Abstract
2,4,6-trinitrotoluene (TNT), a nitro-aromatic explosive commonly used for defense and several non-violent applications is contributing to serious environmental pollution problems including human health. The current study investigated the remediation potential of a native soil isolate, i.e., Indiicoccus explosivorum (strain S5-TSA-19) isolated from collected samples of an explosive manufacturing site, against TNT. The survivability of I. explosivorum against explosives is indirectly justified through its isolation; thus, it is being chosen for further study. At a TNT concentration of 120 mg/L within an optimized environment (i.e., at 30 °C and 120 rpm), the isolate was continually incubated for 30 days in a minimal salt medium (MSM). The proliferation of the isolate and the concentration of TNT, nitrate, nitrite, and ammonium ion were evaluated at a particular time during the experiment. Within 168 h (i.e., 7 days) of incubation, I. explosivorum co-metabolically degraded 100% TNT. The biodegradation procedure succeeded the first-order kinetics mechanism. Formations of additional metabolites like 2,4-dinitrotoluene (DNT), 2,4-diamino-6-nitrotoluene (2-DANT), and 2-amino-4,6-dinitrotoluene (2-ADNT), were also witnessed. TNT seems to be non-toxic for the isolate, as it reproduced admirably in TNT presence. To date, it is the first report of Indiicoccus explosivorum, efficiently bio-remediating TNT, i.e., a nitro-aromatic compound via different degradation pathways, leading to the production of simpler as well as less harmful end products. Further, at the field-scale application, Indiicoccus explosivorum may be explored for the bioremediation of TNT (i.e., a nitro-aromatic compound)-contaminated effluents.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Numerous applications of military and supplementary civil areas, such as engineering, construction, extracting, mining, and rocket propellants, explosives, i.e., organic energetic compounds (nitro-amines and nitro-aromatic), are being used (Chatterjee et al. 2017; Nagar et al. 2018, 2021). 2,4,6-trinitrotoluene (TNT), is a reported universally utilized secondary explosive (Ayoub et al. 2010; Kao et al. 2016). As per Alothman et al. 2020, TNT, being a teratogenic, cytotoxic nitro-aromatic compound, may affect cellular mutations in humans, animals, plants, and microorganisms (Juhasz and Naidu 2007; Fahrenfeld et al. 2013; Kao et al. 2016). Throughout the two World Wars, because of the immense use of TNT, the ecosystem of soil and water has turned out to be utterly polluted (Lewis et al. 2004). The concentration of 10,000 mg per kg and 100 mg per liter of TNT was spotted in nitro-aromatic contaminated soil and water respectively. The lethal and mutagenic effect of TNT is being exhibited on eukaryotes and prokaryotes (Khan et al. 2013; Kao et al. 2016). Subsequently, TNT’s detrimental impacts on soil and groundwater are critical issue concerning environmental protection which is required to be controlled or minimalize as per the demand of the hour.
Plentiful reports have been recorded with numerous remediating methods for TNT polluted spots (Ayoub et al. 2010; Gümüscü and Tekinay 2013; Kao et al. 2016). Conventional treatment involves physical and chemical approaches, like incineration, adsorption, and advanced oxidation procedures, which are expensive and result in lethal by-products (Rodgers and Bunce 2001; Gümüscü and Tekinay 2013; Kao et al. 2016). On the other hand, biological-based remediation approaches are promising both ways; i.e., economically and organically (Chien et al. 2014; Kao et al. 2016). Bioremediation explores the potential characteristics of microorganisms to profitably bio-transform or biodegrades contaminants (Fahrenfeld et al. 2013; Kao et al. 2016). Bioremediation can be studied in situ and is frequently a cheap, less expertise practice which is up to the satisfaction of the general public (Kao et al. 2016). Microbial biotransformation can be approached for TNT biodegradation (Chien et al. 2014; Kao et al. 2016). Hence, TNT bioremediation through bacteriological degradation is a very optimistic practice.
Quite a lot of reviews concise widespread exploration of TNT biodegradation by fungi and bacteria (Van Aken and Agathos 2001; Heiss and Knackmuss 2002; Zhao et al. 2004; Serrano-González et al. 2018. In TNT, the existence of three electron-withdrawing nitro groups generates steric constraints accompanying elevation in electron shortage to the aromatic ring. Thus, the molecule resists oxidative degradation by microbes, nitro groups of TNT are used as a source of nitrogen by microbes (Claus et al. 2007). Anaerobic and aerobic degradation of TNT through a diversity of microorganisms have been testified, along with some anticipated metallic pathways (Esteve-Nunez et al. 2001; Kao et al. 2016). These comprised rigid anaerobic bacteria (such as Clostridium and Desulfovibrio), aerobic bacteria (such as Achromobacter, Bacillus, Citrobacter, and Pseudomonas), and fungi (Phanerochaete) (Duque et al. 1993; Montpas et al. 1997; Kalafut et al. 1998; Oh and Kim 1998; Esteve-Nunez et al. 2001; Lee et al. 2002; Kao et al. 2016).
The bacterium under study is a novel species and has not been used for TNT treatment. However, in the recent past, the authors have treated nitramine (octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine also known as HMX) using the same species but with the nomenclature Planomicrobium flavidum (Nagar et al. 2018) as submitted with accession number LT63187.2 (https://www.ncbi.nlm.nih.gov/nuccore/lt631687.2). However, in Jan 2019, the sequence has been updated and the nomenclature changed to Indiicoccus explosivorum gen. nov., sp. (https://www.ncbi.nlm.nih.gov/nuccore/LT631687.3; Pal et al. 2019). The degradation ability of I. explosivorum against nitro-aromatic-based explosives under aerobic conditions has not yet been explored. In the present study, for the first time native soil bacteria, i.e., Indiicoccus explosivorum (strain S5-TSA-19) was analyzed to get characterized for its ability to bio-transform TNT through bioremediation technique at a laboratory scale under aerobic conditions.
Materials and methods
Experimental preparations
TNT (> 99% purity) was from a reliable manufacturing unit in India and was obtained only for the study purpose. During the current study, all analytical and gradient grade chemicals together with the standard of 2-ADNT were from Sigma-Aldrich. Extracted soil samples from an explosive manufacturing site located in India were sent to the Institute of Microbial Technology (IMTECH), Chandigarh for the isolation and characterization process through 16 s rRNA sequencing (Krishnamurthi et al. 2009; Pal et al. 2019).
Initially, tryptone soya agar (TSA) plates were used to maintain the primary cultures of Indiicoccus explosivorum. Throughout the aqueous phase study, minimal salt medium (MSM) for microbial proliferation and associated experimentations was favored (Cook and Huetter 1981; Thompson et al. 2005). Essential media enhancement was done through 40 mM potassium phosphate buffer (pH 7.2), MgSO4.7H2O (61.61 mg), NH4Cl (50 mg), and trace elements. Trace element mixture supplementation was done with a composition (per liter) as reported earlier by Lamba et al. 2021, with an aim to study the proliferation and degrading potential of I. explosivorum, TNT (120 mg/L) solution was prepared in MSM medium.
Characteristics and tolerance assessment of the microbial strain
Strain S5-TSA-19 (Microbial type culture collection number 12608) with GenBank accession number LT631687 was received in lyophilized form and was characterized through 16S ribosomal RNA sequence practice. The optical density (OD) of the bacterial biomass was observed at 600 nm of absorbance with a UV–Vis spectrophotometer from Perkin Elmer, Model Lambda 650S. For obtaining culture, bacteria were initially nurtured in the minimal salt medium till the third generation. For the degradation study, the cell suspension in the log phase through an OD 0.8 (≈ 108 cells/mL) was used.
The isolated strain was assessed regarding its tolerance level against TNT by growing it in minimal salt media comprising different concentrations of TNT (60, 80, 100, and 120 ppm) and also with an additional nitrogen source in 2 sets along with pH and temperature variations.
Aqueous phase experiment preparation
Under sterile conditions, the isolate I. explosivorum (in its log phase) was inoculated in MSM medium in 250-mL Erlenmeyer flasks already spiked with 120 mg/L of TNT along with 3 replications per study. 5% (v/v) of the bacterium was inoculated in TNT containing MSM. Two sets of controls, viz., MSM containing TNT but excluding bacterial strain and MSM with isolate excluding TNT were also prepared. Flasks were nurtured for 30-day duration (at 30 °C and 120 rpm) in an orbital incubator shaker. Throughout the incubation period, regularly (i.e., on each alternative day) aliquots (2 mL) were drawn off from individual experimental arrangements and were centrifuged (10,000 rpm; 10 min). Further, the supernatant solution was filtered with a 0.45-μm Teflon filter before quantification of TNT and intermediates concentrations (USEPA 2007) with a photodiode array detector of model e2695, Perkin Elmer Inc., high-performance liquid chromatography (HPLC). C18 reversed-phase column (4.6 mm × 150 mm) was employed with acetonitrile and water (at a ratio of 1:1, v/v) as mobile phase and a flow rate of 1 mL/min.
Nitrite, nitrate, ammonia, TNT, and converted compounds analysis
Further, the conversion, disappearance, and tolerance against TNT were evaluated and quantified. To get brief evidence about the disappearance and conversion of TNT along with the formation of secondary metabolites, liquid chromatography–mass spectrophotometric (LC–MS) analysis was carried out. LC–MS analyses were accomplished via Waters, Micro mass Q-TOF microsystem (Waters Alliance 2795) comprising Waters X Bridge Column C18 with negative ion (ES-). 20 µL injection volume through a flow rate of 0.8 mL/min was utilized to analyze individual TNT peaks and its degradation by-products. For solvent purposes, acetonitrile, formic acid (0.1%), and methanol were utilized. Apart from spectrophotometric analyses, strain’s tolerance toward explosive was also viewed using scanning electron microscopy (SEM) detailed earlier by Prasad et al., 2016. Au–Pd layering was done through Sputter Coater (Quorum SC7620 Mini) and detected via ZEISS EV˚18 scanning electron microscope.
Nitrate and nitrite, being important metabolites of TNT degradation, were analyzed regularly (every alternate day). The amount of nitrite ion was measured at OD 540 nm and nitrate ion was analyzed at OD 220 and 275 nm via UV–VIS Spectrophotometer (Mercimek et al. 2013; Lamba et al. 2021).
Using UV–VIS Spectrophotometer at 490 nm of absorbance, the concentration of ammonium ion was evaluated at regular intervals of time. Quantification of ammonium ion was done using Nessler’s reagent. The supernatant (0.5 mL) was incubated at 37 ̊C for 3 h, succeeding with Nessler’s reagent (0.1 mL) addition (Mackie and MacCartney 1989; Lamba et al. 2021).
Statistical analysis
Through OriginPro 8.5 software, one-way analysis of variance (ANOVA) was taken out to significantly examine the differences between sample data’s mean obtained from MSM comprising I. explosivorum with and without the explosive. At 0.05, a significant level descriptive statistical normality test of the sample data was evaluated. Shapiro–Wilk data specified that samples were drawn from a normally distributed population because the significant value was greater than 0.05. Further, at a 0.05 significance level, the means of the data were compared employing Tukey’s post hoc test and homogeneity of variance was derived with Levene’s test.
Results and discussion
A key emphasis of the current study was to explore the efficiency of a native bacterial isolate in remediating nitro-aromatic explosive i.e., TNT aerobically. The strain S5-TSA-19 is a soil bacterium named Indiicoccus explosivorum isolated from the sample collected from an explosive manufacturing site containing up to a concentration of 900–1000 mg/Kg TNT. I. explosivorum can sustain in temperature between 4 and 37 ˚C.
Tolerance level of P. flavidum
The tolerance or sustaining capacity of the isolate with the explosive was evaluated in MSM at various concentrations of TNT (60, 80, 100, 120 mg/L), temperature (20 to 35 ˚C), and pH (5 to 9). Satisfactory results were detected up to higher concentrations of TNT i.e., 120 mg/L at 30 ˚C (± 5 ˚C), and neutral pH i.e., 7. Survival of the isolate at the higher concentration was indicative of its potential for further study.
Isolate’s morphology
SEM images of S5-TSA-19 isolate (SEM images have been detailed in Online Resource 1) with and without the nitro-aromatic explosive depicted that (a) strain is coccoid-shaped which is comparable to the morphology of Gram-positive Indiicoccus explosivorum (Pal et al. 2019), (b) no variation was observed in the isolate’s shape neither in presence or absence of TNT, confirming that I. explosivorum thrives flexibly well in the minimal medium comprising TNT and (c) 120 mg/L TNT was non-lethal to the strain.
Co-metabolism factor
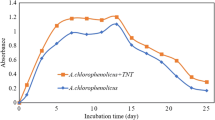
Cells of I. explosivorum exhibited alike growth patterns in MSM with and without TNT (Fig. 1) and proliferate very well in both circumstances. This depicts the unaffected behavior of the isolate S5-TSA-19 against the nitro-aromatic explosive and co-metabolic pathway for degrading TNT as mentioned elsewhere (Dalton et al.1982; Nagar et al. 2018, 2020; Lamba et al. 2021). It attained extreme proliferation in the 12 days as is confirmed by the rise in absorbance at 600 nm from 0.086 initially to 1.073 on the 12th day (Fig. 1). Levene’s test for homogeneity of variance of growth of S5-TSA-19 depicted that there is an insignificant difference in the variances in the isolate’s population with and without TNT at 0.05 level since the p value is 0.7857 which means p < 0.05. Similarly, no significant difference among the pair of means was detected in Tukey’s test for paired mean comparison at a 0.05 level.
Degradation kinetics
To evaluate the degradation rate of TNT with bacterial strain S5-TSA-19, the kinetics model, with first order was exercised against residual TNT concentrations (in treatment sets). Equation (1) depicted the “First-order rate equation”.
where Ao is the nitro-aromatic explosive concentration initially, A is TNT concentration at specified incubation duration, ‘t’ and ‘k’ represent rate constant for degradation. t0.5 is represented as follows:
Through the rate of reaction, the value of k = 0.027 per day and t0.5 = 25.67 h.
Within the first 7 days of the incubation period (30 days), 120 ppm TNT was observed to be 100% degraded by isolate S5-TSA-19. In the control samples containing 120 mg/L TNT in MSM but no bacterial strain, no explosive degradation was noticed (Fig. 3b).
Correlation between nitrite, nitrate, ammonium, and TNT degradation
The end product of TNT degradation is composed of ammonium ions; thus, detection and analysis of the concentration of nitrite, nitrate, and ammonium ions and further correlating them along are important in TNT degradation. According to Mercimek et al. 2015, TNT’s demineralization in the culture with the advances in bacterial growth corresponds to the liberation of nitrite ions. In the present experiment too, an assemblage of nitrite ions was detected with the rise of S5-TSA-19 strain in the media which corresponds to TNT degradation escalation till complete explosive degradation (Fig. 2). Likewise, a consistent pattern was observed in the case of ammonium ion concentration, i.e., after incubation originally, concentration unswervingly amplified through the first 3 days, which subsequently becomes persistent (Fig. 2). Earlier reports support the observation made for nitrite, nitrate, and ammonium ions production along with the pattern of nitro-aromatic explosive degradation (Lotufo and Lydy 2005; Mercimek et al. 2015; Lamba et al. 2021).
A reductive pathway is responsible for TNT degradation where electron-withdrawing nitro groups (03) of TNT expedite the nucleophilic attack of a hydride ion. This leads to the development of a changeable Meisenheimer complex among TNT and the hydride ion (Esteve-Nunez et al. 2001; Conder et al. 2004). Further, the formation of DNT is confirmed with the release of nitrite ions. Within 7 days of TNT degradation through I. explosivorum (Fig. 3a), the formation of 2ADNT was depicted in the HPLC chromatogram. Furthermore, the LC–MS study confirms the TNT degradation pathway along with the formation of transitional compounds (Fig. 4). As per Lachance et al. 2004, nitro group conversion to an amino group during TNT degradation leads to the formation of 2-amino-4,6-dinitrotoluene (2-ADNT) and the same was evident in the current study (Fig. 4). A steady rise in nitrite concentration is indicative of the emergence of DNT as the initial intermediate detected at the preliminary phase of the catabolism of TNT (Lamba et al. 2021) but in the concluding phase, no DNT was noticed that put forward the increased metabolism and bacterium accelerating potential in degrading the compound ultimately producing toluene. Earlier, diverse researches recorded the transformation of DNT to toluene continuing to the TCA cycle via toluene cis-dihydrodiol formation (Esteve-Nunez et al. 2001; Serrano-González et al. 2018) and here in this study also LC–MS peaks confirmed the same (Fig. 4).
Nitrate ions concentration showed an escalation at the early phase of the study followed by a gradual decrease which might be because of the primary transformation of NO2− ions to NO3−. But due to the instability of NO3− ions, they further get converted to NH4+ ions.
The disappearance of TNT concurrently through different pathways
TNT mineralization by pure bacterial system as compared to mixed or undefined bacterial cultures under aerobic or anaerobic conditions is missing in the literature (Kalderis et al. 2011; Serrano-González et al. 2018). The degradation rate of TNT in soil bacteria through nitrate is the best in contrast with other electron acceptors, such as sulfate and carbon (Boopathy et al.1993). TNT is transformed through mutually occurring aerobic and anaerobic circumstances reducing to amino-derivatives via non-specific NAD(P)H-dependent nitro-reductase (Kalderis et al. 2011). Although most of the bacteria follow a lone initial metabolic pathway for TNT transformation, some of them follow two different pathways at the same time (Vorbeck et al. 1994; HaÏdour and Ramos 1996).
Direct reduction of two-electron pathway
With the increase of time in the incubation period, a noticeable variation in the color of the TNT-enriched medium was detected. Explosive-enriched medium changed its color from white to reddish brown (darkened gradually till the end). The conversion of the culture’s color from red to brown is the validation for dihydride Meisenheimer complex formation (Pak et al. 2000; Nyanhongo et al. 2009; Gün Gök et al. 2019; Lamba et al. 2021). The product of direct reduction of two electrons of the aromatic ring via hydride addition reaction forms dihydride Meisenheimer complex, hydride Meisenheimer complex, and protonated tautomers (Serrano-González et al. 2018). This confirms the release of nitro groups during TNT degradation from the nitro-aromatic structural ring of the explosive. The unstable nature of the dihydride Meisenheimer complex contributes to the release of intermediate products like nitrite ions and DNT during TNT degradation (Serrano-González et al. 2018). The consecutive reduction in TNT concentration was in synergy with Meisenheimer complex formation and isolate’s activity which steadily led to the darkening of MSM color comprising TNT (Lamba et al. 2021).
Consecutive reduction of two-electron pathway
This metabolic pathway of TNT degradation initiates via successive reduction of two electrons of the nitro groups of the aromatic ring. Removal of electrons in the nitro groups produces reduced intermediates like di-amino-nitrotoluene (DANT), 2-amino-4,6-dinitrotoluene (2-ADNT), 4-amino-2,6-dinitrotoluene (4-A-2,6-DNT), isomers, and azo and azoxy dimers (Serrano-González et al. 2018).
Collective aerobic and anaerobic degradation of TNT
Aerobic degradation followed two initial biochemical routes. During the initial reduction process, TNT transforms to 2-hydroxylamino-4,6-dinitrotoluene or 2-hydroxylamino-2,6-dinitrotoluene. This is intervened by nitro-reductase/ nitrobenzene nitro-reductase/ dihydropteridinereductase, N-ethylmaleimidereductase, and nitrobenzene nitro-reductase (Serrano-González et al. 2018) and this led to the formation of 4-amino-2,6-dinitrotoluene or 2-amino-4,6-dinitrotoluene along with tetranitro-azoxybenzene compound. The aerobic route leads to the production of 2-amino-4-nitrosotoluene and 2,6-diamino-4-nitrotoluene as it progresses, further continuing with anaerobic degradation producing 4-amino-2,6-dinitrotoluene and 2-ADNT to conclude the process. Through NAD(P)H, nitro-reductase (non-specific) conversion of both 4-amino-2,6-dinitrotoluene and 2-ADNT to DANT takes place with anaerobic bacteria. With the involvement of hydrogenase or carbon monoxide dehydrogenase, DANT converts to 2,4-diamino-6-hydroxyl-aminotoluene (DAHAT), along with TAT formation by dissimilatory sulfite reductase. On the other hand, DANT get directly converted to TAT as also reported earlier in presence of C. sordelli, C. bifermentans, and C. sporogenes (Serrano-González et al. 2018). TAT is sequentially reduced to toluene and then to toluene cis-dihydrodiol. Toluene cis-dihydrodiol is a transitional compound within the pathway of toluene degradation. Further, toluene cis-dihydrodiol gets transformed into 4-hydroxybenzaldehyde as confirmed through the LC–MS chromatogram. Finally, 4-hydroxybenzaldehyde followed the pathway resulting in the trichloroacetate cycle, i.e., the TCA cycle (Serrano-González et al. 2018). Figure 5 depicts the proposed pathway.
Conclusion
The present study depicts the first reporting of both aerobic and anaerobic degradation of TNT in the presence of a novel soil isolate I. explosivorum, strain S5-TSA-19 in an aerobic environment. Throughout the study, satisfactory multiplication of the bacterium was observed in the growth medium containing TNT (120 mg/L). TNT, as known to be a xenobiotic compound, showed complete degradation after I. explosivorum action. Irrespective of the fact that the study was planned for 30 days, the complete disappearance of TNT (120 mg/L) was observed on the 7th day of incubation. Through the elucidation of the degradation pathway, DNT and 2-ADNT were observed to be the two key metabolites of this bioconversion process. SEM results confirmed the adaptable nature of the isolates, as the cell morphology did not change in presence of the toxic compound TNT. Admirable outcomes of the study prove the outstanding efficiency of I. explosivorum for TNT degradation. Therefore, the bacterium can be used on a large field scale along with suitable organic and inorganic amendments.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available as they also form part of an ongoing study but are available from the corresponding author on reasonable request.
References
Van Aken B, Agathos SN (2001) Biodegradation of nitro-substituted explosives by white-rot fungi: a mechanistic approach. In: Laskin AI, Bennett JW, Gadd G (eds) Advances in applied microbiology. San Diego Academic Press, San Diego, pp 1–77. https://doi.org/10.1016/s0065-2164(01)48000-2
Alothman ZA, Bahkali AH, Elgorban AM, Al-Otaibi MS, Ghfar AA, Gabr SA, Wabaidur MS, Habila AM, Ahmed AYBH (2020) Bioremediation of Explosive TNT by Trichoderma viride. Molecules 25(6):1393. https://doi.org/10.3390/molecules25061393
Ayoub K, Hullebusch VDE, Cassir M, Bermond A (2010) Application of advanced oxidation processes for TNT removal: a review. J Hazard Mater 178(1–3):10–28. https://doi.org/10.1016/j.jhazmat.2010.02.042
Boopathy R, Wilson M, Kulpa CF (1993) Anaerobic Removal of 2,4,6-Trinitrotoluene (TNT) under Different Electron Accepting Conditions: Laboratory Study. Water Environment Research 65(3): 271–275. http://www.jstor.org/stable/25044298
Chatterjee S, Deb U, Datta S, Walther C, Gupta DK (2017) Common explosives (TNT, RDX, HMX) and their fate in the environment: emphasizing bioremediation. Chemosphere 184:438–451. https://doi.org/10.1016/j.chemosphere.2017.06.008
Chien CC, Kao MC, Chen YD, Chen CS, Chen CC (2014) Biotransformation of trinitrotoluene (TNT) by Pseudomonas spp isolated from a TNT-contaminated environment. Environ Toxicol Chem 33(5):1059–1063. https://doi.org/10.1002/etc.2553
Claus H, Bausinger T, Lehmler I, Perret N, Fels G, Dehner U, Preuß J, König H (2007) Transformation of 2,4,6-trinitrotoluene (TNT) by Raoultella. Biodegradation 18(1):27–35. https://doi.org/10.1007/s10532-005-9033-7
Conder JM, Point TW, Bowen AT (2004) Preliminary kinetics and metabolism of 2,4,6-trinitrotoluene and its reduced metabolites in an aquatic oligochaete. Aquat Toxicol 69(3):199–213. https://doi.org/10.1016/j.aqua-tox.2004.04.013
Cook AM, Huetter R (1981) s-Triazines as nitrogen sources for bacteria. J Agric Food Chem 29(6):1135–1143. https://doi.org/10.1021/jf00108a009
Dalton H, Stirling DI, Quayle RJ (1982) Co-metabolism philosophical transactions of the royal society B. Biol Sci 297(1088):481–496. https://doi.org/10.1098/rstb.1982.0056
Duque E, Haidour A, Godoy F, Ramos LJ (1993) Construction of a pseudomonas hybrid strain that mineralizes 2,4,6-trinitrotoluene. J Bacteriol 175(8):2278–2283. https://doi.org/10.1128/jb.175.8.2278-2283.1993
Esteve-Nunez A, Caballero A, Ramos JL (2001) Biological degradation of 2,4,6-Trinitrotoluene. Microbiol Mol Biol Rev 65(3):335–352. https://doi.org/10.1128/mmbr.65.3.335-352.2001
Fahrenfeld N, Zoeckler J, Widdowson AM, Pruden A (2013) Effect of bio stimulants on 2,4,6-trinitrotoluene (TNT) degradation and bacterial community composition in contaminated aquifer sediment enrichments. Biodegradation 24:179–190. https://doi.org/10.1007/s10532-012-9569-2
Gümüscü B, Tekinay T (2013) Effective biodegradation of 2,4,6-trinitrotoluene using a novel bacterial strain isolated from TNT-contaminated soil. Int Biodeterior Biodegrad 85:35–41. https://doi.org/10.1016/j.ibiod.2013.06.007
Gün Gök Z, İnal M, Yiğitoğlu M (2019) Biodegradation of 2,4,6-trinitrotoluene (TNT) with bacteria isolated from TNT-polluted waste pink water. Periodica Polytechnica Chem Eng 63(3):459–468. https://doi.org/10.3311/ppch.13390
HaÏdour A, Ramos JL (1996) Identification of products resulting from the biological reduction of 2,4,6-Trinitrotoluene, 2,4-Dinitrotoluene, and 2,6-Dinitrotoluene by Pseudomonas spp. Environ Sci Technol 30(7):2365–2370. https://doi.org/10.1021/es950824u
Heiss G, Knackmuss HJ (2002) Bioelimination of trinitroaromatic compounds: immobilization versus mineralization. Curr Opin Microbiol 5:282–287. https://doi.org/10.1016/S1369-5274(02)00316-8
Juhasz LA, Naidu R (2007) Explosives: Fate, dynamics, and ecological impact in terrestrial and marine environments. Rev Environ Contam Toxicol 191:163–215. https://doi.org/10.1007/978-0-387-69163-3_6
Kalafut T, Wales ME, Rastogi VK, Naumov PR, Zaripova KS, Wild RJ (1998) Biotransformation patterns of 2,4,6-Trinitrotoluene by aerobic bacteria. Curr Microbiol 36:45–54. https://doi.org/10.1007/s002849900278
Kalderis D, Juhasz LA, Boopathy R, Comfort S (2011) Soils contaminated with explosives: environmental fate and evaluation of state-of-the-art remediation processes (IUPAC technical report). Pure Appl Chem 83(7):1407–1484. https://doi.org/10.1351/PAC-REP-10-01-05
Kao MC, Lin HB, Chen CS, Wei FS, Chen CC, Yao LC, Chien CC (2016) Biodegradation of trinitrotoluene (TNT) by indigenous microorganisms from TNT-contaminated soil, and their application in TNT bioremediation. Bioremediation J 20(3):165–173. https://doi.org/10.1080/10889868.2016.1148007
Khan MI, Lee J, Park J (2013) A toxicological review on potential microbial degradation intermediates of 2,4,6-trinitrotoluene, and its implications in bioremediation. KSCE J Civ Eng 17:1223–1231. https://doi.org/10.1007/s12205-013-0305-1
Krishnamurthi S, Bhattacharya A, Mayilraj S, Saha P, Schumann P, Chakrabarti T (2009) Description of Paenisporosarcina quisquiliarum gen.nov., sp. nov., and reclassification of Sporosarcina macmurdoensis Reddy, et al 2003 as Paenisporosarcina macmurdoensis comb nov. Int J Syst Evol Microbiol 59:1364–1370. https://doi.org/10.1099/ijs.0.65130-0
Lachance B, Renoux AY, Sarrazin M, Hawari J, Sunahara GI (2004) Toxicity and bioaccumulation of reduced TNT metabolites in the earthworm Eisenia andrei exposed to amended forest soil. Chemosphere 55(10):1339–1348. https://doi.org/10.1016/j.chemosphere.2003.11.049
Lamba J, Anand S, Dutta J, Chatterjee S, Nagar S, Celin SM, Rai PK (2021) Study on aerobic degradation of 2,4,6-trinitrotoluene (TNT) using Pseudarthrobacter chlorophenolicus collected from the contaminated site. Environ Monit Assess 193(2):80. https://doi.org/10.1007/s10661-021-08869-7
Lee SM, Chang WH, Kahng YH, So SJ, Oh HK (2002) Biological removal of explosive 2,4,6-trinitrotoluene by Stenotrophomonas sp. OK-5 in bench-scale bioreactors. Biotechnol Bioprocess Eng 7:105–111. https://doi.org/10.1007/BF02935888
Lewis TA, Newcombe DA, Crawford RL (2004) Bioremediation of soils contaminated with explosives. J Environ Manag 70(4):291–307. https://doi.org/10.1016/j.jenvman.2003.12.005
Lotufo GR, Lydy MJ (2005) Comparative toxico kinetics of explosive compounds in sheepshead minnows. Arch Environ Contam Toxicol 49(2):206–214. https://doi.org/10.1007/s00244-004-0197-7
Mackie, MacCartney (1989) Practical Medical Microbiology, Collee JG, Duguid JP, Fraser AG, Marmion BP, (Eds.),13th edn Churchill Livingstone, Edinburgh
Mercimek HA, Dincer S, Guzeldag G, Ozsavli A, Matyar F (2013) Aerobic biodegradation of 2,4,6-Trinitrotoluene (TNT) by Bacillus cereus isolated from contaminated soil. Microb Ecol 66(3):512–521. https://doi.org/10.1007/s00248-013-0248-6
Mercimek HA, Dincer S, Guzeldag G, Ozsavli A, Matyar F, Arkut A, Ozdenefe MS (2015) Degradation of 2,4,6-trinitrotoluene by P aeruginosa and characterization of some metabolites. Braz J Microbiol 46(1):103–111. https://doi.org/10.1590/s1517-838246120140026
Montpas S, Samson J, Langlois E, Lei J, Piché Y, Chênevert R (1997) Degradation of 2,4,6-trinitrotoluene by Serratia marcescens. Biotechnol Lett 19(3):291–294. https://doi.org/10.1023/A:1018326228448
Nagar S, Shaw AK, Anand S, Celin SM, Rai PK (2020) Biodegradation of octogen and hexogen by Pelomonas aquatica strain WS2-R2A-65 under aerobic condition. Environ Technol. https://doi.org/10.1080/09593330.2020.1812731
Nagar S, Anand S, Chatterjee S, Rawat DC, Lamba J, Rai KR (2021) A review of toxicity and biodegradation of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) in the environment. Environ Technol Innov 23(2–3):101750. https://doi.org/10.1016/j.eti.2021.101750
Nagar S, Shaw AK, Anand S, Celin SM, Rai PK (2018) Aerobic biodegradation of HMX by Planomicrobium flavidum. 3 Biotech 8(11):45. https://doi.org/10.1007/s13205-018-1479-5
Nyanhongo GS, Aichernig N, Ortner M, Steiner W, Guebitz GM (2009) Incorporation of 2,4,6-trinitrotoluene (TNT) transforming bacteria into explosive formulations. J Hazard Mater 165(1–3):285–290. https://doi.org/10.1016/j.jhazmat.2008.09.107
Oh HK, Kim JY (1998) Degradation of explosive 2,4,6- trinitrotoluene by s-Triazine degrading bacterium isolated from contaminated soil. Bull Environ Contam Toxicol 61(6):702–708. https://doi.org/10.1007/s001289900818
Pak JW, Knoke KL, Noguera DR, Fox BG, Chambliss GH (2000) Transformation of 2,4,6-trinitrotoluene by purified xenobiotic reductase B from Pseudomonas fluorescens I-C. Appl Environ Microbiol 66(11):4742–4750. https://doi.org/10.1128/aem.66.11.4742-4750.2000
Pal Y, Mayilraj S, Paul M, Schumann P, Krishnamurthi S (2019) Indiicoccus explosivorum gen nov, sp nov, isolated from an explosives waste contaminated site. Int J Syst Evol Microbiol. https://doi.org/10.1099/ijsem.0.003541
Prasad R, Shabnam N, Pardhasaradhi P (2016) Immobilization on cotton cloth pieces is ideal for storage and conservation of microalgae. Algal Res 20:172–179
Rodgers DJ, Bunce JN (2001) Treatment methods for the remediation of nitroaromatic explosives. Water Res 35(9):2101–2111. https://doi.org/10.1016/s0043-1354(00)00505-4
Serrano-González YM, Chandra R, Castillo-Zacarias C, Robledo-Padilla F, Rostro-Alanis JM, Parra-Saldivar R (2018) Biotransformation and degradation of 2,4,6-trinitrotoluene by microbial metabolism and their interaction. Def Technol 14(2):151–164. https://doi.org/10.1016/j.dt.2018.01.004
Thompson KT, Crocker FH, Fredrickson HL (2005) Mineralization of the cyclic nitramine explosive hexahydron 1,3,5-Trinitro-1,3,5-Triazine by Gordonia and Williamsia spp. Appl Environ Microbiol 71(12):8265–8272. https://doi.org/10.1128/aem.71.12.8265-8272.2005
USEPA (2007) Nitroaromatics and nitroamines by high performance liquid chromatography (HPLC) revision 1 method 8330A. Office of Solid Waste and Emergency Response, DC
Vorbeck C, Lenke H, Fischer P, Knackmuss HJ (1994) Identification of a hydride Meisenheimer complex as a metabolite of 2,4,6-trinitrotoluene by a Mycobacterium strain. J Bacteriol 176(3):932e-e934. https://doi.org/10.1128/jb.176.3.932-934.1994
Zhao JS, Greer CW, Thiboutot S, Ampleman G, Hawari J (2004) Biodegradation of the nitramine explosives hexahydro1,3,5-trinitro-1,3,5-triazine and octahydro- 1,3,5,7-tetranitro-1,3,5,7-tetrazocine in cold marine sediment under anaerobic and oligotrophic conditions. Can J Microbiol 50(2):91–96. https://doi.org/10.1139/w03-112
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
JL: Conceptualization, Investigation, Methodology, Software, Formal analysis, Writing – original draft. SA: Conceptualization, Methodology, Writing – review & editing, Supervision. JD: Writing – review & editing, Supervision. PKR: Review and editing, Resources, Team Head.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lamba, J., Anand, S., Dutta, J. et al. 2,4,6-trinitrotoluene (TNT) degradation by Indiicoccus explosivorum (S5-TSA-19). Arch Microbiol 204, 447 (2022). https://doi.org/10.1007/s00203-022-03057-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03057-8