Abstract
Bacteria have potential to tolerate and reduce metals. This study evaluated the potential of selected bacterial strains in tolerating and reducing chromium (Cr). Six bacterial strains (Rhizobium miluonense LCC01, LCC04, LCC05, and LCC69; Rhizobium pusense LCC43; and Agrobacterium deltaense LCC50) showed tolerance to Cr(VI) (16 and 32 μg mL−1), reduction potential of Cr(VI) (from 50 to 80%), and efficiency in producing exopolysaccharides. Rhizobium pusense LCC43 exhibited the highest tolerance (128 μg mL−1), reduction potential of Cr(VI) (from 80 to 100%), and efficiency in producing exopolysaccharides. These results suggested that this strain may have the potential to be used in the bioremediation of soils contaminated with Cr(VI).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The production of solid wastes increases annually, and it is estimated that approximately 4.0 billion tons of solid waste would be released by 2050 (Kaza et al. 2018). Therefore, it is necessary to develop alternatives to recycle and reuse these wastes for decreasing their amounts in the environment, particularly in soils. Furthermore, these wastes can have high concentrations of metals that could promote soil contamination. For example, tannery wastes contain a high concentration of chromium (Cr) that can accumulate in the soil (Araujo et al. 2020) and promote negative effects on soil microbial biomass and diversity (Miranda et al. 2018; Araujo et al. 2020). However, this accumulation of Cr drives the selection of specific microbes that can tolerate its presence (Rocha et al. 2019).
The potential microbial tolerance to Cr is due to microbes having mechanisms that can overcome Cr toxicity, such as expression of genes involved in the reduction of Cr(VI), particularly the chromate reductase gene (ChR) (Cheung and Gu 2007), and production and release of exopolysaccharides. Exopolysaccharides (EPSs) are compounds that consist of proteins and polysaccharides and are released by microbes in response to abiotic stressors, such as metal toxicity (Sheng et al. 2010). The action of EPSs is mediated by sorption of metals, which decrease the availability and toxicity of metals; therefore, it is interesting for bioremediation strategies in metal-contaminated sites (Gupta and Diwan 2017). The ChR gene is efficient in reducing Cr(VI) to Cr(III), a less mobile and less toxic version of Cr, and thus reduces Cr toxicity (Cheung and Gu 2007). Some well-known bacteria carrying the ChR gene and having the ability to reduce Cr(VI) are Bacillus sp. (Wani et al. 2018) and Rhizobium sp. (Karthik et al. 2017).
Recently, a preliminary study examining soils that were highly contaminated by Cr found bacterial strains with potential biochemical abilities (Rocha et al. 2019). However, it did not determine if those bacterial strains could tolerate and reduce Cr(VI). Thus, the present study aimed to identify and evaluate six potential bacterial strains, suggested by Rocha et al. 2019, for their potential in tolerating and reducing Cr(VI).
Materials and methods
Bacterial strains
Six bacterial strains (LCC01, LCC04, LCC05, LCC43, LCC50, and LCC69) with potential biochemical ability, measured by enzymes catalase, gelatinase, urease, lipase, phosphate solubilization, and cellulase, by Rocha et al. (2019) were used in our study. These strains were grown in tryptone Yeast (TY) broth (pH 7.2) under shaking incubation conditions (86×g) for 24 h at 28 °C. The bacterial cells were collected at log phase growth by centrifugation (Centrifuge Eppendorf™ 5418R with rotor FA-45-18-11) at 10,000 rpm for 10 min.
16S rRNA and ChR genes sequencing
The genomic DNA of each strain was extracted and the 16S rRNA and ChR gene were amplified using primers described by Weisburg et al. 1991 and Patra et al. 2010, respectively. All the sequences were deposited to NCBI. Phylogenetic analyses based on 16S rRNA and the ChR gene were performed through MEGA-X (Felsenstein, 1988) based on the maximum likelihood statistical method (Saitou and Nei 1987) and the branching support of 1000 bootstrap (Felsenstein 1988).
Minimum inhibitory concentration (MIC)
First, in each evaluation, Cr(VI) was used in the form of K2Cr2O7. The method of plate dilution proposed by Alam and Malik (2008) was applied to verify both bacterial growth and Cr(VI) MIC. For this study, each strain was exposed to Cr(VI) concentrations varying from 16 to 1024 μg mL−1. The lowest concentration of Cr(VI) without bacterial growth was considered the MIC (μg mL−1).
Reduction potential of Cr(VI)
The reduction potential of Cr(VI) was evaluated according to the method proposed by Baldiris et al. (2018). Here, each strain was exposed to varying concentrations of Cr(VI) (0, 25, 50, and 100 μg mL−1). The control was TY broth without bacterial inoculation. The remaining Cr(VI) concentration was determined by the 1,5-diphenylcarbazide method (Pattanapipitpaisal et al. 2001), and the results were read using a spectrophotometer (OD 540 nm). The reduction of Cr(VI) was calculated as follows: Remaining Cr (VI) = (Crs/Crc) × 100%, where Crs and Crc are the Cr(VI) found in supernatants from each strain and control, respectively.
Production of biofilm and exopolysaccharides (EPSs)
Each strain was subjected to varying Cr(VI) concentrations (0, 25, 50, and 100 μg mL−1). Crystal violet staining was used to evaluate biofilm formation (Baldiris et al. 2018). The production of biofilm was determined by spectrophotometer at OD 570 nm.
The production of EPSs was determined according to the method proposed by Castellane et al. (2014). Each strain was subjected to Cr(VI) concentrations of 0 and 25 μg mL−1. After biofilm growth, EPSs produced were separated, washed, and determined gravimetrically.
Statistical analysis
All experiments were performed in triplicate, and the results are presented as mean values. The values of cell biomass, total EPSs, and efficiency were subjected to analysis of variance, and the means were compared by Tukey’s test at 5% probability.
Results and discussion
16S rRNA and ChR genes sequencing
In this study, six potential bacterial strains (Rocha et al. 2019) were evaluated to verify if they could grow efficiently in the presence of Cr(VI), produce EPSs, and reduce Cr(VI). The phylogenetic classification (16S rRNA) evidenced three bacterial species, Rhizobium miluonense (LCC01, LCC04, LCC05, and LCC69), Rhizobium pusense (LCC43), and Agrobacterium deltaense (LCC50) (Table S1; Figure S1). Notably, ChR amplification demonstrated the presence of the gene only in R. pusense (LCC43) (Figs. S2, S3).
The sequencing of the 16S rRNA gene identified Rhizobium (R. miluonense and R. pusense) and Agrobacterium (A. deltaense) as bacterial species in this study. The identification of Rhizobium and Agrobacterium suggested that both the bacterial species have the potential to tolerate Cr(VI) and validates previous studies that reported Rhizobium and Agrobacterium as the main genera found in soils contaminated with Cr(VI) (Raaman et al. 2012; Chaudhary et al. 2021; Gutiérrez et al. 2010).
MIC
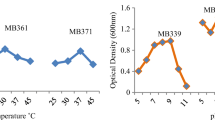
All strains grew under Cr(VI) concentrations of 0 and 25 μg mL−1 (Fig. 1). However, R. miluonense (LCC01, LCC04, LCC05, and LCC69) and A. deltaense (LCC50) did not grow under Cr(VI) concentrations of 50, 100, and 200 μg mL−1, while R. pusense LCC43 grew under a Cr(VI) concentration of 100 μg mL−1. R. miluonense (LCC69) and A. deltaense (LCC50) showed tolerance to Cr(VI) concentrations of 16 and 32 μg mL−1, respectively, while R. miluonense (LCC01, LCC04, and LCC05) showed tolerance to a Cr(VI) concentration of 64 μg mL−1 (Table 1). R. pusense (LCC43) showed the highest tolerance to Cr(VI), showing growth while exposed to a Cr(VI) concentration of 128 μg mL−1.
All bacterial strains showed tolerance to low concentrations of Cr(VI) (25 μg mL−1). However, R. pusense LCC43 tolerated a higher concentration of Cr(VI) (100 μg mL−1). These results suggested that these bacterial strains have mechanisms to tolerate Cr, such as the removal of the metal from the solution (Karthik et al. 2017) and extracellular reduction of Cr(VI) (Chovanec et al. 2012). In addition, the higher tolerance to Cr(VI) found in R. pusense LCC43 suggested that this strain could possess important microbial traits to tolerate Cr(VI), such as distinct proteins, functional genes involved in the cell wall formation, and metabolism of aromatic compounds (Chaudhary et al. 2021).
Reduction potential of Cr(VI)
All strains had potential to reduce Cr(VI). Specifically, R. miluonense (LCC01, LCC04, LCC05, and LCC69) and A. deltaense (LCC50) reduced 80% of Cr(VI) under a Cr(VI) concentration of 25 μg mL−1, and reduced 50% of Cr(VI) under a Cr(VI) concentration of 50 μg mL−1 (Fig. 2). These strains did not reduce Cr(VI) under a Cr(VI) concentration of 100 μg mL−1. However, considering absolute values per ug of reduced Cr(VI), the reducing potential of the strains LCC01, LCC04, and LCC05 remained unchanged or narrowly increased in the presence of 25 and 50 μg Cr(VI), since 80% of 25 μg nearly corresponds to 20 μg and 50% of 50 μg correspond to 25 μg Cr(VI). In addition, the strain LCC50 showed an increased ability to reduce Cr(VI), while LCC69 showed a decreased ability. However, R. pusense (LCC43) showed high potential in reducing Cr under Cr(VI) concentrations of 25, 50, and 100 μg mL−1, reaching a reduction rate higher than 90% under Cr(VI) concentrations of 25 and 50 μg mL−1.
The results showed that each strain has a mechanism to tolerate and reduce Cr(VI); thus, its ability to reduce Cr could be the basis for bacterial strains selection for bioremediation purposes in soils contaminated with Cr(VI) (Karim et al. 2020). We highlighted R. pusense LCC43 for its ability to promote higher reduction of Cr(VI) compared to the other bacterial strains. The genus Rhizobium seems to have high potential in reducing Cr(VI) as reported by Karthik et al. (2017) who reported Rhizobium sp. reducing Cr(VI) by approximately 80 and 100% under concentrations of 50 and 150 μg mL−1 Cr, respectively. Recently, a strain of R. pusense isolated from water containing Cr showed the ability to reduce Cr(VI), thereby showing promising Cr(VI) detoxification applications (Sahoo et al. 2022).
Production of biofilms and EPSs
We observed variations in the production of biofilms (Fig. 3), where both R. miluonense (LCC01) and R. pusense (LCC43) produced more biofilms than R. miluonense (LCC04, LCC05, and LCC69) and A. deltaense (LCC69) under Cr(VI) concentrations of 25 and 50 μg mL−1. The highest values of biofilm biomass and EPSs were from R. miluonense (LCC69) (Fig. 4A). However, R. pusense (LCC43) showed the highest production and efficiency in producing EPSs in the presence of Cr(VI) (Fig. 4B, C).
Production of microbial biomass, exopolysaccharides and efficiency by R. miluonense LCC01, R. miluonense LCC04, R. miluonense LCC05, R. pusense LCC43, A. deltaense LCC50 and R. miluonense LCC69 with and without Cr(VI). Different letters indicate a significant difference (p < 0.05) between treatments. The bars represent the standard deviation
All strains showed efficiency in producing biofilms and EPSs. The ability to produce biofilms is important since they protect bacteria against metals (Haque et al. 2021) by assisting bacteria in metal sorption (Pan et al. 2014). Similarly, EPSs are efficient in protecting bacterial cells and removing metals from solutions by flocculation (Aljuboori et al. 2013). Ayangbenro et al. (2019) reported the presence of EPSs as an important factor to reduce Cr. In our study, R. pusense LCC43 showed a higher ability to produce EPSs in the presence of Cr(VI) compared to the other strains, which was in accordance with previous studies reporting Rhizobium tropici and Ochrobactrum intermedium producing EPSs in response to Cr(VI) (Leonel et al. 2019). Thus, R. pusense LCC43 is highly efficient in producing EPSs. Consequently, this is an important finding for the development of approaches targeting bioremediation of soils contaminated with Cr. Finally, R. pusense LCC43 was found to be the unique strain amplifying ChR gene fragment that can confer higher tolerance and has mechanisms to reduce Cr(VI) (Soni et al. 2013; Zhu et al. 2019).
Conclusions
This study demonstrated that bacterial strains isolated from soil contaminated with Cr presented different abilities to tolerate and reduce Cr(VI). Rhizobium pusense LCC43 had higher potential in tolerating and reducing Cr(VI), efficiently producing EPSs, and carrying the ChR gene. These findings suggested that Rhizobium pusense LCC43 has potential to be used in the bioremediation of soils contaminated with Cr(VI).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alam MZ, Malik A (2008) Chromate resistance, transport and bioreduction by Exiguobacterium sp. ZM-2 isolated from agricultural soil irrigated with tannery effluent. J Basic Microbiol 48:416–420. https://doi.org/10.1002/jobm.200800046
Aljuboori AHR, Idris A, Abdullah N, Mohamad R (2013) Production and characterization of a bioflocculant produced by Aspergillus flavus. Bioresour Technol 127:489–493. https://doi.org/10.1016/j.biortech.2012.09.016
Araujo ASF, Melo WJ, Araujo FF, Van den Brink PJ (2020) Long-term effect of composted tannery sludge on soil chemical and biological parameters. Environ Sci Pollut Res Int 27:41885–41892. https://doi.org/10.1007/s11356-020-10173-9
Ayangbenro AS, Babalola OO, Aremu OS (2019) Bioflocculant production and heavy metal sorption by metal resistant bacterial isolates from gold mining soil. Chemosphere 231:113–120. https://doi.org/10.1016/j.chemosphere.2019.05.092
Baldiris R, Acosta-Tapia N, Montes A, Hernández J, Vivas-Reyes R (2018) Reduction of hexavalent chromium and detection of chromate reductase (ChrR) in Stenotrophomonas maltophilia. Molecules. https://doi.org/10.3390/molecules23020406
Castellane TCL, Lemos MVF, Lemos EGM (2014) Evaluation of the biotechnological potential of Rhizobium tropici strains for exopolysaccharide production. Carbohyd Polym 111:191–197. https://doi.org/10.1016/j.carbpol.2014.04.066
Chaudhary T, Gera R, Shukla P (2021) Deciphering the potential of Rhizobium pusense MB-17a, a plant growth-promoting root endophyte, and functional annotation of the genes involved in the metabolic pathway. Front Bioeng Biotechnol 8:617034. https://doi.org/10.3389/fbioe.2020.617034
Cheung KH, Gu J-D (2007) Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int Biodeterior Biodegradation 59:8–15. https://doi.org/10.1016/j.ibiod.2006.05.002
Chovanec P, Sparacino-Watkins C, Zhang N, Basu P, Stolz JF (2012) Microbial reduction of chromate in the presence of nitrate by three nitrate respiring organisms. Front Microbiol. 3:416. https://doi.org/10.3389/fmicb.2012.00416
Felsenstein J (1988) Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet 22:521–565
Gupta P, Diwan B (2017) Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep (Amst). 13:58–71. https://doi.org/10.1016/j.btre.2016.12.006
Gutiérrez AM, Cabriales JJP, Vega MM (2010) Isolation and characterization of hexavalent chromium-reducing rhizospheric bacteria from a wetland. Int J Phytoremediation. 12:317–334. https://doi.org/10.1080/15226510902968118
Haque MM, Mosharaf MK, Haque MA, Tanvir MZH, Alam MK (2021) Biofilm formation, production of matrix compounds and biosorption of copper, nickel and lead by different bacterial strains. Front Microbiol. 12:615113. https://doi.org/10.3389/fmicb.2021.615113
Karim ME, Sharmin SA, Moniruzzaman M, Fardous Z, Das KC, Banik S, Salimullah M (2020) Biotransformation of chromium (VI) by Bacillus sp. isolated from chromate contaminated landfill site. Chemistry and Ecology, 36(10):922–937. https://doi.org/10.1080/02757540.2020.1799992
Karthik C, Oves M, Sathya K, Sri Ramkumar V, Arulselvi PI (2017) Isolation and characterization of multi-potential Rhizobium strain ND2 and its plant growth-promoting activities under Cr(VI) stress. Arch Acker Pflanzenbau Bodenkd. 63:1058–1069. https://doi.org/10.1080/03650340.2016.1261116
Kaza S, Congyuan YL (2018) What A waste 2.0: A global snapshot of solid waste management to 2050. World Bank Publications, Washington DC
Leonel TF, Moretto C, Castellane TCL et al (2019) The Influence of Cooper and Chromium Ions on the Production of Exopolysaccharide and Polyhydroxybutyrate by Rhizobium tropici LBMP-C01. J Polym Environ 27:445–455. https://doi.org/10.1007/s10924-018-1359-4
Miranda ARL, Antunes JEL, Araujo FF, Melo VMM, Bezerra WM, Van den Brink PJ, Araujo ASF (2018) Less abundant bacterial groups are more affected than the most abundant groups in composted tannery sludge-treated soil. Sci Rep. 8:1–9. https://doi.org/10.1038/s41598-018-30292-1
Pan X, Liu Z, Chen Z, Cheng Y, Pan D, Shao J, Lin Z, Guan X (2014) Investigation of Cr(VI) reduction and Cr(III) immobilization mechanism by planktonic cells and biofilms of Bacillus subtilis ATCC-6633. Water Res. 55:21–29. https://doi.org/10.1016/j.watres.2014.01.066
Patra RC, Malik S, Beer M, Megharaj M, Naidu R (2010) Molecular characterization of chromium (VI) reducing potential in gram positive bacteria isolated from contaminated sites. Soil Biol Biochem. 42:1857–1863. https://doi.org/10.1016/j.soilbio.2010.07.005
Pattanapipitpaisal P, Brown NL, Macaskie LE (2001) Chromate reduction and 16S rRNA identification of bacteria isolated from a Cr(VI)-contaminated site. Appl Microbiol Biotechnol. 57:257–261. https://doi.org/10.1007/s002530100758
Raaman N, Mahendran B, Jaganathan C, Sukumar S, Chandrasekaran V (2012) Removal of chromium using Rhizobium leguminosarum. World J Microbiol Biotechnol. 28:627–636. https://doi.org/10.1007/s11274-011-0856-6
Rocha SMB, Antunes JEL, Silva AVCR, Oliveira LMS, Aquino JPA, Melo WJ, Figueiredo MVB, Araujo ASF (2019) Nodulation, nitrogen uptake and growth of lima bean in a composted tannery sludge-treated soil. Cienc Rural. https://doi.org/10.1590/0103-8478cr20190301
Sahoo H, Kisku K, Varadwaj KSK et al (2022) Mechanism of Cr(VI) reduction by an indigenous Rhizobium pusense CR02 isolated from chromite mining quarry water (CMQW) at Sukinda Valley. Environ Sci Pollut Res, India. https://doi.org/10.1007/s11356-022-22264-w
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Sheng GP, Yu HQ, Li XY (2010) Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnol Adv. 28:882–894. https://doi.org/10.1016/j.biotechadv.2010.08.001
Soni SK, Singh R, Awasthi A, Singh M, Kalra A (2013) In vitro Cr(VI) reduction by cell-free extracts of chromate-reducing bacteria isolated from tannery effluent irrigated soil. Environ Sci Pollut Res Int. 20:1661–1674. https://doi.org/10.1007/s11356-012-1178-4
Wani PA, Wahid S, Singh R, Kehinde AM (2018) Antioxidant and chromium reductase assisted chromium (VI) reduction and Cr (III) immobilization by the rhizospheric Bacillus helps in the remediation of Cr (VI) and growth promotion of soybean crop. Rhizosphere. 6:23–30. https://doi.org/10.1016/j.rhisph.2018.01.004
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 173:697–703. https://doi.org/10.1128/jb.173.2.697-703.1991
Zhu Y, Yan J, Xia L, Zhang X, Luo L (2019) Mechanisms of Cr(VI) reduction by Bacillus sp. CRB-1, a novel Cr(VI)-reducing bacterium isolated from tannery activated sludge. Ecotoxicol Environ Saf. 186:109792. https://doi.org/10.1016/j.ecoenv.2019.109792
Funding
This work was supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq Grant 305069/2018-1) and Fundacao de Amparo a Pesquisa do Estado do Piaui (FAPEPI). Sandra M. B. Rocha has received scholarship from FAPEPI. Erika V. Medeiros and Ademir S.F. Araujo are fellow of CNPq.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SMBR, MRdaA, MKLC, TCdaSS, RMC, JELA, and LMdeSO, The first draft of the manuscript was written by FdeAN, EVdeM, APdeAP, and ASFA: and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rocha, S.M.B., do Amorim, M.R., Costa, M.K.L. et al. Tolerance and reduction of chromium by bacterial strains. Arch Microbiol 204, 730 (2022). https://doi.org/10.1007/s00203-022-03329-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03329-3