Abstract
In this study a novel fungus, Penicillium ochrochloron ZH1 was isolated from soil and identified by 18S rDNA gene sequence technology. Various process parameters affecting CMCase production were optimized through one factor at a time. Results revealed that maximum enzyme production was obtained when medium was supplemented with 3% rice straw powder, 0.4% ground nut meal as nitrogen source, 0.050% CaCl2, with medium pH of 3.0 and 8% inoculum at 30 °C for 144 h of fermentation period. The purified CMCase had molecular weight of 29.7 kDa determined by SDS-PAGE. CMCase had pH and optima of 5.0 and 40 °C and retained 90% CMCase activity after 3 h at 50 °C. Ca2+ and K+ stimulated CMCase activity while Cu, Co and Ca2+ found to be inhibitors of CMCase. The purified CMCase had V max of 18.18 mg/ml/min and K m of 18.45 mg/ml for CMC as substrate specificity.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The success of exciting applications of microbial cellulose depends on the ability to produce cellulase economically. Optimization of media for high productivity will release the strain on cost of cellulase production which ultimately allows high application in various fields [1]. Lignocellulose could be utilized by conversion through cellulolytic enzymes. Cellulose is degraded by combined of endo-β-1,4-glucanase (EC 3.2.1.4), cellobiohydrolase (EC 3.2.1.91) and β-glucosidase (EC 3.2.1.21). All these three enzymes act in synergistic way on cellulose and broken down into simpler sugar like glucose [2].
Fungi can be easily grown in a medium comprising inorganic nutrients and a protein source for healthy growth and spore formation [3]. In recent years, cellulolytic and hemicelulolytic fungal and bacterial cultures are extensively studied for utilizing forest waste, sugarcane bagasse, avicel, xylan, rice straw, CMC, lichenan and wheat straw as a source of cellulose. Among these microbial cultures, fungus strains in the genus Aspergillus, Trichoderma, Penicillium and Fusarium are extensively studied because of their higher activities [4]. This study was planned to screen and identification of potential novel cellulolytic fungus and further production optimization, purification and characterization of carboxymethyl cellulase in submerged fermentation.
2 Materials and Methods
2.1 Isolation and Screening of Cellulolytic Fungus
Samples were taken from soil of changbaishan natural reserve areas and haystack field. All samples were collected in sterilized polyethylene bags, labeled and stored at 4 °C for further analytical work. Samples were incubated at 28 °C in enrichment medium [5] for 7 days in triplicates. Sterilized potato dextrose agar (PDA) plates were used for isolation purpose and all isolates after purification were kept on PDA slants at 4 °C. Then the palm fiber medium [6], filter paper medium [7], CMC medium [8] and avicel medium [9] for screening purpose using plating method. To enable clearly visible transparent hydrolytic ring, we have adopted a double-plate method: first covered with a layer of transparent solid basic agar medium in a petri dish, and then wait until covered with a thin layer of solidified mechanized containing cellulose media, both to ensure the screening of selective media, but not so difficult to filter strain needed. The strain were streaked on medium plates and incubated at 30 °C for 5 days and then 0.5% congo red stain was flooded on plates for 15 min and washed with 1 M NaCl solution to remove excess dye. The appearance of clear zone around colonies showed positive result for cellulase production.
2.2 Molecular Identification Isolated Fungus
The isolated strain was morphologically identified by fungal identification manual. For further identification, 18S rDNA gene sequencing was done from genomic DNA of fungal strain by universal primer pair ITS 1/ITS 4 using the fungal specific primer set amplified The ITS region of fungal DNA : ITS 1 (TCCGTAGGTGAACCTGCGG) and ITS 4 (TCCTCCGCTTATTGATATGC). PCR reactions were performed as per manufacturer’s instructions (TIANGEN, Beijing, China). Sequencing of the amplified product done through Sangon Biotech limited Shanghai China. The sequence was compared by the BLAST program for the identification of the species from the National Center for Biotechnological Information (NCBI) [10].
2.3 Enzyme Assay
The activity of cellulase was estimated by measuring the release of reducing sugars. The clear liquid obtained after centrifugation was used as the crude enzyme source. Carboxymethyl cellulase (CMCase) was estimated using 1.0 ml substrate (1% CMC in citrate buffer pH 4.8) was added in 1 ml of enzyme extract and incubated at 50 °C for 30 min. Adding 2.0 ml DNS stopped the reaction and reducing sugars were estimated [11]. One enzyme unit is described as 1.0 μg of glucose released from substrate per minute by specific amount of enzyme.
2.4 Optimization of Parameters on Cellulase Production
Various process parameters such as fermentation time (24–168 h), initial medium pH (3.0–8.0), incubation temperature (22–41 °C), inoculum size (2–12% substrate), CaCl2 concentration (0.05–0.150%), different carbon source (rice hull power, straw power, peanut straw, maize straw, soybean straw, avicel, filter paper, cotton, CMC) and different nitrogen sources (NaNO3, (NH4)2SO4, KNO3, Peanut meal, peptone and yeast extract) were optimized for maximum production of cellulases by Penicillium ochrochloron ZH1 in submerged fermentation.
2.5 Enzyme Purification
The whole purification procedure and protein determination was adopted from our earlier reports [12].
2.6 Characterization of Cellulase
The optimum temperature of the purified CMCase from Penicillium ochrochloron ZH1 were determined by setting temperature range from 30 to 80 °C. Thermal stability of the purified CMCase was checked by pre-incubating enzyme solution at various temperatures for 4 h. After that residual activity was measured using DNS method. To test the effect of pH, each reaction was performed in appropriate buffer (containing 1% w/v of CMC) that adjusted pH to tested condition. Optimum pH of the purified CMCase was determined by preparing CMC in different pH buffers (0.05 M citrate buffer pH 3.0, 4.0, 5.0 0.05 M phosphate buffer pH 6.0 and 7.0, 0.05 M Tris-HCl buffer pH 8.0). pH stability was measured by incubating enzyme with respective pH buffers for 24 h at room temperature (25 °C). After that enzyme activity was measured as per standard method. The effect of metal ions on the CMCase activity was checked using 10 mM (final concentration) of metal ions (Na+, Cu2+, Fe3+, K+, Zn2+, Mn2+, Co2+ and Ca2+). the enzyme activity was measured by DNS method. Substrate specificity of the CMCase was also determined using various substrates such as microcrystalline cellulose, carboxymethylcellulose, salicin, filter paper, p-NPC and chitin. This test was performed in similar way as described in previous sections.
2.7 Enzyme Kinetic Parameters
Kinetic parameters like K m and V max of the purified CMCase were determined by Lineweaver–Burk plot between 1/[S] versus 1/[V]. Substrate (CMC) concentrations of 2–10 mg/ml was used for this purpose.
2.8 Statistical Analysis
Each experiment was conducted in triplicates and the values mentioned were in mean values. The analysis of variance (ANOVA) was done using the SPSS software version 11.5 (SPSS Inc., Chicago, IL, USA).
3 Results and Discussion
3.1 Identification of Fungal Strain
Most fungal isolates have been identified using traditional taxonomy keys and macroscopic observations [13]. Therefore, in this study, the strain was inoculated on PDA medium and cultured for 5 days at 28 °C for morphological identification. The colonial morphology was coarse with a neat edge and close. Sporangia were spherical or pyriform, and the spores were gray green. These observations indicated that this cellulase-producing fungus was a Penicillium sp. As per widely used method of 18S rRNA analysis [14], we sequenced the 18S rRNA of this cellulase-producing strain. The 18S rRNA sequence of the fungus was 551 bp long. Homology analysis showed that the sequence similarity of this strain to some Penicillium species exceeded 99% (Fig. 1). Phylogenetic tree was constructed by MEGA6.06 showed that the strain was closely related to Penicillium ochrochloron strain IHB F 2914, with a similarity of 99%. Based on the evolutionary distances and the phylogenetic tree resulting from the partial sequencing of Penicillium sp. gene and neighbor-joining, this strain was identified as a Penicillium sp.
3.2 Effect of Carbon Source on Enzyme Production
The presence of different carbon source (rice hull powder, rice straw powder, peanut straw, maize straw, soybean straw, avicel, filter paper, cotton, CMC),significantly influenced the production of the enzyme. The results showed that maximum cellulase activity was observed with rice straw powder as carbon source (Fig. 2a). Cellulose, lactose and sawdust was potentially reported for cellulase production as carbon source by Penicillium sp [15]. Han et al. [16] reported wheat straw (carbon source) for cellulase production by Penicillium waksmanii F10-2. Pumpkin oil cake has been reported as potential substrate by Penicillium roqueforti for cellulase production under solid state fermentation [17].
3.3 Effect of Substrate Concentration on Cellulase Production
In case of native Penicillium ochrochloron, maximum enzyme production was achieved when straw powder (3%) was used as sole carbon source in fermentation medium. It was also found that as the concentration of straw powder increased, enzyme production decreased drastically (Fig. 2b).This decreased in enzyme production was in fact due to the substrate feedback inhibition which activated due to the high concentration of rice straw powder which might be due to the high viscosity of growing medium resulting in improper circulation; hence low fungal growth and enzyme production This indicated that substrate (carbon source) significantly affects the microbial growth [18].
3.4 Effect of Nitrogen Source on Enzyme Production
The results stated that strain exhibit ability to utilize various organic nitrogen sources efficiently, and the maximum enzyme activity was observed when KNO3 was used as nitrogen source. However, the enzyme activity was almost zero when inorganic nitrogen sources (urea) were used as the sole nitrogen sources (Fig. 2c). This suggested that medium composition and environmental conditions had strong influence on enzyme production by various strains [19]. Inorganic nitrogen sources can fulfil the demand of cellulase production by T. reesei [20]. Yeast extract proved to a suitable source of nitrogen for cellulase production by Penicillium sp [15] and Penicillium fellutanum [21]. Some studies suggested that peptone and ammonium nitrate was best nitrogen sources for cellulase production by Penicillium waksmanii F10-2 [17] and Penicillium K-p strain [22].
3.5 Effect of Ratio of Organic and Inorganic Nitrogen Source
Nitrogen has a significant impact on the growth and secretion of metabolites of microorganisms [23]. Microbes can directly use the organic carbon in the amino acid and other organic nitrogen compounds in various structures of the carbon skeleton to synthesize organism required protein, vitamins and trace elements and other substances. However, inorganic nitrogen source as sole nitrogen source, as nitrogen is gradually utilized for cell growth, pH value of the medium also fluctuate to inhibit microbial enzyme. However, due to the inorganic nitrogen was first utilized by the microorganisms more easily, and often as a fast-acting nitrogen source. So choose peanut powder and potassium nitrate were used as organic nitrogen and inorganic nitrogen sources, according to a certain proportion with the use of better [24]. Filter results optimum nitrogen source is shown in Fig. 2d, when the organic nitrogen and inorganic nitrogen ratio of 2:3, cellulase activity was the highest ability.
3.6 Effect of Different Concentration of Nitrogen Source on Cellulase Production
Different level of enzyme production from Penicillium ochrochloron found at different concentrations of nitrogen sources in fermentation medium. Various concentrations of nitrogen sources were added in separate fermentation medium and best enzyme yield was achieved when 0.4% nitrogen sources was incorporated in the fermentation medium. On other hand, media having more nitrogen sources concentration beyond 0.4% showed decreased in enzyme production and continue to decrease as the nitrogen sources concentration of medium increased (Fig. 2e). It was also reported that beside carbon and nitrogen sources some other factors such as temperature, pH, different salt concentrations are also affected the maximum production of enzymes [25].
3.7 Effect of CaCl2 Concentration on Cellulase Production
Effect of CaCl2 concentration on cellulase production During current study, it was found that as the concentration of CaCl2 increased in fermentation medium, increased in enzyme production was observed and maximum enzyme production was achieved at 0.05% (Fig. 2f). supplementation of 0.5% CaCO3 concentration in the fermentation medium favored maximum production of cellulase [26].This positive effect of calcium chloride on enzyme production and stabilization might be due to nature of CaCl2 which in the liquid state splits to provide bioavailable calcium and chloride ions which resists against pH change during fermentation process and keep the enzyme intact and stable. Pachauri et al. [27] reported that 1.932 mg/ml CaCl2 favored Trichoderma longibrachiatum to produce cellulase in solid state fermentation using sugarcane bagasse as a substrate. Addition of 3mM calcium chloride in fermentation medium favored cellulase production by Aspergillus terreus [28].
3.8 Effect of Initial pH on Cellulase Production
The optimal pH varies with different microorganisms and enzymes, for fungal cellulase in most cases it ranges from 3.0 to 6.0. In the present study, the optimum pH for maximum cellulase production was reported at pH 3 (Fig. 2g). Moreover, it was found that when the pH level increased or decreased than the optimum pH, the enzyme production was reduced. Initial medium pH of 5.0 was favorable for CMCase production by Penicillium sp [15]. Kathiresan and Manivannan [21] reported initial medium pH of 6.5 was good for cellulase production by Penicillium fellutanum. Karthikeyan et al. [22] suggested that some strains of Pencillium sp produced cellulase under acidic pH (3.0).
3.9 Effect of Inoculum on Cellulase Production
To study the effect of inoculum size, initial concentration of inoculum was adjusted to 2, 4, 6, 8, 10 and 12%. As shown in Fig. 2h, inoculum concentration of 8% gave better cellulase activity. Increased concentration of inoculum resulted decreased enzyme production, which might affect the log phase of growth. Inoculum size of 5% has been reported for cellulase production in solid state fermentation by Trichoderma viride FBL1 [29].
3.10 Effect of Temperature on Cellulase Production
Temperature is a crucial parameter that affects both growth and cellulase production. The temperature normally employed in SFP is in the range of 25–35 °C [10, 30]. In the present study, optimal temperature for maximum cellulase production by the Penicillium ochrochloron strain was reported at 30 °C (Fig. 2i), and reduced at higher or lower temperature. In accordance with our research, Prasanna et al. [15] stated that incubation temperature of 30 °C was optimum for maximum production of CMCase by Penicillium sp in submerged fermentation.
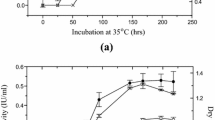
3.11 Effect of Incubation Time
Incubation time was tested in range from 48 to 168 h and peak enzyme production was noted at 144 h (Fig. 2j). Enzyme production becoming increased with increase in growth period, and further increased from 144 h resulted reduced enzyme yield. The decreased enzyme production due to increased time period might be due to the death of cells, denaturation or variation in pH [31].
3.12 Purification of CMCase
The crude CMCase enzyme was fractionated by ammonium sulphate followed by dialysis and size-exclusion chromatography. The specific activity of CMCase was increased from 468.85 to 956.07 U/mg during purification process (Table 1). Purification fold of 2.04 was achieved with yield of 10.63 using Sephadex G-100 column chromatography. Active fractions (9–21) having CMCase activity (Fig. 3) were pooled and further checked for purity and molecular weight determination by SDS-PAGE. CMCase fraction (Nos. 16–21) having activity was pooled and concentrated, then subjected to SDS-PAGE and CMC-zymogram for molecular size determination (Fig. 4). SDS-PAGE revealed single band showing homogeneity and approximate molecular weight of about 29.7 kDa. Cellulases produced by most of the fungi are monomeric having molecular masses range from 20 to 60 kDa [32, 33]. Sajith et al. [34] reported low molecular weight (17 kDa) endoglucanase from Penicillium verruculosum BS3.
3.13 Characterization of Cellulase
3.13.1 Effect of pH on Activity and Stability
To find the optimum pH of purified CMCase, the reaction (containing 1% w/v of CMC) was performed in different pH range. Maximum enzyme activity was recorded at pH 5.0 and retained more than 80% activity over pH range of 4.0–5.0 (Fig. 5). CMCase enzyme produced from Penciliium simplicissimum H 11 exhibited optimum pH of 3.2 and stability in wide range of pH 2.8–5.6 [12]. The endoglucanase produced from Penicillium pinophilum MS20 had optimum pH of 5.0 and stable is wide range of pH 4–7.0 [35]. CMCase produced from Penicillium funiculsoum exhibit optimum pH of 4.0 [36]. Picart et al. [37] reported optimum pH of 4.5 for carboxymethyl cellulase produced from Penicillium sp. CR316 and remained stable at this pH for 3 h.
3.13.2 Effect of Temperature on Activity and Stability
The purified CMCase had optimum temperature of 50 °C. For stability study, more than 80% activity retained in the range of 40–50 °C. The purified enzyme retained 50% activity at 60 °C (Fig. 6). The enzyme was stable in temperature range of 30–50 °C for 4 h. CMCase produced from Penciliium simplicissimum H 11 had optimum temperature of 60 °C and stability at 50 °C for 4 h [12]. Optimum temperature of 65 °C for CMCase has been reported from Penicillium funiculsoum [36]. Thermophilic cellulase had been reported from Penicillium sp. CR316 having optimum temperature of 65 °C and activity remained stable at 65 °C for 3 h [38].
3.13.3 Effect of Metal Ions
Several metal ions affected the activity of CMCase at various levels (Table 2). At 10 mmol/l concentration K+ and Ca2+ stimulated CMCase by 110%. Ca 2+ was found to be activator of cellulases from fungal origin [38, 39] which ultimately enhances the substrate-binding affinity. In contrast, 10 mmol/l Cu 2+, Mn2+ and Co2+ were potent inhibitors of cellulase. Similar kind of inhibitory effects of these metal ions have been reported from results of Picart et al. [36]. Bai et al. [12] also reported that Cu 2+ strongly inhibit the CMCase activity.
3.13.4 Substrate Specificity
The substrate specificity analysis of purified CMCase (Table 3) revealed high preference for CMC as substrate and showed moderate activity on MCC and filter paper. The purified CMCase had no activity against p-NPC, salicin or chitin. Picart et al. [36] also assessed the substrate specificity of crude CMCase produced from Penicillum sp. CR316 showed similar results. CMCase produced from Penciliium simplicissimum H 11 had activity against microcrystalline cellulose and CMC [12].
3.14 Kinetics of CMCase
The kinetic parameters Km and Vmax of the purified CMCase enzyme were determined using Lineweaver–Burk double reciprocal plots with various concentrations of CMC as substrate (2–10 mg/ml) (Fig. 7). The CMCase showed a maximum velocity (Vmax) of 18.18 mg/ml/min and a Michaelis–Menten constant (Km) of 18.45 mg/ml against CMC as substrate. Bai et al. [12] reported Km and Vmax of 14.881 mg/ml and 0.364 mg/ml/min for CMCase from Penciliium simplicissimum H 11 in submerged fermentation. Pol et al. [35] described K m and V max of 4.8 mg/ml and 72.5 U/mg using carboxymethyl cellulose as substrate for CMCase from Penicillium pinophylum MS20.
References
Mohite BV, Kamalja KK, Patil SV (2012) Cellulose 19:1655–1666
Watanabe H, Tokuda G (2010) Annu Rev Entomol 55:609–632
Mandels M, Reese ET (1999) J Ind Microbiol Biotechnol 22:225–240
Ahmad A, Vermette P (2008) Biochem Engin J 40:399–407
Yang L (2013) Screening of strains for degrading cellulose and cellulose degradation capability of mixed bacteria. North West Agriculture and Forestry University
Qu Y (2011) J Shandong Univ 46(10):161–162
Yin Z (2010) Screening on straw cellulose degrading Strain and preliminary study on degradation of straw. The Chinese Academy of Agricultural Sciences
Yang W, Meng F, Peng J (2014) Electronic J Biotechnol 17:262–267
Qi F, Zhang S, Gao P (1999) J Shandong Univ 34(4):484–485
El Bergadi F, Laachari F, Elabed S (2014) Ann Microbiol 64:815–822
Irfan M, Gulsher M, Abbas S (2011) Songklanakarin J Sci Technol 33(4):397–404
Bai H, Wang H, Sun J, Irfan M, Han M, Huang Y, Han X, Yang Q (2013) Bioresources 8(3):3657–3671
Wei J (1979) Fungi identification manual, vol 780. Shanghai Science and Technology Press, Shanghai
Varga I, Poczai P, Cernák I (2014) SpringerPlus 3:569
Prasanna HN, Ramanjaneyulu G, Reddy BR (2016) 3 Biotech 6:162
Pericin D, MaðarevPopovic S, RaduloviPopovic L, Škrinjar M (2008) Roum Biotechnol Lett 13(4):38153820
Han L, Feng J, Zhu C, Zhang X (2009) Afr J Biotechnol 8(16):3879–3886
Mandels M, Reese ET (1985) Dev Ind Microbiol 5:5–20
Rajoka MI (2004) Electron J Biotechnol 7:256–263
Rodriguez-Gomez D, Hobley TJ (2013) World J Microbiol Biotechnol 29:2157–2165
Kathiresan K, Manivannan S (2006) Res J Microbiol 1:438–442
Karthikeyan N, Sakthivel M, Palani P (2010) J Ecobiotechnol 2/10:4–7
Soni R, Nazir A, Chadha BS (2010) Ind Crops Prod 31:277–283
Liu HQ, Feng Y, Zhao DQ, Jiang JX (2012) Biodegradation 23:465–472
Gautam SP, Bundela PS, Pandey AK, Khan J, Awasthi MK, Sarsaiya S (2011) Biotechnol Res Int 2011:810425
Tanskul S, Amornthatree K, Jaturonlak N (2013) Carbohydrate Polym 92:421–428
Pachauri P, Sullia SB, Deshmukh S (2016) J Sci Ind Res 75:181–187
Shahriarinour M, Wahab MNA, Mohamad R, Mustafa S, Ariff AB (2011) Afr J Biotechnol 10(38):7459–7467
Irfan M, Syed Q, Yousaf M, Nadeem M, Baig S, Jafri SA (2010) Acad Arena 2(7):18–30
Sajith S, Sreedevi S (2014) Ann Microbiol 64:763–771
Krishna C (2005) Critical Rev Biotechnol 25:1–30
Yang R, Li J, Teng C (2016) J Mol Catal B Enzy 131:85–93
Asha BM, Sakthivel N (2014) Ann Microbiol 64:1839–1848
Sajith S, Sreedevi S, Priji P, Unni K, Benjamin S (2015) British Microbiol Res J 9(1):1–12
Pol D, Laxman RS, Rao M (2012) Indian J Biochem Biophy 49:189–194
Karboune S, Geraert PA, Kermasha S (2008) J Agr Food Chem 56:903–909
Picart P, Diaz P, Pastor FIJ (2007) Lett Appl Microbiol 45:108–113
Boonchuay P, Takenaka S, Kuntiya A (2016) J Mol Catal B Enzy 129:61–68
Rong Y, Zhang L, Chi Z, Wang X (2015) J Ocean Univ China 14:213–921
Acknowledgements
The authors thanks to the technical staff of the microbial biotechnology laboratory, College of Land and Environmental science, Shenyang Agricultural University China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bai, H., Zi, H., Huang, Y. et al. Catalytic Properties of Carboxymethyl Cellulase Produced from Newly Isolated Novel Fungus Penicillium ochrochloron ZH1 in Submerged Fermentation. Catal Lett 147, 2013–2022 (2017). https://doi.org/10.1007/s10562-017-2119-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2119-0