits protein tyrosine kinases activity in vitro [6, 7]. One such tyrosine kinase protein is the epidermal growth factor (EGF)-receptor. The EGF-receptor and its ligands play essential roles in normal and pathological mammary gland development. Early in development, the EGF-signaling pathway plays an essential role in cell differentiation, development, and ductal morphogenesis [44, 45]. Accordingly, we investigated the potential of genistein to regulate the EGF-receptor in vivo. In 21 day old rats treated prepubertally with genistein, we found increased EGF-receptor expression in mammary terminal end buds (Fig. 2.6A & B) [40]. Not only was this finding contrary to the in vitro reports [6, 7], but this was surprising to us because we expected a chemoprevention agent to down-regulate the expression of this cancer-related growth factor signaling pathway. However, as discovered later, in mammary glands of 50 old rats exposed prepubertally to genistein the expression of the EGF-receptor was down-regulated (Fig. 2.6C & D) [40, 46]. We surmise that exposure to genistein early in postnatal life alters the “molecular program” from which the mammary gland of 50 day old rats responds to later. This will be discussed in more detail in the next section.
Immunohistochemical staining for the EGF-receptor in mammary terminal end buds. Photographs (A, B) show staining for the EGF-receptor in terminal end buds of 21 day old rats treated prepubertally with (A) vehicle, or (B) genistein. Photographs (C, D) show staining for the EGF-receptor in terminal end buds of 50 day old rats treated prepubertally with (A) vehicle, or (B) genistein. Note the dark immunohistochemical staining for the EGF-receptor in panels B and C (40). Reprinted with permission from Brown NM, Wang J, Cotroneo MS, Zhao, Y-X, Lamartiniere CA (1998) Prepubertal genistein treatment modulates TGF-α, EGF and EGF-receptor mRNAs and proteins in the rat mammary gland. Mol Cell Endocrinol 144:149–165 (Elsevier)
To increase the number of mammary biomarkers, we used two-dimensional gel electrophoresis coupled with mass spectrometry to separate, quantitate, and identify mammary gland proteins that are changed in response to prepubertal genistein treatment [46, 47]. For those proteins that we were able to obtain commercially available antibodies, we pursued validation via western blot analysis, and extended our use of immuno-blots to identify and quantitate proteins that play a role in cell proliferation, apoptosis, or other signaling pathways related to carcinogenesis. In mammary glands of 21 day old rats exposed prepubertally to genistein, expression of phospho-Akt and annexin A2 were increased compared to controls (Table 2.2) [46]. Like the EGF-receptor, these two proteins have been associated with mitotic activity, and their actions are believed to play a significant role in the cell proliferation observed in the presence of genistein in mammary glands of prepubertal rats. In mammals, cell proliferation is required for embryogenesis, growth, and differentiation of cells and tissues, including the mammary gland. Metabolic effects of the PI3K/Akt/mTOR pathway include enhanced uptake of glucose and essential amino acids and protein translation that can contribute to cell motility, and cell survival [48]. While not prominent in the field of mammary cancer, annexin A2 has been shown to play a role in DNA synthesis, cell differentiation and neoangiogenesis [49].
GTP-cyclohydrolase-1 (GTPCH-1) and gelsolin are two other proteins that were found to be up-regulated in mammary glands of 21 day old rats treated prepubertally with geinistein [46, 47]. GTPCH-1 is the rate limiting enzyme in the production of tetrahydrobiopterin (BH4) and to the production of catecholamines, which regulate differentiation and development of cells [50]. Gelsolin is an actin filament-capping protein that has been shown to play a key role in cell migration. It is required in the mammary stroma for proper ductal morphogenesis and promotes mammary ductal growth through stromal-epithelial communication [51]. Also, gelsolin has been reported to be an inhibitor of apoptosis, and overexpression of gelsolin results in the lack of activation of caspase-3 [52].
Gelsolin being up-regulated is consistent with our finding that cleaved caspace-3 protein expression was reduced in mammary glands of 21 day old rats exposed prepubertally to genistein [43]. Previously, Qian et al. [53] showed that genistein treatment reversed ischemia-induced mitochondrial dysfunction by decreasing mitochondrial reactive oxygen species, preventing cytochrome C release, and inhibiting caspase 3 activation. Protein disulfide-isomerase A3 (PDIA3), also known as glucose-regulated protein or GRP58/ERp57, was reduced in mammary glands of 21 day old rats exposed prepubertally to genistein via the diet [54]. PDIA3 is reported to play a role in protein folding and differentiation [52]. We found decreased PDIA3 expression in mammary glands of 21 day old rats, but unchanged at day 50. We speculate that up-regulated PDIA3 in mammary glands of prepubertal rats supports cell differentiation and gland maturation.
Day 50 mammary glands. Turning our attention to proteins in 50 day old rats exposed to genistein during the prepubertal period, we found higher β-casein protein expression in mammary glands of 50 day old rats exposed prepubertally to genistein (Table 2.3) [28]. This finding is consistent with our reports of increased number of lobules (more differentiated terminal ductal structures) in 50 day old animals exposed prepubertally to genistein [22, 23, 25, 28, 30, 40]. β-Casein is a milk protein and considered a marker of differentiation in mammary glands [34]. This confirms that prepubertal genistein exposure enhances mammary gland cell differentiation.
Also, we were able to confirm by western blot analysis that EGF-receptor expression was decreased in mammary glands of 50 day old rats exposed prepubertally to genistein compared to those with no genistein in the diet [46]. This was previously demonstrated by immunohistochemical staining [42]. Importantly, in comparing increased mammary EGF-receptor expression from the 21 day old rats being exposed to genistein to that of reduced mammary EGF-receptor expression in 50 day old rats where genistein treatment had been discontinued since day 22 postpartum, we see the dramatic effect of direct genistein action in the prepubertal period to that of an apparent delayed, but permanent effect on specific protein expression. We interpret this to mean that early in postnatal life (days 1–21) genistein up-regulated the EGF-receptor to stimulate mammary gland development and cell differentiation that resulted in enhanced mammary gland maturation later in life. Reduced EGF signaling and decreased cell proliferation at day 50, at which time the DMBA was given, coincides with the more mitotically inert lobules and thus reduced susceptibility to chemical carcinogenesis [21, 22, 25, 30, 40]. Developmental modifications by a hormonally-active chemical that results in altered biochemical or behavioral responses later in life has been defined as organizational, imprinting or programming effects [55–57]. We speculate that down-regulated EGF-receptor signaling in mammary terminal end buds at the time of carcinogen exposure plays a role in reduced mammary cancer development.
Not only is the EGF signaling pathway important for cell proliferation and differentiation, and mammary gland development and carcinogenesis, but so are estrogen receptors. Regulation of steroid receptor action is complex due to a number of transcriptional regulatory molecules, including steroid receptor co-activators (SRCs), which can determine signaling specificity and intensity, resulting in pleiotropic biological effects, including cancer causation [58–60]. We assessed the expression of ER-α, ER-β and SRC proteins known to play a role in estrogen signaling and breast cancer in mammary glands of 50 day old rats exposed prepubertally to genistein in the diet. No significant differences were observed in ER-α and ER-β expression (data not shown), but we found all three members of the p160 family of steroid receptor coactivator proteins, SRC-1, SRC-2 (GRIP-1: glucocorticoid receptor-interacting protein) and SRC-3 (AIB1: amplified in breast cancer) to be significantly down-regulated in mammary glands of 50 day old rats following prepubertal genistein exposure when compared controls (Table 2.3) [43]. Just as a large variety of steroid hormone dependent cancers overexpress SRCs, down regulation of steroid receptor-coactivators is viewed as consistent with suppressing cancer development.
In mammary glands of 50 day old rats with prepubertal exposure to genistein, we found annexin A2 expression to be decreased [46]. As opposed to the decreased expression seen here, increases in annexin A2 have been reported in cancer invasion and progression processes, and observed in cancers of the breast and prostate [61, 62]. This suggests that annexin A2 possess cancer promoting properties. On the other hand, the opposite result, reduced expression of annexin A2 as noted in mammary glands of 50 day old rats could be viewed as contributing to reduced cell proliferation and thus reduced potential for cancer. This pattern is similar to what was evidenced with the EGF-receptor. Both protein expressions were increased in mammary glands of 21 day old rats and decreased at day 50. Likewise, in the presence of genistein during the prepubertal period, both may contribute to cell proliferation and cell differentiation, and in the more differentiated and mature mammary glands (in the absence of genistein) there is less annexin A2 and EGF-receptor signaling, properties that are less conducive for carcinogenesis.
The caspases are a family of evolutionarily conserved cysteinyl proteases that mediate both apoptosis and inflammation through aspartate-specific cleavage of a wide number of cellular substrates. Caspase biology has been extended to cellular responses such as cell differentiation and proliferation. We selected two caspases as potential biomarkers, caspace-3 and caspace-9. Caspases involved in apoptosis have been classified by their mechanism of action and are either initiator caspases (caspase-9) or executioner caspases (caspase-3) [63–65]. We also measured c-PARP, a nuclear enzyme involved in DNA repair which is a well-established substrate for caspase-3 [66]. Cleaved PARP is considered to be a hallmark of apoptosis [66–68]. Activated-caspace-3, activated-caspace-9 and activated PARP were all up-regulated at day 50 [43]. These results are consistent with increased potential for apoptosis, which was determined by an in situ apoptosis assay in mammary terminal end buds of 50 day old rats exposed prepubertally to genistein [43]. This would suggest that DNA damaged cells would undergo apoptosis and serve as another level of chemoprevention.
Fetuin B has been reported to possess anti-angiogenic properties, and its overexpression in skin squamous carcinoma cells leads to suppression of tumor growth in nude mice [69]. We found that prepubertal genistein exposure resulted in increased fetuin expression by 67 % compared to controls in mammary glands of 50 day old rats [46]. Therefore, fetuin B could be acting as a tumor suppressor in the rat mammary gland. Also, Cabanes et al. [70] reported that injections of prepubertal genistein resulted in increases in the tumor suppressor BRAC1 mRNA in prepubertal and 8 week old rats. It would be interesting to determine if the mRNA of this tumor suppressor gene is translated to the protein in a dietary model.
Genistein exposure decreased the levels of PGK1 by 54 % compared to control [46]. PGK1 is involved in the glycolytic pathway. PGK1, like annexin A2 is a component of the primer recognition complex that stimulates the activity of DNA polymerase [71, 72]. Therefore, decreased expression of PGK1 could explain, in part, the reduced rate of cell proliferation observed in the mammary gland.
As a follow-up to our finding that GTPCH-1 protein expression was up-regulated in mammary glands of 21 day old rats treated with genistein, we investigated GTPCH-1 and tyrosine hydrolase expression in mammary glands of 50 day old rats. At 50 days, there was no change in GTPCH-1 protein, but tyrosine hydroxylase expression was increased [47], a factor that could lead to increased dopamine. Interestingly, Teunis et al. [73] reported that rats with high dopaminergic activity had a reduction in tumor size compared with rats with low dopaminergic activity. Associating elevated dopamine levels with suppressed mammary tumorigenesis, they noted that the angiogenic response to the vascular endothelial growth factor receptor (VEGFR) could be inhibited by administration of dopaminergic agonists. Basu et al. [74] showed that dopamine acts through the dopamine 2 receptor to induce the endocytosis of VEGFR and thereby inhibit or prevent VEGF binding, receptor phosphorylation, and subsequent signaling steps. They reported that immunohistochemical studies did not find tyrosine hydroxylase–positive nerves in tumors, and the dopamine concentration in malignant tumors was significantly reduced compared with concentrations in controls. Furthermore, Ferguson et al. [75] reported that lifetime exposure to genistein could potentiate dopamine levels in striata of amphetamine-exposed animals. In addition, researchers found that genistein decreased both transcription and protein levels of VEGF and that this decrease is involved in the loss of angiogenesis. Heffelfinger et al. [76] demonstrated that inhibition of VEGFR2 will prevent DMBA-induced mammary tumors in rats. As a follow-up, we found VEGFR2 expression to be decreased in mammary glands of 50 day old rats exposed prepubertally to genistein [46, 47]. These results complemented the finding that tyrosine hydroxylase levels were elevated in the mammary glands of 50 day old rats from prepubertal genistein treatment. We speculate that increased tyrosine hydroxylase expression results in dynamic up-regulation of catecholamines, which, in turn, decrease the VEGFR2 levels, resulting in decreased ability to promote angiogenesis. Ortega et al. [77] implicated VEGFR2 in mediating cell proliferation. Therefore, a decrease in VEGFR2 may decrease the overall proliferative potential of the mammary gland. The absence of a demonstrable change in dopamine concentrations may mean that the concentration is dynamic or that changes in concentration within the microenvironment may not manifest in detectible or significant change in the larger sample (whole mammary gland). Observed decrease in VEGFR2 at the time of carcinogenesis could reduce cell proliferation, angiogenesis, and cell invasion, and favor breast cancer prevention.
Not surprisingly, we found the Ki-67 protein to be down-regulated in mammary glands of 50 day old rats [46], thus adding validity to differentially regulated proteins involved in cell proliferation. The fact that the Ki-67 protein is present during all active phases of the cell cycle, but is absent from resting, makes it an excellent marker for determining the so-called growth fraction of a given cell population [78]. Ki-67 protein expression is an absolute requirement for progression through the cell-division cycle and is an excellent indicator of cell proliferation.
2.12 Programming Against Breast Cancer
Programming or differentiation in the mammary gland is to be contrasted to activational effects that are often described in conventional mechanisms of action following exposure to a chemical modulator. For example, when a chemical such as genistein is provided to an animal and the result is an up or down expression of a specific protein, followed by reversal to previous expression level when the chemical is withdrawn, this is an activational effect, i.e. an effect that is reversible. On the other hand, when hormones of pregnancy or genistein is given to a young female rat, not only can activational effects take place, but so can organization effects that can be termed programming or imprinting [55–57]. An example is cell and mammary gland differentiation whereby terminal end buds are transformed to the more mature lobules that can eventually produce milk. These lobules can now be characterized as having different gene and protein expressions from those of terminal end buds. Some of these changes are permanent and irreversible changes at the molecular level. Hence, we refer to these permanent changes in gene and protein expressions as the “blue-print” from which these mammary cells respond. When the term imprinting is used, this most often refers to an epigenetic modification, i.e. as a consequence of changes in DNA methylation or histone acetylation. But, proteins can undergo post-translational modifications that can alter function. Because we do not yet know the exact nature of genistein’s action in promoting mammary cell and gland differentiation, and subsequent biological actions that render the mammary to be resistant to cancer, we use the term programming. In our proteomic studies we observed several examples of activational effects. In mammary glands of 21 day old rats exposed to genistein, we found that p-AKT, gelsolin, GTPCH-1 and PDIA3 were differentially regulated in the presence of genistein. However, in adults, in the absence of genistein, the expression of these specific proteins were similar to those of controls, i.e. the effect was not sustained at 50 days.
On the other hand, in mammary glands of 50 day old rats exposed prepubertally to genistein we observed 14 proteins whose expressions were different from controls (Table 2.3). The importance of this observation is that neither group of adult animals was in the presence of genistein at the time of measurement. Hence, exposure to genistein during the prepubertal period, a critical time for mammary gland development, set the biological “blue print” or the stage for permanent manifestations that determine how the mammary gland develops and responds later to chemical exposures such as hormones, (pro)carcinogens and effectors of cell proliferation and apoptosis. Critically, programming can in the mammary predetermine susceptibility for disease, including breast cancer.
Does the programming mechanism apply to all tissues? No. For example, we investigated the potential of dietary genistein exposure in transgenic mice designed to spontaneously develop prostate tumors (TRAMPs), and chemically-induced prostate cancer in Lobund-Wistar rats. Our prostate cancer studies demonstrated that genistein exposure during the neonatal and prepubertal periods only did not suppress prostate cancer development in adult TRAMPs [79]. On the other hand, genistein in the diet to adult TRAMP mice resulted in a 29% decrease in poorly-differentiated adenocarcinomas. More effective was life-time (weeks 1–28 postpartum) genistein treatment. It resulted in a 50% decrease in poorly differentiated prostate tumors. With both of these genistein treatment groups, the chemoprevention was associated with suppressing the rate of cancer development as evidenced by increased percentage of prostate cancer manifested as moderately differentiated tumors, (44–60%). Similar results were obtained with N-methylnitroso urea (NMU)-induced prostate cancer in Lobund-Wistar rats [80], i.e. adult exposure was more effective than neonatal/prepubertal genistein exposure only, and life-time use of genistein, starting in the first week was more effective in suppressing prostate cancer. In both prostate cancer models, the direct presence of genistein was necessary to suppress prostate cancer development.
2.13 Toxicology Studies
In laboratory studies, genistein has been reported to stimulate tumor growth in athymic ovariectomized mice subcutaneously implanted with MCF-7 breast cancer cells, albeit six fold lower than positive controls provided by estrogen [81]. But, the latter is contradictory to the report where genistein administered to intact athymic mice orthotopically implanted with MCF-7 cells suppressed the growth of resulting tumors [82]. The significance of ovariectomy as a model of menopause and the use of mice lacking proper cellular surveillance immunity remains to be discerned.
Since the perinatal period is the most sensitive time for toxicity to the endocrine and reproductive systems, we have carried out toxicology studies in rats exposed perinatally to genistein. We chose to administer genistein via the diet, which is the primary means of soy and genistein exposure. The dietary genistein doses were selected on the basis of a previous report that rats fed 25 mg genistein/kg AIN-76A diet (phytoestrogen-free) resulted in total genistein concentrations of 252 pmol/ml in the serum [26]. This was comparable with the total genistein concentration (276 pmol/ml) in the blood of Asian men eating a traditional diet high in soy [27]. A dose one order of magnitude higher was also selected for the purpose of investigating potential toxicity of dietary genistein and for dose-response and bioavailability studies. The numbers of male and female offspring, anogenital distances, time of testes descending and vaginal opening were not significantly different from controls [25]. The body weight, uterine weights and mammary gland size were not significantly different compared to control exposed animals at postnatal days 21 and 50. Perinatal genistein in the diet did not alter percent of time in each phase of the estrous cycle of the female offspring. The numbers of primordial normal follicles and corpora lutea were not significantly different in females exposed perinatally to genistein. Also, histomorphological analysis of vaginal, uterine and ovarian tissues in 50 and 100 day old female rats exposed perinatally to genistein did not reveal significant alterations compared to controls [25].
Flynn et al. [83] have carried out toxicology investigations using dose response studies and evaluated morphometric measurements and sexually dimorphic behaviors in rats. They reported that dietary genistein at 25 and 250 mg/kg AIN-76A diet fed to pregnant rats, beginning on gestational day 7 and the offspring continued until postnatal day 77, did not significantly alter gestational duration, total offspring/litter, total sex ratio, live pups/litter, live sex ratio, and average weight/live pup.
Studies with humans show that isoflavonic phytoestrogens are normal constituents of human urine from subjects consuming large amounts of soy products (tofu, soy flour, soy milk, tempeh, soy nuts, soy bars, etc). Yet, little or no toxicity is associated with soy/genistein consumption [84]. Infants are able to absorb isoflavones, and infants fed soy formula were demonstrated to have plasma isoflavone blood levels exceeding those of Japanese adults several-fold [85]. Soy-based infant formula can result in plasma concentrations of isoflavones in infants that are 13,000–22,000 times higher than endogenous estrogen concentrations in infants [86]. Infants consuming soy-based formula are exposed to 6–11 mg isoflavones /kg per day (4–7 mg total genistein/kg) that result in circulating total genistein levels of approximately 1–5 μM. In contrast, adults consuming a moderate to large amount of soy in the diet are exposed to ~1 mg total genistein/kg per day resulting in circulating total genistein levels of approximately 0.5 μM [87]. Even though infants ingesting soy milk are exposed to high concentrations of genistein, little toxicity has been reported. The most noted consequence is hypothyroidism in infants with already compromised thyroid function, a situation that is remedied by fortifying soy milk with thyroid hormone supplement [88]. On the other hand, a plethoria of publications have investigated the potential of soy and it components for health benefits.
To address the potential of soy formula to result in toxicity to children, the National Toxicology Program convened an expert panel to determine the level of concern for soy infant formula on infants and child development. The Expert Panel of the 2010 NTP Brief on Soy Infant Formula focused on soy infant formula and the potential developmental toxicity of its major isoflavone components, e.g. genistein, daidzein (and estrogenic metabolite, equol), and glycitein. They expressed minimal concern for adverse developmental effects in infants fed soy infant formula. The NTP concurred with the expert panel that there is minimal concern for adverse effects on development in infants who consume soy infant formula [89].
2.14 Epidemiology
Early chemoprevention work with soy and genistein has been driven by epidemiology reports of high soy diets being protective against breast cancer in women [12, 13]. Since then, a multitude of epidemiology publications have supported these publications, and some have not. On the other hand, none report that soy or genistein promote new estrogen-dependent breast or reproductive cancers. One of the most comprehensive meta-analyses of soy and risk for breast cancer was carried out by Trock et al. [90]. They performed a meta-analysis of 18 epidemiology studies (12 case–control and six cohort or nested case–control) published from 1978 through 2004 that examined soy exposure and breast cancer risk. Pooled relative risk estimates were based on either the original soy exposure measure defined in each study or on an estimate of daily soy protein intake. They found that risk estimates, levels and measures of soy exposure, and control for confounding factors varied considerably across studies. In a pooled analysis, among all women, high soy intake was modestly associated with reduced breast cancer risk (odds ratio (OR) = 0.86, 95 % confidence interval [CI] = 0.75–0.99); the association was not statistically significant among women in Asian countries (OR = 0.89, 95 % CI = 0.71–1.12). Among the ten studies that stratified by menopausal status the inverse association between soy exposure and breast cancer risk was somewhat stronger in premenopausal women (OR = 0.70, 95 % CI = 0.58–0.85) than in postmenopausal women (OR = 0.77, 95 % CI = 0.60–0.98). However, eight studies did not provide menopause-specific results, six of which did not support an association. When exposure was analyzed by soy protein intake in grams per day, a statistically significant association with breast cancer risk was seen only among premenopausal women.
More intriguing, but convincing, are the four epidemiology reports showing an association between soy intake of adolescents and reduction in breast cancer that are consistent with the laboratory demonstrations that genistein exposure during the prepubertal period suppresses chemically-induced mammary cancer in rats [22–25, 28, 30]. In 2001, Shu et al. [91] analyzed data from a population-based case-control of 1459 breast cancer cases and 1556 age-matched controls and showed that high soy food intake during adolescence (age 13–15) resulted in an inverse association with breast cancer risk in both pre- and postmenopausal Chinese women. Shortly thereafter, Wu et al. [92] reported a population-based, case-control study of breast cancer risk among Chinese, Japanese and Filipino women in Los Angeles County to investigate the role of soy, focusing on soy intake during adolescence and adult life among Asian-American women. Women who reported soy intake at least once per week during adolescence showed a significantly reduced risk of breast cancer, and there was a significant trend of decreasing risk with increasing soy intake during adult life. Furthermore, high soy intake during both adolescence and adult life showed the lowest risk for breast cancer.
Also, Korde et al. [93] reported Asian-American women with high soy intake as children (between the ages of 5 and 11 years) with the greatest reduction in breast cancer risk (58 %), followed by exposures at adolescence (age 12–19), and as young adults age 20 to approximately 27, furthermore illustrating how important early postnatal development for reduction in breast cancer risk. The epidemiologic reports by Wu et al. [92] and Korde et al. [93] support our laboratory report that female rats exposed to genistein via the diet from parturition through day 21 and then from day 100 until the end of the study at day 180 had fewer mammary tumors than those provided genistein only during the prepubertal period or as adults only [28]. More recently, a population-based case–control study evaluated the association between adolescent dietary phytoestrogen intake and adult breast cancer risk among women in Ontario, Canada. Higher phytoestrogen intake during adolescence was associated with a reduced breast cancer risk, and a monotonic trend was observed from the lowest to the highest quartile [94]. Frankly, it is remarkable how consistent the prepubertal genistein laboratory data and the adolescent soy epidemiology data are. Furthermore, the reports of adolescents exposed to soy having reduced breast cancer risk [91–94] explain why many earlier epidemiology reports had less than stellar results, i.e. adult only soy exposure matters only if adolescent plus adult exposure takes place.
Realizing that the most likely way towards cancer prevention is via early exposure to soy or genistein, Maskarinec et al. [95] investigated the compliance of young girls to soy intervention. They used an eight week dietary intervention, and urine samples were collected from eight to 14-year-old girls. The girls were asked to consume one daily serving of soymilk, soy nuts, or tofu. 17/20 of the girls completed the study. The serving sizes provided at least 30 mg isoflavones/day. Daily soy intake logs indicated a mean intake of 6.28 servings out of a maximum of seven servings per week. The food records revealed a six-fold increase in isoflavone intake during the study period (P < 0.01) which was confirmed by urinary isoflavone concentrations of 23.3 nmol/mg creatinine prior to intervention and 142.1 nmol/mg creatinine during intervention. The adolescent girls demonstrated compliance, and no health complications related to soy consumption were reported.
2.15 Blood Proteomics of Prepubertal Girls
Our focus on cancer biomarkers breaks from the accepted dogma of using genomic markers and moves to a more practical aspect of biomarkers that actually reflects function, proteins. Proteins, as enzymes, cofactors and regulators, actually carryout the enzymatic actions and support many metabolic processes. Although there are a plethora of papers that examine gene expression, the latter may not always translate into protein action. Recently, we developed methods to identify protein biomarkers of effect and susceptibility from blood using Isobaric Tandem Mass Tags and quantitative mass spectrometry (TMT-MS) combined with MudPIT technology. We used blood sera from prepubertal girls whose urine had been subjected to mass spectrometry analysis for soy isoflavones, phenols and phthalates. In prepubertal girls, urine concentrations of genistein, bisphenol A (BPA), mono-ethyl hexylphthalate (MEHP) and mono-benzyl phthalate (MBzP) were used to identify girls in the top quintile of exposure for each of these environmental chemicals, and age-matched prepubertal girls with urine analyte concentrations below the median [96]. Blood samples of these girls were depleted of the seven most abundant proteins using human-specific affinity spin columns. Using TMT-MS, 34, 51, 57 and 47 differentially expressed proteins were identified from the blood of prepubertal girls with high urine concentrations of genistein, BPA, MEHP and MBzP, respectively, compared to controls. Using bioinformatics and focusing on cancer as a disease, we also identified cancer biomarkers of susceptibility for genistein and BPA exposures. The differentially regulated cancer associated proteins in genistein and BPA girls are especially convincing in light of divergent functions and the literature demonstrating that genistein and BPA exposures are associated with mammary cancer prevention and causation, respectively.
In blood of girls with high genistein concentrations in their urine, two proteins with cancer associations were down-regulated: endothelin-converting enzyme (ECE-1) and eukaryotic translation initiation factor 3 subunit J (EIF-3) [96]. ECE-1 has been implicated in the pathogenesis of a range of disease states including breast, gynecological and urological cancers, cardiovascular disease and Alzheimer’s disease [97]. EIF-3 has been found elevated in human breast, cervical, esophageal, and lung cancers, suggesting a potential role in malignant transformation and cell growth control [98]. On the other hand, nucleolar 7 and PR domain zinc finger 5 (PRDM5) are proteins that are up-regulated in genistein girls. Nucleolar 7 and PRDM5 have been reported to regulate the cell cycle. The nucleolar 7 gene is reported to be a candidate tumor suppressor gene in cervical cancer that modulates the angiogenic phenotype [99]. PRDM5 has growth suppressive activities and is silenced in breast, ovarian, liver, lung, colon, and other cancers [100]. All four proteins could be considered as biomarkers of susceptibility for genistein/soy and cancer prevention. Interestingly, from PANTHER analysis of biological functions, the genistein group had the highest response on apoptotic process [96], a finding that corresponds very well with our report of apoptosis being increased in mammary glands of rats exposed prepubertally to genistein [43]. In fact, the differentially regulated cancer associated proteins in girls with high concentrations of genistein and BPA (details not provided for BPA here) are consistent with reported roles in mammary cancer prevention and causation, respectively.
2.16 Summary and Conclusions
The concept of programming against breast cancer is both intriguing and challenging. Intriguing, because the mechanism of action is unique, but it is based on solid research that has been well documented, and the critical experiments have been confirmed by different laboratories. Proving it via the scientific method in humans will be the biggest challenge. It is not always easy to carryout clinical studies in humans, especially when it means children.
To summarize, (1) dietary genistein provided during the prepubertal period suppresses chemically-induced mammary cancer in adult rats, and this has been independently confirmed, (2) four epidemiological studies show that adolescent girls eating a diet high in soy are at reduced risk for breast cancer, (3) in rats, the cellular mechanism of action has been described as early cell and mammary terminal ductal structure differentiation, a mechanism similar to mammary gland differentiation that follows from early pregnancy in young women, (4) identification of genistein mechanisms of action at the molecular level (Seven proteins are identified as playing a role in enhancing cell and mammary gland differentiation, cell turnover and tissue remodeling in presence of genistein. On the other hand, 13 proteins are associated with increased apoptosis, decreased cell turnover, and potential for carcinogenesis in mammary glands of mature animals.), and (5) in vivo toxicology studies with genistein in animal models and epidemiology reports in humans demonstrate little or no toxicity.
Accordingly, the time has come for soy/genistein to be tested in adolescent girls for prevention of mammary cancer. Let’s consider the facts. One in eight women will be diagnosed with breast cancer in their lifetime. Breast cancer is the most commonly diagnosed cancer in women, and it is the second leading cause of death among women. Each year it is estimated that over 220,000 women in the United States will be diagnosed with breast cancer and more than 40,000 will die. The protocol for programming against breast cancer may sound unusual, but children consuming soy milk, tofu, soy nuts or soy bars is not unusual, and they can easily incorporate soy or genistein in one or two meals a day.
References
Gilbert ER, Liu D. Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic β-cell function. Food Funct. 2013;4:200–12. doi:10.1039/C2FO30199G.
Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333:276–82.
Wang C, Kurzer MS. Effects of phytoestrogens on DNA synthesis in MCF-7 cells in the presence of estradiol or growth factors. Nutr Cancer. 1998;31(2):90–100.
Zava DT, Duwe G. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro. Nutr Cancer. 1997;27(1):31–40.
Kuiper GG, Kuiper GG, Lemmen JG, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–63.
Akiyama T, Ishida J, Nakagawa S, et al. Genistein, a specific inhibitor of tyrosine specific protein kinases. J Biol Chem. 1987;262:5592–6.
Dean NM, Kanemitsu M, Boynton AL. Effects of the tyrosine-kinase inhibitor genistein on DNA synthesis and phospholipid-induced second messenger generation in mouse 10T1/2 fibroblasts and rat liver T51B cells. Biochem Biophys Res Commun. 1989;165:705–801.
Okura A, Arakawa H, Oka H, et al. Effect of genistein on topoisomerase activity and on the growth of [Val12] H-ras-transformed N1H 3T3 cells. Biochem Biophys Res Commun. 1988;157:183–9.
Fotsis T, Pepper M, Adlercreutz H, et al. Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis. J Nutr. 1995;125(3):790S–7.
Allred CD, Allred KF, Ju YH, et al. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61(13):5045–50.
Mizunuma H, Kanazawa K, Ogura S, et al. Anticarcinogenic effects of isoflavones may be mediated by genistein in mouse mammary tumor virus-induced breast cancer. Oncology. 2002;62(1):78–84. doi:10.1159/000048250.
Lee HP, Gourley L, Duffy SW, et al. Dietary effects on breast cancer risk in Singapore. Lancet. 1991;337:1197–200.
Ziegler RG, Hoover RN, Hildeshein RN, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–27.
Welsch CW. Host factors affecting the growth of carcinogen-induced rat mammary carcinomas: a review and tribute to Charles Brenton Huggins. Cancer Res. 1985;45:3415–43.
Barnes S, Grubbs C, Setchell KD, et al. Soybeans inhibit mammary tumors in models of breast cancer. Prog Clin Biol Res. 1990;347:239–53.
Baggott JE, Ha T, Vaughn WH, et al. Effect of miso (Japanese soybean paste) and NaCl on DMBA-induced rat mammary tumors. Nutr Cancer. 1990;14:103–9.
Barnes S, Peterson G, Grubbs C, et al. Potential role of dietary isoflavones in the prevention of cancer. Adv Exp Med Biol. 1994;354:135–47.
Shellabarger CJ, Soo VA. Effects of neonatally administered sex steroids on 7, 12-dimethylbenza[a]anthracene-induced mammary neoplasia in rats. Cancer Res. 1973;33:1567–9.
Nagasawa H, Yanai R, Shodono M, et al. Effect of neonatally administered estrogen or prolactin on normal and neoplastic mammary growth and serum estradiol-17 beta level in rats. Cancer Res. 1974;34:2643–6.
Lamartiniere CA, Moore JB, Holland M, et al. Neonatal genistein chemoprevents mammary cancer. Proc Soc Exper Biol Med. 1995;208:120–3.
Lamartiniere CA, Moore JB, Brown NM, et al. Genistein suppresses mammary cancer in rats. Carcinogenesis. 1995;16:2833–40.
Murrill WB, Brown NM, Zhang J-X, et al. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis. 1996;17:1451–7. doi:10.1093/carcin/16.11.2833.
Lamartiniere CA, Zhang J-X, Cotroneo MS. Genistein studies in the rat: potential for breast cancer prevention and reproductive and developmental toxicology. Amer J Clin Nutr. 1998;168:1400S–5.
Hilakivi-Clarke L, Onojafe I, Raygada M, et al. Prepubertal exposure to zearalenone or genistein reduces mammary tumorigenesis. Br J Cancer. 1999;80:1682–8.
Fritz WA, Coward L, Wang J, et al. Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis. 1998;19:2151–8.
Dalu A, Haskell JF, Coward L, et al. Genistein, a component of soy, inhibits the expression of the EGF and ErbB/Neu receptors in the rat dorsolateral prostate. Prostate. 1998;37:36–43. doi:10.1002/(SICI)10970045(19980915)37.
Adlercreutz H, Markkanen H, Watanabe S. Plasma concentration of phyto-oestorogens in Japanese men. Lancet. 1993;342:1209–10.
Lamartiniere CA, Cotroneo MS, Fritz WA, et al. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–8.
Hilakivi-Clarke L, Cho E, Onojafe I, et al. Maternal exposure to genistein during pregnancy increases carcinogen-induced mammary tumorigenesis in female rat offspring. Oncol Rep. 1999;6(5):1089–184. doi:10.3892/or.6.5.1089.
Lamartiniere CA, Zhao Y-X, Fritz WA. Genistein: mammary cancer chemoprevention, in vivo mechanisms of action, potential for toxicity, and bioavailability in rats. J Women’s Cancer. 2000;2:11–9.
Cotroneo MS, Lamartiniere CA. Pharmacologic, but not dietary genistein supports endometriosis in a rat model. Toxicol Sci. 2001;61:68–75.
Russo IH, Russo J. Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12-dimethylbenzanthracene. J Natl Cancer Inst. 1978;61:1439–49.
Russo J, Russo IH. DNA labeling index and structure of the rat mammary gland as determinants of its susceptibility to carcinogenesis. J Natl Cancer Inst. 1978;61:1451–9.
Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2(1):5–73.
Grubbs CJ, Juliana MM, Hill DL, et al. Suppression by pregnancy of chemically induced preneoplastic cells of the rat mammary gland. Anticancer Res. 1986;6:1395–400.
Russo J, Russo IH. Development of the human breast. Maturitas. 2004;49(1):2–15. PMID:15351091.
Boice JD, Monson RR. Breast cancer in women after repeated fluoroscopic examinations of the chest. J Natl Cancer Inst. 1977;59:823–32. doi:10.1093/jnci/59.3.823.
McGregor DH, Land CE, Choi K, et al. Breast cancer incidence among atomic bomb survivors, Hiroshima and Nagasaki, 1950–69. J Natl Cancer Inst. 1977;59(3):799–811. doi:10.1093/jnci/59.3.799.
Brown NM, Lamartiniere CA. Xenoestrogens alter mammary gland differentiation and cell proliferation. Environ Health Persp. 1995;103:708–13.
Brown NM, Wang J, Cotroneo MS, et al. Prepubertal genistein treatment modulates TGF-α, EGF and EGF-receptor mRNAs and proteins in the rat mammary gland. Mol Cell Endocrinol. 1998;144:149–65.
Lamartiniere CA, Wang J, Smith-Johnson M, et al. Daidzein: bioavailability, potential for reproductive toxicity and breast cancer chemoprevention. Toxicol Sci. 2002;65:228–38.
Brown NM, Belles CA, Lindley SL, et al. Mammary gland differentiation by early life exposure to enantiomers of the soy isoflavone metabolite equol. Food Chem Toxicol. 2010;48(11):3042–50. doi:10.1016/j.fct.2010.07.042.
Wang J, Jenkins S, Lamartiniere CA. Cell proliferation and apoptosis in rat mammary glands following combinational exposure to bisphenol A and genistein. BMC Cancer. 2014;14:379. doi:10.1186/1471-2407-14-379.
Nandi S. Endocrine control of mammary-gland development and function in the C3H/HeCrgl mouse. J Natl Cancer Inst. 1958;21:1039–63.
Sebastian J, Richards RG, Walker MP, et al. Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis. Cell Growth Differ. 1998;9(9):777–85.
Wang J, Betancourt AB, Mobley JA, et al. Proteomic discovery of genistein action in the rat mammary gland. J Proteome Res. 2011;10(4):1621–31. doi:10.1021/pr100974w.
Rowell C, Carpenter DM, Lamartiniere CA. Chemoprevention of breast cancer, proteomic discovery of genistein action in the rat mammary gland. J Nutr. 2005;135:2953S–9.
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, et al. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20.
Schwartz-Albiez R, Koretz K, Möller P, et al. Differential expression of annexins I and II in normal and malignant human mammary epithelial cells. Differentiation. 1993;52(3):229–37.
Tatham AL, Crabtree MJ, Warrick N, et al. GTP cyclohydrolase I expression, protein, and activity determine intracellular tetrahydrobiopterin levels, independent of GTP cyclohydrolase feedback regulatory protein expression. J Biol Chem. 2009;284:13660–8. doi:10.1074/jbc.M807959200.
Crowley MR, Head KL, Kwiatkowski DJ, et al. The mouse mammary gland requires the actin-binding protein gelsolin for proper ductal morphogenesis. Dev Biol. 2000;225(2):407–23.
Koya RC, Fujita H, Shimizu S, et al. Gelsolin inhibits apoptosis by blocking mitochondrial membrane potential loss and cytochrome c release. J Biol Chem. 2000;275(20):15343–9. doi:10.1074/jbc.275.20.15343.PMID10809769.
Qian Y, Guan T, Huang M, et al. Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochem Int. 2012;60(8):759–67. doi:10.1016/j.neuint.2012.03.011.
Jessop CE, Chakravarthi S, Garbi N, et al. ERp57 is essential for efficient folding of glycoproteins sharing common structural domains. EMBO J. 2007;26(1):28–40. PMCID: PMC1782378.
Gorski RA. The neuroendocrine regulation of sexual behavior, Advances in psychobiology. New York: Wiley; 1974. p. 1–58.
McEwen BS. Interactions between hormones and nerve tissue. Sci Am. 1976;235:48–58.
Lamartiniere CA, Sloop CA, Clark J, et al. (1982) Organizational effects of hormones and hormonally-active xenobiotics on postnatal development. 12th Conference on Environmental Toxicology. Dayton: U.S. Air Force Publication: AFAMRL-TR-81-149 1982:96-121
An J, Tzagarakis-Foster C, Scharschmidt TC, et al. Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J Biol Chem. 2001;276:17808–14. doi:10.1074/jbc.M205355200.
Lydon JP, O'Malley BW. Steroid receptor coactivator-3: a multifarious coregulator in mammary gland metastasis. Endocrinology. 2011;152:19–25.
O’Malley BW, Kumar R. Nuclear receptor coregulators in cancer biology. Cancer Res. 2009;69:8217–22.
Sharma MR, Koltowski L, Ownbey RT, et al. Angiogenesis associated protein annexin II in breast cancer: selective expression in invasive breast cancer and contribution to tumor invasion and progression. Exp Mol Pathol. 2006;81:146–56.
Shiozawa Y, Havens AM, Jung Y, et al. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105:370–80.
Thorneberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281(5381):1312–6.
Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–70. doi:10.1101/gad.12.11.1551.
Lamkanfi M, Festjens N, Declercq W, et al. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55. doi:10.1038/sj.cdd.4402047.
D'Amours D, Germain M, Orth K, et al. Proteolysis of poly (ADP-ribose) polymerase by caspase 3: kinetics of cleavage of mono(ADP-ribosyl)ated and DNA-bound substrates. Radiat Res. 1998;150:3–10.
O'Brien MA, Moravec RA, Riss TL. Poly (ADP-Ribose) polymerase cleavage monitored in situ in apoptotic cells. Biotechniques. 2001;30:886–91.
Duriez PJ, Shah GM. Cleavage of poly (ADP-ribose) polymerase: a sensitive parameter to study cell death. Biochem Cell Biol. 1997;75:337–49.
Hsu SJ, Nagase H, Balmain A. Identification of Fetuin-B as a member of a cystatin-like gene family on mouse chromosome 16 with tumor suppressor activity. Genome. 2004;47:931–46.
Cabanes A, Wang M, Olivo S, et al. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004;25(5):741–8. doi:10.1093/carcin/bgh065.
Jindal HK, Vishwanatha JK. Functional identity of a primer recognition protein as phosphoglycerate kinase. J Biol Chem. 1990;265:6540–3.
Vishwanatha JK, Jindal HK, Davis RG. The role of primer recognition proteins in DNA replication: association with nuclear matrix in HeLa cells. J Cell Sci. 1992;101(1):25–34.
Teunis MA, Kavelaars A, Voest E, et al. Reduced tumor growth, experimental metastasis formation, and angiogenesis in rats with a hyperreactive dopaminergic system. FASEB J. 2002;16:1465–7.
Basu S, Nagy JA, Pal S, et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med. 2001;7:569–74.
Ferguson SA, Flynn KM, Delclos KB, et al. Effects of lifelong dietary exposure to genistein or nonylphenol on amphetamine-stimulated striatal dopamine release in male and female rats. Neurotoxicol Teratol. 2002;24:37–45.
Heffelfinger SC, Yan M, Gear RB, et al. Inhibition of VEGFR2 prevents DMBA-induced mammary tumor formation. Lab Invest. 2004;84:989–98.
Ortega N, Hutchings H, Plouet J. Signal relays in the VEGF system. Front Biosci. 1999;4:D141–52.
Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–22.
Wang J, Eltoum I-E, Lamartiniere CA. Genistein chemoprevention of prostate cancer in TRAMP mice. J Carcinog. 2007;6:3. doi:10.1186/1477-3163-6-3.
Wang J, Eltoum I-E, Carpenter M, et al. Genistein mechanisms and timing of prostate cancer chemoprevention in Lobund-Wistar rats. Asian Pac J Cancer Prev. 2009;10:1–4.
Ju YH, Allred CD, Allred KF, et al. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J Nutr. 2001;131:2957–62.
Shao ZM, Wu J, Shen ZZ, et al. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998;58:4851–7.
Flynn KM, Ferguson SA, Delclos KB, et al. Effects of genistein exposure on sexually dimorphic behaviors in rats. Toxicol Sci. 2000;55:311–9.
Adlercreutz H, Honjo H, Higashi A, et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr. 1991;54(6):1093–100.
Strom BL, Schinnar R, Ziegler EE, et al. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA. 2001;286(7):807–14.
Third National Report on Human Exposure to Environmental Chemicals. Department of Health and Human Services (DHHS). Centers for Disease Control and Prevention (CDC); National Center for Environmental Health. 2005.
Setchell KD, Zimmer-Nechemias L, Cai J, et al. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998;68(6S):1453S–61.
Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16(3):249–58.
McCarver G, Bhatia J, Chambers C, et al. NTP-CERHR expert panel report on the developmental toxicity of soy infant formula. Birth Defects Res B Dev Reprod Toxicol. 2011;92(5):421–68. doi:10.1002/bdrb.20314.
Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98(7):459–71.
Shu XO, Jin F, Dai Q, et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–8.
Wu AH, Wan P, Hankin J, et al. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–6. doi:10.1093/carcin/23.9.1491.
Korde LA, Wu AH, Fears T, et al. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1050–9. doi:10.1158/1055-9965.EPI-08-0405.
Thanos J, Cotterchio M, Boucher BA, et al. Adolescent dietary phytoestrogen intake and breast cancer risk (Canada). Cancer Causes Control. 2011;17(10):1253–61.
Maskarinec G, Oshiro C, Morimoto Y, et al. Urinary isoflavone excretion as a compliance measure in a soy intervention among young girls: a pilot study. Eur J Clin Nutr. 2005;59(3):369–75. doi:10.1038/sj.ejcn.1602083.
Wang J, Betancourt A, Jenkins S, et al. Altered blood proteome in girls with high urine concentrations of bisphenol A, genistein, mono-ethyl hexylphthalate and mono benzyl phthalate. MOJ Proteom Bioinform. 2015;2(2):40. doi:10.15406/mojpb.2015.02.00040.
Smollich M, Wulfing P. Targeting the endothelin system: novel therapeutic options in gynecological, urological and breast cancers. Expert Rev Anticancer Ther. 2008;8:1481–93. doi:10.1016/j.febslet.2012.06.020.
Dong Z, Liu LH, Han B, et al. Role of eIF3 p170 in controlling synthesis of ribonucleotide reductase M2 and cell growth. Oncogene. 2004;23(21):3790–801.
Hasina R, Pontier AL, Fekete MJ, et al. NOL7 is a nucleolar candidate tumor suppressor gene in cervical cancer that modulates the angiogenic phenotype. Oncogene. 2006;25(4):588–98. PubMed: 16205646.
Deng Q, Huang S. PRDM5 is silenced in human cancers and has growth suppressive activities. Oncogene. 2004;23(28):4903–10. doi:10.1038/sj.onc.1207615.
Acknowledgements
The authors acknowledge the following grants: NIH-RO1-CAG1742, NIH-RO1-ES07273, NIH-U01-ES012771, NIH-UO1-ES/CA-ES019482, NIH-1U01-ES016003, AICR 92SG12, Susan G. Komen Foundation (DISS0201242), DOD BC 17-03-1-0433, DOD BC-17-00-1-0119, DOD DAMD 17-98-1-8582 and DOD DAMD 17-00-1-0118.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lamartiniere, C.A., Jenkins, S.B., Wang, J. (2016). Genistein: Programming Against Breast Cancer. In: Russo, J. (eds) Trends in Breast Cancer Prevention. Springer, Cham. https://doi.org/10.1007/978-3-319-27135-4_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-27135-4_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27133-0
Online ISBN: 978-3-319-27135-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)