Abstract
The present work was designed to isolate and characterise the actinobacteria in the Polar Front region of the Southern Ocean waters and species of Nocardiopsis and Streptomyces were identified. Among those, the psychrophilic actinobacterium, Nocardiopsis dassonvillei PSY13 was found to have good cellulolytic activity and it was further studied for the production and characterisation of cold-active cellulase enzyme. The latter was found to have a specific activity of 6.36 U/mg and a molar mass of 48 kDa with a 22.9-fold purification and 5% recovery at an optimum pH of 7.5 and a temperature of 10 °C. Given the importance of psychrophilic actinobacteria, N. dassonvillei PSY13 can be further exploited for its benefits, meaning that the Southern Ocean harbours biotechnologically important microorganisms that can be further explored for versatile biotechnological and industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antarctica in the South Pole is less studied compared to other habitats concerning microbes. Temperatures in these cold environments ranged from −1 to 4 °C, which is expected to be severe (Deming 2002). In general, extreme conditions lower the diversity of organisms. Hence, this environment remains less explored but can offer vast options to the researchers to study and explore the microbial diversity and ecological functions. Archer et al. (2015) have explored the Antarctic environment to find out the presence of actinobacteria and describe their distribution pattern. Most of the studies have reported on Antarctic ice, benthic region and glacier waters. However, studies on the community of bacterioplankton in the Southern Ocean, mainly, actinobacteria are infrequent compared to the ice and soil reports (Murray and Grzymski 2007). Goodfellow and Haynes (1984) have described several specific isolation strategies to enumerate the actinobacterial population from different habitual environments. The Antarctic environment requires a significant emphasis on the elucidation of actinobacteria towards their distribution and ecological evaluation. The isolation media to be used need to have minimal nutrients with required vitamins and amino acids to promote the growth and multiplication of these organisms.
Interestingly, the microbes from the cold environment are provided with unique mechanisms, which favour their survival in extreme conditions. These special mechanisms aid in producing novel molecules such as cold-active enzymes having biotechnological applications as they possess higher catalytic activities. D’Amico et al. (2003) have reported that the enzymes of the cold-adapted organisms are more active than their mesophilic counterparts. These enzymes show ten times higher activities than the enzymes obtained from terrestrial microorganisms (Margesin and Miteva 2011). As a consequence of these features, microbes have been commonly used for many eco-biotechnology viewpoints (Kasana 2010). Plants primarily consist of cellulosic carbohydrates and are recognized to be the earth’s most reproducible source of energy. Cellulosic components are significantly involved in the global carbon cycle. Cold-active cellulases are responsible for more than 80% of the cellulose recycling in the cryosphere (Grant et al. 2004).
Medie et al. (2011) explained the three types of cellulases, such as endoglucanase, exogluconase and glucosidase, which are are involved in the catalytic breakdown of the cellulose complex. Hayashi et al. (1996) have reported the novel cold-active cellulase from Acremonium alcalophilum, which was active at 40 °C. The enzymes derived from the cold environment had 20–40 °C as the optimum temperatures for higher activities (Zeng et al. 2006). Cellulases are secreted by different types of microorganisms. Psychrophiles are proper candidates for the production of enzymes that are active at low temperatures. Such psychrophilic enzymes are active in alkaline conditions and detergents and therefore have the characteristics to be used in the preparations of laundry additives. In addition, these enzymes can also be used for the bioremediation of biomass-derived from domestic and agricultural practices (Kasana and Gulati 2011). In particular, psychrophilic cellulases have been shown to have higher degradation efficiencies for lignocellulosic biomass and may have enormous potential in the bioenergy sector (Cavicchioli et al. 2011; Bai et al. 2016).
Nevertheless, cellulose is usually converted into bioethanol at relatively high temperatures (50–60 °C) and may, therefore, raise consumption and production costs (Tiwari et al. 2015). Alternatively, cold-active cellulases can be used in the biofuel industry to reduce energy consumption as they have been proven to convert cellulose into ethanol at low temperatures. In addition, cold-active cells can also be used in various sectors, such as pulping in the paper industry, biopolishing in textiles and silage in food and feed formulations (Kasana and Gulati 2011; Bai et al. 2016). Given the importance, the study was intended to identify the potential for cold-active cellulase of actinobacteria isolated from the Southern Ocean's Polar Front region.
Materials and methods
Sample collection
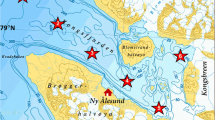
Water samples were obtained from the SOE-7 expedition (SOE-2012–13). The samples were obtained at the Polar Frontal region (PF1: Lat −53.14; Long 47.82, PF2: Lat −56.76; Long 57.72) of the Southern Ocean, using Conductivity/ Temperature/ Depth (CTD), a profile (SEABIRD 911 plus, USA) sampler. Seawater was filtered using a membrane filter (0.22 µm), stored in 20% glycerol suspension, and used to isolate actinobacteria.
Isolation and identification of actinobacteria
A 100 µL of the sample was spread on various media such as Starch Casein Nitrate Agar (SCNA), International Streptomyces Project-2 (ISP2), and Minimal Antarctic Medium (AMM), Actinomycete Isolation Agar (AIA) and Arginine-Vitamin Agar (AV) added with Mycostatin (50 µg/mL) and cycloheximide (50 µg/mL). Later, the plates were kept for incubation at 10 and 20 °C, for the recovery of psychrophilic and psychrotolerant actinobacteria in 60 days (Lo Giudice et al. 2007). Morphologically distinct actinobacterial strains were subcultured on AV agar and subjected to further analyses. The morphological, physiological and chemotaxonomical characteristics were assessed to identify the selected actinobacterial strains (Shirling and Gottlieb 1966; Lechevalier and Lechevalier 1970). The obtained results were verified using Nonomura Key (Nonomura 1974) and justified at the genus level. In addition, the taxonomic position of the strains was studied by sequencing the 16S rRNA gene. Genomic DNA was extracted (Everest et al. 2013) and amplified using the high G+C gram-positive primers (27F-5′-AGAGTTTGATCCTGGCTCAG-3′, 1492R-5′-TACGGCTACCTTGTTACGACTT-3′); the PCR conditions were followed as per the method (Karuppiah et al. 2011). The amplified product was purified using the Qiagen PCR purification kit, followed by sequencing (Applied Biosystems-3100, Macrogen Inc.-Republic of Korea). Two-way sequencing was done and assembled in an EZ-Taxon server, and the closest neighbour details were obtained in BLAST. N-J (Neighbour-joining) algorithm was used to construct the phylogenetic tree to delineate the lineage of the actinobacteria. 1000 replicate bootstrapping was used to evaluate the phylogenetic tree topology.
Cardinal temperature determination
The selected actinobacterial strains were tested for cardinal temperatures to justify the optimum growth temperature to differentiate the psychrophiles from psychrotolerants. The strains isolated at 10 and 20 °C were grown separately in AV liquid medium at various temperatures (0, 5, 10, 15, 20 and 25 °C for psychrophiles and 5, 10, 15, 20, 25 and 30 °C for psychrotolerants, respectively) for 25 days to ascertain their optimal temperature requirements. After incubation, aliquots were plated on AV agar medium and were incubated for 25 days at their respective temperatures to assess the viability of the actinobacteria (Helmke and Weyland 2004). The experiments were conducted as triplicates, and the mean values were reported.
Cold-active cellulase screening
Fresh cultures of the actinobacterial strains were cross streaked on basal mineral salt medium (BSM) (composed of CMC sodium salt 2.0, NaNO3 2.0, K2HPO4 1.0, MgSO4 0.5, KCl 0.5, peptone 0.2 and agar 18 (g/L)) and kept at 10 °C for 25 days. Then, the cellulolytic activities of the strains were detected by flooding with 1% iodine solution (1% iodine in 2% KOI) and were kept for 10 min under room temperature; then, de-stained with 1 M NaCl to visualize the zones of clearance around the actinobacterial colonies. Strains found with potential cellulolytic activities were used for further enzyme production.
Production and purification of cold-active cellulase enzyme
Nocardiopsis sp. spores aseptically collected and suspended in sterile distilled water were used as inoculum for the cold-active cellulase enzyme production. 10 mL of the spore suspension (5.6 × 107 spores/mL) was inoculated in 500 mL of BSM containing 2% CMC (pH 7.0) for submerged fermentation, and the flask was incubated at 10 °C for 25 days by providing with intermittent shaking. After production, the flasks were spun in a centrifuge at 10,000 rpm for 15 min at 4 °C. Cell-free solutions were added with ammonium sulphate (saturated up to 70%) and kept overnight at 4 °C to precipitate the enzyme. The precipitate obtained by centrifugation (9,000 rpm for 10 min at 4 °C) was suspended in 10 mM Tris–HCl buffer (pH 8.0) and kept overnight for dialysis. Then, the dialysate was loaded on a Sephadex column G-50 (2.5 × 50 cm) preequilibrated with 50 mM Tris–HCl buffer (pH 7.5) and the bound enzyme was eluted using the same buffer at 0.2 mL/min flow rate. Fractions with cellulase activity were pooled and precipitated using ammonium sulphate and dialysed with 10 mM Tris–HCl buffer (pH 7.0). Then, the dialysate was loaded on a Fast Flow Q-Sepharose column (1.6 × 10 cm, GE Healthcare), preequilibrated with 50 mM Tris–HCl buffer (pH 9.0). Elution was done using linear-gradient NaCl (0.1–0.5 M) at 0.3 mL/min flow rate. Fractions showing higher cellulase activities were pooled and used for SDS-PAGE analysis.
Enzyme assay and protein determination
Dinitro salicylic acid (DNS) method was employed to test the cellulose hydrolytic activity of the cold-active cellulase on carboxymethyl cellulose (CMC) as a substrate, and glucose was used as the standard (Miller et al. 1960). Enzyme activity was estimated by observing the reducing sugars liberated from the CMC prepared in Tris–HCl buffer (50 mM) at pH 8.0. The reaction was performed by incubating the solution at 25 °C for 10 min and terminated it by adding the DNS solution. The enzyme-treated sample was boiled, cooled and the optical density was measured at 540 nm. The protein concentration of the purified enzyme was determined according to the method of Bradford (1976) using a microplate reader. Bovine Serum Albumin (BSA) was used as the standard to generate the standard curve. The absorbance of the reaction mixture was read at 595 nm. The test was carried out in triplicates, and the mean values were calculated.
Molecular weight determination using SDS-PAGE
The purity and the molar mass of the enzyme were estimated using SDS-PAGE (12% gel). The purified enzyme sample was added with Laemmli sample buffer, boiled for 5 min, and then loaded onto 12% gel. A 6.5–97.4 kDa protein ladder from Genaxy Scientific was used as the molecular weight marker. After electrophoresis, the gel was stained with Coomassie Brilliant Blue (CBB) R-250 (0.25%), and the molecular weight was determined using the Total lab imaging software.
Determination of optimal pH and temperature for activity and stability of the cold-active cellulase
The optimal pH and temperature were determined for enzyme activity and stability as per the standard protocol, using CMC (1%) as the substrate. Different buffer systems were used for different pH values such as pH 3.0–6.0 (citrate buffer-10 mM), pH 6.0–7.5 (sodium phosphate buffer-10 mM), pH 7.5–9.0 (Tris–HCl-10 mM), and pH 9.0–11.0 (glycine–NaOH-10 mM). After incubation at 10 °C for 60 min, the standard DNS method estimated the reducing sugar products. The enzyme was incubated for 60 min at 10 °C as stated above to assess the enzyme’s stability at different pH levels. The effects of temperature on enzyme activity and stability were determined using 1% CMC (10 mM Tris–HCl, pH 8.0) as the substrate. The purified enzyme was added to CMC and was incubated at different temperatures ranging from 0 to 80 °C for 60 min, and finally, DNS solution was added to stop the reaction, and the residual activity was recorded as stated above. Upon determining the optimal temperature, the purified cellulase was incubated on a CMC agar plate at 10 °C for one hour to justify the enzyme activity.
Statistical analysis
All experimental data were subjected to a one-way Analysis of Variance (ANOVA). Dunnett's multiple comparisons (GraphPad Prism v7.0) was also used to determine the difference among means at the level of 0.05.
Results
Isolation and recovery of the actinobacteria from Polar Frontal waters
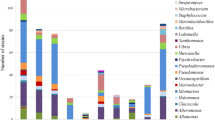
Isolation showed a higher psychrophilic actinobacterial population density (0.3 × 102 CFU mL−1) in the PF1 sample, followed by lower psychrophilic actinobacterial population density (0.2 × 102 CFU mL−1) in the PF2 sample in the AV agar medium. Whereas the psychrotolerant actinobacterial forms were found to be the same (0.6 × 102 CFU mL−1) at the PF1 and PF2 samples in the AV agar medium. The other media could not capture actinobacteria when the AV medium was able to report higher population density (Fig. 1a).
a Extracellular cellulolytic activity of the psychrophilic and psychrotolerant actinobacteria. b Proven cellulolytic activity of the psychrophilic actinobacterium N. dassonvillei PSY13 and strong cellulolytic activity demonstrated by the purified cold-active cellulase enzyme from N. dassonvillei PSY13 at 10 °C in 1 h
Identification of actinobacterial strains
Actinobacterial strains have been identified by conventional and molecular methods. Morphological analysis showed that the cold-loving strains had white, olive green and white yellow aerial mycelia. The PSY and PST 16S genes have been sequenced and deposited in the NCBI GenBank repository (Accession Nos: KY120275, KY120276, KY120277, KY120278, KY120279, KY120280, KY120281, KY120282, KY120283, KY120279). The phylogenetic association of the nine strains is shown in Table 1. All nine strains are phylogenetically classified into actinobacteria and phylogenetic neighbors of PSY13, PSY15, PSY21, PSY25, PST1, PST2, PST3, PST4 and PST5 were found to be Nocardiopsis dassonvillei, N. prasina, N. alba, S. albus, S. albidoflavus, S. exfoliates, S. pactum, S. griseorubens and S. althioticus, respectively (Supplementary Fig. 1).
Effect of temperature on the growth of psychrophilic and psychrotolerant actinobacteria
The ability to adapt to temperature fluctuations allows organisms to survive, if not grow, at temperatures close to the minimums and maximums (Scholze et al. 2021). The strains PSY13, PSY15, PSY21 and PSY25 were observed to grow well at an optimum temperature of 10 °C and are therefore classified as psychrophiles (Fig. 1b). The strains PST1, PST2, PST3, PST4 and PST5 were observed to grow well at an optimum temperature of 20 °C and are therefore classified as psychrotolerants (Fig. 1c). This temperature reliant classification would significantly help discriminate the organisms and design experiments to explore their bioactive potentials. Cavicchioli (2016) has reported the cardinal temperature of the cold loving organisms (psychrophiles) with 5 °C as the minimum and 20 °C as the maximum and 10 °C as the optimum. However, the cardinal temperatures of the cold-tolerant organisms (psychrotolerants) were determined with 10 °C as the minimum and 25 °C as the maximum and 20 °C as the optimum.
Cellulolytic activity
In the cellulolytic screening test, the psychrophilic strain PSY13 was found to possess higher cellulolytic activity than the other strains (Fig. 2a). The cellulose hydrolytic zone measured around 10 cm (Fig. 2b) and thus, justifying the cold-active cellulase potential of the psychrophilic actinobacterial strain in less than an hour.
Purification and molecular weight determination of cold-active cellulase
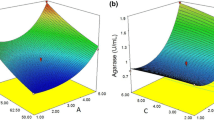
The cold-active enzyme was recovered from the N. dassonvillei PSY13 culture supernatant and was purified as per the steps given in Table 2. The enzyme from the culture supernatant was processed through ammonium sulphate recovery and gel filtration chromatography using Sephadex G-50 followed by ion-exchange chromatography (Q-Sepharose Fast Flow column). The active fraction (0.4 M) was collected from the Q-Sepharose FF fractionation (Fig. 3a) and was concentrated; 5% recovery was achieved with 22.9-fold purification with a specific activity of 6.36 U/mg. The enzyme was further spotted on the CMC agar plate and incubated at 10 °C, and a robust cellulolytic activity was witnessed (Fig. 2b). The purified cold-active cellulase enzyme was observed with a mass of 48 kDa as a distinct band in the SDS-PAGE analysis (Fig. 3b). The mass of the cellulase enzyme was higher than the others (29.7 and 36.6 kDa), and in most of the cases, the cellulase will be having a mass ranging between 20 and 60 kDa (Bai et al. 2017).
Optimal pH and temperature ranges for better stability and activity of the enzyme
Optimal pH for cellulase activity was studied, and a higher activity (>90%) was recorded at pH 7.5 followed by pH 8.0 (80%) and pH 9.0 (70%), respectively. The enzyme was stable and active between pH 6.5 and pH 9.0 (Fig. 4a). The purified cellulase enzyme was found to be stable between 0 and 30 °C, and the optimal working temperature was recorded as 10 °C with nearly 80% of the relative activity (Fig. 4b). However, the relative activity of the cold-active cellulase enzyme was maintained at >70% between the range 10 and 40 °C. Beyond 40 °C, the enzyme’s relative activity reduced gradually due to the instability of the enzyme in the high-temperature range.
Discussion
Pearce et al. (2005) research has suggested that supplementation nutrients in the isolation media would help increase the bacterial population. ISP2 showed no growth among the five-isolation media used, yet it was reported to improve actinobacteria growth in other aquatic and terrestrial counterparts (Lee et al. 2014). Streptomyces were also dominant in the Southern Ocean waters, as in the other environmental counterparts (Kamala et al. 2015). Studies by Lavin et al. (2016)_ENREF_28 have also reported the presence of Streptomyces in the Fildes Peninsula soils of King George Island, Antarctica. Streptomyces usually are present in the oceanic habitats (Ramesh et al. 2006) and are found to be the important member among the actinobacteria, which are prevalent in the cryo environment with 80% recoverability (Babalola et al. 2009). Streptomyces fildesensis sp. nov. and Streptomyces hypolithicus sp. nov. have been recovered successfully from Antarctica and have been described as new species (Le Roes-Hill et al. 2009; Li et al. 2011). Streptomyces are shown to have higher bioactivity as they dominate the environment despite increasing salinity and lowering temperatures under extreme conditions (Encheva-Malinova et al. 2014).
Psychrophilic bacteria contain specific proteins, especially enzymes, which can still function at low temperatures (about 0 °C) (although at a slower rate) (Bowman 2017; Pavankumar et al. 2021). These psychrophilic proteins do not function at normal atmospheric temperatures and cannot grow even at moderate temperatures (Zhang and Gross 2021). Since they are active at low temperatures, psychrophilic bacteria and psychrotrophic bacteria are important decomposers in cold climates (Papale et al. 2018; Misiak et al. 2021). The cold-active cellulase production was justified by the supporting data reported by Buchon et al. (2000), who have also proposed that temperature was highly influencing the cold-adapted microorganisms for the production of cold-active enzymes. As these enzymes have a high biotechnological value, the focus on amylases, esterases, agarases and proteases is increasing. Thus, will pave a way to utilize these novel enzymes in various industrial applications (Tomova et al. 2014). Particularly, cellulases posing cold-active capabilities and obtained from the microbes of Antarctic environments have vast industrial applications viz. food, brewery, feed, paper pulp and so on. In addition, the cellulases have high values as they are abundantly utilised in the production of biofuels in the refining industry (Juturu and Wu 2014).
The cellulase purification can vary depending on the methods and matrix used for purification. Islam and Roy (2018) has purified the cellulase from Bacillus sp. and reported that it has 9.7-fold purification in the CM-cellulose fractionation, which is comparatively low than the present study. Another study has reported 39.1-fold purification attained by the Sephadex G-75 column purification for the cellulase produced by B. vallismortis (Gaur and Tiwari 2015), which is relatively higher than the present study. Pachauri et al. (2017) has also worked on fungal cellulase and reported that he had obtained 14.82-fold purification with a 25.8% yield. As mentioned in the previous statement, the purification fold and the specific activity of the enzymes depends significantly on the matrix/resin used in the purification process and thus helps to unfold the novel enzyme activity (Kumar et al. 2018).
The enzyme was stable and active between pH 6.5 and 9.0 (Fig. 4a). Beyond this range, the enzyme was not stable, and the activity was reduced. This was in agreement with the previous report, which showed relatively higher activity at pH 7.5 (Kim et al. 2009). More recently, Shajahan et al. (2017) have reported the cellulase produced by B. licheniformis. NCIM 5556 was stable and active between pH 4.5 and 9.5, and the enzyme was highly active at pH 6.5. However, cellulase produced by the psychrotolerant yeast had a different optimal pH of 6.4 and thus, reported to be highly stable and active in that range (Carrasco et al. 2016).
The higher catalytic nature of the cold-active enzymes (<25 °C) makes them novel biological catalysts. Li et al. (2016) have reported the maximum activity of cellulase obtained at 10.4 °C and in the present study, cold-active cellulase exhibited a strong cellulolytic activity at 10 °C. In general, most cold-active enzymes have been reported to have a temperature optimum of 20–40 °C. However, enzymes having higher catalytic activities (80%) at 10 °C are considered novel (Santiago et al. 2016). Such low-temperature dependency of enzymes makes them useful in various beverage and food industries where the process is performed under low temperatures. Therefore, the cold-active enzymes hold more than 80% of the market share. It is known that different types of actinomycetes belonging to a wide range of habitats and active in different environmental conditions produce hemicellulolytic enzymes (Suriya et al. 2016). Attempts can be made to reduce the production cost of these enzymes through the use of highly efficient actinomycetal enzyme systems with a wider range of tolerance and activity in various environmental conditions.
Psychrophilic enzymes are usually characterized by a higher degree of structural flexibility, lower thermal stability, and higher specific activity at low temperatures than their mesophilic counterparts (Georlette et al. 2004). In particular, several mechanisms are used to increase the flexibility and activity of enzymes, as well as to decrease thermal stability, and not all mechanisms are applicable to this psychrophilic protein (Brininger et al. 2018; Collins and Margesin 2019). It's possible that this enhanced adaptability will reduce activation energy while also boosting the substrate turnover rate since it increases complementarity between the catalytic site and the substrate. Cold-active microbial enzymes offer significant biotechnological potential in a variety of areas, including detergents, food and beverage processing, and textiles, and can be employed for specialized applications in biology / molecular research, medicines, and diagnostics.
Conclusion
Actinobacterial strains were isolated from the Southern Ocean's Polar Frontal Waters. Nocardiopsis and Streptomyces were identified; among all the strains studied, the psychrophilic strain N. dassonvillei PSY13 showed a higher hydrolysing activity and their cold-active cellulase found to have specific activity of 6.36 U/mg and a molar mass of 48 kDa with 7.5 and 10 ºC as the optimum pH and temperature respectively.
References
Archer SDJ, Mcdonald IR, Herbold CW et al (2015) Benthic microbial communities of coastal terrestrial and ice shelf Antarctic meltwater ponds. Front Microbiol 6:1–11. https://doi.org/10.3389/fmicb.2015.00485
Babalola OO, Kirby BM, Le Roes-Hill M et al (2009) Phylogenetic analysis of actinobacterial populations associated with Antarctic Dry Valley mineral soils. Environ Microbiol 11:566–576. https://doi.org/10.1111/j.1462-2920.2008.01809.x
Bai X, Yuan X, Wen A et al (2016) Cloning, expression and characterization of a cold-adapted endo-1, 4-β-glucanase from Citrobacter farmeri A1, a symbiotic bacterium of Reticulitermes labralis. PeerJ 4:e2679. https://doi.org/10.7717/peerj.2679
Bai H, Zi H, Huang Y et al (2017) Catalytic properties of carboxymethyl cellulase produced from newly isolated novel fungus Penicillium ochrochloron ZH1 in submerged fermentation. Catal Lett 147:2013–2022. https://doi.org/10.1007/s10562-017-2119-0
Bowman JP (2017) Genomics of psychrophilic bacteria and archaea. In: Margesin R (ed) Psychrophiles from biodivers to biotechnology, 2nd edn. Springer, Cham, pp 345–387
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brininger C, Spradlin S, Cobani L, Evilia C (2018) The more adaptive to change, the more likely you are to survive: protein adaptation in extremophiles. Semin Cell Dev Biol 84:158–169. https://doi.org/10.1016/J.SEMCDB.2017.12.016
Buchon L, Laurent P, Gounot AM, Guespin-Michel JF (2000) Temperature dependence of extracellular enzymes production by psychrotrophic and psychrophilic bacteria. Biotechnol Lett 22:1577–1581. https://doi.org/10.1023/A:1005641119076
Carrasco M, Villarreal P, Barahona S et al (2016) Screening and characterization of amylase and cellulase activities in psychrotolerant yeasts. BMC Microbiol 16:21. https://doi.org/10.1186/s12866-016-0640-8
Cavicchioli R (2016) On the concept of a psychrophile. ISME J 10:793–795. https://doi.org/10.1038/ismej.2015.160
Cavicchioli R, Charlton T, Ertan H et al (2011) Biotechnological uses of enzymes from psychrophiles. Microb Biotechnol 4:449–460. https://doi.org/10.1111/j.1751-7915.2011.00258.x
Collins T, Margesin R (2019) Psychrophilic lifestyles: mechanisms of adaptation and biotechnological tools. Appl Microbiol Biotechnol 1037(103):2857–2871. https://doi.org/10.1007/S00253-019-09659-5
D’Amico S, Marx JC, Gerday C, Feller G (2003) Activity-stability relationships in extremophilic enzymes. J Biol Chem 278:7891–7896. https://doi.org/10.1074/jbc.M212508200
Deming JW (2002) Psychrophiles and polar regions. Curr Opin Microbiol 5:301–309. https://doi.org/10.1016/S1369-5274(02)00329-6
Encheva-Malinova M, Stoyanova M, Avramova H et al (2014) Antibacterial potential of streptomycete strains from Antarctic soils. Biotechnol Biotechnol Equip 28:721–727. https://doi.org/10.1080/13102818.2014.947066
Everest GJ, le Roes-Hill M, Omorogie C et al (2013) Amycolatopsis umgeniensis sp. nov., isolated from soil from the banks of the Umgeni River in South Africa. Antonie Van Leeuwenhoek 103:673–681. https://doi.org/10.1007/s10482-012-9851-7
Gaur R, Tiwari S (2015) Isolation, production, purification and characterization of an organic-solvent-thermostable alkalophilic cellulase from Bacillus vallismortis RG-07. BMC Biotechnol 15:19. https://doi.org/10.1186/S12896-015-0129-9
Georlette D, Blaise V, Collins T et al (2004) Some like it cold: biocatalysis at low temperatures. FEMS Microbiol Rev 28:25–42. https://doi.org/10.1016/J.FEMSRE.2003.07.003
Goodfellow M, Haynes JA (1984) Actinomycetes in marine sediments. Biological, biochemical, and biomedical aspects of actinomycetes. Elsevier, Orlando, pp 453–472
Grant S, Sorokin DY, Grant WD et al (2004) A phylogenetic analysis of Wadi el Natrun soda lake cellulase enrichment cultures and identification of cellulase genes from these cultures. Extremophiles 8:421–429. https://doi.org/10.1007/s00792-004-0402-7
Hayashi K, Nimura Y, Ohara N et al (1996) Low-temperatureactive cellulase produced by Acremonium alcalophilum JCM 7366. Seibutsu-Kogaku Kaishi 74:7–10
Helmke E, Weyland H (2004) Psychrophilic versus psychrotolerant bacteria–occurrence and significance in polar and temperate marine habitats. Cell Mol Biol (Noisy-le-grand) 50:553–61
Islam F, Roy N (2018) Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC Res Notes 11:445. https://doi.org/10.1186/s13104-018-3558-4
Juturu V, Wu JC (2014) Microbial cellulases: Engineering, production and applications. Renew Sustain Energy Rev 33:188–203. https://doi.org/10.1016/J.RSER.2014.01.077
Kamala K, Sivaperumal P, Gobalakrishnan R et al (2015) Isolation and characterization of biologically active alkaloids from marine actinobacteria Nocardiopsis sp. NCS1. Biocatal Agric Biotechnol 4:63–69. https://doi.org/10.1016/j.bcab.2014.10.005
Karuppiah V, Aarthi C, Sivakumar K (2011) Enhancement of PCR amplification of actinobacterial 16S rRNA gene using an adjuvant, dimethyl sulphoxide. Curr Sci 101:22–23
Kasana RC (2010) Proteases from psychrotrophs: an overview. Crit Rev Microbiol 36:134–145. https://doi.org/10.3109/10408410903485525
Kasana RC, Gulati A (2011) Cellulases from psychrophilic microorganisms: a review. J Basic Microbiol 51:572–579. https://doi.org/10.1002/jobm.201000385
Kim B-K, Lee B-H, Lee Y-J et al (2009) Purification and characterization of carboxymethylcellulase isolated from a marine bacterium, Bacillus subtilis subsp. subtilis A-53. Enzyme Microb Technol 44:411–416. https://doi.org/10.1016/j.enzmictec.2009.02.005
Kumar B, Bhardwaj N, Alam A et al (2018) Production, purification and characterization of an acid/alkali and thermo tolerant cellulase from Schizophyllum commune NAIMCC-F-03379 and its application in hydrolysis of lignocellulosic wastes. AMB Express 8:173. https://doi.org/10.1186/s13568-018-0696-y
Lavin PL, Yong ST, Wong CMVL, De Stefano M (2016) Isolation and characterization of Antarctic psychrotroph Streptomyces sp. strain INACH3013. Antarct Sci 28:433–442. https://doi.org/10.1017/S0954102016000250
Le Roes-Hill M, Rohland J, Meyers PR et al (2009) Streptomyces hypolithicus sp. nov., isolated from an Antarctic hypolith community. Int J Syst Evol Microbiol 59:2032–2035. https://doi.org/10.1099/ijs.0.007971-0
Lechevalier MP, Lechevalier H (1970) Chemical composition as a criterion in the classification of aerobic actinomycetes. Int J Syst Bacteriol 20:435–443. https://doi.org/10.1099/00207713-20-4-435
Lee L, Zainal N, Azman A et al (2014) Diversity and antimicrobial activities of actinobacteria isolated from tropical mangrove sediments in Malaysia. Sci World J. https://doi.org/10.1155/2014/698178
Li J, Tian X-P, Zhu T-J et al (2011) Streptomyces fildesensis sp. nov., a novel streptomycete isolated from Antarctic soil. Antonie Van Leeuwenhoek 100:537–543. https://doi.org/10.1007/s10482-011-9609-7
Li D, Feng L, Liu K (2016) Optimization of cold-active CMCase production by psychrotrophic Sphingomonas sp. FLX-7 from the cold region of China. Cellulose 23:1335–1347. https://doi.org/10.1007/s10570-016-0859-4
Lo Giudice A, Brilli M, Bruni V et al (2007) Bacterium-bacterium inhibitory interactions among psychrotrophic bacteria isolated from Antarctic seawater (Terra Nova Bay, Ross Sea). FEMS Microbiol Ecol 60:383–396. https://doi.org/10.1111/j.1574-6941.2007.00300.x
Margesin R, Miteva V (2011) Diversity and ecology of psychrophilic microorganisms. Res Microbiol 162:346–361. https://doi.org/10.1016/J.RESMIC.2010.12.004
Medie F, Vincentelli R, Drancourt M, Henrissat B (2011) Mycobacterium tuberculosis Rv1090 and Rv1987 encode functional β-glucan-targeting proteins. Protein Expr Purif 75:172–176. https://doi.org/10.1016/j.pep.2010.08.015
Miller GL, Blum R, Glennon WE, Burton AL (1960) Measurement of carboxymethylcellulase activity. Anal Biochem 1:127–132. https://doi.org/10.1016/0003-2697(60)90004-X
Misiak M, Goodall-Copestake WP, Sparks TH et al (2021) Inhibitory effects of climate change on the growth and extracellular enzyme activities of a widespread Antarctic soil fungus. Glob Chang Biol 27:1111–1125. https://doi.org/10.1111/GCB.15456
Murray AE, Grzymski JJ (2007) Diversity and genomics of Antarctic marine micro-organisms. Philos Trans R Soc Lond B Biol Sci 362:2259–2271. https://doi.org/10.1098/rstb.2006.1944
Nonomura H (1974) Key for classification and identification of 458 species of the streptomycetes included in ISP. J Ferment Technol 52:8–92
Pachauri P, Aranganathan V, More S et al (2017) (2017) Purification and characterization of cellulase from a novel isolate of Trichoderma longibrachiatum. Biofuels. https://doi.org/10.1080/17597269.2017.1345357
Papale M, Conte A, Mikkonen A et al (2018) Prokaryotic assemblages within permafrost active layer at Edmonson Point (Northern Victoria Land, Antarctica). Soil Biol Biochem 123:165–179. https://doi.org/10.1016/J.SOILBIO.2018.05.004
Pavankumar TL, Mittal P, Hallsworth JE (2021) Molecular insights into the ecology of a psychrotolerant Pseudomonas syringae. Environ Microbiol 23:3665–3681. https://doi.org/10.1111/1462-2920.15304
Pearce DA, van der Gast CJ, Woodward K, Newsham KK (2005) Significant changes in the bacterioplankton community structure of a maritime Antarctic freshwater lake following nutrient enrichment. Microbiology 151:3237–3248. https://doi.org/10.1099/mic.0.27258-0
Ramesh S, Jayaprakashvel M, Mathivanan N (2006) Microbial status in seawater and coastal sediments during pre- and post-tsunami periods in the Bay of Bengal, India. Mar Ecol 27:198–203. https://doi.org/10.1111/j.1439-0485.2006.00110.x
Santiago M, Ramírez-Sarmiento CA, Zamora RA, Parra LP (2016) Discovery, molecular mechanisms, and industrial applications of cold-active enzymes. Front Microbiol 7:1408. https://doi.org/10.3389/fmicb.2016.01408
Scholze C, Jørgensen BB, Røy H (2021) Psychrophilic properties of sulfate-reducing bacteria in Arctic marine sediments. Limnol Oceanogr 66:S293–S302. https://doi.org/10.1002/LNO.11586
Shajahan S, Moorthy IG, Sivakumar N, Selvakumar G (2017) Statistical modeling and optimization of cellulase production by Bacillus licheniformis NCIM 5556 isolated from the hot spring, Maharashtra, India. J King Saud Univ - Sci 29:302–310. https://doi.org/10.1016/j.jksus.2016.08.001
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340. https://doi.org/10.1099/00207713-16-3-313
Suriya J, Bharathiraja S, Manivasagan P, Kim SK (2016) Enzymes from rare actinobacterial strains. Adv Food Nutr Res 79:67–98. https://doi.org/10.1016/BS.AFNR.2016.08.002
Tiwari R, Nain PKS, Singh S et al (2015) Cold active holocellulase cocktail from Aspergillus niger SH3: process optimization for production and biomass hydrolysis. J Taiwan Inst Chem Eng 56:57–66. https://doi.org/10.1016/J.JTICE.2015.04.026
Tomova I, Gladka G, Tashyrev A, Vasileva-Tonkova E (2014) Isolation, identification and hydrolytic enzymes production of aerobic heterotrophic bacteria from two Antarctic islands. Int J Environ Sci 4:614–625
Zeng R, Xiong P, Wen J (2006) Characterization and gene cloning of a cold-active cellulase from a deep-sea psychrotrophic bacterium Pseudoalteromonas sp. DY3. Extremophiles. https://doi.org/10.1007/s00792-005-0475-y
Zhang Y, Gross CA (2021) Cold shock response in bacteria. Annu Rev Genetics 55:377–400
Acknowledgements
The Science and Engineering Research Board—Department of Science and Technology (DST-SERB), National Postdoctoral Fellowship Scheme (N-PDF) (Grant number PDF/2016/003905) and the Department of Biotechnology (DBT) (Grant number BT/PR5426/AAQ/3/599/2012), Government of India, have supported the work. The author (A.M.A) thanks for being funded by the Researchers Supporting Project Number (RSP-2021/293) King Saud University, Riyadh, Saudi Arabia.
Funding
The Science and Engineering Research Board—Department of Science and Technology (DST-SERB), National Postdoctoral Fellowship Scheme (N-PDF) (Grant number PDF/2016/003905) and the Department of Biotechnology (DBT) (Grant number BT/PR5426/AAQ/3/599/2012), Government of India, have supported the work. The author (A.M.A) thanks for being funded by the Researchers Supporting Project Number (RSP-2021/293) King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sivasankar, P., Poongodi, S., Sivakumar, K. et al. Exogenous production of cold-active cellulase from polar Nocardiopsis sp. with increased cellulose hydrolysis efficiency. Arch Microbiol 204, 218 (2022). https://doi.org/10.1007/s00203-022-02830-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02830-z