Abstract
Echinococcosis is a common and endemic disease that affects both humans and animals. In this study, the in vitro activities of methanolic extracts of Ruta graveolens, Peganum harmala aerial parts, and Citrullus colocynthis seeds against protoscolosis and isolated bacterial strains from hydatid cysts were assessed using disc diffusion methods and Minimum Inhibitory Concentration (MIC). The chemical composition of three methanolic extracts was studied using LC–MS. After 3 h of exposure to 40 mg/mL R. graveolens extract, a tenfold protoscolocidal effect was seen when compared to the convintional medication (ABZ) for the same duration (P < 0.05). The bacteria listed below were isolated from hydatid cyst fluid collected from a variety of sick locations, including the lung and liver. Micrococcus spp., E. coli, Klebsiella oxytoca, Enterobacter aerogenes, Enterobacter amnigenus, Pseudomonas aeruginosa, Staphylococcus xylosus, and Achromobacter xylosoxidans are among the bacteria that have been identified. The most effective extract was R. graveolens, followed by P. harmala and C. colocynthis, according to the results of antibacterial activity using the disc diffusion method. R. graveolens extract had the lowest MIC values (less than 2 mg/mL) against all microorganisms tested. This shows that the R. graveolens extract has additional properties, such as the ability to be both scolocidal and bactericidal. Because these bacteria are among the most prevalent pathogenic bacteria that increase the risk of secondary infection during hydatid cysts, the results of inhibitory zones and MICs of the R. graveolens methanol extract are considered highly promising.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystic Echinococcosis (CE) is a parasitic disease that occurs in all mammals but mainly sheep and cattle. It also occurs in humans, caused by the larval stage of the tapeworm genus Echinococcus (Ali et al. 2012; Malekifard and Keramati 2018). Echinococcosis is a common and endemic health problem in humans and animals in most of the Mediterranean basin, including Jordan (Nasrieh et al. 2003). Canines serve as definitive hosts for the parasite, whereas herbivores serve as intermediate hosts for the hydatid cyst. After excretion by the definitive host, infection occurred due to intake of food or water contaminated with Echinococcus spp. eggs (Hijjawi et al. 2018).
The fertile hydatid cysts are typically filled with a clear fluid contains protoscolices which are mostly bacteriologically sterile. Sometimes, liver and lung hydatid cysts can be infected with bacteria. Outside-released protoscolices have the ability to differentiate into secondary hydatid cysts in viscera. Cystic differentiation of protoscolices can probably be triggered by altered physiological conditions, such as bacterial diffusion into the cyst fluid causes the concerted effort between parasites and bacteria that cause some human and animal pathologies (Aitken et al. 1978). According to Boes and Helwigh (2000), there are two types of synergy between parasites and bacteria: first, indirect synergy, that causes an increase in the pathogenic effects of the bacteria and makes the host susceptible to the bacterial disease, especially when the bacteria and parasites occur in the same tissue or organ; and second, a direct synergy that occurs when bacteria transported into the host by the parasite after invading stages of the parasite present in the environment (Ziino et al. 2009).
In animals, the synergy between CE and bacteria results in significant economic losses due to decreased meat, wool, and milk production, as well as the condemnation of infected organs (Jahed et al. 2013), whereas in people, the economic issues are due to the amplified costs of therapy and surgery (Ahmed et al. 2021). Antibiotics, whether synthetic or natural, are important biochemicals produced by living organisms and widely employed in medical use. In spite of producing a large number of new antibiotics by the pharmaceutical industries within the last three decades, microbial resistance to these drugs has increased, as well (Al-Asoufi et al. 2017; dos Santos et al. 2001).
Uncontrolled use of commercial drugs by either patients or prescriptions that are made without susceptibility tests increases the resistance of bacteria and parasites (De Queiroz et al. 2014; Friedman et al. 2002). Therefore, more attention needs to be paid to increase the interest in plant extracts as antibacterial and anti-parasitic agents. Plant-based products are thought to account for 30% of all medicine sales worldwide. Jordan's check list of medicinal plants includes 2552 flowering plant species, and 363 of them are medicinal plants (Oran and Al-Eisawi 1998; Oran 2014), giving scientists encouragement to study and investigate their biological activities.
The plant R. graveolens belongs to the family Rutaceae and is commonly known as Rue (Oran 2014). It is a herbaceous perennial that was originally native to the Mediterranean region (Asgarpanah and Khoshkam, 2012). In Jordan, the plant R. graveolens is used as a spice (Oran and Al-Eisawi 1998). In folk medicine, it is used as an aphrodisiac and fertility-promoting agent (Asgarpanah and Khoshkam 2012), and is used to treat several diseases, including parasitic infections, inflammation, ulcers, hypotension, reproductive disorders, menstrual problems, and wounds. R. graveolens has anticancer and schistosomicidal activity (Amabye 2015; Asgarpanah and Khoshkam 2012; Carvalho et al. 2019; De Queiroz et al. 2014; Pathak et al. 2003). The R. graveolens extracts and essential oil showed good antibacterial and antifungal properties (Al-Shuneigat et al. 2015; Amabye 2015). According to Nabaei et al. (2014), no toxic effect was reported using different doses of hydro-alcoholic extract of R. graveolens on the histopathology of the liver. The plant P. harmala is commonly known as Syrian Rue and has the Arabic names of Harmal and Harjal (Oran and Al-Eisawi 1998). It belongs to the family Zygophyllaceae, and is widely used in folk medicine. P. harmala alkaloids are used as anti-parasidal, antifungal, antibacterial, insecticidal, anti-leishmanial effects and anticancer by exhibiting a cytotoxic effect on leukemia cell lines (Mamedov et al. 2018; Moazeni et al. 2014, 2017; Moloudizargari et al 2013; Niroumand et al. 2015; Rezaee and Hajighasemi 2019; Sohrabi et al. 2018; Wink 2012).
Citrullus colocynthis belongs to the family of Cucurbitaceae (Oran and Al-Eisawi 1998). It is distinguished by the occurrence of many constituents such as flavonoids, alkaloids, carbohydrates, tannins, gums, and mucilage. C. colocynthis has been used in the traditional medicine as anticancer, antibacterial, insecticidal, anti-diabetic, and anti-parasitic including Leishmania, Plasmodium and Haemonchus contortus (Ahmed et al. 2019; Dhakad et al. 2017; Uma and Sekar 2014).
The goal of this study was to find out how common bacterial infection is in hydatid cysts and to identify the most common bacterial species found in hydatid fluid. In addition, the effects of methanolic extracts of P. harmala aerial parts, R. graveolens, and seeds of C. colocynthis on the sustainability of bacterial strains and protoscolices isolated from hydatid cysts were studied in vitro.
Materials and methods
Microbial analysis for cyst fluid
An entire of 3725 animals (sheep and goats) including 1675 native and 2050 imported have been collected between 1/8/2020 to 1/10/2020, from slaughter houses in the area of Karak. The infected organs (liver or/and lung) were collected and transported to the laboratory within an hour of collection under refrigerated conditions. The infected organ surface was sterilized with 70% ethanol and washed with sterile distilled water. The hydatid fluid was aspirated by a sterile syringe, the protoscolices were isolated, and the hydatid fluid cultured for isolation and identification of bacteria.
Bacterial isolation and identification
Initially the hydatid fluid was inoculated on three different types of media: blood agar for the bacterial isolation of aerobic and facultative anaerobic Gram-positive, Eosin Methylene Blue (EMB) agar, and MacConkey agar for the isolation of Gram-negative bacteria. Then, the grown colonies were picked and inoculated on tryptone soy agar and nutrient agar to get pure culture.
To identify the bacterial isolates, colonies and cells characteristics were determined microscopically. The Gram-positive isolates were further characterized using standard biochemical tests including oxidase, DNase, catalase, phosphatase, coagulase, and fermentation of mannitol, starch and sodium hippurate, pyrrolidonyl arylamidase (PYR) and Christie-Atkins, aesculin hydrolysis, Munch-Petersen (CAMP) tests, and novobiocin sensitivity. The Gram-negative isolates were further characterized using standard biochemical tests including motility, methyl red, urease, indole production, Voges-Proskauer, o-nitrophenyl-β-d-galactopyranoside (ONPG) potassium cyanide (KCN) and H2S production, triple sugar iron agar (TSI), reactions of phenylalanine and lysine, lactose fermentation, and ornithine decarboxylase tests. In addition, the identification was confirmed using API 20E and API Staph diagnostic systems (Khleifat et al. 2008).
Plant materials
R. graveolens, P. harmala, and C. colocynthis were collected in June, 2020. R. graveolens was collected from Irbid, northern of Jordan. P. harmala and C. colocynthis were collected from AL Karak, southern of Jordan. The plants were identified to species level by Prof. Sawsan Al Oran, Biology Department, Faculty of Science, Jordan University. The freshly gathered materials were washed, air-dried in the shade at room temperature, and then ground into a reasonable powder using a mixer.
Extraction
A 100 g sample of both R. graveolens and P. harmala plants aerial parts, as well as C. colocynthis seeds, were steeped in 1000 mL methanol for 3 days at room temperature with continuous shaking. The solvent was extracted using a rotary evaporator at 45 °C with reduced pressure after filtration. The extracts were kept in sealed glass vessels at − 20 °C.
Determining the protoscolices' mortality
The mortality of protoscolices was determined by measuring cell motility while staining with 0.1 percent aqueous eosin solution. Dead protoscolices stained with eosin and appear in reddish color using a microscope, whereas alive protoscolices do not permeate the eosin and thus remain unchanged (Al-Arabi et al. 2019; Smyth and Barrett 1980). The rate of mortality was considered by taking the number of dead protoscolices divided by the number of predicted headings.
Extracted protoscolices were kept in a sterile Roswell Park Memorial Institute (RPMI) 1640 medium provided with fetal bovine serum (10%) under incubation of 37 °C. To control the contamination (Wang et al. 2015), penicillin (100 U/mL) and 100 µg/mL streptomycin sulfate were added to the medium (Malekifard and Keramati 2018; Monteiro et al. 2017). The impact of P. harmala, R. graveolens, and seeds of C. colocynthis methanol extracts on the percentage of mortality of protoscolices in vitro was conducted. Protoscolices were treated with 10–40 mg/ml with being 10 as intervals of the three plants by taking one milliliter of protoscolices suspension containing about 2 × 103 protoscolices/mL in test tubes. The length of exposure periods was 1, 3, 6, 12, 18, and 24 h. To test the viability of protoscolices, 100 µl of pooled protoscolices were mixed with 100 µl of 0.1 percent eosin on a slide for 15 min; dead protoscolices stained red, while surviving protoscolices remained colorless, as observed under a compound microscope. Only samples with 100 percent viable protoscolices were used for the in vitro studies (Yones et al. 2011). For comparison, stock solution of albendazole (ABZ) (protoscolicidal agent available for treatment of human hydatid disease) prepared by dissolving 0.5 g in 1 mL of 30% DMSO; the drug was filtered before using through a 0.22 µm filter. The efficacy of methanolic plants extract on the viability of protoscolices was compared with positive and negative control groups which received 20 mg/mL ABZ and normal saline, respectively (Blanton et al. 1998). Experiments were carried out in triplicates. For obtaining images, digital camera type (Pro-MicroScan, 8 M Pixels High-Speed) and light microscope (100x) model (OLYMPUS CX21FS1) were used.
Dual staining with acridine orange–ethidium bromide (AO/EB)
In seek to monitor the cellular and nuclear morphological changes, the protoscolices were treated with different concentrations of tested plants extracts and ABZ for 48 h, washed with PBS, and dual stained with an equal volume of AO (100 µg/ml) and EB (100 µg/ml) for 2 min (Durgadevi et al. 2019). The preparation was examined using a fluorescent microscope, in which green-colored cells were indicative of viable protoscolices, while those in red color are dead protoscolices.
Antibacterial assay
Bacterial strains
Methanolic extracts of aerial sections of R. graveolens and P. harmala plants, as well as seeds of C. colocynthis, were tested against eight bacterial species isolated from hydatid cysts. Two were Gram-positive bacteria (Micrococcus spp. and S. xylosus) and six were Gram-negative bacteria (P. aeruginosa, A. xylosoxidans, E. coli, E. amnigenus, E. aerogenes, and K. oxytoca). In addition, strains of S. aureus, B. cereus, B. subtilis, E. coli, and P. aeruginosa with known ATCC identity were employed.
Disc diffusion method
The disc diffusion method was used to investigate the antibacterial activity of the various extracts (Alzoreky and Nakahara 2003). The cell number was adjusted to 2 106 CFU/mL through mixing the investigated bacteria's broth cultures with sterile nutritional agar that was cooled at 45–50 °C. The inoculated agar was then placed on sterilized Petri plates for 45–60 min to harden. Then, under aseptic conditions, a disc containing 1 mg, 2 mg of plant extracts, 10% DMSO (negative control), or tetracycline (positive control) was deposited on the surface of the agar plate. The growth inhibitory action was measured by measuring the diameter of the clear zone around the disc with a ruler after 24 h at 37 °C (Khleifat et al. 2006; Romero et al. 2005). Triplicates of each sample were evaluated.
Minimum inhibitory concentration (MIC)
Plant extracts' minimum inhibitory concentration (MIC) against bacterial growth was determined. A bacterial inoculum of 2 × 106 CFU/mL was inoculated into tubes containing a serial dilution of plant extract (0–2 mg/mL) in 5 mL Muller-Hinton broth. The MIC was considered as the lowest concentration of extracts that inhibited observable bacterial growth (Khleifat et al. 2010; Patel et al. 2011).
Liquid chromatography–mass spectrometry (LC–MS)
At a flow rate of 0.5 ml/min, HPLC separation was performed with the mobile phase containing solvents A and B in gradient, where A was 0.1 percent (v/v) formic acid in water and B was 0.1 percent (v/v) formic acid in acetonitrile, for the following gradient: 5 percent B for 5 min, 5–100 percent B in 15 min, and 100 percent for 5 min. The Agilent Zorbax Eclipse XDB-C18 column was used (2.1 × 150 mm × 3.5 um). The sample injection volume was 1 l (18 mg/mL in methanol) and the oven temperature was 25 °C. The eluent was scanned from 100 to 1000 m/z and secondary scan from 50 to 100 m/z MRM mode using a Shimadzu LC–MS 8030 with an electrospray ion mass spectrometer (ESI–MS) in positive-ion mode. The ESI was performed with a 125 V fragment or and a 65 V skimmer. At a flow rate of 10 L/min, a nebulizer at 45 pressure, and a capillary temperature of 350 C, high-purity nitrogen (99.999 percent) was used as the drying gas. A blank of 0.1 percent formic acid was utilized in parallel. The Shimadzu CBM-20A system controller, the LC-30AD pump, the SIL-30AC autosampler with cooler, and the CTO-30 column oven were used to inject a sample into the mass detector.
Results
During inspections from January 8, 2020 to January 10, 2020, 3,725 butchered animals were checked. Hydatid cysts were found in 25 of the 3,725 animals killed.
Isolation of protoscolice-associated bacteria
A total of 8% of the cysts were found in the lung, 16% in the liver, and 76% in both the liver and the lung. Nineteen of the 25 hydatid cyst containing animals were infected with one or more bacterial species representing a bacterial infection rate of 76% (Table 1). Thirty-one bacterial isolates were collected from hydatid cysts fluid of lung, liver or lung and liver. Interestingly, 78.9% of the isolated bacteria were isolated from samples of bi-organs infected animals. Gram-negative bacteria were found in six of the isolates, while Gram-positive bacteria were found in two. Staphylococcus xylosus, Achromobacter xylosoxidans, and Pseudomonas aeruginosa have been the most prevalent bacteria detected in hydatid cysts fluid retrieved from the lungs, whereas Micrococcus spp. and E. coli were recovered from hepatic hydatid cysts fluid. Klebsiella oxytoca, Enterobacter aerogenes, Pseudomonas aeruginosa, and Enterobacter amnigenus were identified from the fluid of hydatid cysts in the lungs and livers. Pseudomonas aeruginosa was the most commonly isolated species, with 9 isolates; other species included 6, 5, 4, 2, and 2 isolates, respectively, for K. oxytoca, E. amnigenus, E. aerogenes, A. xylosoxidans, and E. coli. Micrococcus spp. was identified from two samples of fluid collected from the liver, whereas S. xylosus was recovered from one hydatid cyst fluid sample collected from the lung.
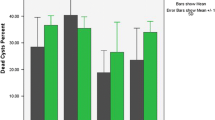
Treatment of protoscolices in vitro with methanolic extracts of R. graveolens, P. harmala, and C. colocynths
The hydatid cyst protoscolices were treated with methanolic extracts of R. gravelens, P. harmala, and C. colocynths for various periods of time (1, 3, 6, 12, 18, and 24 h). The extract concentrations used in this study were 10, 20, 30, and 40 mg/mL (Table 2 and Fig. 1a–c). When protoscolices were treated with R. graveolens methanol extract, the killing of protoscolices was dramatically increased. This study revealed that using different concentrations of methanol extracts from R. graveolens, P. harmala, and C. colocynthis resulted in a significant effect (P < 0.05), with mortality rates of 100%, 50%, and 15% after treatment for periods of 1.25 h, 24 h, and 24 h, respectively, and using the maximum concentration (40 mg/mL). The survivability testing findings were in line with the morphological changes and structural damages seen in protoscolices. As shown in structural and morphological investigations involving SEM, there was a positive association between the intensity of injury and the extract concentration (Fig. 2a–d). Alterations in protoscolices included loss of motility, paralysis, tegument bleb formation, contraction of the soma area, rostellar disarray, and loss of hooks and microtriches. Normal protoscolices showed green fluorescence as a result of acridine orange penetrating the normal cell membrane, but apoptotic protoscolices showed orange colored apoptotic bodies occurring as a result of nuclear shrinkage, damage, and blebbing (Fig. 3). When studied under a fluorescent microscope, dead protoscolices showed red hue fluorescence due to their loss of membrane integrity. These structural and morphological changes were identical to those seen in protoscolices that had been treated with ABZ in vitro.
Images of living, dead, and partially dead E. granulosus protoscolices following staining with 0.1 percent eosin; (a), protoscolices untreated with plant extract. (b), effect of 20 mg/mL ABZ on protoscolices after 24 h of exposure (c), total mortality of protoscolices after 1.25 h (75 min) of exposure to 40 mg/mL R. graveolens extract 1c. Blebs formation in tegument, 2c. Rostellar disorganization and loss of hooks and microtriches (Total magnification 100X)
The ultrastructural damages observed with scanning electron microscopy when treated with R. graveolens extract. (A) Evaginated control protoscolex, (B) Invaginated protoscolices, clearly altered after culture in the presence of R. graveolens extract, collapse of soma region, disorganization in the rosteller cone is visible with damage in scolex and sucker region. (C) Loosening of hooks, disorganization of hooks was also observed at rosteller cone (D) Collapse of the soma region, shedding of microtriches of the scolex region and damage at the surface teguments is also observed. (Magnification A, B, C 686X, D, 340X)
Antibacterial activity
The disc diffusion method was used to test the antibacterial activity of methanolic extracts of R. graveolens, P. harmala, and C. colocynthis at two different concentrations. In general, all of the extracts examined had varying antibacterial activity that was dose-dependent. R. graveolens was the most effective extract, followed by P. harmala and C. colocynthis. The Gram-negative A. xylosoxidans strains were the most sensitive, with the largest inhibition zone (18.3 mm). All bacterial strains were inhibited by R. graveolens methanolic extract at both concentrations tested, with the exception of S. xylosus at 1 mg/disc. Strong antibacterial activity of R. graveolens extract against A. xylosoxidans, E. amnigenus, S. aureus ATCC 43,300, B. subtilis ATCC 6633, E. aerogenes, and B. cereus ATCC 11,778 was seen at the maximum dosage tested (2 mg), with inhibition zones ranging from 14.0 to 18.3 mm.
R. graveolens methanolic extract had a moderate antibacterial activity against E. coli, P. aeruginosa ATCC 27,853, Micrococcus spp, S. xylosus, P. aeruginosa, and K. oxytoca, with an inhibition zone ranging from 10.3 to 13.3 mm (Table 3). S. aureus was the most susceptible strain to P. harmala extract, with inhibition zones of 12.0 and 14.3 mm at dosages of 1 and 2 mg/disc, respectively. P. harmala extract inhibited B. cereus ATCC 11,778, B. subtilis ATCC 6633, P. aeruginosa ATCC 27,853, P. aeruginosa, E. coli ATCC 25,922, Micrococcus spp, K. oxytoca, and A. xylosoxidans at the maximum dose tested, with inhibition zones ranging from 13.0 to 9.7 mm. Because no inhibitory zones were found, E. amnigenus, E. aerogenes, and S. xylosus appear to be resistant to P. harmala extract. The disc diffusion method also revealed that the extract of C. colocynthis has antibacterial activity against Gram-positive bacteria. S. aureus ATCC 43,300, B. cereus ATCC11778, B. subtilis ATCC 6633, and Micrococcus spp. were inhibited by C. colocynthis extracts in inhibition zones ranging from 13.3 to 8.7 mm. Against all Gram-negative bacteria tested, no inhibition zones were found.
Table 4 shows the MIC of MeOH extracts of R. graveolens, P. harmala, and C. colocynthis. In general, the MIC results were consistent with the inhibitory zones seen, with R. graveolens being the most potent extract, followed by P. harmala, and then, C. colocynthis. B. cereus ATCC 11,778 was the most sensitive bacterial strain. All of the extracts studied are still more efficient against Gram-positive bacteria than Gram-negative bacteria. All of the tested bacteria showed MIC values of less than 2 mg/mL against R. graveolens extract. The extract MIC value for B. cereus ATCC 11,778 (0.25 mg/mL) was the lowest, followed by S. aureus ATCC43300 (0.5 mg/mL), B. subtilis ATCC 6633 (0.5 mg/mL), A. xylosoxidans (0.5 mg/mL), and K. oxytoca (1.5 mg/mL). P. harmala extract had MIC values of 0.25 mg/mL against B. cereus ATCC 11,778 and B. subtilis ATCC 6633, respectively. P. harmala extract had MIC values of 1 mg/mL against Micrococcus spp., S. aureus ATCC 43,300, E. coli, E. coli ATCC 25,922, and P. aeruginosa ATCC 27,853, but 1.5 mg/mL against P. aeruginosa ATCC 27,853. S. xylosus E. amnigenus, E. aerogenes, and K. oxytoca were resistant to P. harmala extract with MIC values less than 2 mg/mL, according to disc diffusion technique data. C. colocynthis extract was more efficient against Gram-positive bacteria than Gram-negative bacteria, similar to the results of the disc diffusion approach. C. colocynthis extract has an MIC of less than 2 mg/mL against all Gram-negative bacteria tested. B. cereus ATCC 11,778 and B. subtilis ATCC 6633 had the lowest MIC values (0.5 mg/mL and 2.0 mg/mL, respectively), followed by Micrococcus spp. (1.5 mg/mL) and S. xylosus and S. aureus ATCC 43,300 (2.0 mg/mL, respectively).
Chemical composition of R. graveolens, P. harmala, and C. colocynthis using LC–MS
LC–MS was used to examine the chemical composition of methanolic extracts of R. graveolens, P. harmala aerial parts and the C. colocynthis seeds (data not shown). In the methanolic extract of the aerial portion of R. graveolens, a total of 26 compounds were identified. The main components of R. graveolens methanolic extract were rutin (13.7%), quercetin (9.3%), isoquinoline (6.9%), methoxypsoralen (6.8%), procyanidin (6.3%), and tropane (6.3%). (5.5 percent). In the aerial component of P. harmala methanolic extract, 31 compounds were identified using LC–MS. The primary components were discovered as harmaline (10.6 percent), harmine (6.3 percent), and pinene (6.3 percent). Linalool (5.9%), squalene (5.8%), terpineol (5.5%), catechin (5.4%), limonene (5.3%), terpinene (5.35), flavan (4.9%), and anthraquinone (4.9%) were found abundant in the extract (4.7%). The methanolic extract of C. colocynthis seeds comprised 32 compounds. Lactic acid was found in the highest concentration (10.2%), followed by xylitol (7.4%), glycerol (7.2%), proline (6.2%), inositol (5.4%), glucitol (5.4%), lauric acid (5.4%), linoleic acid (5.3%), phytol (5.3%), and campesterol (5.3%).
Discussion
E. granulosus causes cystic echinococcosis, a parasitic cestode illness. It causes a medically and veterinary-important persistent infection (Ahmed et al. 2021; Zeghir-Bouteldja et al. 2009). After three hours of treatment, R. graveolens extract produced a result that was incomparable to conventional ABZ treatment, which produced a tenfold extra deadly effect than ABZ. The viability test results corroborate morphological and structural changes detected using a compound, fluorescence, and scanning electron microscopy. Tissue injury was assessed at the ultrastructural level using a scanning electron microscope (SEM). After culture in the presence of R. graveolens extract and ABZ, invaginated protoscolices showed evident changes, including the collapse of the soma region, disarray in the rosteller cone, damage to the scolex and sucker region and damage to the surface teguments. In comparison to the control group, the damage was decreased but still significant at the highest concentration of C. colocynthis extract.
The percentage of bacterial infection of hydatid cysts in this study was 76 percent. Several previous studies found a high incidence (88 percent) of bacterial infection in hydatid cysts isolated from cattle, goats, and sheep (Hadadi et al. 2020; Khleifat et al. 2010; Ziino et al. 2009). Furthermore, P. aeruginosa, K. oxytoca, E. amnigenus, and E. aerogenes were the most common bacterial isolates, indicating that Gram-negative bacteria are the most common bacterial invaders in hydatid cysts. According to similar studies, Gram-negative bacteria were found to be prevalent in hepatic and lung hydatid cyst fluids in sheep, with E. coli and K. pneumoniae being the most common isolates (Abdullah et al. 2021; Fallah et al. 2014; Khleifat et al. 2010). In contrast, S. aureus was recovered from hepatic hydatid cyst fluids in a recent test, demonstrating that the types of bacteria isolated from the cyst fluids are highly varied (Najim et al. 2020). This could be due to the infective stages ability to live in the outside environment, as well as the life cycle of Echinococcus spp., which entails tissue translocation into the intermediate host.
Despite the idea that hydatid cyst fluid is a sterile fluid, bacterial pathogens from the respiratory and gastrointestinal tract were found in high numbers in bi-organ cyst fluid samples in this study. In addition to being harmful, these isolates are naturally widespread in the environment and are part of the usual flora of warm-blooded animals. According to one theory, intermediate animals such as sheep and goats swallow bacteria-infected Echinococcus spp. eggs (Ahmed et al. 2021; Fallah et al. 2014). When these eggs reach the colon, they hatch, and the bacterially tainted oncosphere embryo penetrates the mucosa, eventually forming hydatid cysts in the liver and lungs. According to certain views, the infection may have entered by the bile duct or enterohepatic circulation. Although only to a limited extent, hydatid cysts can be infected through the bronchial tree or a hematogenous pathway (Ahmed et al. 2021; Wani et al. 2010; Ziino et al. 2009).
According to the current study's findings, the plants studied have varying levels of antibacterial activity. The inhibitory zone results matched the MIC values for the different plant extracts. The most effective extract was R. graveolens, followed by P. harmala and C. colocynthis. The most sensitive strains of A. xylosoxidans were Gram-negative, with the largest inhibition zone (18.3 mm). The methanolic extract of R. graveolens inhibited all of the bacterial strains tested. S. xylosus demonstrated an inhibiting effect only at a concentration of 2 mg/disc. At the highest dose tested (2 mg), R. graveolens extract demonstrated strong antibacterial activity against A. xylosoxidans, E. amnigenus, S. aureus ATCC 43,300, B. subtilis ATCC 6633, E. aerogenes, and B. cereus ATCC 11,778, with inhibition zones ranging from 14.0 to 18.3 mm. With inhibition zones ranging from 10.3 to 13.3 mm, R. graveolens methanolic extract had moderate antibacterial activity against E. coli, P. aeruginosa ATCC 27,853, Micrococcus spp, S. xylosus, P. aeruginosa, and K. oxytoca. The inhibitory zone results matched the MIC values for R. graveolens extract. This demonstrates that the R. graveolens extract has both scolocidal and bactericidal properties. Because these bacteria are among the most common pathogenic bacteria that increase the risk of secondary infection during hydatid cysts, the results of the R. graveolens extract inhibition zones and MICs are deemed highly promising. These findings are consistent with those of (Pavić et al. 2019), who found that R. graveolens extract had excellent antibacterial activity against gram-positive bacteria such Staphylococcus aureus, Streptococcus pyogenes, Listeria monocytogenes, and Bacillus subtilis. Molnar et al. (Molnar et al. 2018) found that R. graveolens methanolic extracts had good antibacterial activity against E. coli, P. aeruginosa, B. subtilis, and S. aureus, which is consistent with our findings. Rutin, quercetin, isoquinoline, tropane, procyanidin, and hydroxyl benzoic acids are phenolic chemicals found in the aerial portions of R. graveolens that have antibacterial and antifungal properties (Wolters and Eilert 1981). Acridone alkaloids and coumarin, phytochemical substances found in R. graveolens aerial portions, showed the strongest antibacterial action against Gram-positive and Gram-negative bacteria (Ivanova et al. 2005). Flavonoids such as rutinand quercetin, phenolic compounds, alkaloids, and terpenoides isolated from R. graveolens showed antibacterial activity against Staphylococcus aureus and Bacillus subtilis (Amabye 2015), the high antimicrobial activity of this plant may be due to presence of these compounds. The MIC values of R. graveolens extract against all tested bacteria were less than 2 mg/mL, with B. cereus and K. oxytoca having the lowest (0.25 mg/mL) and highest (1.5 mg/mL) MIC values, respectively.
In this study, P. harmala extract was more effective against Gram-positive bacteria than Gram-negative bacteria, with the latter being moderately sensitive to P. harmala extract (Sohrabi et al. 2018; El-Zayat et al. 2021). According to another study, P. harmala chloroform extract has strong antibacterial activity against P. aeruginosa and S. aureus, with an MIC value of 1.56 ug/mL (Hadadi et al. 2020). A flavonoid-rich extract of P. harmala leaves had excellent antibacterial activity against E. coli but not against S. aureus (Fatma et al. 2016). The methanolic extract of P. harmala aerial parts was shown to be high in beta-carboline, harmaline, and harmine in this study. The beta-carboline molecules are one of P. harmala's most potent components (Moloudizargari et al. 2013). Harmane, harmine, harmaline, and harmalol have been found to be bacteriostatic against E. coli, Proteus vulgaris, S. aureus, and B. subtilis (Nenaah 2010). In vivo and in vitro, these core chemicals may be responsible for antibacterial, anti-parasitic, and other biological actions (Doskaliyev et al. 2021). Other chemicals identified from P. harmala extracts, such as catechin, apigenin, rutin, anthraquinone, and flavan, may have diverse biological actions, as demonstrated in various investigations (Allaq et al. 2021; Darabpour et al. 2011; Elansary et al. 2020; Mounira et al. 2021). Most bacteria isolated from hydatid cysts were resistant to P. harmala extract at high concentrations (MIC > 2 mg/ml), while MIC results for different ATCC bacteria ranged between 0.25 and 1.5 mg/mL. The varying quantities of antibiotics provided to afflicted sheep may have contributed to the high resistance of bacteria isolated from hydatid cysts.
C. colocynthis seed extract has been shown to have weak antibacterial activity. However, against Gram-positive bacteria, C. colocynthis seed extract was more efficient than against gram negative bacteria. It was mentioned that all Gram-negative bacteria, as well as S. aureus was resistant to C. colocynthis seed extracts (Bourhia et al. 2021). Gram-positive and Gram-negative bacteria were inhibited by C. colocynthis extracted using high polarity solvents such as water and acetone. Cucurbitacin B, E, and I, among the most potent components of C. colocynthis seed, have been shown to have antibacterial action against Staphylococcus aureus, Bacillus cereus, and K. pneumonia (Ali et al. 2013). Cucurbitacin E has also been shown to have antibacterial properties against M. tuberculosis H37Rvat (Bourhia et al. 2020). The biological activity of plant extracts is dependent on the solvent and extraction method used, and the antimicrobial effectiveness of plant extracts is dependent on the active substances, selected bacterial strains, and plant parts tested, so antimicrobial activity may differ from one bacterium Gram-negative to another Gram-positive (Qaralleh et al. 2019). This low activity could be due to acquired resistance through mutations, or to infected animals' failure to respond to all applicable treatments. The findings of variances in antibacterial outcomes could be related to differences in plant collecting time or phytochemical concentration during season growth (Kumar et al. 2006; Esiyok et al. 2004).
Conclusion
The current study demonstrated the antibacterial activity of R. graveolens, P. harmala and C. colocynthis against pathogenic bacteria isolated from hydatid cysts. The results of this study provide evidence to use and develop naturally occurring agents such as R. graveolens and P. harmala aerial part extracts as antibacterial agents. Scolocidal and antibacterial properties of methanolic extracts of R. graveolens may have the ability to reduce the in vivo appearance of secondary infection in hydatid cysts. This can be applied to protecting and treating humans as well as animals. However, further investigation is required, including studying their toxicity effects of these extracts.
References
Abdullah M, Ali I, Haleem KS, Rehman AU, Qayyum S, Niaz Z, Sultana N (2021) Molecular and biochemical characterization of Echinococcus spp. In hydatid cyst fluid collected from human and livestock in northern khyber pakhtunkhwa and gilgit baltistan. J Anim Plant Sci 31(5):1293–1301
Ahmed CN, Hamad KK, Qadir FA (2019) Haemonchus contortus as a model in assessing activity of Citrullus colocynthis fruit extract to control benzimidazole-resistant parasitic nematodes. ZANCO J Pure Appl Sci 31(5):61–70
Ahmed AB, Ras R, Mahmoud AF, El-Ghazaly E, Widmer G, Dahshan H, Elsohaby I (2021) Prevalence and bacterial isolation from hydatid cysts in dromedary camels (Camelus dromedarius) slaughtered at Sharkia abattoirs. Egypt J Parasit Dis 45(1):236–243
Aitken MM, Jones PW, Hall GA, Hughes DL, Collis KA (1978) Effects of experimental Salmonella dublin infection in cattle given Fasciola hepatica thirteen weeks previously. J Comp Pathol 88(1):75–84
Al-Arabi FY, Mehdi MAH, Farooqui M, Pradhan V (2019) The effect of Aloe vera extracts on the viability of Echinococcus granulosus protoscolices. Int Res J Pharm 10(4):184–189
Al-Asoufi A, Khlaifat A, Tarawneh AA, Alsharafa K, Al-Limoun M, Khleifat K (2017) Bacterial quality of urinary tract infections in diabetic and non diabetics of the population of Ma’an province. Jordan Pak J Biol Sci 20(4):179–188
Ali SA, Dawood KA, Al-Oumashi GB (2012) Hydatidosis of cattle with secondary bacterial invaders. Kufa J Vet Med Sci 3(2):104–110
Ali AA, Alian MA, Elmahi HA (2013) Phytochemical analysis of some chemical metabolites of Colocynth plant [Citrullus colocynthis L.] and its activities as antimicrobial and antiplasmidial. J Basic Appl Sci Res 3(5):228–236
Allaq AA, Sidik NJ, Abdul-Aziz A, Alkamil AMA, Elengoe A, Yahya EB, Abdulsamad MA (2021) Epidemiological studies of the novel Coronavirus (COVID-19) in Libya. Pak J Biol Sci 18(1–2):7–16
Al-Shuneigat JM, Al-Tarawneh IN, Al-Qudah MA, Al-Sarayreh SA, Al-Saraireh YM, Alsharafa KY (2015) The chemical composition and the antibacterial properties of Ruta graveolens L. essential oil grown in Northern Jordan. Jordan J Biological Sci 8(2):139–143
Alzoreky NS, Nakahara K (2003) Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int J Food Microbiol 80(3):223–230
Amabye TG, Shalkh TM (2015) Phytochemical screening and evaluation of antibacterial activity of Ruta graveolens L.—A medicinal plant grown around Mekelle, Tigray, Ethiopia. Nat Prod Chem Res 3:195. https://doi.org/10.4172/2329-6836.1000195
Asgarpanah J, Khoshkam R (2012) Phytochemistry and pharmacological properties of Ruta graveolens L. J Med Plants Res 6(23):3942–3949
Blanton RE, Wachira TM, Zeyhle EE, Njoroge EM, Magambo JK, Schantz PM (1998) Oxfendazole treatment for cystic hydatid disease in naturally infected animals. Antimicrob Agents Chemother 42(3):601–605
Boes J, Helwigh AB (2000) Animal models of intestinal nematode infections of humans. Parasitology 121(S1):S97–S111
Bourhia M, Messaoudi M, Bakrim H, Mothana RA, Sddiqui NA, Almarfadi OM, Benbacer L (2020) Citrullus colocynthis (L.) Schrad: chemical characterization, scavenging and cytotoxic activities. Open Chem 18(1):986–994
Bourhia M, Bouothmany K, Bakrim H, Hadrach S, Salamatullah AM, Alzahrani A, Laglaoui A (2021) Chemical profiling, antioxidant, antiproliferative, and antibacterial potentials of chemically characterized extract of Citrullus colocynthis L. Seeds Sep 8(8):114
Darabpour E, Bavi AP, Motamedi H, Nejad SMS (2011) Antibacterial activity of different parts of Peganum harmala L. growing in Iran against multi-drug resistant bacteria. EXCLI J 10:252
de Carvalho LSA, Queiroz LS, Alves Junior IJ, Almeida AC, Coimbra ES, de Faria Pinto P, Da Silva Filho AA (2019) In vitro schistosomicidal activity of the alkaloid-rich fraction from Ruta graveolens L (Rutaceae) and its characterization by UPLC-QTOF-MS. Evid-Based Complement Altern Med 2:2
De Queiroz AC, Dias T, Da Matta CBB, Cavalcante Silva LHA, de Araújo-Júnior JX, de Araújo GB, Alexandre-Moreira MS (2014) Antileishmanial activity of medicinal plants used in endemic areas in northeastern Brazil. Evid-Based Complement Altern Med 2:2
Dhakad PK, Sharma PK, Kumar S (2017) A review on phytochemical studies and biological potential of Citrullus colocynthis (L.) Schrad (Cucurbitaceae). J Bioeng Biosci 5(4):55–64
dos Santos PRD, de Lima Moreira D, Guimarães EF, Kaplan MAC (2001) Essential oil analysis of 10 Piperaceae species from the Brazilian Atlantic forest. Phytochemistry 58(4):547–551
Doskaliyev A, Seidakhmetova R, Tutai DS, Goldaeva K, Surov VK, Adekenov SM (2021) Alkaloids of Peganum harmala L. and their pharmacological activity. Open Access Maced J Med Sci 9:766–775
Durgadevi PSKS, Saravanan A, Uma S (2019) Antioxidant potential and antitumour activities of Nendran banana peels in breast cancer cell line. Indian J Pharm Sci 81(3):464–473
Elansary HO, Szopa A, Kubica P, Ekiert H, El-Ansary DO, Al-Mana A, Mahmoud EA (2020) Polyphenol content and biological activities of Ruta graveolens L. and Artemisia abrotanum L. in northern Saudi Arabia. Processes 8(5):531
El-Zayat MM, Eraqi MM, Alfaiz FA, Elshaer MM (2021) Antibacterial and antioxidant potential of some Egyptian medicinal plants used in traditional medicine. J King Saud Univ Sci 33(5):101466
Esiyok D, Otles S, Akcicek E (2004) Herbs as a food source in Turkey. Asian Pac J Cancer Prev 5(3):334–339
Fallah M, Kavand A, Mashouf RY (2014) Infected hydatid cysts bacteria in slaughtered livestock and their effects on protoscoleces degeneration. Jundishapur J Microbiol 7(6):e10135
Fatma B, Fatiha M, Elattafia B, Noureddine D (2016) Phytochemical and antimicrobial study of the seeds and leaves of Peganum harmala L. against urinary tract infection pathogens. Asian Pac J Trop Dis 6(10):822–826
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Walton AL (2002) Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137(10):791–797
Hadadi Z, Nematzadeh GA, Ghahari S (2020) A study on the antioxidant and antimicrobial activities in the chloroformic and methanolic extracts of 6 important medicinal plants collected from North of Iran. BMC Chem. 14(1):1–11
Hijjawi NS, Al-Radaideh AM, Rababah EM, Al-Qaoud KM, Bani-Hani KE (2018) Cystic echinococcosis in Jordan: a review of causative species, previous studies, serological and radiological diagnosis. Acta Trop 179:10–16
Ivanova A, Mikhova B, Najdenski H, Tsvetkova I, Kostova I (2005) Antimicrobial and cytotoxic activity of Ruta graveolens. Fitoterapia 76(3–4):344–347
JahedKhaniki GR, Kia EB, Raei M (2013) Liver condemnation and economic losses due to parasitic infections in slaughtered animals in Iran. J Parasit Dis 37(2):240–244
Khleifat K, Abboud M, Al-Shamayleh W, Jiries A, Tarawneh K (2006) Effect of chlorination treatment on gram negative bacterial composition of recycled wastewater. Pak J Biol Sci 9:1660–1668
Khleifat KM, Tarawneh KA, Ali Wedyan M, Al-Tarawneh AA, Al Sharafa K (2008) Growth kinetics and toxicity of Enterobacter cloacae grown on linear alkylbenzene sulfonate as sole carbon source. Currt Microbiol 57(4):364–370
Khleifat KM, Halasah RA, Tarawneh KA, Halasah Z, Shawabkeh R, Wedyan MA (2010) Biodegradation of linear alkylbenzene sulfonate by Burkholderia sp.: effect of some growth conditions. Int J Agr Biol 12:17–25
Kumar VP, Chauhan NS, Padh H, Rajani M (2006) Search for antibacterial and antifungal agents from selected Indian medicinal plants. J Ethnopharm 107(2):182–188
Malekifard F, Keramati F (2018) Susceptibility of protoscoleces of hydatid cyst to various concentrations of oak gall (quercus infectoria olivier) extract at different exposure times in vitro. Zahedan. J Res Med Sci 20:5
Mamedov NA, Pasdaran A, Mamadalieva NZM (2018) Pharmacological studies of Syrian rue (Peganum harmala L., Zygophyllaceae). Int J Second Metab 5(1):1–6
Moazeni M, Larki S, Saharkhiz MJ, Oryan A, AnsaryLari M, MootabiAlavi A (2014) In vivo study of the efficacy of the aromatic water of Zataria multiflora on hydatid cysts. Antimicrob Agents Chemother 58(10):6003–6008
Moazeni M, SaadatyArdakani ZS, Saharkhiz MJ, Jalaei J, Khademolhoseini AA, Abad SE (2017) In vitro ovicidal activity of Peganum harmala seeds extract on the eggs of Fasciola hepatica. J Paras Dis 41(2):467–472
Molnar M, Tomić M, Pavić V (2018) Coumarinyl thiosemicarbazides as antimicrobial agents. Pharm Chem J 51(12):1078–1081
Moloudizargari M, Mikaili P, Aghajanshakeri S, Asghari MH, Shayegh J (2013) Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev 7(14):199
Monteiro DU, Azevedo MI, Weiblen C, Botton SDA, Funk NL, Da Silva CDB, De La Rue ML (2017) In vitro and ex vivo activity of Melaleuca alternifolia against protoscoleces of Echinococcus ortleppi. Parasitology 144(2):214–219
Mounira K, Farah R, Rachida B, Halima A (2021) Preliminary phytochemical screening, quantification of phenolic compounds, of plant extract from Chenopodium quinoa. Alger J Biosci 2(01):42–45
Nabaei M, Mesbah AR, Ghavami H, Saeidinia A, Keihanian F (2014) Effects of Ruta graveolens extract on histopathologic changes in mice livers. Int J Pharm Res Schol 3(2):675–680
Najim TM, Shahooth MA, Khamees AS (2020) Isolation of bacteria associated with hepatic hydatid cyst of iraqi sheep. Eur J Mol Clin Med 7(11):972–976
Nasrieh MA, Abdel-Hafez SK, Kamhawi SA, Craig PS, Schantz PM (2003) Cystic echinococcosis in Jordan: socioeconomic evaluation and risk factors. Parasitol Res 90(6):456–466
Nenaah G (2010) Antibacterial and antifungal activities of (beta)-carboline alkaloids of Peganum harmala (L) seeds and their combination effects. Fitoterapia 81(7):779–782
Niroumand MC, Farzaei MH, Amin G (2015) Medicinal properties of Peganum harmala L. in traditional Iranian medicine and modern phytotherapy: a review. J Tradit Chin Med 35(1):104–109
Oran SA (2014) The status of medicinal plants in Jordan. J Agric Sci Technol A 4:6
Oran SA, Al-Eisawi DM (1998) Check-list of medicinal plants in Jordan. Dirasat 25(2):84–112
Patel MH, Patel AM, Patel SM, Ninama GL, Patel KR, Lavingia BC (2011) Antifungal susceptibility testing to determine mic of amphotericine b, fluconazole and ketoconazole against ocular fungal infection. Nat J Comm Med 2:302–305
Pathak S, Multani AS, Banerji P, Banerji P (2003) Ruta 6 selectively induces cell death in brain cancer cells but proliferation in normal peripheral blood lymphocytes: A novel treatment for human brain cancer. Int J Oncol 23(4):975–982
Pavić V, Flačer D, Jakovljević M, Molnar M, Jokić S (2019) Assessment of total phenolic content, in vitro antioxidant and antibacterial activity of Ruta graveolens L. extracts obtained by choline chloride based natural deep eutectic solvents. Plants 8(3):69
Qaralleh H, Khleifat KM, Al-Limoun MO, Alzedaneen FY, Al-Tawarah N (2019) Antibacterial and synergistic effect of biosynthesized silver nanoparticles using the fungi Tritirachium oryzae W5H with essential oil of Centaurea damascena to enhance conventional antibiotics activity. Adv Nat Sci Nanosci. Nanotechnol 10(2):025016
Rezaee M, Hajighasemi F (2019) Sensitivity of hematopoietic malignant cells to Peganum harmala seed extract in vitro. J Basic Clin Physiol Pharmacol 7(1):21–26
Romero CD, Chopin SF, Buck G, Martinez E, Garcia M, Bixby L (2005) Antibacterial properties of common herbal remedies of the southwest. J Ethnopharm 99(2):253–257
Smyth JD, Barrett NJ (1980) Procedures for testing the viability of human hydatid cysts following surgical removal, especially after chemotherapy. Trans R Soc Trop Med Hyg 74(5):649–652
Sohrabi R, Moghaddam MT, Maghsood AH, Matini M, Moradkhani S, Fallah M (2018) Scolicidal effects of barberry (Berberis vulgaris), wild rue seed (Peganom harmala) and shirazian thyme (Zataria multiflora) extracts on protoscolices of hydatid cysts Zahedan. J Res Med Sci 20:12
Uma C, Sekar KG (2014) Phytochemical analysis of a folklore medicinal plant Citrullus colocynthis L. (bitter apple). Int J Pharmacogn Phytochem Res 2:6
Wang B, Jiang Y, Wang Z, Li F, Xing G, Peng X, Lv H (2015) Arsenic trioxide negatively affects Echinococcus granulosus. Antimicrob Agents Chemother 59(11):6946–6951
Wani I, Bhat Y, Khan N, Mir F, Nanda S, Shah OJ (2010) Concomitant rupture of hydatid cyst of liver in hepatic duct and gallbladder: case report. Gastroenterol Res 3(4):175
Wink M (2012) Medicinal plants: a source of anti-parasitic secondary metabolites. Molecules 17(11):12771–12791
Wolters B, Eilert U (1981) Antimicrobial substances in callus cultures of Ruta graveolens. Planta Med 43(10):166–174
Yones DA, Taher GA, Ibraheim ZZ (2011) In vitro effects of some herbs used in Egyptian traditional medicine on viability of protoscolices of hydatid cysts. Korean J Parasitol 49(3):255
Zeghir-Bouteldja R, Amri M, Aitaissa S, Bouaziz S, Mezioug D, Touil-Boukoffa C (2009) In vitro study of nitric oxide metabolites effects on human hydatid of Echinococcus granulosus. J Parasitol Res 2:1
Ziino G, Giuffrida A, Panebianco A, Bilei S (2009) Bacteria isolated from 25 hydatid cysts in sheep, cattle and goats. Vet Rec 165(8):234–236
Funding
The authors has received no funding for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al Qaisi, Y.T., Khleifat, K.M., Oran, S.A. et al. Ruta graveolens, Peganum harmala, and Citrullus colocynthis methanolic extracts have in vitro protoscolocidal effects and act against bacteria isolated from echinococcal hydatid cyst fluid. Arch Microbiol 204, 228 (2022). https://doi.org/10.1007/s00203-022-02844-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02844-7