Abstract
Mayaro virus (MAYV), first isolated in 1954 in Trinidad and Tobago islands, is the causative agent of Mayaro fever, a disease characterized by fever, rashes, headaches, myalgia, and arthralgia. The infection can progress to a chronic condition in over 50% of cases, with persistent arthralgia, which can lead to the disability of the infected individuals. MAYV is mainly transmitted through the bite of the female Haemagogus spp. mosquito genus. However, studies demonstrate that Aedes aegypti is also a vector, contributing to the spread of MAYV beyond endemic areas, given the vast geographical distribution of the mosquito. Besides, the similarity of antigenic sites with other Alphavirus complicates the diagnoses of MAYV, contributing to underreporting of the disease. Nowadays, there are no antiviral drugs available to treat infected patients, being the clinical management based on analgesics and non-steroidal anti-inflammatory drugs. In this context, this review aims to summarize compounds that have demonstrated antiviral activity against MAYV in vitro, as well as discuss the potentiality of viral proteins as targets for the development of antiviral drugs against MAYV. Finally, through rationalization of the data presented herein, we wish to encourage further research encompassing these compounds as potential anti-MAYV drug candidates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
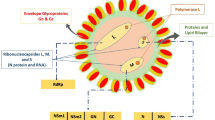
The Mayaro virus (MAYV) belongs to the Togaviridae family and Alphavirus genus, which is composed of a positive single-stranded RNA of 11,5 kb in length, covered by an icosahedral capsid and viral envelope (Fig. 1) (Diagne et al. 2020). It was first isolated in 1954 in Trinidad and Tobago islands from the blood of five febrile rural workers (Anderson et al. 1957). Since then, sporadic outbreaks have been identified in countries with tropical forests, including Brazil, Bolivia, Suriname, Peru, Equator, Venezuela, and recently Haiti (Aguilar-Luis et al. 2020). In addition, cases in North America (Taylor et al. 2005) and Europe (Theilacker et al. 2013) were reported in citizens who traveled to South America.
Schematic structure of MAYV virion. Electron microscopy data show that the viral particle is about 70 nm in diameter. The virion is composed of a positive single-stranded RNA with an icosahedral capsid formed by the capsid protein (C), covered by an envelope membrane inserted of the E1, E2, and E3 glycoproteins. The E3 protein is not shown since it detaches from the E2 protein at the viral surface during maturation (Ribeiro-Filho et al. 2021). Viral structure was based on Cryo-EM structure of mature MAYV (PDB ID: 7KO8) (Ribeiro-Filho et al. 2021)
MAYV belongs to the Semliki Complex, a subgroup of the Alphavirus genus that includes Bebaru virus, Chikungunya virus (CHIKV), Getah virus, Una Forest virus (UNAV), O’nyong-nyong virus, among others. Viruses from this complex are classified into the same serological group, sharing common antigenic sites, resulting in cross-reactivity in conventional serological tests, complicating the accurate diagnosis of MAYV infections and the underreporting of the disease (Acosta-Ampudia et al. 2018).
MAYV infection is the causative agent of the Mayaro Fever, a disease characterized by acute fever, rashes, headache, retro-orbital pain, nausea, diarrhea, myalgia, and arthralgia (Acosta-Ampudia et al. 2018) that remain for months or years in over 50% of cases (Mackay and Arden 2016; Li et al. 2019), culminating in physical disability (Ferreira et al. 2018). In severe cases, Mayaro fever can cause intermittent fever, neurological complications, myocarditis, and ultimately death (Acosta-Ampudia et al. 2018).
MAYV is transmitted through the bite of the Haemagogus spp. mosquito, usually from infected non-human primates, birds, rodents, and small mammals to susceptible humans (Esposito and Fonseca 2017). The vector competence of Aedes aegypti mosquitoes in the transmission of MAYV is also known, and considering the wide geographic distribution of the Aedes genus, there is a concern about virus spread beyond endemic areas (Long et al. 2011; Choi et al. 2019).
The MAYV replication begins with virus entry into the host cells via specific receptors on the cell surface, such as MXRA8, triggering clathrin-mediated endocytosis or, alternatively, through a caveolin-coated pit (Carvalho et al. 2017; Zhang et al. 2018). Through pH changes in the endosome, the interaction between the endosome membrane and the viral envelope leads to the viral RNA release into the cytoplasm. MAYV RNA is composed of two open reading frames (ORFs). The first ORF is translated in the non-structural viral proteins (nsP) nsP1, nsP2, nsP3, and nsP4, which form the replicative complex (RC), responsible to produce a negative strand from the viral genome, that serves as templates for the synthesis of new positive-sense strands, besides the sub-genomic 26S RNA (Diagne et al. 2020). The other ORF is transcribed from the negative strand, and translated into a polyprotein which is cleaved to the structural proteins C, E1, E2, E3, and 6K (viroporin). Additionally, a frameshift event occurs during translation of the 6K gene, yielding the production of the transframe (TF) protein, comprised of a C-terminal extension of the 6K protein in the − 1 ORF. TF protein has been associated with alphavirus budding efficiency and/or assembly (Firth et al. 2008; Snyder et al. 2013). The replication process is followed by the assembling of the viral components and virus release through budding in mammalian cells membrane (Jose et al. 2017; Brown et al. 2018) (Fig. 2), or through an alternative exocytosis pathway in insect cells (Acosta-Ampudia et al. 2018).
Schematic representation of MAYV replicative cycle. The replicative cycle and genomic structure of MAYV. E envelope, nsP nonstructural protein, UTR untranslated region, nt nucleotides. MAYV attaches to the host cells through the interaction between the host receptors and glycoproteins. Endocytosis occurs through a clathrin-coated pit or, alternatively, a caveolin-coated pit; virus internalization starts after interaction with host cell receptors; the low pH in the endosome leads to structural modifications in the viral envelope that reveal the E1. The latter mediates cell membrane-virus fusion, and later, endosomal cell membrane-virus fusion. Viral uncoating results in the release of the viral genome, and the replication stage occurs (translation and transcription). From the RNA of the virus, nsP precursors are generated; the replication complex is obtained from nsP proteins. This complex thus allows the synthesis of a minus-strand RNA, and it is used as the template to generate both genomic and subgenomic RNAs. Polyprotein precursor is cleaved by an autoproteolytic serine protease; and genomic RNA involved in nucleocapsid core assembly and genomic RNA packaging. Maturation of glycoproteins pE2 and E1 occurs. In the Golgi, processed glycoproteins are associated. Then they are transported into the cell membrane. At the cell membrane, the pE2 is split into E2 and E3; there is also the association of the viral RNA with the capsid C and the recruitment of E1 allows viral assembly. Last, particles of MAYV associated with the core are released outside of the host cell through the membrane. Compounds with antiviral activity against MAYV are indicated in each step of the virus replication cycle: (i) Viral Entry; (ii) Post-Entry (Maturation, RNA and Protein Synthesis); and (iii) Protective Effect on Host Cells. Viral cycle was based on the work of Diagne and colleagues (Diagne et al. 2020)
Despite the chronic condition of a high number of MAYV infections, there is no licensed antiviral against Mayaro fever. The treatment is palliative and consists in managing the symptoms using analgesics and/or non-steroidal anti-inflammatory drugs (Sun and Wu 2019). Therefore, research data have been produced on the potential of natural and synthetic molecules against MAYV. Here we summarize the natural and synthetic compounds previously described to possess antiviral activity against MAYV (Table 1). Thus, we critically compare molecules that could be further investigated in vivo and suggest protein targets to be further evaluated as anti-MAYV molecules. Finally, we aim to encourage further research encompassing these compounds as potential anti-MAYV drug candidates to treat infected patients.
Natural compounds as inhibitors of the replicative cycle of MAYV
Some of the first natural molecules tested for their potential to inhibit MAYV replication in vitro were Prostaglandin A1 (PGA1) and Prostaglandin B2 (PGB2) (Ishimaru et al. 1998). PGA1, a natural molecule that possesses a hormone-like lipid structure, is produced by cells in the presence of injury and/or inflammation (Caldas et al. 2018). Alternatively, PGB2 is a lipid mediator that acts as an auxiliary signal to T lymphocyte activation (Ishimaru et al. 1998; Raulin 2002). The antiviral effects of PGA1 and PGB2 against MAYV were investigated by using the plaque reduction assay in infected green monkey kidney cells (Vero cells) (Ishimaru et al. 1998). Cells were infected with MAYV at a concentration of 1 plaque-forming unit (pfu) per cell for 1 h, followed by the administration of PGs at concentrations ranging from 0 to 10 µg/ml for 1 h. The EC50 was observed with doses of 3 µg/ml for PGA1 and 8 µg/ml for PGB2. A higher antiviral effect was observed when compounds were added up to 2 h post-infection (h.p.i.) (Ishimaru et al. 1998). PGA1 was more effective than PGB2, being able to inhibit the production of glycoproteins E1 and E2 by over 50% at 10 µg/ml, resulting in a decrease of infectious particles (Ishimaru et al. 1998).
Later, Burlandy and Rebello corroborated the anti-MAYV activity of PGA1 that reduced 99% of MAYV replication in vitro at 10 µg/mL through a reduction plaque assay (Burlandy and Rebello 2001). Moreover, to suggest which stage of the replicative cycle was affected, a time-of-drug addition assay was performed. The results showed that PGA1 possesses the strongest effect when added to the cells simultaneously to the virus. The authors also identified a reduction in the synthesis of E1, E2, and C, and in the precursors of virus proteins, which suggests the effect in the early stages of viral replication. Additionally, an increase in the heat shock protein 70 (HSP70) was detected, and since this molecule is responsible for protein folding, disaggregation, and degradation to ensure proper cell function (Holbrook et al. 1992), it is possible to suggest a mode of action for PGA1(Burlandy and Rebello 2001). Caldas and coworkers also assessed the effect of PGA1 on MAYV replication and the relevance of HSP70 in this process (Caldas et al. 2018). Human cervix carcinoma cells (Hep-2 cells) were infected with MAYV with a multiplicity of infection (MOI) of 1 for 1 h and treated with different concentrations of the compound. According to the authors, the PGA1 was not cytotoxic, presented an EC50 of 1 μg/mL, and inhibited up to 95% of viral production at 6 μg/mL. In agreement with the previous studies, PGA1 impaired p110, p62, E1/E2, and C synthesis, as well as a significantly increased HSP70 expression (Ishimaru et al. 1998; Caldas et al. 2018). The HSP70 increase seems to be related to the PGA1 antiviral effect since the inhibition of viral replication was only observed in PGA1 concentrations that induced the production of this HSP. In this context, the authors proposed that PGA1 may act in different stages of viral replication due to the different strategies presented by the molecule.
Alternatively, Carvalho and colleagues proposed the natural macromolecule Lactoferrin, a globular glycoprotein, as a candidate for treatment against MAYV (Carvalho et al. 2014). This molecule is produced by exocrine glands, such as mammary and lacrimal glands (Kell et al. 2020) and has been used as apolactoferrin, its apo form found in bovine whey (bLf). To assess the effect of Lactoferrin against MAYV, Vero cells were infected with viral particles labeled with DiD (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine,4-chlorobenzenesulfonate salt), a lipophilic carbocyanine marker that expresses red fluorescence, and then incubated with bLf at concentrations ranging from 0.2 to 1.0 mg/mL. BLf inhibited viral replication in a dose-dependent manner, with 1 mg/mL being able to inhibit 85% of viral infection. Furthermore, a time-of-drug addition assay was also carried out to investigate which stage of the replication cycle was affected (Carvalho et al. 2014). The bLf mainly acted on virus entry, suggesting that this molecule may interact with the sulfate groups of the glycosaminoglycans layer (GAGs) of the host cells, preventing the attachment of the virus. However, since the specific MAYV receptor at the time of the study was unknown (Carvalho et al. 2014), further studies involving the Mrxa8 are needed to corroborate this mechanism of action.
Other potential antiviral candidates tested against MAYV were the flavonoids 4′-O-methylepigallocatechin (MEP) and the proanthocyanidin [(−)-epicatechin-(4β → 8)-(−)-4′-methylepigallocatechin] (PAC), an MEP dimerized with the epicatechin, isolated from Maytenus imbricata roots (Ferraz et al. 2019). The viability of MEP and PAC was evaluated through an MTT ([(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide)] assay using Vero cells, and both demonstrated no cytotoxic effect, with CC50 over 1640 µM. To assess the antiviral effect, cells were infected with MAYV and treated with concentrations of MEP and PAC. No activity was observed against MAYV with MEP, even at the highest tested concentration (1000 μM). However, the treatment of MAYV-infected cells with PAC resulted in EC50 of 37.9 μM and a SI (selectivity index) higher than 43. The treatment at different stages of the virus cycle demonstrated the virucidal activity of PAC, which was confirmed by a dialysis membrane assay. The authors proposed that the mechanism of action may be related to the interaction of proanthocyanins with the envelope glycoproteins, decreasing the infectivity of viral particles (Ferraz et al. 2019).
The antiviral effect of the flavonoid Quercetin, as well as the EtOAc, and n-BuOH fractions, isolated from Bauhinia longifólia, was investigated by employing the viral yield inhibition assay. Vero cells with MAYV (MOI of 0.1) for 1 h, and then treated for 24 h with the substances (0–100 μg/ml). Subsequently, the culture supernatants were recovered and tittered to calculate the extracellular infectious viral particles using the plaque reduction assay. Among the tested compounds, quercetin and the fractions of EtOAc, and n-BuOH had an EC50 of 10 µM, 5 µM, and 3 µM, respectively. EtOAc had the highest SI (623), followed by n-BuOH (208) and quercetin (94). The mechanisms of action of these substances still need to be elucidated. It has been suggested that quercetin may act inhibiting viral RNA polymerase (dos Santos et al. 2014).
The antiviral activity of Quercetin against MAYV was also studied by Bakhache and coworkers, in addition to the compounds Cerulenin, Orlistat, and CAY10566 (Bakhache et al. 2019). These molecules are known to interfere with fatty acid synthase (FASN) or stearoyl-CoA desaturase (SCD1), cofactors of MAYV infection. To assess the their antiviral activity, human embryonic epithelial kidney cells (HEK293T) were infected with the MAYV-Luc reporter virus in an MOI of 1, and Quercetin, Cerulenin, Orlistat, and CAY10566 were added 1.5 h before or 1.5 h after the cell infection at concentrations of 0–150 μM, 0–25 μM, 0–100 μM, and 0–7.5 μM, respectively. Luciferase activity was then monitored for 6, 16, and 24 h.p.i.. As an outcome, the inhibitors induced a dose-dependent inhibitory effect on MAYV, when added before or after the infection. Among the tested drugs, Cerulenin presented the highest antiviral activity, demonstrating an increased ability to inhibit MAYV infectivity when added before the viral infection. The authors also screened these molecules against CHIKV, which demonstrated to decrease CHIKV genome replication due to the interference with FASN or SCD1 enzymatic activity, proposing that these compounds act in a similar mechanism against MAYV.
Similarly, the antiviral activity of EtOAc, n-BuOH, and EtOAc-Pp, extracted from Cassia australis, against MAYV were evaluated (Spindola et al. 2014). The production of MAYV in Vero cells decreased by about 70% and 85% in the presence of EtOAc and n-BuOH at 25 μg/mL, respectively, while EtOAc-Pp at 10 μg/mL decreased 90% the viral progeny production. The authors proposed that the highest antiviral effect of EtOAc-Pp may be due to the condensed tannins, which are present only in this substance. Tannins are a class of polyphenols, characterized by having different molecular sizes and degrees of polymerization, which affect their pharmacokinetics and antiviral activity (Maugeri et al. 2022). Condensed tannins have previously demonstrated antiviral activity against other viruses, such as the respiratory syncytial virus (RSV), influenza A virus (FLU-A), and parainfluenza virus (PIV) (Bruyne et al. 1999). The authors suggested that the mechanism of action of EtOAc-Pp is related to the binding of tannins with viral envelope proteins, resulting in the inhibition of viral binding and penetration into host cells.
What is more, the EtOH fruit extract from Punica granatum (Pomegranate), and its two main fractions, one containing the α and β anomers of Punicalagin and another the Pomegranate ethanol extract, were assessed by their anti-MAYV activity (Salles et al. 2021). To this, Vero cells were infected with MAYV at an MOI of 0.1 for 1 h, and then added of medium-containing substances at concentrations of 12.5, 25, 50, and 100 µg/ml. Virus was titrated 24 h.p.i. and titrated by the TCID50. As a result, P. granatum ethanolic extract demonstrated strong antiviral activity against MAYV infection, with a CC50 of 590.8 µg/mL and an EC50 of 12.3 µg/mL. The fraction containing punicalagin as the main component also showed strong antiviral activity, with a CC50 of 523.1 µg/mL and an EC50 of 62.5 µg/mL. The authors performed a virucidal assay, incubating the virus with each substance at 200 µg/mL for 1 h at 37 °C. Pomegranate ethanol extract showed 98% virucidal activity against MAYV particles, and punicalagin showed no visible virucidal activity, being the results confirmed by immunofluorescence microscopy.
Another natural compound identified to possess anti-MAYV activity is Epicatechin, isolated from extracts of Salacia crassifolia, which belongs to a genus of plants known by its use against diabetes, and as an anti-inflammatory, in folk medicine (Ferreira et al. 2018). Ferreira and coworkers selected Epicatechin for in vitro analysis after performing in silico screening tests that suggested a potential binding interaction with MAYV C protein. To assess the in vitro antiviral activity, cells were pretreated for 30 min with dilutions of the compound (0.013 to 0.574 μmol/mL), and then infected with MAYV at an MOI of 0.1 or 5 for 48 h. Epicatechin presented EC50 of 0.247 μmol/mL, and prevented approximately 100% of the cytopathic effect in MAYV-infected cells at 0.430 μmol/mL. A time-of-drug addition assay was also performed, and when added after infection, epicatechin reduced virus production by two logs in the first 12 h. The authors suggested that the compound acts in the second cycle of infection, when mature virions are released into the extracellular environment, but did not exclude an intracellular action in the late stages of viral replication, considering the MOI used in the assay, concluding that epicatechin acts by binding to components of the viral particle, but not to cellular components.
Ginkgolic Acid (GA), a compound isolated from Ginkgo biloba, is widely used in traditional Chinese medicine (Campos et al. 2020), reported to possess biological activities, such as anti-tumor (Ma et al. 2015; Qiao et al. 2017), antibacterial (Hua et al. 2017), anti-parasitic (Chen et al. 2008), and antiviral (Lü et al. 2012). Campos and colleagues evaluated the effects of GA on CHIKV, MAYV, and UNAV replication. Through a plaque reduction assay using epithelial cervical cancer cells (HeLa cells), it was found that GA at 10 µM reduced the MAYV viral progeny in almost 2 log10 at 16 h.p.i.. To confirm these results, an immunofluorescence assay was performed, demonstrating that treated cells presented a decrease in viral antigens. Additionally, a time-of-drug-addition assay was performed by adding GA to the cells hourly after adsorption, and supernatants were collected 24 h.p.i. to measure viral progeny yields. As a result, the compound was able to mainly inhibit the initial stages of the viral cycle, which was confirmed by the suppression of E1 and E2 viral proteins in western blot (Campos et al. 2020). Despite the promising effects of GA against MAYV in vitro, the molecule demonstrated to be toxic in other cell lines and animal studies (Berg et al. 2015; Qian et al. 2017). Therefore, the authors suggest that further investigations are needed to confirm the safety of the treatment, and the antiviral activity against MAYV.
Another promising inhibitor of MAYV is Silymarin, a compound extracted from Silybum marianum. This molecule is widely used for the treatment of liver disorders (Camini et al. 2018), and was described to have antiviral activity against the Hepatitis C virus (Wagoner et al. 2010) and CHIKV (Lani et al. 2015). Camini and coworkers assessed the effects of Silymarin using hepatocarcinoma cells (HepG2 cells), which resulted in CC50 of 103.5 µg/mL and EC50 of 3.58 µg/mL. Then, HepG2 cells were treated with 25 μg/mL of Silymarin and simultaneously infected with MAYV at an MOI of 5. Supernatants were collected 48 h.p.i. and used to infect naïve Vero cells. The results demonstrated a reduction of up to two logs in MAYV replication, inhibiting almost 100% of the cytopathic effect. The production of reactive species of oxygen (ROS) was measured by employing infected HepG2 cells with a fluorogenic marker, confirming that MAYV infection increased ROS generation in a time-dependent curve. Other oxidative stress biomarkers were quantified, such as malondialdehyde (MDA) and carbonyl protein, in the presence of MAYV and Silymarin, which culminated in a drop of these biomarkers, suggesting a protective effect on host cells, associated with the antiviral activity (Camini et al. 2018).
Finally, Valdés-Torres and collaborators investigated the potential of Honokiol and α-Mangostin against MAYV (Valdés-Torres et al. 2022). Primary human dermal fibroblasts (HDFs), Vero-E6, and HeLa cells were infected with MAYV at an MOI of 1 for 1 h, and then treated with Honokiol (5 to 10 μM) or α-Mangostin (1 to 5 μM) for 24 h. Honokiol and α-Mangostin reduced MAYV-induced cytopathic effects in a dose-dependent manner and inhibited the production of viral progeny in pretreatment assay. MAYV-infected HDFs revealed that α-Mangostin at 1 µM affected MAYV binding to the host cells, while Honokiol showed a modest effect at 10 µM. The viral entry was partially disturbed by α-Mangostin, but not by Honokiol. Both compounds affected the post-entry stage of MAYV infection. The authors also evaluated the expression of Interferon (IFN) type I and specific genes stimulated by IFN in treated or untreated HeLa cells through quantitative RT-PCR. As an outcome, treatment with Honokiol and α-Mangostin promoted a significant increase in IFN type I and interferon-stimulated genes. In addition, the compounds decreased the expression of MAYV E1 and nsP1, and affected viral RNA replication. The authors pointed out that Honokiol and α-Mangostin are post-entry inhibitors impairing MAYV replication through different mechanisms of action, and may act as potential broad-spectrum antivirals, also inhibiting UNAV, CHIKV, and Zika virus (ZIKV).
Synthetic and semi-synthetic inhibitors of MAYV replicative cycle
Natural compounds may present limitations in terms of large-scale production and patentability, and therefore, the production of synthetic and semi-synthetic compounds is an alternative and attractive approach to the development of antiviral candidates (Ortholand and Ganesan 2004).
Among the synthetic molecules, the Thienopyridine derivatives were described as antimicrobial agents against bacteria (Aly et al. 2011), protozoa (Pinheiro 2012), viruses (Bernardino et al. 2004), and parasites (Rolim Bernardino et al. 2006). In this context, Amorim and coworkers conducted a study based on the antiviral activity of thieno[2,3-b]pyridine derivatives 101 to 113 on the MAYV replicative cycle in vitro, using Vero cells (Amorim et al. 2017). Among the non-cytotoxic compounds, molecule 104 was the most viable compound, presenting CC50 of 2500 µM, EC50 of 20.0 µM, and an SI of 125. To further assess the effect of molecule 104 on viral protein synthesis, Vero cells were infected with MAYV at an MOI of 0.05 and treated with 100 µM of the compound in a time-of-drug addition assay. Through the quantification of the plaque-forming assay, it was observed that 104 presented a higher effect when added prior or during the virus infection. Therefore, a virucidal assay was performed by incubating the virus and the compound for 1 h prior to the infection, confirming the virucidal effect of 104 at concentrations higher than 25 µM. The authors also showed a decrease in protein synthesis in the presence of 104, by labeling viral proteins with 35S-methionine, confirmed by transmission electron microscopy, which demonstrated a reduction in the number of intracytoplasmic nucleocapsids and mature virus particles in treated cells.
Porphyrins are organic molecules that can bear a metal ion in the center of their tetrapyrrolic structure (Assunção-Miranda et al. 2016). These molecules are capable of absorbing light and are widely used in photodynamic therapies. The porphyrins have also been shown to inactivate viral particles by targeting the viral envelope of Dengue virus (DENV) and Yellow Fever virus (YFV) (Neris et al. 2018). Neris and coworkers evaluated the antiviral potential of the heme porphyrins Co-protoporphyrin IX (CoPPIX) and Sn-protoporphyrin IX (SnPPIX) in the presence or absence of light against MAYV using Baby Hamster Kidney Fibroblast (BHK-21) or Vero cells (Neris et al. 2018). With no light stimulation, CoPPIX and SnPPIX presented a CC50 of 762.3 µM and 816 µM, respectively, while in the presence of light, the compounds demonstrated a CC50 of 1018 µM and 20.7 µM, respectively. Later, 107 PFU of viruses were pre-incubated with different concentrations of each compound in the absence of light for 1 h at 37 °C, and then used to infect the cells with an MOI of 0.1. CoPPIX and SnPPIX at 300 μM were able to completely inactivate MAYV, with an EC50 of 5.94 µM and 5.99 µM, respectively. Additionally, light-stimulated for 10 min before incubation for 1 h in the dark resulted in EC50 of 0.09 µM for SnPPIX and 7.48 µM for CoPPIX. To further investigate the CoPPIX and SnPPIX inhibition, MAYV was labeled with DiD to follow fusion between virus and endosomal membranes during endocytosis. After treatment with CoPPIX or SnPPIX at 300 μM with no light stimulation, and SnPPIX at 10 μM with light stimulation, MAYV was unable to efficiently fuse with the endosomal membrane. The authors highlighted that the porphyrins are hydrophobic molecules, that might interact with viral envelope lipids, impairing the attachment and entry of the virus into the cells. Furthermore, the photoactivation of SnPPIX generates ROS, which can modify viral proteins in the envelope environment, producing an antiviral activity. The authors emphasized that a single intensity of light stimulation was performed and suggested that an increase in luminous intensity might increase SnPPIX virucidal efficiency (Neris et al. 2018).
Another promising compound is β-D-N4-hydroxycytidine (EIDD-1931), a ribonucleoside analogue which previously demonstrated antiviral activity against RNA viruses, such as SARS-COV-2, Equine Encephalitis Virus, and CHIKV (Yousefi et al. 2021; Langendries et al. 2021). EIDD-1931 can rapidly reach plasma, is efficiently distributed in mice organs, and also presents a high genetic barrier for the development of viral resistance (Painter et al. 2019; Yousefi et al. 2021). A study conducted by Langendries and coworkers screened compounds with previously reported antiviral activity against other arboviruses, such as EIDD-1931, Favipiravir, and Suramin, against MAYV (strain TC625, MOI of 0.01), using Vero cells (Langendries et al. 2021). The experiments demonstrated CC50 of > 100 µM, > 2000 µM, and 2837 µM, and EC50 of 1.6 µM, 124 µM, and 79 µM for EIDD-1931, Suramin, and Favipiravir, respectively. To evaluate which stage of the replicative cycle was affected by these compounds, a time-of-drug-addition assay was carried out. Vero cells were incubated with 300 µM Favipiravir, 500 µM Suramin, and 25 µM EIDD-1931 2 h before infection, and 0, 2, 4, 6, and 8 h.p.i. at an MOI of 1. Through end-point titration at 10 h.p.i and qRT-PCR quantification, the authors demonstrated that EIDD-1931 impaired viral replication when added at 0, 2, and 4 h.p.i., with a moderated effect if added at 6 h.p.i. Differently, Suramin and Favipiravir reduced viral titers at 0 and 2 h.p.i., respectively. From the results and previous data in the literature, the authors suggested that EIDD-1931 and Favipiravir impair viral RNA synthesis, resulting in non-functional copied viruses due to a large number of mutations in the viral genome (Joshi et al. 2021; Langendries et al. 2021). It was also proposed that Suramin acts during the early steps of the MAYV replicative cycle, more specifically in the viral entry, in a similar mechanism demonstrated by studies with CHIKV (Albulescu et al. 2020) and SARS-CoV-2 (Salgado-Benvindo et al. 2020).
Cyclic ketones are natural and synthetic compounds with antiviral (Sivropoulou et al. 1997; Chung 2017), antioxidant (Pombal et al. 2017), and antifungal (Pizzolitto et al. 2015) properties. The antiviral activity of 24 cyclic ketones was assessed by Fernandes and coworkers, by screening these compounds, using MAYV-infected Vero cells (MOI 1), treated with each compound at concentrations ranging from 1 to 100 µM for 24 h. Among them, 8 compounds showed viability ≥ 50% and were selected for viral inhibition assay. A plaque-forming assay was performed, and among the 8 compounds, the Xanthenodione 9-(5-(4-chlorophenyl]furan-2-yl)-3,6-dimethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2))-dione inhibited the MAYV with EC50 of 21.5 µM and SI of 15.8. The authors also performed a time-of-drug addition assay and found that the compound inhibits viral replication in the prior and post-treatment assays (Fernandes et al. 2021).
The Proteasome inhibitors MG132 and Lactacystin are also antiviral candidates against MAYV (Llamas-González et al. 2019). MG132 acts as a reversible inhibitor of the chymotryptic activity of the proteasome, and Lactacystin is an irreversible inhibitor capable of binding to the catalytic β subunits of the 20S proteasome (Llamas-González et al. 2019). The anti-MAYV activity of these compounds was evaluated by Llamas-González and colleagues. Vero-E6 or HeLa cells were treated with MG132 at 10 μM or Lactacystin at 25 μM for 1 h, and then infected with MAYV (MOI of 1 or 10) for 1 h, when the inoculum was replaced by fresh medium-containing compounds. Viruses were titrated by plaque-forming assay 24 h.p.i.. MG132 and Lactacystin low cytotoxicity and significant antiviral effect using either cell lines. The authors also performed immunofluorescence of MAYV-infected HeLa cells, which demonstrated a significant reduction in the percentage of MAYV-positive cells under treatment, resulting in < 5% for MG132 and < 10% for Lactacystin, versus > 20% in the untreated control group. Viral titers were lower when compounds were added in the early stages of infection, and the treatment with MG132 or Lactacystin affected the production of viral proteins nsP1 and E1, showed by the reduced expression of MAYV proteins under the treatment with these proteasome inhibitors.
Exploiting host factors, the Src family of protein tyrosine kinases (SFK) are involved in intracellular signaling pathways related to virus elimination (Broeckel et al. 2019). However, during infection, the virus can interact with kinases favoring viral replication. Therefore, cell signaling inhibitors, including SFK inhibitors, have been tested for their antiviral activity against Alphavirus in human fibroblasts (Broeckel et al. 2019). Since the knowledge of the signaling profile of SFK during Alphavirus is limited, the authors carried out a range of experiments using CHIKV infection, as a base to identify the kinase pathways involved in this genus infection. The SFK-phosphatidylinositol 3-kinase (PI3K)-Akt-mTOR signaling was demonstrated to be the main pathway during CHIKV infection. Then, the authors evaluate the effects of dasatinib and Torin, inhibitors of this pathway, on fibroblasts infected with MAYV. Through the viral titration of the supernatant collected from infected and treated cells, it was shown that MAYV was significantly reduced by these inhibitors. The authors demonstrated that the SFK inhibitors blocked the subgenomic RNA translation, resulting in the impairment of Alphaviruses replication (Broeckel et al. 2019).
A non-natural interferon (cIFN) that was designed according to the analysis of the amino acid sequences present in several subtypes of IFN-α was investigated by its anti-MAYV activity by Grabarz et al. (2021). Vero cells were treated with increasing concentrations of cIFN for two hours and then infected with MAYV (MOI = 0.1) for 48 h. MAYV infection was inhibited by cIFN inhibited, with EC50 of 35.7 fg/mL, being possible to obtain about 80% and 100% cell confluency with concentrations of 81.4 fg/mL, and 162.8 fg/mL of the compound, respectively. In comparison, untreated cells showed almost complete destruction of the cell monolayer 24 h.p.i. with MAYV. In this study, cIFN was also able to inhibit the cytopathic effect of other viruses, such as ZIKV, CHIKV, and SARS-CoV-2.
Another promising compound is N-(methylcarbamoyl)-2-{[5-(4-methylphenyl)-1,3,4-oxadiazol-2-yl]sulfanyl}-2-phenylacetamide (known as C11), a molecule that induces type I IFN-dependent activity in human cell lines (Gall et al. 2018). In this context, Gall and collaborators produced THF cells and MM6 cells with deletion of the stimulator of interferon genes (STING), such as the RIG-1 mitochondrial antiviral signaling (MAVS) and the TIR-domain-containing adapter-inducing interferon-β gene (TRIF), related to the activation of the immune response. To assess the antiviral activity of C11, these cells were treated for 2 h with C11, IFN-β, or DMSO at non-cytotoxic concentrations and then infected with MAYV at an MOI of 1. The results demonstrated that C11 had an effective concentration of 90% (EC90) of 25.19 μM against MAYV. STING-deleted cells showed no inhibition of virus replication in the presence of the compound. C11 was also evaluated against CHIKV, VEEV, and RRV viruses in human cells, resulting in reductions in the virus titer, but when evaluated using non-human cells (RAW264.7 monocytic cells), a lack of antiviral activity in the murine RAW264.7 cell line was noticed. The authors proposed that C11 acts by activating specifically the human type I IFN response through a STING-dependent process, leading to the generation of an IFNAR-mediated antiviral cellular state.
Molecular target to drug development against MAYV
Drug development can be a time-consuming process, which can result in unexpected outcomes (Adamson et al. 2021). Additionally, the lack of treatment against viral infections, mainly for neglected diseases, may jeopardize the control of the disease, as well as its consequences, such as the loss of human lives (Young et al. 2019). In this context, several strategies can be applied to accelerate the progress toward the development of novel molecules. Therefore, this section focuses on the MAYV proteins which could be exploited as a target for antiviral development, considering the factors: (i) previously described compounds with antiviral activity against MAYV and/or other Alphaviruses, (ii) resolved structure of viral proteins deposited in databanks, and (iii) structural similarity and conservation among viruses.
The glycoprotein E1 possesses an ectodomain, subdivided into subdomains I, II, and III, and a transmembrane subdomain (TM). Similarly, the E2 ectodomain is composed of subdomains A, B, C, and D, and a TM portion (Filho et al. 2020). The ectodomains of E1 and E2 interact with each other to form a portion of the spike structure outside the envelope: the glycoproteins E1 and E2 interact forming a dimeric unit (Fig. 3A) that is further associated with additional two dimers to form the spike (Fig. 3B–D). The TM portions of E1/E2 bind the spike to the lipid membrane, while E2 C-terminal connects the spike to the capsid protein, through a well-described TPY consensus motif (West et al. 2006; Ribeiro-Filho et al. 2021), forming an E1–E2-capsid unit (Fig. 3C, E). As observed for other Alphaviruses, MAYV possesses an asymmetric unit formed by the assembling of four E1–E2-capsid units resulting in 80 spikes (Filho et al. 2020). MAYV belongs to the arthritogenic class of viruses, and, therefore, it is possible to suggest that Mxra8 is the main receptor related to the MAYV entry into host cells (Fig. 3E) (Powell et al. 2020). In this context, considering that the glycoprotein protein complex interacts with Mxra8, mainly through E2, this interaction could be impaired by active molecules through interference or destabilization of E2 binding sites (Zhang et al. 2018; Filho et al. 2020). Molecules were previously described to bind to viral glycoproteins of other arthritogenic viruses, such as CHIKV, consequently inhibiting viral entry to the host cells. For instance, Oliveira and coworkers described the in vitro virucidal activity of a coordinated organic compound against CHIKV, and demonstrated that in silico analysis suggested interactions between the compound and CHIKV E2 domain (Oliveira et al. 2020). Additionally, studies encompassing the compounds PAC (Ferraz et al. 2019), bLf (Carvalho et al. 2014), CoPPIX, and SnPPIX (Neris et al. 2018), which are discussed here, also demonstrated a virucidal activity against MAYV infection. Even though these molecules were not rationally designed for specific targets, further studies could investigate whether their mode of action is related to these hint targets.
Schematic structure of MAYV glycoprotein. The glycoproteins E1 and E2 interact forming a dimeric unit (A), that is further associated to two additional dimeric units to form a trimer (B). The localization of the trimers is demonstrated in (C), and a diagram of the E1–E2 trimer is shown in (D). The interaction between the glycoproteins and the MRXA8 receptor in host cells is demonstrated in E. MAYV E1 and E2 structure were based on Cryo-EM structure of mature MAYV (PDB ID: 7KO8) (Ribeiro-Filho et al. 2021), and the receptor was based on human MXRA8 (PDB ID: 6JO8) (Powell et al. 2020)
Another spot protein in MAYV replication is the nsp4, an RNA-dependent RNA polymerase (RdRp) essential to the viral replication (Rubach et al. 2009). Therefore, it is an attractive target to the development of antiviral drugs. To the best of our knowledge, the RdRp crystal structure of MAYV has not been determined and characterized yet, which might postpone drug design applied to this target. Regardless, the RdRp amino acid sequence is commonly conserved among viruses from the Alphaviruses genus, and especially between the Semliki Forest serocomplex members (Rupp et al. 2015). Therefore, the RdRp structure from the Ross River virus (RRV) (Fig. 4), another member of the Alphavirus genus might be employed as a template for drug development. Its structure is composed of fingers, the palm containing the GDD active site, and thumb domains (Rubach et al. 2009). Therefore, it is possible to hypothesize that compounds with antiviral activity described against the RdRp of other viruses could act against MAYV infection (Chen et al. 2017). Indeed, most of the described and approved drugs are nucleoside and non-nucleoside inhibitors that impair viral infections through RNA synthesis inhibition. Among them, Remdesivir, Azidothymidine (AZT), Sofosbuvir, and Lopinavir/Ritonavir, are licensed antivirals used to treat Ebola virus, Hepatitis C virus, and Human immunodeficiency virus infections (Tian et al. 2021).
Schematic structure of MAYV genome and the crystal structure of nsP3 macrodomain and nsP4. Nsp3 is a modular protein with three domains: the N-terminal macro domain, a central zinc-binding domain, and the C-terminal hypervariable domain, being the macro domain constituted by four alpha-helices and six beta-strands. The nsP4 protein acts as an RNA-dependent RNA polymerase (RdRp), and its structure is composed of fingers, the palm containing the GDD active site, and thumb domains. Since RdRp crystal structure of MAYV has not been determined and characterized yet, the template used in the figure was obtained from Ross River virus RdRp (PDB ID: 7F0S) (Tan et al. 2022). The nsP3 structure was based on MAYV macro domain structure (PDB ID: 5IQ5) (Tsika et al. 2019)
The MAYV nsP3 is another protein that represent a target for antiviral development (Tsika et al. 2019). It is a modular protein with three domains: the N-terminal macro domain, a central zinc-binding domain, and the C-terminal hypervariable domain (Fig. 4) (Götte et al. 2018). Each domain plays a different role in viral replication and interferes with the virulence among Old and New World Alphaviruses, being necessary for RNA synthesis (Rupp et al. 2015). The MAYV macro domain possesses four alpha-helices and six beta-strands (Malet et al. 2009; Melekis et al. 2015). On the other hand, the central zinc-binding domain plays an undefined, but essential role in minus-strand RNA synthesis, through an association with the macro domain, forming a ring-like structure to RNA binding (Shin et al. 2012). Alternatively, the hypervariable domain is a large protein that upholds several insertions and deletions, and is responsible for interactions with multiple host proteins during viral replication, probably favoring virulence (Davis et al. 1989; Dé et al. 2003; Uversky 2013). However, the macro domain is the most conserved site in the protein structure and could be exploited for drug development, particularly as a broad-spectrum antiviral. The macro domain site binds to ADP-ribose, dephosphorylate ADP-ribose-1′-phosphate, and possesses a de-ADP-ribose hydrolase activity, which is essential to viral replication (McPherson et al. 2017). Several compounds were virtually screened employing the nsP3 macrodomain of CHIKV (Nguyen et al. 2014; Shimizu et al. 2020; Subudhi et al. 2018), SARS-CoV-2 (Jung et al. 2020), and the Venezuelan equine encephalitis virus (VEEV) (Atasheva et al. 2014) as targets. Shimizu and collaborators selected compounds that targeted the nsP3 macro domain of CHIKV by in silico analysis, and validated their antiviral activity performing in vitro assays (Shimizu et al. 2020). Therefore, it is possible to suggest the nsP3 macrodomain of MAYV as a relevant target for future antiviral discovery.
Furthermore, components of virus lipidic membrane are also potential targets yet to be explored. Among membrane components, the cholesterol is an important molecule related to alphavirus stability, infectivity, and assembly (Sousa et al. 2020). This molecule was recently associated with the successful infection in several viruses (Sun and Whittaker 2003; Huang et al. 2006), being also modulated to increase the infection efficiency (Zhang et al. 2019). Cholesterol can be found in increased proportions in MAYV viral particles isolated from vertebrate cells, being associated with the lateral organization of the viral envelope and binding to the host membrane (Sousa et al. 2020). Additionally, a decrease in alphavirus infectivity is observed when the arrangement of the viral envelope is disturbed, suggesting that the lateral membrane organization is important for the physical stability of the viral particle and, consequently, for virus-cell interactions (Kielian et al. 2010). Cholesterol is also associated with MAYV entry, being used as an alternative pathway for its entry through cholesterol-enriched caveolae-derived vesicles and release (Carvalho et al. 2017). Therefore, targeting cholesterol from the viral lipidic membrane represent an interesting approach for antiviral drug development. However, designing and identifying active compounds focusing on these components can be challenging, due to their similarity to the host cell membrane (Sousa et al. 2020). Interestingly, the compound 25-hydroxycholesterol (25HC), a reactive oxysterol catalyzed by cholesterol-25-hydroxylase, is a promising molecule that can interact with lipids on viral membrane, and demonstrated broad-spectrum antiviral activity, low toxicity, and ability to reduce viremia and protect embryonic mice against ZIKV in vivo (Li et al. 2017; Mao et al. 2022). In this context, molecules focused on interacting with cholesterol could be employed to drug development against MAYV. ed to drug development against MAYV.
Perspectives
MAYV infection can impact the quality of life of infected patients, due to the chronic condition that can result in progressive arthralgia and disabling disease. Besides that, the lack of approved antiviral treatment and vaccines against MAYV, associated with the presence of susceptible vectors in tropical and subtropical countries, can lead to future outbreaks.
In this context, this review summarized and discussed the literature concerning the compounds with anti-MAYV activity, as well as presented possible viral proteins that could be further exploited as targets for drug development. To the best of our knowledge, only the studies described here reported antiviral activity against MAYV infection, demonstrating that further research on treatments against Mayaro fever is urgent. It is important to emphasize that the data presented here employed different methodologies, including cell lines, MOI for infection assays, and the design of the assays. However, these studies present relevant information on the antiviral activity of a range of molecules (natural and synthetic) against MAYV, and suggested possible mechanisms of action (MOA) related to each compound. The characterization of the MOA of these molecules represents an advance for further studies, providing information concerning the interactions among host cells, viruses, and compounds (Iorio et al. 2010; Salters-Pedneault 2014). Besides, the MOA of several compounds against MAYV are still unknown (Figueiredo et al. 2019). From the compounds discussed here, Lactoferrin, molecule 104 (thienopyridine derivative), and Silymarin presented significant antiviral activity against MAYV in vitro, with low or no cytotoxicity. The repurposing antiviral agent EIDD-1931 is also a promising treatment since it was able to completely inhibit MAYV replication in vitro. However, these compounds need to be further investigated in vivo against MAYV. Ginkgolic Acid (GA) was one of the most active compounds, and the only molecule tested in vivo, however demonstrating hepatotoxicity and nephrotoxicity effects, as well cytotoxicity in different cell lines. Qian and collaborators also observed that rats treated with either, high or low doses of GA, showed histopathological changes in the liver and kidneys. In addition, the serum levels of urea nitrogen and creatinine increased in the blood, and total protein and albumin decreased, indicating kidney dysfunction and liver damage (Qian et al. 2017). On the other hand, the genotoxicity evaluation of GA evidenced a low risk of genotoxicity in vivo (Berg et al. 2015). Therefore, more studies in human cells and animal models are necessary to analyze the safety of this compound.
Furthermore, we discussed and highlighted MAYV proteins that could represent relevant targets for drug development, acknowledging that focusing on host factors might produce cytotoxicity in vitro, and adverse effects in vivo. Here, we suggested the glycoproteins complex, the nsP4 (RdRp), and the nsP3 macro domain as targets. The lack of resolved structures of several MAYV proteins, such as nsP1, nsP2, C, and E, invalidate them as targets for drug development.
Finally, licensed drugs employed in the treatment of several diseases have the potential to be repurposed to target the viral molecules underlined here. In this sense, Langendries and colleagues identified EIDD-1931, favipiravir, and suramin, three licensed antivirals, with activity against MAYV (Langendries et al. 2021). Additionally, the potential of Lactacystin (Llamas-González et al. 2019), Cerulenin, Orlistat (Bakhache et al. 2019), and Quercetin (dos Santos et al. 2014), as repurposing therapy against MAYV was also discussed. The compounds were active with low EC50 and low cytotoxicity, and showed potential to be applied as antiviral therapy in the treatment of infections caused by this pathogen, being a prospective target for further research. The use of approved drugs as repurposing therapy may favor patient access to drugs due to the potential to result in shorter clinical studies, since these molecules have their pharmacological profile and adverse effects already described (Santos et al. 2022). Bearing that in mind, it is an inconsistency that repurposing drugs against MAYV have been understudied. Therefore, we further emphasize and encourage researchers to screen licensed drugs against MAYV.
Conclusions
The spread of MAYV infections in tropical and subtropical regions represents a threat to developing countries. Additionally, the lack of epidemiological data on Mayaro fever might underestimate the importance of this disease and its consequences. There is an urgent need for studies to understand the biology of the virus, thus creating paths to combat future outbreaks. In this context, the compounds described to possess anti-MAYV activity, and the possible targets discussed herein, may contribute to the future development of antiviral drugs against MAYV.
Data availability statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Acosta-Ampudia Y, Monsalve DM, Rodríguez Y et al (2018) Mayaro: an emerging viral threat? Emerg Microbes Infect 7:1–11
Adamson CS, Chibale K, Goss RJM et al (2021) Antiviral drug discovery: preparing for the next pandemic. Chem Soc Rev 50:3647. https://doi.org/10.1039/d0cs01118e
Agostini ML, Pruijssers AJ, Chappell JD et al (2019) Small-molecule antiviral β-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J Virol. https://doi.org/10.1128/JVI.01348-19
Aguilar-Luis MA, del Valle-Mendoza J, Silva-Caso W et al (2020) An emerging public health threat: Mayaro virus increases its distribution in Peru. Int J Infect Dis 92:253–258. https://doi.org/10.1016/j.ijid.2020.01.024
Albulescu IC, White-Scholten L, Tas A et al (2020) Suramin inhibits chikungunya virus replication by interacting with virions and blocking the early steps of infection. Viruses. https://doi.org/10.3390/v12030314
Aly HM, Saleh NM, Elhady HA (2011) Design and synthesis of some new thiophene, thienopyrimidine and thienothiadiazine derivatives of antipyrine as potential antimicrobial agents. Eur J Med Chem 46:4566–4572. https://doi.org/10.1016/j.ejmech.2011.07.035
Amorim R, de Meneses MDF, Borges JC et al (2017) Thieno[2,3-b]pyridine derivatives: a new class of antiviral drugs against Mayaro virus. Arch Virol 162:1577–1587. https://doi.org/10.1007/s00705-017-3261-0
Anderson CR, Wattley GH, Ahin NW et al (1957) Mayaro virus: a new human disease agent. Am J Trop Med Hyg 6:1012–1016. https://doi.org/10.4269/ajtmh.1957.6.1012
Arias-Arias JL, Vega-Aguilar F, Picado-Soto D et al (2021) In vitro inhibition of Zika virus replication with amantadine and rimantadine hydrochlorides. Microbiol Res (pavia) 12:727–738. https://doi.org/10.3390/microbiolres12030052
Assunção-Miranda I, Cruz-Oliveira C, Neris RLS et al (2016) Inactivation of dengue and yellow fever viruses by heme, cobalt-protoporphyrin IX and tin-protoporphyrin IX. J Appl Microbiol 120:790–804. https://doi.org/10.1111/jam.13038
Atasheva S, Frolova EI, Frolov I (2014) Interferon-stimulated poly(ADP-ribose) polymerases are potent inhibitors of cellular translation and virus replication. J Virol 88:2116–2130. https://doi.org/10.1128/jvi.03443-13
Bakhache W, Neyret A, McKellar J et al (2019) Fatty acid synthase and stearoyl-CoA desaturase-1 are conserved druggable cofactors of Old World Alphavirus genome replication. Antiviral Res 172:104642. https://doi.org/10.1016/j.antiviral.2019.104642
Berg K, Braun C, Krug I, Schrenk D (2015) Evaluation of the cytotoxic and mutagenic potential of three ginkgolic acids. Toxicology 327:47–52. https://doi.org/10.1016/j.tox.2014.10.001
Bernardino AMR, Pinheiro LCS, Ferreira VF, Azevedo AR (2004) Synthesis and antiviral activity of new 4-(phenylamino)thieno[2,3-b]pyridine derivatives. Heterocycl Comm. https://doi.org/10.1515/HC.2004.10.6.407
Broeckel R, Sarkar S, May NA et al (2019) Src family kinase inhibitors block translation of alphavirus subgenomic mRNAs. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.02325-18
Brown R, Wan J, Kielian M (2018) The alphavirus exit pathway: what we know and what we wish we knew. Viruses 10:89. https://doi.org/10.3390/v10020089
Burlandy FM, Rebello MA (2001) Inhibition of Mayaro virus replication by prostaglandin A1 in Vero cells. Intervirology 44:344–349. https://doi.org/10.1159/000050069
Caldas LA, Ferreira DF, Freitas TRP (2018) Prostaglandin A1 triggers Mayaro virus inhibition and heat shock protein 70 expression in an epithelial cell model. Rev Soc Bras Med Trop 51:584–590. https://doi.org/10.1590/0037-8682-0235-2018
Camini FC, da Silva TF, da Silva Caetano CC et al (2018) Antiviral activity of silymarin against Mayaro virus and protective effect in virus-induced oxidative stress. Antiviral Res 158:8–12. https://doi.org/10.1016/j.antiviral.2018.07.023
Campos D, Navarro S, Llamas-González YY et al (2020) Broad antiviral activity of ginkgolic acid against chikungunya, Mayaro, una, and Zika viruses. Viruses. https://doi.org/10.3390/v12040449
Carvalho CAM, Sousa IP, Silva JL et al (2014) Inhibition of Mayaro virus infection by bovine lactoferrin. Virology 452–453:297–302. https://doi.org/10.1016/j.virol.2014.01.022
Carvalho CAM, Silva JL, Oliveira AC, Gomes AMO (2017) On the entry of an emerging arbovirus into host cells: Mayaro virus takes the highway to the cytoplasm through fusion with early endosomes and caveolae- derived vesicles. PeerJ. https://doi.org/10.7717/peerj.3245
Chen SX, Wu L, Jiang XG et al (2008) Anti-toxoplasma gondii activity of GAS in vitro. J Ethnopharmacol 118:503–507. https://doi.org/10.1016/j.jep.2008.05.023
Chen MW, Tan YB, Zheng J et al (2017) Chikungunya virus nsP4 RNA-dependent RNA polymerase core domain displays detergent-sensitive primer extension and terminal adenylyltransferase activities. Antiviral Res 143:38–47. https://doi.org/10.1016/j.antiviral.2017.04.001
Choi H, Kudchodkar SB, Reuschel EL et al (2019) Protective immunity by an engineered DNA vaccine for mayaro virus. PLoS Negl Trop Dis 13:1–21. https://doi.org/10.1371/journal.pntd.0007042
Chung MS (2017) Antiviral activities of Artemisia princeps var. orientalis essential oil and its α-thujone against norovirus surrogates. Food Sci Biotechnol 26:1457. https://doi.org/10.1007/S10068-017-0158-3
Davis NL, Willis LV, Smitht JF, Johnston RE (1989) In vitro synthesis of infectious venezuelan equine encephalitis virus RNA from a cDNA clone: analysis of a viable deletion mutant. Virology 171:189–204. https://doi.org/10.1016/0042-6822(89)90526-6
Dé I, Fata-Hartley C, Sawicki SG, Sawicki DL (2003) Functional analysis of nsP3 phosphoprotein mutants of Sindbis virus. J Virol 77:13106–13116. https://doi.org/10.1128/jvi.77.24.13106-13116.2003
de Bruyne T, Pieters L, Deelstra H, Vlietinck A (1999) Condensed vegetable tannins: biodiversity in structure and biological activities. Biochem Syst Ecol 27:445–459. https://doi.org/10.1016/S0305-1978(98)00101-X
de Oliveira DM, de Santos IA, Martins DOS et al (2020) Organometallic complex strongly impairs Chikungunya virus entry to the host cells. Front Microbiol. https://doi.org/10.3389/fmicb.2020.608924
Diagne CT, Bengue M, Choumet V et al (2020) Mayaro virus pathogenesis and transmission mechanisms. Pathogens 9:1–23
dos Santos AE, Kuster RM, Yamamoto KA et al (2014) Quercetin and quercetin 3-O-glycosides from Bauhinia longifolia (Bong.) Steud. show anti-Mayaro virus activity. Parasit Vectors 7:130. https://doi.org/10.1186/1756-3305-7-130
Elm JL, Halstead SB, Koff WC (1981) Suppression of dengue virus replication in vitro by rimantadine hydrochloride *. Am J Trop Med Hyg 30:184–189. https://doi.org/10.4269/ajtmh.1981.30.184
Esposito DLA, da Fonseca BAL (2017) Will Mayaro virus be responsible for the next outbreak of an arthropod-borne virus in Brazil? Brazil J Infect Dis 21:540–544
Fernandes LS, da Silva ML, Dias RS et al (2021) Evaluation of antiviral activity of cyclic ketones against Mayaro virus. Viruses 13:2123. https://doi.org/10.3390/V13112123
Ferraz AC, de Moraes T, FS, Nizer WS da C, et al (2019) Virucidal activity of proanthocyanidin against Mayaro virus. Antiviral Res 168:76–81. https://doi.org/10.1016/j.antiviral.2019.05.008
Ferreira PG, Ferraz AC, Figueiredo JE et al (2018) Detection of the antiviral activity of epicatechin isolated from Salacia crassifolia (Celastraceae) against Mayaro virus based on protein C homology modelling and virtual screening. Arch Virol 163:1567–1576. https://doi.org/10.1007/s00705-018-3774-1
Figueiredo CM, da Neris RLS, Gavino-Leopoldino D et al (2019) Mayaro virus replication restriction and induction of muscular inflammation in mice are dependent on age, type-I interferon response, and adaptive immunity. Front Microbiol 10:1–11. https://doi.org/10.3389/fmicb.2019.02246
Filho HR, Coimbra LD, Cassago A et al (2020) Cryo-EM structure of the mature and infective Mayaro virus at 44 Å resolution reveals new features of arthritogenic alphaviruses. bioRxiv. https://doi.org/10.1101/2020.11.06.371773
Firth AE, Chung BY, Fleeton MN, Atkins JF (2008) Discovery of frameshifting in alphavirus 6K resolves a 20-year enigma. Virol J 5:108. https://doi.org/10.1186/1743-422X-5-108
Gall B, Pryke K, Abraham J et al (2018) Emerging alphaviruses are sensitive to cellular states induced by a novel small-molecule agonist of the STING pathway. J Virol. https://doi.org/10.1128/JVI.01913-17
Gescher K, Hensel A, Hafezi W et al (2011) Oligomeric proanthocyanidins from Rumex acetosa L. inhibit the attachment of herpes simplex virus type-1. Antiviral Res 89:9–18. https://doi.org/10.1016/j.antiviral.2010.10.007
Götte B, Liu L, McInerney GM (2018) The enigmatic alphavirus non-structural protein 3 (nsP3) revealing its secrets at last. Viruses. https://doi.org/10.3390/v10030105
Grabarz F, Lopes APY, Barbosa FF et al (2021) Strategies for the Production of Soluble Interferon-Alpha Consensus and Potential Application in Arboviruses and SARS-CoV-2. Life 11:460. https://doi.org/10.3390/life11060460
Holbrook NJ, Carlson SG, Choi AM, Fargnoli J (1992) Induction of HSP70 gene expression by the antiproliferative prostaglandin PGA2: a growth-dependent response mediated by activation of heat shock transcription factor. Mol Cell Biol 12:1528–1534. https://doi.org/10.1128/mcb.12.4.1528
Hosseinimehr SJ, Rostamnejad M, Ghaffari-rad V (2013) Epicatechin enhances anti-proliferative effect of bleomycin in ovarian cancer cell. Res Mol Med 1:25–28. https://doi.org/10.18869/acadpub.rmm.1.3.25
Hua Z, Wu C, Fan G et al (2017) The antibacterial activity and mechanism of ginkgolic acid C15:1. BMC Biotechnol 17:1–12. https://doi.org/10.1186/s12896-016-0324-3
Huang H, Li Y, Sadaoka T et al (2006) Human herpesvirus 6 envelope cholesterol is required for virus entry. J Gen Virol 87:277–285. https://doi.org/10.1099/vir.0.81551-0
Iorio F, Bosotti R, Scacheri E et al (2010) Discovery of drug mode of action and drug repositioning from transcriptional responses. Proc Natl Acad Sci 107:14621–14626. https://doi.org/10.1073/pnas.1000138107
Ishida YI, Takeshita M, Kataoka H (2014) Functional foods effective for hepatitis C: identification of oligomeric proanthocyanidin and its action mechanism. World J Hepatol 6:870–879. https://doi.org/10.4254/wjh.v6.i12.870
Ishimaru D, Marcicano FGP, Rebello MA (1998) Inhibition of Mayaro virus replication by prostaglandin A1 and B2 in Vero cells. Braz J Med Biol Res 31:1119–1123. https://doi.org/10.1590/S0100-879X1998000900003
Jose J, Taylor AB, Kuhn RJ (2017) Spatial and temporal analysis of alphavirus replication and assembly in mammalian and mosquito cells. Mbio. https://doi.org/10.1128/mBio.02294-16
Joshi S, Parkar J, Ansari A et al (2021) Role of favipiravir in the treatment of COVID-19. Int J Infect Dis 102:501–508
Jung LS, Gund TM, Narayan M (2020) Comparison of binding site of remdesivir and its metabolites with NSP12-NSP7-NSP8, and NSP3 of SARS CoV-2 virus and alternative potential drugs for COVID-19 treatment. Protein J 39:619–630. https://doi.org/10.1007/s10930-020-09942-9
Karupiah G, Xie QW, Buller RML et al (1993) Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science (1979) 261:1445–1448. https://doi.org/10.1126/science.7690156
Kell DB, Heyden EL, Pretorius E (2020) The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front Immunol 11:1–15. https://doi.org/10.3389/fimmu.2020.01221
Kielian M, Chanel-Vos C, Liao M (2010) Alphavirus entry and membrane fusion. Viruses 2:796–825. https://doi.org/10.3390/v2040796
Kim S, Chen J, Cheng T et al (2021) PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res 49:D1388–D1395. https://doi.org/10.1093/nar/gkaa971
Langendries L, Abdelnabi R, Neyts J, Delang L (2021) Repurposing drugs for Mayaro virus: Identification of eidd-1931, favipiravir and suramin as Mayaro virus inhibitors. Microorganisms. https://doi.org/10.3390/microorganisms9040734
Lani R, Hassandarvish P, Chiam CW et al (2015) Antiviral activity of silymarin against chikungunya virus. Sci Rep 5:11421. https://doi.org/10.1038/srep11421
Li C, Deng Y-Q, Wang S et al (2017) 25-hydroxycholesterol protects host against Zika virus infection and its associated microcephaly in a mouse model. Immunity 46:446–456. https://doi.org/10.1016/j.immuni.2017.02.012
Li X, Zhang H, Zhang Y et al (2019) Development of a rapid antiviral screening assay based on eGFP reporter virus of Mayaro virus. Antiviral Res 168:82–90. https://doi.org/10.1016/j.antiviral.2019.05.013
Llamas-González YY, Campos D, Pascale JM et al (2019) A functional ubiquitin-proteasome system is required for efficient replication of new world Mayaro and Una alphaviruses. Viruses 11:370. https://doi.org/10.3390/v11040370
Long KC, Ziegler SA, Thangamani S et al (2011) Experimental transmission of Mayaro virus by Aedes aegypti. Am J Trop Med Hyg 85:750–757. https://doi.org/10.4269/ajtmh.2011.11-0359
Lü JM, Yan S, Jamaluddin S et al (2012) Ginkgolic acid inhibits HIV protease activity and HIV infection in vitro. Med Sci Monit 18:293–298. https://doi.org/10.12659/MSM.883261
Ma J, Duan W, Han S et al (2015) Ginkgolic acid suppresses the development of pancreatic cancer by inhibiting pathways driving lipogenesis. Oncotarget 6:20993–21003. https://doi.org/10.18632/oncotarget.3663
Mackay IM, Arden KE (2016) Mayaro virus: a forest virus primed for a trip to the city? Microbes Infect 18:724–734
Malet H, Coutard B, Jamal S et al (2009) The crystal structures of chikungunya and Venezuelan equine encephalitis virus nsP3 Macro domains define a conserved adenosine binding pocket. J Virol 83:6534–6545. https://doi.org/10.1128/jvi.00189-09
Mao S, Ren J, Xu Y et al (2022) Studies in the antiviral molecular mechanisms of 25-hydroxycholesterol: disturbing cholesterol homeostasis and post-translational modification of proteins. Eur J Pharmacol 926:175033. https://doi.org/10.1016/j.ejphar.2022.175033
Maugeri A, Lombardo GE, Cirmi S et al (2022) Pharmacology and toxicology of tannins. Arch Toxicol 96:1257–1277. https://doi.org/10.1007/s00204-022-03250-0
McPherson RL, Abraham R, Sreekumar E et al (2017) ADP-ribosylhydrolase activity of Chikungunya virus macrodomain is critical for virus replication and virulence. Proc Natl Acad Sci USA 114:1666–1671. https://doi.org/10.1073/pnas.1621485114
Melekis E, Tsika AC, Lichière J et al (2015) NMR study of non-structural proteins—part I: 1H, 13C, 15N backbone and side-chain resonance assignment of macro domain from Mayaro virus (MAYV). Biomol NMR Assign 9:191–195. https://doi.org/10.1007/s12104-014-9572-0
Neris RLS, Figueiredo CM, Higa LM et al (2018) Co-protoporphyrin IX and Sn-protoporphyrin IX inactivate Zika, Chikungunya and other arboviruses by targeting the viral envelope. Sci Rep 8:1–13. https://doi.org/10.1038/s41598-018-27855-7
Nguyen PTV, Yu H, Keller PA (2014) Discovery of in silico hits targeting the nsP3 macro domain of chikungunya virus. J Mol Model. https://doi.org/10.1007/s00894-014-2216-6
Ortholand JY, Ganesan A (2004) Natural products and combinatorial chemistry: back to the future. Curr Opin Chem Biol 8:271–280
Painter GR, Bowen RA, Bluemling GR et al (2019) The prophylactic and therapeutic activity of a broadly active ribonucleoside analog in a murine model of intranasal Venezuelan equine encephalitis virus infection. Antiviral Res. https://doi.org/10.1016/j.antiviral.2019.104597
Pinheiro LCS (2012) Searching for new antileishmanial lead drug candidates: synthesis, biological and theoretical evaluations of promising thieno[2,3-b] pyridine derivatives. J Microbiol Antimicrob. https://doi.org/10.5897/JMA11.109
Pizzolitto RP, Herrera JM, Zaio YP et al (2015) Bioactivities of ketones terpenes: antifungal effect on F. verticillioides and repellents to control insect fungal vector, S. Zeamais. Microorganisms 3:851–865. https://doi.org/10.3390/MICROORGANISMS3040851
Pombal S, Hernández Y, Diez D et al (2017) Antioxidant activity of carvone and derivatives against superoxide ion. Nat Prod Commun 12:653–655. https://doi.org/10.1177/1934578X1701200502
Powell LA, Miller A, Fox JM et al (2020) Human mAbs broadly protect against arthritogenic alphaviruses by recognizing conserved elements of the Mxra8 receptor-binding site. Cell Host Microbe 28:699-711.e7. https://doi.org/10.1016/j.chom.2020.07.008
Qian Y, Peng Y, Shang E et al (2017) Metabolic profiling of the hepatotoxicity and nephrotoxicity of Ginkgolic acids in rats using ultra-performance liquid chromatography-high-definition mass spectrometry. Chem Biol Interact 273:11–17. https://doi.org/10.1016/j.cbi.2017.05.020
Qiao L, Zheng J, Jin X et al (2017) Ginkgolic acid inhibits the invasiveness of colon cancer cells through AMPK activation. Oncol Lett 14:5831–5838. https://doi.org/10.3892/ol.2017.6967
Raulin J (2002) Human immunodeficiency virus and host cell lipids. Interesting pathways in research for a new HIV therapy. Prog Lipid Res 41:27–65. https://doi.org/10.1016/S0163-7827(01)00019-4
Ribeiro-Filho HV, Coimbra LD, Cassago A et al (2021) Cryo-EM structure of the mature and infective Mayaro virus at 4.4 Å resolution reveals features of arthritogenic alphaviruses. Nat Commun 12:3038. https://doi.org/10.1038/s41467-021-23400-9
Rolim Bernardino AM, da Silva Pinheiro LC, Rodrigues CR et al (2006) Design, synthesis, SAR, and biological evaluation of new 4-(phenylamino)thieno[2,3-b]pyridine derivatives. Bioorg Med Chem 14:5765–5770. https://doi.org/10.1016/j.bmc.2006.03.013
Rubach JK, Wasik BR, Rupp JC et al (2009) Characterization of purified Sindbis virus nsP4 RNA-dependent RNA polymerase activity in vitro. Virology 384:201–208. https://doi.org/10.1016/j.virol.2008.10.030
Rupp JC, Sokoloski KJ, Gebhart NN, Hardy RW (2015) Alphavirus RNA synthesis and non-structural protein functions. J Gen Virol 96:2483–2500. https://doi.org/10.1099/jgv.0.000249
Salgado-Benvindo C, Thaler M, Tas A et al (2020) Suramin inhibits SARS-CoV-2 infection in cell culture by interfering with early steps of the replication cycle. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00900-20
Salles TS, Meneses MDF, Caldas LA et al (2021) Virucidal and antiviral activities of pomegranate (Punica granatum) extract against the mosquito-borne Mayaro virus. Parasit Vectors 14:443. https://doi.org/10.1186/s13071-021-04955-4
Salters-Pedneault K (2014) Definition of Mechanism of Action. In: About Health. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/mechanism-of-action
Santos IA, dos Pereira AKS, Guevara-Vega M et al (2022) Repurposing potential of rimantadine hydrochloride and development of a promising platinum(II)-rimantadine metallodrug for the treatment of Chikungunya virus infection. Acta Trop 227:106300. https://doi.org/10.1016/j.actatropica.2021.106300
Shimizu JF, Martins DOS, McPhillie MJ et al (2020) Is the ADP ribose site of the Chikungunya virus NSP3 Macro domain a target for antiviral approaches? Acta Trop 207:105490. https://doi.org/10.1016/j.actatropica.2020.105490
Shin G, Yost SA, Miller MT et al (2012) Structural and functional insights into alphavirus polyprotein processing and pathogenesis. Proc Natl Acad Sci USA 109:16534–16539. https://doi.org/10.1073/pnas.1210418109
Sivropoulou A, Nikolaou C, Papanikolaou E et al (1997) Antimicrobial, cytotoxic, and antiviral activities of Salvia fructicosa essential oil. J Agric Food Chem 45:3197–3201. https://doi.org/10.1021/JF970031M
Snyder JE, Kulcsar KA, Schultz KLW et al (2013) Functional characterization of the alphavirus TF protein. J Virol 87:8511–8523. https://doi.org/10.1128/JVI.00449-13
Sousa IP, Carvalho CAM, Gomes AMO (2020) Current understanding of the role of cholesterol in the life cycle of alphaviruses. Viruses 13:35. https://doi.org/10.3390/v13010035
Spindola KCW, Simas NK, Salles TS et al (2014) Anti-Mayaro virus activity of Cassia australis extracts (Fabaceae, Leguminosae). Parasit Vectors 7:537. https://doi.org/10.1186/s13071-014-0537-z
Subudhi B, Chattopadhyay S, Mishra P, Kumar A (2018) Current strategies for inhibition of chikungunya infection. Viruses 10:235. https://doi.org/10.3390/v10050235
Sun X, Whittaker GR (2003) Role for influenza virus envelope cholesterol in virus entry and infection. J Virol 77:12543–12551. https://doi.org/10.1128/JVI.77.23.12543-12551.2003
Sun J, Wu D (2019) Mayaro virus, a regional or global threat? Travel Med Infect Dis 32:101462
Sun W, Tanaka TQ, Magle CT et al (2015) Chemical signatures and new drug targets for gametocytocidal drug development. Sci Rep 4:3743. https://doi.org/10.1038/srep03743
Tan YB, Lello LS, Liu X et al (2022) Crystal structures of alphavirus nonstructural protein 4 (nsP4) reveal an intrinsically dynamic RNA-dependent RNA polymerase fold. Nucleic Acids Res 50:1000–1016. https://doi.org/10.1093/nar/gkab1302
Taylor SF, Patel PR, Herold TJS (2005) Recurrent arthralgias in a patient with previous mayaro fever infection. South Med J 98:484–485. https://doi.org/10.1097/01.SMJ.0000145879.14102.F4
Terlizzi ME, Occhipinti A, Luganini A et al (2016) Inhibition of herpes simplex type 1 and type 2 infections by Oximacro®, a cranberry extract with a high content of A-type proanthocyanidins (PACs-A). Antiviral Res 132:154–164. https://doi.org/10.1016/j.antiviral.2016.06.006
Theilacker C, Held J, Allering L et al (2013) Prolonged polyarthralgia in a German traveller with Mayaro virus infection without inflammatory correlates. BMC Infect Dis 13:2011–2014. https://doi.org/10.1186/1471-2334-13-369
Tian L, Qiang T, Liang C et al (2021) RNA-dependent RNA polymerase (RdRp) inhibitors: The current landscape and repurposing for the COVID-19 pandemic. Eur J Med Chem 213:113201
Tsika AC, Melekis E, Tsatsouli SA et al (2019) Deciphering the nucleotide and RNA binding selectivity of the Mayaro virus macro domain. J Mol Biol 431:2283–2297. https://doi.org/10.1016/j.jmb.2019.04.013
Tsukuda S, Watashi K, Hojima T et al (2017) A new class of hepatitis B and D virus entry inhibitors, proanthocyanidin and its analogs, that directly act on the viral large surface proteins. Hepatology 65:1104–1116. https://doi.org/10.1002/hep.28952
Uversky VN (2013) Intrinsic disorder-based protein interactions and their modulators. Curr Pharm Des 19:4191–4213. https://doi.org/10.2174/1381612811319230005
Valdés-Torres P, Campos D, Bhakta M et al (2022) Honokiol and alpha-Mangostin inhibit Mayaro virus replication through different mechanisms. Molecules 27:7362. https://doi.org/10.3390/molecules27217362
Wagoner J, Negash A, Kane OJ et al (2010) Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology 51:1912–1921. https://doi.org/10.1002/hep.23587
West J, Hernandez R, Ferreira D, Brown DT (2006) Mutations in the endodomain of Sindbis virus glycoprotein E2 define sequences critical for virus assembly. J Virol 80:4458–4468. https://doi.org/10.1128/JVI.80.9.4458-4468.2006
Young LS, Ruschel S, Yanchuk S, Pereira T (2019) Consequences of delays and imperfect implementation of isolation in epidemic control. Sci Rep 9:1–9. https://doi.org/10.1038/s41598-019-39714-0
Yousefi H, Mashouri L, Okpechi SC et al (2021) Repurposing existing drugs for the treatment of COVID-19/SARS-CoV-2 infection: a review describing drug mechanisms of action. Biochem Pharmacol 183:114269
Zhang R, Kim AS, Fox JM et al (2018) Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 557:570–574. https://doi.org/10.1038/s41586-018-0121-3
Zhang Z, He G, Filipowicz NA et al (2019) Host lipids in positive-strand RNA virus genome replication. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00286
Acknowledgements
ACGJ is grateful to FAPEMIG (Minas Gerais Research Foundation APQ-01487-22 and APQ-04686-22), and to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)-Brasil-Prevention and Combat of Outbreaks, Endemics, Epidemics and Pandemics-Finance Code #88881.506794/2020-01. MP thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the scholarship # 23117.018764/2020-00. MSM thanks CNPq and FAPEMIG for the scholarships # 129422/2021-5 and # 12152. IAS thanks to CNPq for the scholarship # 142495/2020-4 and CAPES.Print scholarship # 88887.700246/2022-00. VRG and DOSM thanks to CAPES for the scholarships # 88887.505971/2020–00 and # 88887.505880/2020-00, respectively.
Author information
Authors and Affiliations
Contributions
Literature research: MP, MdSM, IAS, VRG, and DOSM. Drafting of the manuscript: MP, MdSM, and DOSM. Figures: VRG. Design and Critical revision: IAS, RBR, and ACGJ. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential direct or indirect conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paschoalino, M., Marinho, M.d., Santos, I.A. et al. An update on the development of antiviral against Mayaro virus: from molecules to potential viral targets. Arch Microbiol 205, 106 (2023). https://doi.org/10.1007/s00203-023-03441-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03441-y