Abstract
A Gram-positive, aerobic, rod-shaped, spore-forming bacterium, designated NE201T, was isolated from a freshwater pond in Village Nerur, India. Growth was observed in the range of 15–45 °C temperature with optimum at 30 °C, pH range of 5–9 (optimum at 7.0), and at concentrations of NaCl ranging between 0 and 14% (optimum 0%, w/v). The 16S rRNA gene sequence showed the highest similarity with Fictibacillus enclensis NIO-1003T (JF893461) at 99.01% followed by F. rigui WPCB074T (EU939689) at 98.9% and F. solisalsi CGMCC 1.6854T (EU046268) at 98.66%. The digital DNA–DNA hybridization (dDDH) and orthoANI values for strain NE201T against F. enclensis NIO-1003T (GCA_900094955.1) were 33.7% and 87.68%, respectively. The phylogenetic analysis based on the 16S rRNA gene, 92 core genes derived from the genome, and 20 proteins involving over 20,236 amino acid positions revealed the distinct phylogenetic position of strain NE201T and the formation of a clearly defined monophyletic clade with F. enclensis. The strain NE201T showed a unique carbon utilization and assimilation pattern that differentiated it from F. enclensis NIO-1003T. The major fatty acids were anteiso -C15:0 (51.42%) and iso-C15:0 (18.88%). The major polar lipids were phosphatidylglycerol (PG), phosphatidylethanolamine (PE, and diphosphatidylglycerol (DPG). The antiSMASH analyzed genome of NE201T highlighted its diverse biosynthetic potential, unveiling regions associated with terpene, non-ribosomal peptide synthetases (NRPS), lassopeptides, NI-siderophores, lanthipeptides (LAP), and Type 3 Polyketide Synthases (T3PKS). The overall phenotypic, genotypic, and chemotaxonomic characters strongly suggested that the strain NE201T represents a novel species of genus Fictibacillus for which the name Fictibacillus fluitans sp. nov. is proposed. The type strain is NE201T (= MCC 5285 = JCM 36474).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditionally, bacteria that are Gram-positive, aerobic, and capable of forming endospores have been classified within the genus Bacillus. However, subsequent analyses of 16S rRNA gene sequences have unveiled distinct phylogenetic lineages within the Bacillus genus (Claus 1986). Consequently, numerous Bacillus species have been subject to reclassification and reassignment to other genera. These include Alicyclobacillus (Wisotzkey et al. 1992), Salibacillus (Wainø et al. 1999), Marinibacillus (Yoon et al. 2001), Lysinibacillus (Ahmed et al. 2007), Rummeliibacillus (Vaishampayan et al. 2009), and few others.
In 2013, Glaeser proposed the creation of a new genus, Fictibacillus, based on phylogenetic analysis and lipid profiles, as certain Bacillus species formed a monophyletic cluster with significantly low sequence similarities (< 94%) to the type species of the genus Bacillus, Bacillus subtilis. This reclassification resulted in the renaming of these species as Fictibacillus nanhaiensis, Fictibacillus barbaricus, Fictibacillus arsenicus, Fictibacillus rigui, Fictibacillus macauensis, Fictibacillus solisalsi, and Fictibacillus gelatine (Glaeser et al. 2013). Currently, the genus Fictibacillus comprises total 12 validly published species. During our investigation of bacterial diversity from a freshwater pond, we discovered a Gram-stain-positive bacterium capable of forming endospores. This strain designated as NE201T was obtained from a freshwater sample collected from a pond at Village Nerur (Maharashtra) India and was subjected to polyphasic taxonomic investigation. This description forms a segment of a larger ecological study investigating the bacterial populations in freshwater bodies across western Maharashtra, India.
Materials and methods
Isolation and culture conditions
The freshwater sample was collected from a pond at Nerur, Maharashtra. The water sample was serially diluted and spread on various growth media (data not shown), and incubated at 30 °C for 24–72 h to observe the growth of bacterial colonies. Morphologically distinct isolates were selected and sub-cultured on their respective media until pure colonies were achieved and were further subjected for their identification. The strain NE201T was purified and cultivated on Nutrient Agar (M001; HiMedia, India), and preserved in 20% glycerol prepared in PBS and stored at − 80 °C and in liquid nitrogen.
Phenotypic characterizations
The Gram-staining characteristics of strain NE201T were ascertained using a Gram-staining kit (K001-KT; HiMedia, India) and light microscopy (Olympus Model BX53, USA). The cellular dimensions and morphology were subsequently analyzed using a scanning electron microscope (Carl Zeiss, EVO 18, Version 6.02).
The NE201T strain was cultivated on Tryptic Soy Agar (M1938; HiMedia, India), Reasoner’s 2A Agar (M1743; HiMedia, India), Nutrient Agar (M001; HiMedia, India), and Luria Agar (M557; HiMedia, India) to determine the most conducive medium for its optimal growth. For temperature optimization, cell growth was checked at various temperatures ranging between 4 and 50 °C (4, 10, 15, 20, 25, 28, 30, 37, 45, and 50 °C) after 24 h using a spectrophotometer at 600 nm on nutrient broth. The culture growth capacity under varying pH conditions was evaluated across a pH range spanning from pH 4 to pH 11 units (with intervals of a value of 1.0). Its response to salt tolerance was evaluated by inoculating it into Nutrient broth containing a salinity gradient ranging from 0 to 15% NaCl, with increments of 1%. The samples were then incubated at 30 °C, and after 24 h, the growth was assessed using a spectrophotometer at a wavelength of 600 nm.
Spore formation was assessed using the modified Schaeffer-Fulton staining technique (Hussey and Zayaitz 2007), employing Methylene blue as the primary stain and a 0.5% Saffron solution as the secondary stain. This staining technique allowed for the visualization of spores (data not shown). Cell motility was observed using the Hanging-drop method. Oxidase and catalase activity were investigated using oxidase discs (DD018; Himedia, India) and bubble production in 3% (v/v) H2O2, respectively.
Strain NE201T was further tested for utilization and assimilation of different carbon sources and enzyme activity against different substrates by Analytical profile index, Biomerieux (API 20NE, API ZYM, API50CH, API20E) and Biolog GEN III Microplate as per manufacturer’s instructions.
Chemotaxonomic characterization
For cellular fatty acid analysis, the cell mass of NE201T was harvested from NA plates after incubation for 24 h at 30 °C. The fatty acids were extracted and converted into fatty acid methyl esters (FAME) following established standards. The process began with the collection and lysis of microbial cells to release their intracellular contents. Subsequently, the fatty acids were isolated using organic solvents. FAME preparation involved derivatization, transforming the fatty acids into methyl esters, following the MIDI/Hewlett Packard protocol (1995) to ensure accuracy. The prepared FAME samples were then analyzed using gas chromatography.
For polar lipid analysis, cell mass was harvested from cultures at the logarithmic phase. Methanol/Chloroform/0.3% Sodium Chloride (2:1:0.8, by vol.) was used for the extraction of polar lipids (Bligh and Dyer 1959) in addition to the modifications of (Card 1973). Two-dimension chromatography was used for separation using chloroform–methanol–water (65:25:4 by vol.) in the first dimension and Chloroform–Acetic acid–Methanol–Water (40:7.5:6:2, by vol.) in the second dimension on silica gel TLC (Kieselgel 60 F254; Merck) (Minnikin et al. 1984). The plates were dried and sprayed with 5% ethanolic phosphomolybdic acid for visualization of total lipids. Further characterization was done by spraying the plates with ninhydrin (for amino groups), molybdenum blue (for phosphates), Dragendorff (for quaternary nitrogen), or α-naphthol (for sugars) (Card 1973).
The diagnostic diamino acid for the peptidoglycan within the cell wall of cell NE201T was determined using thin-layer chromatography (Schumann 2011). In brief, the process began with the extraction of peptidoglycan from bacterial cell walls, followed by hydrolysis with hydrochloric acid to break it into individual amino acids. These amino acids were then derivatized to make them suitable for TLC analysis. The derivatized mixture was applied to a TLC plate, and as a solvent moved up the plate, it carried the amino acids, causing them to separate based on their polarities. Visualization was achieved through exposure to a ninhydrin and o-phthaldialdehyde (OPA) reagent.
Sequencing and phylogenetic analysis
The genomic DNA was isolated using the CTAB method (Doyle and Doyle 1987). The 16S rRNA gene was amplified using primers 27F and 1492R (Lane et al. 1985). The 1.5 kb PCR amplicon was purified by the PEG-NaCl method (Green and Sambrook 2017) and was sequenced on ABI 3730XL DNA Analyzer (Applied Biosystems, USA) using bacterial universal primers 343R, 704F, 907F, and 1028F (Baker et al. 2003). The sequences were assembled and curated manually and were in the DDBJ/EMBL/GenBank database. The phylogenetically closest relatives were searched on the EzBioCloud database (Yoon et al. 2017). Phylogenetic trees for the 16S RNA gene were constructed using neighbor-joining (NJ), maximum-likelihood (ML), and maximum-parsimony (MP) methods to infer the position of the NE201T among the members of genus Fictibacillus using the MEGA7 (Kumar et al. 2016). The closest phylogenetic relative Fictibacillus enclensis NIO-1003T (Dastager et al. 2014) was procured from their respective culture collections for comparative polyphasic characterization and maintained on NA at pH 7 with a temperature of 30 °C.
The genomic DNA of strain NE201T was subjected to whole genome sequencing using Illumina HiSeq (150 × 2). The raw reads were checked for quality using FastQC v0.11.9 (Brown et al. 2017). The quality-filtered reads were assembled using Unicycler v0.5.0 (Wick et al. 2017) to obtain genome assembly of the strain NE201T. The quality and completeness of the assembled genome were checked using QUAST v4.6.3 (Gurevich et al. 2013) and CheckM v1.2.2 (Parks et al. 2015). The digital DNA–DNA hybridization (dDDH) values were calculated using the Genome-to-Genome Distance Calculator (GGDC) webserver following the recommended formula 2. The average nucleotide identity (ANI) values were calculated using an orthoANI calculator (Auch et al. 2010; Meier-Kolthoff et al. 2014; Yoon et al. 2017). Further, the genome was annotated PGAP (Zhao et al. 2012). The functional groups of strain NE201T were analyzed using Clusters of Orthologous Groups (COG) in eggNOG-mapper v2 (Cantalapiedra et al. 2021). A comparison of orthologous gene clusters among the genomes under study was made using OrthoVenn2 (Xu et al. 2019).
To validate and confirm the taxonomic position of the new strain NE201T, a phylogenetic tree based on the core genome was constructed using BPGA, version 1.3.0 (Chaudhari et al. 2016) and UBCG, version 3.0 (Na et al. 2018). BPGA pipeline utilized 20 orthologous protein clusters generated using USEARCH. The protein sequences were further aligned and concatenated and the phylogenetic tree was reconstructed using the neighbor-joining method in MEGA 7 (Kumar et al. 2016). A total of 20,236 amino acid positions were utilized in the final dataset. Furthermore, UBCG was employed to construct phylogenetic trees, incorporating 92 core genes identified through HMMER (Potter et al. 2018). The phylogenetic tree was visualized in MEGA 7 and topologies were compared with the phylogeny constructed using BPGA.
The genome of NE201T was examined through antiSMASH version 7.0.1 with 'relaxed' strictness to screen the putative secondary metabolite regions in its genome (Blin et al. 2023).
Results and discussion
Morphological, physiological, and biochemical characteristics
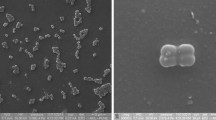
The NE201T strain was a motile, Gram-positive rod, capable of forming endospores. The colonies were 3 mm in diameter and displayed a cream coloration a convex elevation with smooth and intact margin. They appeared opaque, and they exhibited a butyrous consistency. When observed through a Scanning Electron Microscope, elongated bacterial rods ranging from 2.5 to 9 μM in size were detected (Fig. S1).
The cells showed growth after 24 h of incubation at the temperature of 15–45 °C (optimum 30 °C) over the pH range of 5–9 (optimum 7.0) and at concentrations of NaCl ranging between 0 and 14% (optimum 0%, w/v).
The strain NE201T demonstrated contrasting results with its closest phylogenetic relative, F. enclensis NIO-1003T, in API profiles. When assessed using the API 20NE, NE201T exhibited positive results for the reduction of potassium nitrate and assimilation of arabinose, whereas F. enclensis NIO-1003T did not. Conversely, during API 20E testing, NE201T displayed negative results for citrate utilization and positive results for gelatinase activity, setting it apart from F. enclensis NIO-1003T. Furthermore, in API 50CH testing, NE201T demonstrated positive results for the utilization of D-glucose, asculine, and sucrose, further distinguishing it from its phylogenetic relative, F. enclensis NIO-1003T.
Strain NE201T displayed unique carbon utilization and chemical sensitivity patterns in BIOLOG GEN III assays, differing from F. enclensis NIO-1003T, as outlined in Table 1. It also exhibited resistance towards, Aztreonam antibiotic. The strain NE201T tested negative for oxidase and catalase activity.
Chemotaxonomic characterization
The major Fatty Acids present in the strain were anteiso -C15:0 (51.42%), iso-C15:0 (18.88%), anteiso -C17:0 (9.31%), C16:00 (6.3%) (Table S1). For Peptidoglycan detection, NE201T showed the presence of DL- Lysine dihydrochloride and 2,6 dipicolinic acid. The major polar lipids present in strain NE201T comprised phosphatidylglycerol (PG), phosphatidylethanolamine (PE), and diphosphatidylglycerol (DPG), along with traces of unidentified phospholipid lipid (APL) (Figure S2). No glycolipids were found in strain NE201T which was consistent with the lipid profiles of genus Fictibacillus.
The genomic assessment of NE201T using antiSMASH unveiled a spectrum of secondary metabolite regions, underscoring its robust biosynthetic capacity. Particularly noteworthy is a region featuring a non-ribosomal peptide synthetase (NRPS) cluster, exhibiting a 9% similarity to pacidamycin biosynthetic clusters. NRPS clusters are recognized for their adaptability in peptide synthesis with varied structures and functions, encompassing antibiotics, immunosuppressants, and siderophores. Another genomic segment showcased attributes of a lassopeptide cluster, recognized for antimicrobial properties, displaying an 80% similarity to the established paeninodin cluster. Furthermore, a region hinted at the existence of an NI-siderophore cluster, contributing to iron acquisition, with a 60% similarity to the schizokinen cluster. Additional identifications encompassed lanthipeptide (LAP) clusters, renowned for antimicrobial features, highlighting distinctive biosynthetic potentials within the NE201T genome. Moreover, a genomic segment unveiled Type 3 Polyketide Synthase (T3PKS) clusters, simple homodimers of ketosynthases catalyzing the condensation of extender substrates onto a starter substrate. These revelations shed light on NE201T’s genomic prowess in producing varied bioactive compounds, holding implications for ecological and biotechnological applications, and prompting further exploration.
Phylogenetic and genotypic analysis
The 16S rRNA gene of strain NE201T (1485 bp) exhibited the highest sequence similarity with Fictibacillus enclensis NIO-1003T (JF893461) at 99.01% followed by F. rigui WPCB074T (EU939689) at 98.9% and F. solisalsi CGMCC 1.6854T (EU046268) at 98.66%. The phylogenetic analysis of the 16S rRNA gene through the neighbor-joining (NJ), maximum-likelihood (ML), and maximum parsimony (MP) methods in MEGA7 for strain NE201T revealed the formation of a clearly defined monophyletic clade with F. enclensis NIO-1003T (JF893461) (Fig. 1).
Phylogenetic Analysis of Fictibacillus fluitans strain NE201T (MCC 5285) within the Genus Fictibacillus Based on 16S rRNA Gene Sequences. A phylogenetic tree was constructed using 16S rRNA gene sequences to elucidate the relationship between strain NE201T (MCC 5285) and closely related members of the genus Fictibacillus. The tree was generated through neighbor-joining (NJ), maximum-likelihood (ML), and maximum parsimony (MP) methods, employing MEGA 7. Bootstrap analysis was performed with 1000 replicates to assess the tree topologies, and the figures displayed at the nodes represent the percentage of replicates supporting the NJ, ML, and MP methods. The final dataset consisted of 1379 positions, with the 16S rRNA gene sequence of Neobacillus terrae C11T (MN620419) serving as the outgroup. The bar on the tree indicates the number of nucleotide substitutions per site. Non-validly published names are indicated in double inverted commas (“—”)
The genome sequence of strain NE201T using Illumina HiSeq generated 11,831,461 reads. The de novo assembly resulted in 51 scaffolds with depth coverage of 335X and 98.92% completeness. The DNA G + C contents were 44.5 mol% (Table 2). The digital DNA–DNA hybridization (dDDH) and orthoANI values for strain NE201T against F. enclensis NIO-1003T (GCA_900094955.1) were 33.7% and 87.68% respectively. The phylogenetic analysis involving over 20,236 amino acid positions in BPGA and 92 core genes in UBCG strongly supported the phylogeny established by the 16S rRNA gene sequence analysis. (Fig. 2).
Phylogenetic Analysis Fictibacillus fluitans strain NE201T (MCC 5285) Based on Combined Pan-Genome Orthologous Sequences. A comprehensive combined pan-genome phylogenetic tree of strain NE201T was constructed utilizing the BPGA and UBCG tools with available genome sequences. The pan-genome tree generated by BPGA employed the neighbor-joining method with 1000 bootstrap replicates in MEGA7. The dataset used in the analysis consisted of a total of 20,236 positions. Additionally, the UBCG tree was constructed based on data from 92 core genes. Bootstrap values at branch points represent the confidence levels of the tree topologies obtained from both BPGA and UBCG methods. To provide context, the draft genome sequence of Sutcliffiella cohnii HAMBI 2098 T (ASM225005) was employed as an outgroup for comparative analysis. The bar on the tree indicates the number of substitutions per site
A total of 4909 protein-coding genes were predicted including 68 tRNA and 7 rRNAs. A total of 3949 genes had specific functional distributions according to the COG categories. The functional genes were assigned to 18 functional categories. The genes responsible for amino acid transport and metabolism (355 genes), transcription (346 genes), and carbohydrate transport and metabolism (320 genes) were the most abundant followed by genes responsible for inorganic ion transport and metabolism (228 genes) and energy production and conversion (223 genes). The OrthoVenn analysis demonstrated that 34 gene clusters were unique to NE201T while 2440 gene clusters were shared by closely related type strains.
Description of Fictibacillus fluitans sp. nov.
Fictibacillus fluitans (flu’i.tans. L. part. adj. fluitans, floating), refers to the type of pond ecosystem from where the type strain was isolated).
Gram-staining positive, rod shaped, 2.5–9 μM in length; endospore forming, motile; catalase and oxidase negative, colonies cream in color, with convex elevation and a smooth, intact margin, opaque appearance and a butyrous consistency; grow at a temperature range of 15–45 °C (optimum 30 °C) at pH 5.0–10.5 with optimum pH of 7.0 and a salinity range of 0–5.0% (w/v) with optimum salinity at 0%, w/v; positive results for the reduction of potassium nitrate and assimilation of arabinose, gelatinase activity, utilization of d-Glucose, asculine, and sucrose and negative for citrate utilization; positive for utilization of assimilate α-d-Lactose, N-Acetyl-Neuraminic Acid, d-Galactose, 3-Methyl Glucose, d-Sorbito, d-Mannitol, Glycerol, Gelatin, Methyl Pyruvate, l-Lactic Acid, α-Hydroxy-Butyric Acid, β-Hydroxy-d,l-Butyric Acid Negative for assimilation of assimilation of d-Mellibiose, β-methyl-d-Glucoside, d-Fucrose, l-Fucrose, d-Arabitol, myo-Inositol, d-Glucose-6-PO4, Glycyl-l-Proline, l-Arginine, d-Malic Acid; chemical sensitivity for d-Serine, Rifamycin SV and, resistance towards antibiotic Aztreonam; contains major polar lipids as phosphatidylglycerol (PG), diphosphatidylglycerol (DPG), phosphatidylethanolamine (PE) and cellular fatty acids anteiso -C15:0 (51.42%), iso-C15:0 (18.88%), anteiso -C17:0 (9.31%), C16:00 (6.3%). The genomic DNA G + C contents are 44.5 mol %.
The type strain NE201T (= MCC 5285 = JCM 36474) was isolated from a freshwater pond located at Nerur, Maharashtra, India.
Data availability
The GenBank/EMBL/DDBJ accession numbers for the reference 16S rRNA gene sequences of the strain NE201T is OR234369 and the draft genome is NZ_JAUHTR000000000.1.
References
Ahmed I, Yokota A, Yamazoe A, Fujiwara T (2007) Proposal of Lysinibacillus boronitolerans gen. nov. sp. nov., and transfer of Bacillus fusiformis to Lysinibacillus fusiformis comb. nov. and Bacillus sphaericus to Lysinibacillus sphaericus comb. nov. Int J Syst Evol Microbiol 57:1117–1125
Auch AF, von Jan M, Klenk H-P, Göker M (2010) Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genomic Sci 2:117–134
Baker GC, Smith JJ, Cowan DA (2003) Review and re-analysis of domain-specific 16S primers. J Microbiol Methods 55:541–555
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Blin K, Shaw S, Augustijn HE, Reitz ZL, Biermann F, Alanjary M et al (2023) antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res gkad344 51(W1):W46–W50. https://doi.org/10.1093/nar/gkad344
Brown J, Pirrung M, McCue LA (2017) FQC Dashboard: integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 33:3137–3139
Cantalapiedra CP, Hernández-Plaza A, Letunic I, Bork P, Huerta-Cepas J (2021) eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. bioRxiv 38(12):5825–5829
Card GL (1973) Metabolism of phosphatidylglycerol, phosphatidylethanolamine, and cardiolipin of Bacillus stearothermophilus. J Bacteriol 114:1125–1137
Chaudhari NM, Gupta VK, Dutta C (2016) BPGA-an ultra-fast pan-genome analysis pipeline. Sci Rep 6:1–10
Claus D (1986) Genus Bacillus Cohn 1872, 174^. Bergey’s Man Syst Bacteriol 2:1105–1139
Dastager SG, Mawlankar R, Srinivasan K, Tang S-K, Lee J-C, Ramana VV et al (2014) Fictibacillus enclensis sp. nov., isolated from marine sediment. Antonie Van Leeuwenhoek 105:461–469
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19:11–15. https://worldveg.tind.io/record/33886/?ln=en
Glaeser SP, Dott W, Busse H-J, Kämpfer P (2013) Fictibacillus phosphorivorans gen. nov., sp. nov. and proposal to reclassify Bacillus arsenicus, Bacillus barbaricus, Bacillus macauensis, Bacillus nanhaiensis, Bacillus rigui, Bacillus solisalsi and Bacillus gelatini in the genus Fictibacillus. Int J Syst Evol Microbiol 63:2934–2944
Green MR, Sambrook J (2017) Preparation of single-stranded bacteriophage M13 DNA by precipitation with polyethylene glycol. Cold Spring Harb Protoc 2017:pdb-prot093419
Gurevich A, Saveliev V, Vyahhi N, Tesler G (2013) QUAST: quality assessment tool for genome assemblies. Bioinformatics 29:1072–1075
Hussey MA, Zayaitz A (2007) Endospore stain protocol. Am Soc Microbiol 8:1–11
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci 82:6955–6959
Meier-Kolthoff JP, Klenk H-P, Göker M (2014) Taxonomic use of DNA G+ C content and DNA–DNA hybridization in the genomic age. Int J Syst Evol Microbiol 64:352–356
Minnikin DE, O’donnell AG, Goodfellow M, Alderson G, Athalye M, Schaal A et al (1984) An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J Microbiol Methods 2:233–241
Na S-I, Kim YO, Yoon S-H, Ha S, Baek I, Chun J (2018) UBCG: up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J Microbiol 56:280–285
Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW (2015) CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055
Potter SC, Luciani A, Eddy SR, Park Y, Lopez R, Finn RD (2018) HMMER web server: 2018 update. Nucleic Acids Res 46:W200–W204
Schumann P (2011) Peptidoglycan structure. In: Methods in microbiology (Elsevier), 101–129
Vaishampayan P, Miyashita M, Ohnishi A, Satomi M, Rooney A, La Duc MT et al (2009) Description of Rummeliibacillus stabekisii gen. nov., sp. nov. and reclassification of Bacillus pycnus Nakamura et al. 2002 as Rummeliibacillus pycnus comb. nov. Int J Syst Evol Microbiol 59:1094–1099
Wainø M, Tindall BJ, Schumann P, Ingvorsen K (1999) Gracilibacillus gen. nov., with description of Gracilibacillus halotolerans gen. nov., sp. nov.; transfer of Bacillus dipsosauri to Gracilibacillus dipsosauri comb. nov., and Bacillus salexigens to the genus Salibacillus gen. nov., as Salibacillus salexig. Int J Syst Evol Microbiol 49:821–831
Wick RR, Judd LM, Gorrie CL, Holt KE (2017) Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595
Wisotzkey JD, Jurtshuk PJR, Fox GE, Deinhard G, Poralla K (1992) Comparative sequence analyses on the 16S rRNA (rDNA) of Bacillus acidocaldarius, Bacillus acidoterrestris, and Bacillus cycloheptanicus and proposal for creation of a new genus, Alicyclobacillus gen. nov. Int J Syst Evol Microbiol 42:263–269
Xu L, Dong Z, Fang L, Luo Y, Wei Z, Guo H et al (2019) OrthoVenn2: a web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res 47:W52–W58
Yoon J-H, Weiss N, Lee K-C, Lee I-S, Kang KH, Park Y-H (2001) Jeotgalibacillus alimentarius gen. nov., sp. nov., a novel bacterium isolated from jeotgal with l-lysine in the cell wall, and reclassification of Bacillus marinus Rüger 1983. as Mrinibacillus marinus gen nov., comb. nov. Int J Syst Evol Microbiol 51:2087–2093
Yoon S-H, Ha S-M, Kwon S, Lim J, Kim Y, Seo H et al (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67:1613
Zhao Y, Wu J, Yang J, Sun S, Xiao J, Yu J (2012) PGAP: pan-genomes analysis pipeline. Bioinformatics 28:416–418
Funding
The authors acknowledge the funding by the Department of Biotechnology (DBT) through the project, grant no. BT/Coord.II/01/03/2016 for in-house facilities.
Author information
Authors and Affiliations
Contributions
AM, RT, and YB carried out and compiled the polyphasic characterization; KK performed genome sequencing; AB and KK constructed the phylogenetic trees and did bioinformatic analysis; AM and RT carried out peptidoglycan analysis; ED, VT collected, isolated, and identified the bacterial isolate; AM, RT; compiled the overall data for the manuscript; KK analyzed the data; SD and KK curated the manuscript; AY conceptualized, coordinated the overall work, edited, analyzed the data, and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical statement
No human or animal subjects were recruited for this study.
Additional information
Communicated by Wen-Jun Li.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, A., Maurya, A., Bhavsar, Y. et al. Fictibacillus fluitans sp. nov., isolated from freshwater pond. Arch Microbiol 206, 70 (2024). https://doi.org/10.1007/s00203-023-03794-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03794-4